Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has caused a pandemic coronavirus disease-19 (COVID-19) that began in Wuhan city, China, in December 2019. Till 14th April, 19,39,801 people have been affected by this virus, of whom 1,20,897 died. Though respiratory symptoms are the typical manifestation of this disease, gastrointestinal (GI) symptoms such as anorexia, nausea, vomiting, loss of taste sensation, diarrhea, abdominal pain, and discomfort have been reported. The pooled prevalence of GI symptom is 17.6% (95% confidence interval, 12.3%–24.5%), as indicated in a meta-analysis. A few studies suggested that the presence of GI symptoms is associated with poorer prognosis. The virus is excreted in feces during the acute disease, and even after, the nasopharyngeal swab has become negative for viral ribonucleic acid. Fecal viral excretion may have clinical significance because of possible feco-oral transmission of the infection. Nearly, 10.5%–53% of patients with COVID-19, particularly those with severe disease, have been shown to have an elevation of hepatic enzymes though biochemical and clinical jaundice are uncommon. Knowledge about this disease in general and GI involvement, in particular, is currently evolving.
Keywords: coronavirus disease-19, pandemic, hepatitis, diarrhea, betacoronavirus
Abbreviations: ACE2, Angiotensin Receptor 2; CI, Confidence Interval; COVID-19, Coronavirus Disease-19; GI, Gastrointestinal; RNA, Ribonucleic Acid; RT-PCR, Real-time Polymerase Chain Reaction; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2; TMPRSS, Transmembrane Serine Protease
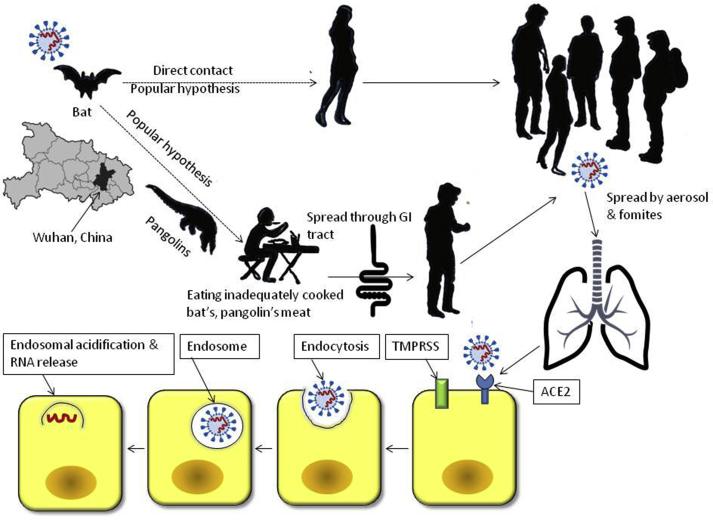
On January 7th, 2020, a novel coronavirus was named and identified as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by the International Committee on Taxonomy of Viruses.1 The current pandemic of coronavirus disease-19 (COVID-19) began in Wuhan city, China, in December 2019.1 Scientists from Wuhan Institute of Virology, Wuhan, China, initially identified the SARS-CoV-2 by metagenomic analysis of bronchoalveolar lavage fluid from a patient with pneumonia.2,3 This virus is an RNA virus belonging to the family of β coronavirus and can cause minor illnesses such as common cold and fatal conditions such as pneumonia and severe acute respiratory syndrome.1 The virus has been characterized, and it has three subtypes: A, B, and C.4 “A” subtype is the ancestral type like bat coronavirus. “A” and “C” subtypes are found in the USA and Europe, and “B” subtype is predominant in East Asia.4 The prevalent hypothesis suggested that this virus came from the bat, possibly through pangolins,5 both of which are consumed in China even in undercooked states. If this hypothesis is correct, it suggests the gastrointestinal (GI) tract be a route for transmission. Subsequently, the infected patients can transmit the virus in the community through fomites and aerosol (Figure 1).
Figure 1.
Schematic diagram showing transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and mechanism of the disease. TMPRSS, transmembrane serine protease; ACE2, angiotensin receptor 2.
