Abstract
This short report is dedicated to the description of the wide antiviral and antibacterial activity of the immune-modulating agent Panavir®. Panavir® is a high-molecular-weight fraction of the polysaccharides extracted from the shoots of the Solanum tuberosum. It demonstrates activity against many types of viruses, including animal coronavirus and also against bacterial infections. These properties look very promising considering the COVID-19 epidemy and allow propose that Panavir® would be effective in the therapy of the SARS-CoV-2 infection.
Keywords: Antiviral activity, Immune modulator, COVID-19
1. Introduction
Panavir® is a high-molecular-weight fraction of the polysaccharides extracted from Solanum tuberosum (potato) shoots. The chemical composition of Panavir® is rather variable: glucose (10–67%), galactose (2–27%), arabinose (3–15%), rhamnose (2–10%), mannose (0.1–5%), and xylose (0.1–3%). It also contains some uronic acids (2–5%), traces of lipids, and peptides and proteins, first of all, RuBisCo (less than 1% in total). The absence of iodine coloring indicates the cross-linking of the hexose polymers, which prevents the formation of the channel-type clathrates responsible required for the characteristic blue coloring [1].
At pH 7 and the temperature of 36 °C, the solubility of the Panavir® is 13 g per 100 g. Panavir® solution is the monodisperse colloidal system, composed of the spherical particles. The zeta potential of the particles was negative and equals about −25 mV. According to the ultra-sonic acoustic spectral analysis, the average particles' diameter was about 140 nm, while according to the results of the DLS examination, it was about 350 nm. EM investigation of a residual after drying of the solution gave an estimate of about 250 nm. The average molecular weight of these particles was estimated as 3·109 Da [1,2].
Panavir® was successfully used as an antiviral immune-modulating drug, as well as an anti-inflammatory and anti-bacterial agent that was reported earlier for other types of polysaccharides [[3], [4], [5]]. The antiviral activity of Panavir® was demonstrated for a number of viral infections in experiment and clinic, such as the recurrent genital herpes [6], papilloma [7,8], influenza [9], tick-borne encephalitis [10], and rabies [11]. The animal analog of Panavir® (Forvet®) was successfully used against the coronaviral peritonitis [12]. Besides that, Panavir® promotes the secretion of the IFNα and IFNγ by the peripheral blood leukocytes [13]. The anti-inflammatory effect of Panavir® was shown in mice and rats [14,15]. Here we report the results on the antibacterial activity of the Panavir® (including anti-pneumonia activity). Given that one of the common complications of the severe cases of COVID-19 infection is the bacterial pneumonia, the wide-spectrum antiviral and antibacterial activities of Panavir® in conjunction with its interferon-inducing ability look quite promising. One of the indicative symptoms caused by SARS-CoV-2 infection is the loss of smell and taste [16], which indicates some damage to the brain. The very recently, Washington Post [17] reported an increase in the number of middle-aged patients with COVID-19 who died of a sudden stroke. In this regard, an additional advantage of our agent is its ability to affect the CNS functioning [18] and act as a neuroprotector, which was shown in the model of Parkinson's decease in mice and rats [19].
2. Methods
2.1. Antiviral activity
The effect against recurrent genital herpes was estimated in adult women suffering from characteristic herpes symptoms such as painfulness, burning, and itch (ALS-MED medical center, St. Petersburg). The experimental group was composed of 33 women, while only 20 women were in the control. The diagnosis was confirmed by PCR. Panavir® was applied in the form of the skin ointment (2 mg per 100 g of the gel) and intravenous injections (0.004% solution, 5 ml once a day) for 14 days [6].
The effect against tick-borne encephalitis was investigated in mice and in human. The white outbred mice of 10–12 g (24 specimens in each group) were infected by Sofin strain of the encephalitis virus (hypodermic injection of 10% brain suspension of the infected mice) and treated with Panavir® in 24 and 72 h after infection (0.01 mg per 1 g of the living mass, intravenously). The concentration of the viral particles in the brain of the infected mice was assessed by the contagiosity of the brain suspension on the pig kidney cell line (SPEV). The initial suspension was sequentially diluted 10 times, and tested in the cell culture. The concentration of the antibodies against the virus was also assessed by the ability of the serum to neutralize the virus and prevent the infection of the same SPEV cells.
