Editor—We read with interest the correspondences by Cubillos and colleagues1 and Au Yong and Chen2 describing two different barrier enclosure designs that attempt to reduce the exposure risk of aerosolised severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in healthcare providers performing aerosol-generating medical procedures. Gould and colleagues3 questioned the merit of these enclosure devices that increased the difficulty in managing the airway and lacked any mechanism to safely remove or clean the barrier enclosures without dispersing high concentrations of aerosolised SARS-CoV-2.
Based on industrial local exhaust ventilation systems that effectively evacuate hazardous particulate matter away from workers in occupations such as surgery,4 a similar evacuation system was recently described.5 A commercially available, disposable adult size oxygen face tent was repurposed and connected to suction to form an aerosol evacuation system. This aerosol evacuation system showed qualitative effectiveness in removing a continuous stream of visible aerosolised saline droplets generated during simulated passive breathing. However, tracheal extubation is a different challenge for healthcare providers as coughing occurs in ∼40%6 of patients undergoing extubation.
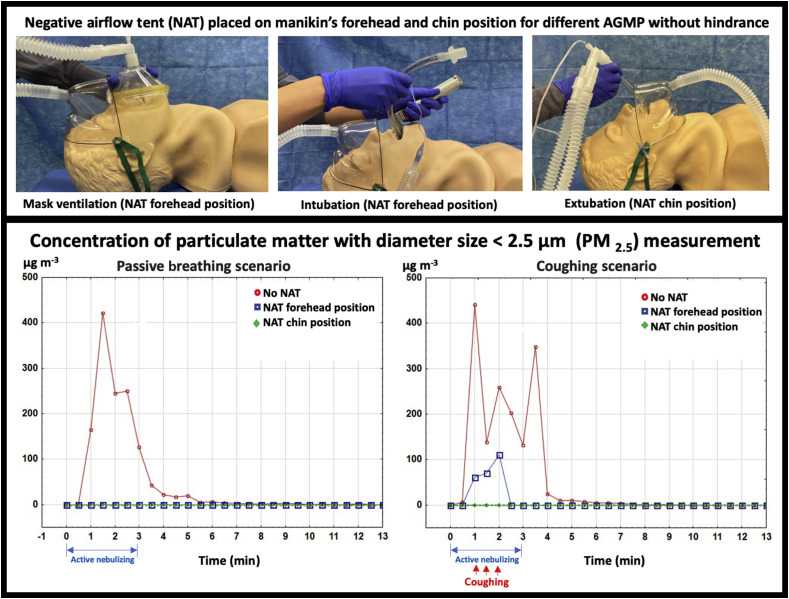
We therefore sampled surrounding air particle concentrations in both simulated passive breathing and coughing scenarios. As shown in Fig 1 and supplementary online video, the same commercially available disposable oxygen face tent was adapted (Salters face tent; Salter Labs, Arvin, CA, USA) and connected to a closed biohazardous smoke evacuation system (Neptune 3, Stryker, Kalamazoo, MI, USA) with an internal high efficiency particulate air (HEPA) filter. This high-efficiency waste management system commonly found in operating rooms can capture aerosolised particles as small as 0.1 μm with 99.99% efficiency with suction power up to 25 ft3 min−1 air exchange. The face tent was placed in one of two positions on an adult high-fidelity airway manikin: below the manikin's chin (chin position) or on top of the manikin's forehead (forehead position). Visible aerosolised saline was introduced into the manikin with the use of a nebuliser (Airlife Misty Max 10 disposable nebuliser; Carefusion, San Diego, CA, USA) and 8 L min−1 of oxygen to simulate passive breathing. A forceful cough was simulated by a 1.8-L resuscitator bag (Hudson RCI, Teleflex, Wayne, PA, USA) that was rapidly emptied over 1 s with the nebulised saline through the airway of the manikin. Assuming the manikin trachea is cylindrical, the air velocity of the cough is calculated as the air volume generated per unit of time divided by the cross-sectional area of the trachea. Assuming laminar flow with a manikin tracheal diameter of 1.5 cm, air velocity of the simulated cough with rapid emptying of 1.8 L over 1 s is about 10 m s−1 (i.e. 1.8 L s−1 ÷ [3.14×(0.75 cm)2]), which is slightly lower than the reported maximum human cough air velocity (∼11.7 m s−1).7
Fig 1.
Simulation illustration and aerosol concentration measurements. The negative airflow face tent evacuation system was placed on an airway manikin in two positions, on the forehead and below the chin. A forceful cough was simulated using an aerosol nebuliser while simultaneously emptying a resuscitator bag. The face tent was attached to a high-efficiency smoke evacuation system which, when turned on, evacuated the visible aerosol into the face tent and away from the simulated healthcare provider (see video). (Top) Different negative airflow tent positions for aerosol-generating medical procedures (AGMP). (Bottom) Results of particle concentration (μg m−3) measurements of particulate matter with diameter size <2.5 μm (PM2.5).
Supplementary video related to this article can be found at https://doi.org/10.1016/j.bja.2020.06.009.