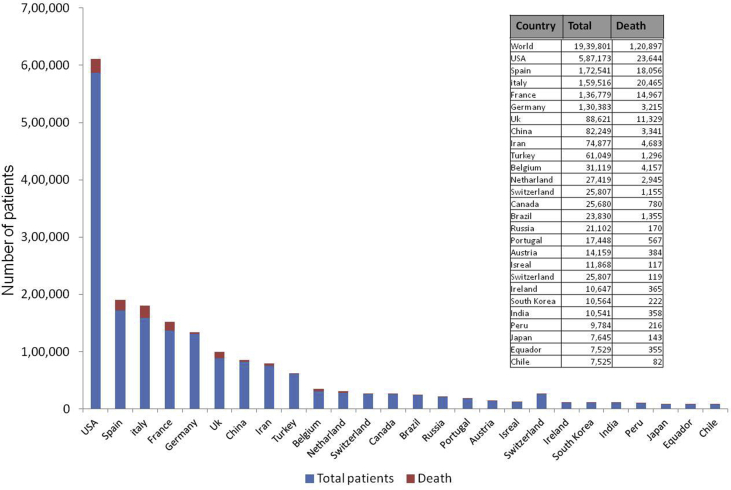
The COVID-19 outbreak has become a pandemic and has led to severe consequences to the global economy.1 More than 1,20,897 people died of coronavirus to date in the world, and more than 19,39,801 people have been affected till 14th April 2020. The mortality of coronavirus is currently 3%–6%. Figure 2 shows the country-wise number of COVID-19 patients and the number of death as of now, though it is increasing every day. Though respiratory symptoms are the most common manifestations of SARS-CoV-2, digestive system involvement is not uncommon.1 This review attempts to give an overview of the spectrum of gastrointestinal system involvement in these patients and its clinical, epidemiological, and public health importance. It is crucial mentioning that the understanding of this disease is currently evolving; a lot is yet to be understood.
Figure 2.
Number of patients (country-wise) and number of death due to coronavirus disease-19 (COVID-19) as on 14th April 2020. (Source: https://www.worldometers.info/coronavirus/?fbclid=IwAR3rf9f1jh4koadadNl6BouRYBaPumhwHPDdbnInjQ5MHfx1HD_Pml8lcig)
GI symptoms in COVID-19 patients
GI symptoms such as nausea, vomiting, anorexia, abdominal pain and discomfort, and diarrhea are common among patients with COVID-19, and their frequency varies widely between 1% and 98%.6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17 Table 1 lists the frequency of these symptoms, as reported in the different studies. In a meta-analysis of 60 studies, including 4243 patients, the pooled prevalence of all gastrointestinal symptoms was 17.6% (95% confidence interval [CI], 12.3%–24.5%).15 Though a few studies suggested that the patients with GI symptoms may have more severe disease and even worse prognosis, the same was not substantiated in this meta-analysis (pooled prevalence in severe disease: 17.1% [95% CI 6.9%–36.7%] vs. in less severe disease: 11.8% [95% CI, 4.1%–29.1%]).15 Since SARS-CoV-2 (also called 2019-nCoV) is largely homologous to an earlier virus (SARS-CoV; 79% sequence identified by next-generation sequencing)18 causing a similar outbreak in 2003 in which 20%–25% had diarrhea,19 frequent occurrence of diarrhea in COVID-19 is not unexpected. One recent study from the USA showed gastrointestinal symptoms to be a predictor of the positive test for SARS-CoV-2 RNA in the nasopharyngeal swab.16
Table 1.
Studies Reporting Digestive System Involvement in Patients With Coronavirus Infection 19 (COVID 19).
| Author, year | Total number of patients | Number & nature of GI symptoms |
|---|---|---|
| Chan JFW, 20206 | 6 | Diarrhea: 2 (33%) |
| Huang C, 20207 | 38 | Diarrhea: 1 (3%) |
| Chen N, 20208 | 99 | Diarrhea: 2 (2%) Nausea and vomiting: 1 (1%) |
| Luo S, 20209 | 1141 | Nausea 134 (73%) Vomiting 119 (65%) Abdominal pain 45 (25%) Diarrhea 68 (37%) Loss of appetite 180 (98%) Both nausea and vomiting 37 (20%) Both abdominal pain and diarrhea 16 (9%) All symptoms 12 (7%) |
| Holshue ML, 202010 | 1 | Nausea, vomiting, diarrhea, abdominal discomfort: 1 (100%) |
| Guan W, 202011 | 1099 | Nausea and vomiting: 55 (5%) Diarrhea 42 (3.8%) |
| Jin X, 202012 | 651 | At least one of the following symptoms: nausea, vomiting, diarrhea: 74 (11.4%) |
| Pan L, 202013 | 204 | Anorexia: 83 (83.8%) Diarrhea: 29 (29.3%) Vomiting: 8 (0.8%) Abdominal pain: 4 (0.4%) |
| Lechien JR, 202014 | 417 | Loss of taste 88% Diarrhea 50% Abdominal pain 30% Nausea, vomiting 22% |
| Cheung KS, 202015 | 59 | 15 (25.4%) |
| Nobel YR, 202016 | 278 | Nausea, vomiting, diarrhea 97 (35%) |
| Cholankeril G, 202017 | 116 | Any GI Symptoms 37 (31.9) Nausea and/or vomiting 12 (10.3) Diarrhea 12 (10.3) Nausea/Vomiting and Diarrhea 5 (4.3) Abdominal Pain 10 (8.8) Loss of Appetite 22 (25.3) |
GI, gastrointestinal.