In human, the activity of Panavir® against the tick-borne encephalitis was investigated in patient (30 people in experimental group and 113 in the control) who suffered of the acute (feverish) stage of the syndrome, were treated with the specific immunoglobulin, and were dismissed. But, over time of several months, returned with headache, adynamia, and hyporeflexia. The leukocyte grouping was made using the monoclonal antibodies obtained from Diagno-Tex (Moscow). The concentration of the interferons was measured using the specific testing kits from Vector-Best (St. Petersburg) [10]. Panavir® was applied in the form of intravenous injections of 5 ml 0.004% solution separated by 18-h intervals (tree times).
The activity of the Panavir® against rhabdovirus infection was demonstrated in mice, guinea pigs, and dogs. In mice, the infection was induced by the ERA-CB-20 M strain of the rhabdovirus injected intramuscularly (0.1 ml of 3% cerebral suspension obtained from the infected mice). There was 20 white outbred animals (12–13 g each) in the experimental and control group. Panavir® (0.04–4 μg per mouse) was applied intramuscularly (directly into the portal of the infection) or abdominally, five times: 2 h prior to the infection and then four times with the interval of 48 h. The control mice obtained the same volume of the saline. The concentration of the anti-rhabdovirus antibodies was measured immunohistochemically using the BIO-RAD kit.
The effect against the influenza A virus was demonstrated in vitro, in the MDCK cell culture, and in young white outbred mice (9–11 g). The MDCK cell culture was infected with the virus suspension (strain A/WSN/1933, subtype H1N1) in the dose of 0.01–10 ID50 per the cell. Panavir® was applied 12 h prior to the infection, or 6 h after infection (10, 100, 500, and 1000 μg per ml). The virus proliferation activity was controlled by the virus concentration in the culture media 24 h after infection. The mice were infected intranasally with Aichi H3N2 2/68 strain of influenza A virus. Panavir® was applied intravenously (0.2 mg per mouse, 24 and 96 h after infection) [9].
The activity of Forvet® (animal analog of Panavir®) was estimated against the coronavirus infection in cats. An investigation was made on the set of 22 outbred homeless cats having the coronavirus infection confirmed by PCR. Sixteen cats were treated with Forvet® (1 ml of 0.005 % solution hypodermically), five times with the interval of one day, and after one week the course was repeated. Six cats were used as the control group. The effect of the treatment was controlled by the concentration of the anti-coronavirus antibodies in the blood since one and five months after application of Forvet®. The concentration of the antibodies was assessed using immunohistochemical kit designed for the diagnosis of the infectious peritonitis in cats (VetBioChim, Russia) [12].
2.2. Antibacterial activity
The protective activity of the Panavir® against bacterial infections was investigated in young (18–20 g) males of the white outbred mice infected by Klebsiella pneumoniae or Shigella flexneri 516; and on the young (190–230 g) females of the white outbred rats with induced fecal peritonitis. The mice in the experimental group (20 animals per set) were treated with the Panavir® twice (0.4 ml of 0.004% solution abdominally): at the first and the second days of the experiment (after the infection). The rats in the experimental group (10 animals per set) were treated with Panavir® only once (0.4 ml 0.004% solution per 100 g of the living mass, abdominally), 4 h prior to the infection. Both mice and rats in the control group were treated by the saline of the same volume as the experimental animals. The mice were infected 4 h after the second abdominal injection of Panavir® or saline. The K. pneumoniae cells' suspension was injected intravenously (~103 and ~104 CFU per mouse), while Sh. flexneri cells were injected abdominally (~2·108 and ~5·108 CFU per mouse). The peritonitis in rats was induced by the abdominal injection of 20% fecal suspension (0.4 ml per 100 g of the living mass).
3. Results
3.1. Antiviral activity
Panavir® demonstrated the prominent effect against recurrent genital herpes in human. In the experimental group of 30 people (113 in the control group), the alleviation of the symptoms was observed after 2.3 ± 0.1 days, while the epithelialization of the erosive focuses achieved after 5.4 ± 0.1 days since the beginning of the treatment. In the control group, the alleviation was registered after 3.8 ± 0.2 days and the epithelialization – only after 8.9 ± 1.1 days [6].