The following are the supplementary data related to this article:
The particle concentration (μg m−3) of particulate matter with diameter <2.5 μm (PM2.5)8 was measured using a particle counter (Digital PM2.5 Air Quality Detector, Geekcreit, Banggood, Guangzhou, China) at the level of the head of the manikin and 2 ft above the manikin's head to approximate the height of a healthcare provider performing an aerosol-generating procedure. To simulate a tracheal intubation scenario with passive breathing, measurements were taken every 30 s for 13 min with the following sequence: (a) at time 0, nebuliser activation for 3 min to simulate passive exhalation during manual ventilation and intubation; and (b) at time 3 min, nebuliser deactivation to simulate a secured airway. To simulate three coughs within 1 min, measurements were also taken in the following sequence: (a) at time 0, nebuliser activation; (b) at time 1 min, first rapid emptying of the resuscitator bag with ongoing nebuliser to simulate coughing; (c) at time 1.5 min, second rapid emptying of the resuscitator bag; (d) at time 2 min, last rapid emptying of the resuscitator bag; and (e) nebuliser deactivation at time 3 min to simulate control of the airway with a face mask. Both sequences of measurements were performed with and without the negative airflow tent in both the forehead and chin positions.
In the absence of the negative airflow tent, aerosolised saline was released into the surrounding environment as expected (Fig 1). With active suction and the face tent in the forehead position during passive breathing, the PM2.5 remained near ambient levels. However, considerable spikes in PM2.5 that rapidly subsided were seen during the cough scenario. These spikes may be attributable to the direction and speed of the airflow from coughing that overwhelmed the suction capacity at the tested suction distance away from the mouth with the negative airflow tent on the forehead. In contrast, when the negative airflow tent was placed below the chin during the cough scenario, no spikes in PM2.5 were encountered and PM2.5 levels remained at baseline. These results suggest the importance of minimising coughing on induction when the negative airflow tent is on the forehead. The negative airflow tent should also be positioned below the chin for extubation to capture aerosols generated by coughs more effectively. Furthermore, the negative airflow tent on the forehead may be uncomfortable for an awake patient and at risk for manual dislodgement by a semiconscious patient.
The adaptability of negative airflow tent positioning allows for flexibility during various aerosol-generating procedures such as tracheal intubation and extubation. The negative airflow tent is comprised of a transparent soft plastic material with a behind-the-neck strap for a secure fit that allows for quick adjustments to accommodate mask ventilation and intubation without hindering performance. The negative airflow tent can also be advanced inferiorly to act as an additional physical barrier to prevent ‘forceful droplets’ from reaching the healthcare provider.9 This evacuation system is an improved alternative to static barrier enclosures to enhance the safety of healthcare providers performing aerosol-generating procedures without compromising patient care. Nonetheless, personal protective equipment (PPE) should remain the main defence during the coronavirus disease 2019 (COVID-19) pandemic.3 With the threat of a potential second wave of infection as the world reopens,10 any additional protective measures should not be overlooked. However, such measures should not trade off patient safety or create further exposure risks to healthcare providers after use.
Declarations of interest
The authors declare that they have no conflicts of interest.
References
- 1.Cubillos J., Querney J., Rankin A., Moore J., Armstrong K. A multipurpose portable negative air flow isolation chamber for aerosol-generating procedures during the COVID-19 pandemic. Br J Anaesth. 2020 doi: 10.1016/j.bja.2020.04.059. Advance Access published on April 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Au Yong P.S., Chen X. Reducing droplet spread during airway manipulation: lessons from the COVID-19 pandemic in Singapore. Br J Anaesth. 2020;125:e176–e178. doi: 10.1016/j.bja.2020.04.007. Advance Access published on April 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gould C., Alexander P., Allen C., McGrath B., Shelton C. Protecting staff and patients during airway management in the COVID-19 pandemic. Br J Anaesth. 2020;125:e294–e295. doi: 10.1016/j.bja.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fencl J.L. Guideline implementation: surgical smoke safety. AORN J. 2017;105:488–497. doi: 10.1016/j.aorn.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Tsui B.C.H. Re-purposing a face tent as a disposable aerosol evacuation system to reduce contamination in COVID-19 patients: a simulated demonstration. Can J Anesth. 2020:1–3. doi: 10.1007/s12630-020-01687-4. Advance Access published on April 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tung A., Fergusson N.A., Ng N., Hu V., Dormuth C., Griesdale D.G.E. Pharmacological methods for reducing coughing on emergence from elective surgery after general anesthesia with endotracheal intubation: protocol for a systematic review of common medications and network meta-analysis. Syst Rev. 2019;8:32. doi: 10.1186/s13643-019-0947-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chao C.Y.H., Wan M.P., Morawska L. Characterization of expiration air jets and droplet size distributions immediately at the mouth opening. J Aerosol Sci. 2009;40:122–133. doi: 10.1016/j.jaerosci.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang H.-K., Mo F., Ma C.-G. Evaluation of fine particles in surgical smoke from an urologist’s operating room by time and by distance. Int Urol Nephrol. 2015;47:1671–1678. doi: 10.1007/s11255-015-1080-3. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y., Ning Z., Chen Y. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020 doi: 10.1038/s41586-020-2271-3. Advance Access published on April 27. [DOI] [PubMed] [Google Scholar]
- 10.Xu S., Li Y. Beware of the second wave of COVID-19. Lancet. 2020;395:1321–1322. doi: 10.1016/S0140-6736(20)30845-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.