VIRAL excretion in feces and its implication on transmission
In an initial study from China on 1070 clinical samples from 205 patients with COVID-19, the frequency of detection of SARS-CoV-2 RNA by real-time polymerase chain reaction (RT-PCR) was 14/15 (93%), 72/104 (72%), 5/8 (63%), 6/13 (46%), 126/398 (32%), 44/153 (29%), 3/307 (1%), and 0 (0%) for bronchoalveolar lavage fluid, sputum, nasal swabs, fiberoptic bronchoscope brush biopsy, pharyngeal swabs, feces, blood, and urine specimens, respectively. Quite a few studies showed that stool samples were more often positive for SARS-CoV-2 RNA in patients with than without diarrhea. The meta-analysis showed a pooled prevalence of stool RNA positivity 48.1% (95% CI, 38.3%–57.9%), even after respiratory specimens were negative in about 70% of patients. Viral excretion in these patients maybe even longer than its presence in the nasopharyngeal samples.20 In a Chinese study on 74 patients in whom both fecal and nasopharyngeal samples were studied by RT-PCR, in 41/74 patients, fecal samples showed the presence of SARS-CoV-2 RNA by RT-PCR; fecal samples remained positive 11·2 ± 9·2 longer than the respiratory samples.20
Based on the above findings that showed the presence of SARS-CoV-2 RNA in multiple clinical samples, the possibility of numerous routes transmission cannot be ignored.21 The popularly believed hypothesis that the virus initially came from the Wuhan wild animal market suggested that the initial human infection occurred because of eating of an uncooked or undercooked bat and pangolin meat, also gave an impression of GI route of transmission and not respiratory route. Hence, multiple studies documenting fecal excretion of the virus are not unexpected. Fecal excretion may have public health importance as it may suggest possible feco-oral transmission of the virus. Moreover, prolonged fecal excretion, even in asymptomatic patients, may contribute to disease burden and potentially infectious carrier pool in the society.22 Feco-oral transmission is well known in several human viral diseases such as poliovirus and hepatitis E virus.23,24 SARS-CoV-2 is also known to survive in the environment for days.25 Hence, prolonged SARS-CoV-2 excretion in the feces may have public health consequences because of possible feco-oral transmission.26 Another exciting aspect of the fecal excretion is the fact that in the future if attenuated virus vaccine can be developed, this may spread the attenuated virus strain in the community leading to wide-spread immunity against the virus in the population. Such herd immunity is one of the mechanisms by which dreaded poliomyelitis has been eradicated in India. Suggestion for such a herd immunity leading to protection against the virus and prevention of severe disease due to SARS-CoV-2 infection has already been hypothesized. However, most vaccines being developed currently against this virus are parenteral mRNA, which is unable to provide such immunity.
Molecular pathogenesis of digestive symptoms in COVID-19
The data on GI tissue involvement in COVID-19 patients are scanty; it might be partly related to the fact that because of the risk of transmission of the virus, nonemergency endoscopy in these patients has been recommended to be withheld. Gastrointestinal endoscopy and multiple biopsies have been performed from the esophagus, stomach, duodenum, and rectum in one patient with COVID-19 that showed normal histology except mild lymphocytic and plasma cell infiltration and interstitial edema.27 Angiotensin receptor 2 (ACE2) was not much expressed in esophageal epithelium though it was abundant in the glandular epithelia. Nucleocapsid protein of the SARS-CoV-2 was found in the cytoplasm of gastric, duodenal, and rectum epithelium but not in the esophagus.27
The receptors for the entry of the virus inside the cell are host cellular transmembrane serine protease and ACE2; the latter is shown to be expressed in the esophageal, small intestinal, and colonic epithelial cells in addition to the pulmonary epithelial cells.28 As ACE2 is known to control intestinal inflammation, its involvement by the virus has been hypothesized to cause diarrhea.29 It has been suggested that once infected by the virus, enterocyte damage may lead to increased GI permeability and malabsorption causing entry of the pathogens resulting in diarrhea30; however, more studies are needed on this issue.