Panavir® demonstrated the prominent positive effect against the tick-borne encephalitis. The mice in the experimental group demonstrated 30 ± 5% better survival than in the control one. The concentration of the viral particles in the brain of the mice treated with Panavir® was significantly (2.5–3.0 lg) lower than in the control group. It caused the statistically significant acceleration of the relief of the headache and giddiness, and also forced the increase of the lymphocytes' amount (CD3+, CD4+, CD8+, and CD16+ cells) and the concentration of the interferons 2α and γ [10].
The activity of the Panavir® against rhabdovirus infection was demonstrated in mice, guinea pigs, and dogs. The intramuscular application of Panavir® into the portal of the infection promoted the significant decrease of the morbidity rate (35–45%), increase in the survival (30–40%), and two-fold increase of the concentration of the anti-rhabdovirus antibodies in the brain and blood of the survived mice. The abdominal application was inefficient [11].
In vitro, Panavir® demonstrated the effect against the influenza A virus in a rather high concentrations (0.5–1.0 mg/ml). At this concentrations and massive infection (10 ID50 per the cell), the concentration of the virus particles in the culture media was two-to-four times smaller than in the control culture. The effect was more prominent in case of the preliminary application of the drug. In mice, Panavir® caused a 25% increase of survivability (from 55 to 70%), while the average lifetime of the dead animals increased from 11.0 ± 0.3 to 13.0 ± 0.6 days [9].
Panavir®/Forvet® demonstrated the prominent effect against the coronavirus infection in cats. Three of six control cats died within five months since the beginning of the experiment, and only one of 16 cats died in the experimental group. In the experimental group, the concentration of the antibodies against the coronavirus decreased 4.8 ± 0.8 and 3.3 ± 0.6 times after one and five months since the treatment, while in the control group there was no changes in the antibodies concentration in the first month of the experiment, and only 1.5 ± 0.6 decrease after five months (in survived animals) [12].
3.2. Activity against bacterial infections
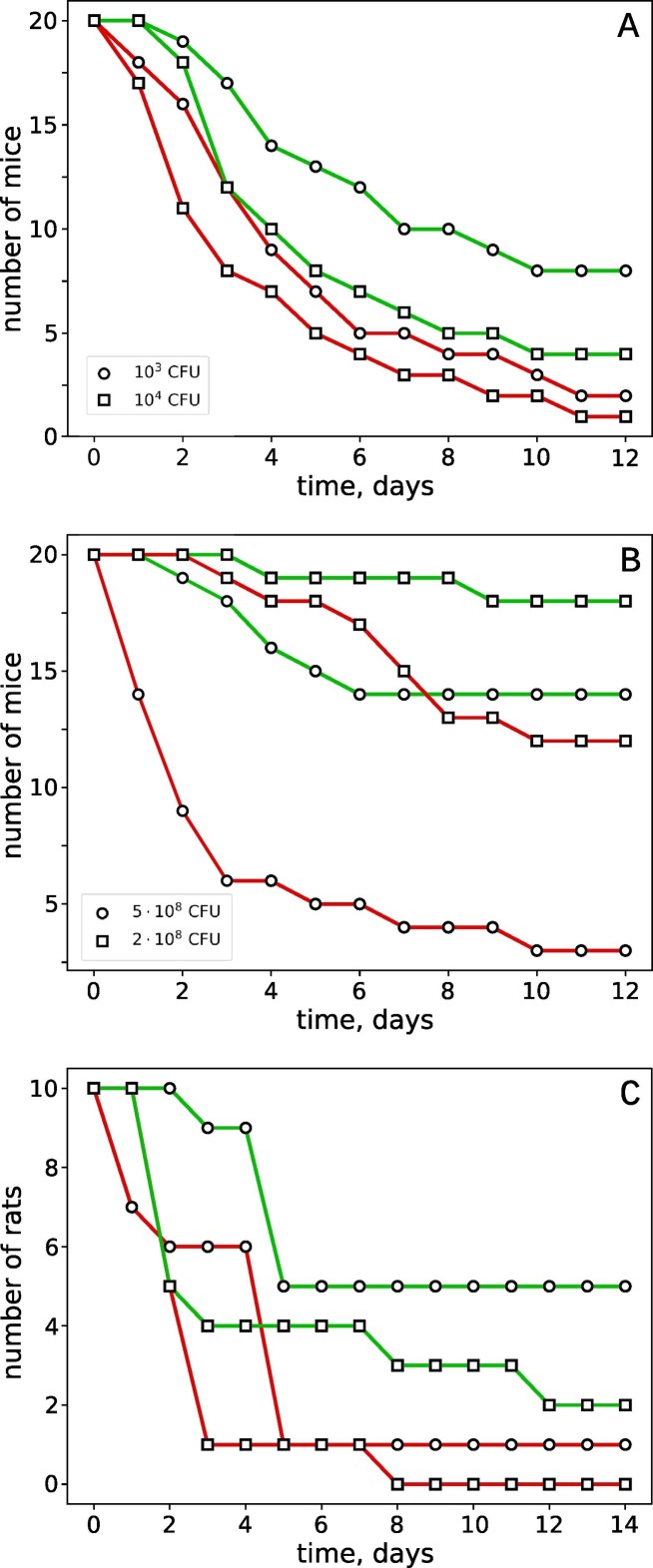