Hepatic involvement in COVID-19 patients
Table 2 lists the available studies on hepatic involvement in patients with COVID-19.7,8,11,17,31, 32, 33, 34, 35, 36, 37, 38 As evident from Table 2, 10.5%–53% of patients with COVID-19 have an elevation of hepatic enzymes though biochemical and clinical jaundice are uncommon (total bilirubin elevated in 5–18% patients). A few studies also reported a reduction in serum albumin, suggesting impairment in the synthetic function of the liver. It is important to note that 2–3% of patients in these series had preexisting liver disease. Patients with more severe COVID-19 had liver function test abnormalities more often. ACE2, to which the SARS-CoV-2 binds before entering into the host cells, is present both in the hepatocytes and cholangiocytes, more in the latter.39 Because cholangiocytes play an essential role in hepatic regeneration and immune response, it has been hypothesized that bile duct epithelial cells may play more role in hepatic injury due to SARS-CoV-2 infection than the cholangiocytes.40 Patients with severe COVID-19 may have sepsis and hypotension, which are well-known contributors to hepatic injury. Patients with severe COVID-19 have associated cytokine storm, which may also contribute to the hepatic injury. Moreover, several drugs used in COVID-19 patients may add to hepatic injury. Several medications, such as chloroquine, hydroxychloroquine, azithromycin, other antibiotics, anti-viral drugs such as lopinavir/ritonavir, and corticosteroids used in the treatment of patients with COVID-19 may also contribute to the liver injury. Since the pandemic of SARS-CoV-2 infection started recently in November 2019, whether it can cause chronic liver disease is not yet known. Data on liver histology in patients with COVID-19 are scanty. Recently, postmortem liver biopsy in a patient who died of COVID-19 has been reported.41 The histology only showed moderate microvascular steatosis and mild lobular and portal activity. More studies on the mechanism of liver injury in patients with COVID-19 are needed.
Table 2.
Studies Reporting a Liver Injury in Patients With Coronavirus Infection 19 (COVID 19).
| Author, year | Total number of patients | Proportion with preexisting liver disease | Manifestations |
|---|---|---|---|
| Guan W, 202011 | 1099 | 23 (2.3%) | Elevation of AST 168/757 (22.2%) ALT 158/741 (21.3%) TB 76/722 (10.5%) |
| Cai Q, 202031 | 298 | 8 (2.7%) | 44 (14.8%) More in patients with severe disease (36.2%) than those with mild disease (9.6%) |
| Fan Z, 202032 | 148 | NA | 75 (50.7%) |
| Wang D, 202033 | 138 | 4 (2.9%) | Mild AST elevation, particularly in patients with severe disease |
| Chen N, 20208 | 99 | NA | Abnormality in ALT 28 (28%) AST 35 (35%) TB 18 (18%) Low albumin 97 (98%) |
| Shi H, 202034 | 81 | 7 (9%) | 43 (53%) |
| Xu X, 202035 | 62 | 7 (11%) | 10 (16.1%) |
| Yang X, 202036 | 52 | NA | 15 (29%) |
| Huang C, 20207 | 41 | 1 (2%) | 15 (31%) |
| Zhang B, 202037 | 82 | 2 (2.4%) | 64 (78%) |
| Huang Y, 202037 | 36 | NA | Elevation of ALT 4/30 (13.33%) AST 18/31 (58.06%) TB 4/31 (12.90%) |
| Cholankeril G, 202017 | 116 | Abnormal LFT 26/65 (40%) | |
| Wu J, 202038 | 80 | NA | 3 (3.75%) |
AST, aspartate aminotransferase; ALT, alanine aminotransferase; TB, total bilirubin; LFT, liver function test; NA, not available.
GI and liver manifestations such as anorexia, dysgeusia, nausea, vomiting, diarrhea, abdominal pain, and discomfort are common among patients with COVID-19. Hepatic involvement in this condition is reflected by abnormal liver enzymes and much less frequently elevated bilirubin values. Quite a few studies showed that patients with the severe disease more often have GI symptoms and hepatic involvement. Though a few studies did suggest that GI manifestations are associated with the worst outcome, this has been refuted by other studies. SARS-CoV-2 has been detected in multiple other clinical samples, including feces, in addition to the nasopharyngeal specimens. Therefore, a possible transmission of the virus by endoscopy cannot be refuted though a recent study suggested this possibility to be rather low.42 Fecal excretion of the virus has clinical implications because of possible feco-oral transmission of the disease.43 A few studies showed that the virus is excreted in the stool even after the nasopharyngeal specimen has become negative. Therefore, the current practice of relieving the patient from isolation after the nasopharyngeal SARS-CoV-2 has become negative may not be correct as such patients may result in feco-oral transmission of the disease. Knowledge about this disease, however, is currently evolving, and hence, a lot remains to be explored.44,45
Conflicts of interest
The authors have none to declare.
Funding
The author thank the Department of Biotechnology, Government of India, for the funding for the study on Gastrointestinal involvement in COVID-19 (project No. T/PR40311/COD/139/9/2020).
Author contribution
UCG reviewed the literature, and drafter the first draft of the paper. UG and RKD edited the paper. All the authors approved the final version of the paper.
References
- 1.Zhai P., Ding Y., Wu X. The epidemiology, diagnosis and treatment of COVID-19. Int J Antimicrob Agents. 2020:105955. doi: 10.1016/j.ijantimicag.2020.105955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Del Rio C., Malani P.N. COVID-19-New insights on a rapidly changing epidemic. J Am Med Assoc. 2020 Feb 28 doi: 10.1001/jama.2020.3072. [DOI] [PubMed] [Google Scholar]
- 3.Del Rio C., Malani P.N. 2019 Novel coronavirus-important information for clinicians. J Am Med Assoc. 2020 Feb 5 doi: 10.1001/jama.2020.1490. [DOI] [PubMed] [Google Scholar]
- 4.Forster P., Forster L., Renfrew C. Phylogenetic network analysis of SARS-CoV-2 genomes. Proc Natl Acad Sci U S A. 2020;117:9241–9243. doi: 10.1073/pnas.2004999117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lam T.T., Jia N., Zhang Y.W. Identifying SARS-CoV-2 related coronaviruses in Malayan pangolins [published online ahead of print, 2020 Mar 26] Nature. 2020 doi: 10.1038/s41586-020-2169-0. [DOI] [PubMed] [Google Scholar]
- 6.Chan J.F., Yuan S., Kok K.H. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luo S., Zhang X., Xu H. Don’t overlook digestive symptoms in patients with 2019 novel coronavirus disease (COVID-19) Clin Gastroenterol Hepatol. 2020;18(7):1636–1637. doi: 10.1016/j.cgh.2020.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holshue M.L., DeBolt C., Lindquist S. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jin X., Lian J.S., Hu J.H. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69(6):1002–1009. doi: 10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan L., Mu M., Ren H.G. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Am J Gastroenterol. 2020;115:766–773. doi: 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lechien J.R., Chiesa-Estomba C.M., De Siati D.R. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Oto-Rhino-Laryngol. 2020;6:1–11. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheung K.S., Hung I.F.N., Chan P.P.Y. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from the Hong Kong cohort and systematic review and meta-analysis [published online ahead of print, 2020 Apr 3] Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.03.065. S0016-5085(20)30448-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nobel Y.R., Phipps M., Zucker J. Gastrointestinal symptoms and COVID-19: case-control study from the United States. Gastroenterology. 2020;12 doi: 10.1053/j.gastro.2020.04.017. S0016-5085(20)30490-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cholankeril G., Podboy A., Aivaliotis V.I. High prevalence of concurrent gastrointestinal manifestations in patients with SARS-CoV-2: early experience from California [published online ahead of print, 2020 Apr 10] Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.04.008. S0016-5085(20)30471-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu R., Zhao X., Li J. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang C., Horby P.W., Hayden F.G. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu Y., Guo C., Tang L. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol. 2020;5:434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang W., Du R.H., Li B. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microb Infect. 2020;9:386–389. doi: 10.1080/22221751.2020.1729071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ianiro G., Mullish B.H., Kelly C.R. Screening of faecal microbiota transplant donors during the COVID-19 outbreak: suggestions for urgent updates from an international expert panel. Lancet Gastroenterol Hepatol. 2020;5:430–432. doi: 10.1016/S2468-1253(20)30082-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aggarwal R., Jameel S., Hepatitis E. Hepatology. 2011;54:2218–2226. doi: 10.1002/hep.24674. [DOI] [PubMed] [Google Scholar]
- 24.Kalkowska D.A., Pallansch M.A., Wassilak S.G.F., Cochi S.L., Thompson K.M. Global transmission of live polioviruses: updated dynamic modeling of the polio endgame [published online ahead of print, 2020 Jan 20] Risk Anal. 2020 doi: 10.1111/risa.13447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Doremalen N., Bushmaker T., Morris D.H. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yeo C., Kaushal S., Yeo D. Enteric involvement of coronaviruses: is faecal-oral transmission of SARS-CoV-2 possible? Lancet Gastroenterol Hepatol. 2020;5:335–337. doi: 10.1016/S2468-1253(20)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiao F., Tang M., Zheng X., Liu Y., Li X., Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158(6):1831–1833.e3. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gui M., Song W., Zhou H. Cryo-electron microscopy structures of the SARS-CoV spike glycoprotein reveal a prerequisite conformational state for receptor binding. Cell Res. 2017;27:119–129. doi: 10.1038/cr.2016.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liang W., Feng Z., Rao S. Diarrhoea may be underestimated: a missing link in 2019 novel coronavirus. Gut. 2020;69(6):1141–1143. doi: 10.1136/gutjnl-2020-320832. [DOI] [PubMed] [Google Scholar]
- 30.Gu J., Han B., Wang J. COVID-19: gastrointestinal manifestations and potential fecal-oral transmission. Gastroenterology. 2020;158(6):1518–1519. doi: 10.1053/j.gastro.2020.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cai Q., Huang D., Ou P. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China [published online ahead of print, 2020 Apr 2] Allergy. 2020 doi: 10.1111/all.14309. [DOI] [PubMed] [Google Scholar]
- 32.Fan Z., Chen L., Li J. Clinical features of COVID-19-related liver functional abnormality. Clin Gastroenterol Hepatol. 2020;18(7):1561–1566. doi: 10.1016/j.cgh.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China [published online ahead of print, 2020 Feb 7] J Am Med Assoc. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi H., Han X., Jiang N. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu X.W., Wu X.X., Jiang X.G. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368 doi: 10.1136/bmj.m606. m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study [published correction appears in Lancet Respir Med. 2020 Apr;8(4):e26] Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu L., Liu J., Lu M., Yang D., Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40(5):998–1004. doi: 10.1111/liv.14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu J., Liu J., Zhao X. Clinical characteristics of imported cases of COVID-19 in Jiangsu Province: a multicenter descriptive study. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chai X., Hu L. bioRxiv. 2020 doi: 10.1101/2020.02.03.931766. 02.03.931766; Available from: [DOI] [Google Scholar]
- 40.Banales J.M., Huebert R.C., Karlsen T. Cholangiocyte pathobiology. Nat Rev Gastroenterol Hepatol. 2019;16:269–281. doi: 10.1038/s41575-019-0125-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu Z., Shi L., Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Repici A., Aragona G., Cengia G. Low risk of COVID-19 transmission in GI endoscopy. Gut. 2020 doi: 10.1136/gutjnl-2020-321341. pii: gutjnl-2020-321341; [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 43.Matthai J., Shanmugam N., Sobhan P. Coronavirus disease (COVID-19) and the gastrointestinal system in children. Indian Pediatr. 2020 doi: 10.1007/s13312-020-1851-5. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chatterjee P., Nagi N., Agarwal A. The 2019 novel coronavirus disease (COVID-19) pandemic: a review of the current evidence. Indian J Med Res. 2020;151:147–159. doi: 10.4103/ijmr.IJMR_519_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kumar A., Arora A., Sharma P. Gastro-intestinal and hepatic manifestations of COVID-19 and their relationship to severe clinical course: a systematic review and meta-analysis. Indian J Gastroenterol. 2020;39 doi: 10.1007/S12664-020-01058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]