According to our results, Panavir® significantly increased the survival of the animals, exposed to the close to the lethal dose of the infection agents. In the model of pneumonia, Panavir® caused four-times increase of the 12-days survivability (Fig. 1A): from 10 to 40% (103 CFU per mouse) and from 5 to 20% (104 CFU per mouse). In the model of the bacterial peritonitis, Panavir® also demonstrated significant protective effect. Under the massive infection (5·108 CFU per mouse) the mortality decreased from 85 to 30%, while under milder conditions (2·108 CFU per mouse), the mortality decreased from 40 to 10% (Fig. 1B). Similar effect was obtained in the model of the fecal peritonitis in rats (Fig. 1C). In the control group, the 14-days mortality of the fecal peritonitis was as high, as 90–100%, while application of Panavir® decreased this value to 50–80%. Therefore, the abdominal application of Panavir® became effective against infections with a high degree of mortality irrespective of the use before or after the infection.
Fig. 1.
The dynamics of mortality of mice infected by Klebsiella pneumoniae (A) or Shigella flexneri (B) and rats suffering from fecal peritonitis (C). Experimental groups (green curves) were treated with Panavir®, while the control (red curves) – were not. In C, the results of two independent experiments are presented.
4. Conclusion
In the conclusion of our short report, we would like to describe our first experience in using of Panavir® against the SARS-CoV-2 infection. By now, we have only twelve subjects (22 to 56 years old), suffered from the severe form of COVID-19, admitted Panavir®, and successfully recovered. The treatment schedule included: 1. Five intravenous injections of Panavir® (5 ml of 0.004% solution), three with an interval of 24 h, and then another two with an interval of 48 h; 2. Azithromycin, 500 mg two times a day, for three days after the first three injections of Panavir®, then 500 mg once a day for another three days. All of the subjects fully recovered within ten days after the first injection of Panavir®. We realize that the current statistics on the anti-COVID-19 activity of Panavir® is insufficient, but we feel that our experience must be announced for all to hear.
Authors' statement
Tatiana S. Kalinina: Investigation, Conceptualization, Writing - Original Draft. Dmitry V. Zlenko: Validation, Writing - Original Draft, Writing - Review & Editing, Visualization. Aleksei V. Kiselev: Investigation. Alexander A. Litvin: Investigation. Sergey V. Stovbun: Conceptualization, Validation, Writing - Original Draft, Supervision, Project administration, Funding acquisition.
Footnotes
The work was completed as a part of the state assignment (theme number AAAA-A20-120013190076-0).
References
- 1.Stovbun S.V., Yakovenko L.V. The physicochemical basis of the biological activity and pharmacological properties of the antiviral agent Panavir. Mosc. Univ. Phys. Bull. 2014;69:542–547. [Google Scholar]
- 2.Stovbun S., Berlin A., Mikhailov A., Sergienko V., Govorun V., Demina I., Kalinina T. Physicochemical properties of high-molecular-weight plant polysaccharide of hexose glycoside class (Panavir) with antiviral activity. Nanotechnologies in Russia. 2012;7:539–543. [Google Scholar]
- 3.Martinez M.J.A., Olmo L.M.B.D., Benito P.B. Antiviral activities of polysaccharides from natural sources. Stud. Nat. Prod. Chem. 2005;30:393–418. [Google Scholar]
- 4.Wang W., Wang S.-X., Guan H.-S. The antiviral activities and mechanisms of marine polysaccharides: an overview. Marine Drugs. 2012;10:2795–2816. doi: 10.3390/md10122795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen L., Huang G. The antiviral activity of polysaccharides and their derivatives. Int. J. Biol. Macromol. 2018;115:77–82. doi: 10.1016/j.ijbiomac.2018.04.056. [DOI] [PubMed] [Google Scholar]
- 6.Melnikova S., Stovbun S., Korobkova E., Kiselev V., Kucherov V. Efficacy of Panavir in patients with recurrent genital herpes. Russian Bulletin of Obstetrician-Gynecologist. 2017;13:97–101. [Google Scholar]
- 7.Kovchur P., Bakhlaev I. Efficacy of Panavir in treatment of chronic papillomavirus infections of the cervix uteri. Bulletin MSRU. 2011;1:24–28. [Google Scholar]
- 8.Perlamutrov Y., Chernovaa N., Stovbun S., Kucherov V., Bagaeva M., Safronov D. Papillomavirus infection in sexually active women of reproductive age: possibilities of safe and effective therapy. Russian Bulletin of Obstetrician-Gynecologist. 2014;14:90–93. [Google Scholar]
- 9.Prokudina E., Galegov G., Semenova N., Grigoryeva T., Kalinina T., Litvin A., Stovbun S., Sergienko V. Effect of Panavir on influenza a virus reproduction. Antibiot. Chemother. 2006;51:7–10. [PubMed] [Google Scholar]
- 10.Lepekhin A., Ratnikova L., Litvin A., Stovbun S., Sergienko V. An experience of using Panavir in therapy of tick-borne encephalitis. Infectious Deseases. 2007;5:41–46. [Google Scholar]
- 11.Gribencha S., Litvin A., Kokhnovich M., Sergienko V., Stovbun S., Yakimchuk P., Bezmen V. Protective activity of Panavir in experimental rabies infection. Antibiot. Chemother. 2009;54:31–36. [PubMed] [Google Scholar]
- 12.Rakhmanina N., Terekhova Y., Tsibezov V., Verkhovsky O., Ulasov V. Efficacy evaluation of the treatment with “Forvet” (drug) for the feline infectious peritonitis development prophylactics. Russian Veterinary Journal. 2015;2:38–41. [Google Scholar]
- 13.Kolbukhina L., Nosik N., Merkulova L., Braginskii D., Lavrukhina L., Kalinina T., Stovbun S., Litvin A., Sergienko V. Time course of leukocyte interferon induction after single and repeated application of Panavir. Cytokines and Inflammation. 2009;8:49–52. [Google Scholar]
- 14.Stovbun S., Safronov D., Kucherov V., Farzaliev T., Chekameyeva V. Anti-inflamatory Panavir effect in simulation experiments and clinical practice. Bulletin MSRU. 2011;3:82–85. [Google Scholar]
- 15.Litvin A., Kalinina T., Sergienko V., Stovbun S. Comparative experimental study of specific anti-inflammatory activities and antipiretic properties of Panavir and Diclofenac in rats. Cytokines and Inflammation. 2012;11:123–127. [Google Scholar]
- 16.Gautier J., Yann R. A new symptom of COVID-19: loss of taste and smell. Obesity. 2020;28(5) doi: 10.1002/oby.22809. 848–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.A.E. Cha. Washington Post; 2020. Young and middle-aged people, barely sick with COVID-19, are dying of strokes. April 25, 2020. [Google Scholar]
- 18.Stovbun S., Safronov D., Farzaliev T., Nerobkova L. Influence of Panavir on electroencephalogram of human brain. Bulletin MSRU. 2011;2:94–101. [Google Scholar]
- 19.Kalinina T., Nerobkova L., Voronina T., Stovbun S., Litvin A., Sergienko V. Study of antiparkinsonic activity of Panavir on a model of parkinson syndrome induced by systemic administration of MPTP to outbred rats and C57Bl/6 mice. Bull. Exp. Biol. Med. 2005;140:55–57. doi: 10.1007/s10517-005-0410-3. [DOI] [PubMed] [Google Scholar]