Editor—During airway management of patient with coronavirus disease 2019 (COVID-19), some authors and guidelines recommend use of a box or tent that covers the head as a part of personal protective equipment (PPE).1, 2, 3, 4 A major problem is that wearing eye goggles and a face shield, and operating within an aerosol box can make tracheal intubation difficult.4 Guidelines and expert recommendations2 , 5 recommend use of a videolaryngoscope for the initial attempt at tracheal intubation to minimise the time required to intubate the trachea. Different videolaryngoscopes might perform differently in this setting and work.6 , 7
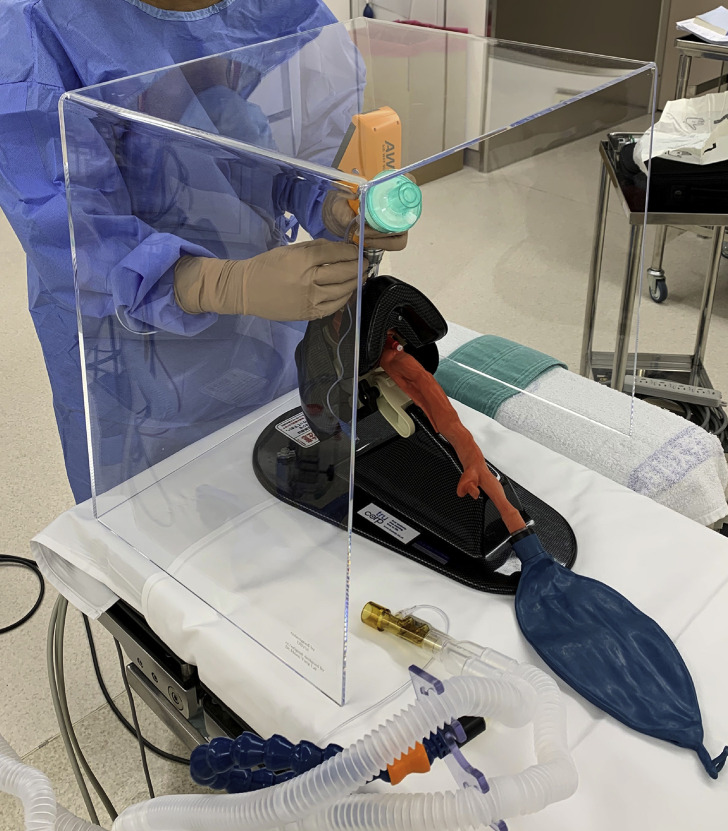
Requirements for a suitable videolaryngoscope include a high success rate of tracheal intubation even in patients with a difficult airway, short intubation time, an introducer (e.g. stylet or gum elastic bougie) is not required, and the device can be disposed of or appropriately disinfected after use. Available videolaryngoscopes with a tube guide that satisfy these requirements include the Airtraq® (Prodol, Vizcaya, Spain), Airwayscope® (Hoya, Tokyo, Japan), and Kingvison® (Ambu, Copenhagen, Denmark). An additional advantage of these videolaryngoscopes is that there is no need to insert a tracheal tube stylet, such that a breathing system filter can be connected to the tracheal tube before intubation, thus minimising the spread of viral particles (Fig 1 ).
Fig 1.
Insertion of a tracheal tube (with a breathing system filter attached) using a videolarygnoscope with a tube guide (Airwayscope®) for a patient with COVID-19.
To determine whether these videolarygoscopes are no less effective than other types of laryngoscopes, we carried out a small randomised simulation trial. Eight anaesthetists with more than 3 yr of clinical experience provided written informed consent to participate. The study included the following five laryngoscopes: Airtraq® AVANT (with a size 3 blade, without a video monitor attached), Airwayscope® S-100 (with the standard P blade), Kingvison® (with a Kingvison® aBlade), McGrath® (Aircraft Medical Ltd, Edinburgh, UK, with size 3 MAC blade), and Macintosh laryngoscope (Penlon, Oxford, UK; with blade 3). To simulate tracheal intubation in a patient with COVID-19, we used an intubation simulator manikin (TruCorp AirSim®; TruCorp Ltd, Belfast, Northern Ireland, UK) with an aerosol box® (Minowa Co., Osaka, Japan) placed over the manikin head (Fig 1). Each participant had previously performed more than 20 patient intubations with each device. They then received a demonstration of the five laryngoscopes by one of the investigators, and then practiced at least 10 times for each device on a manikin without wearing PPE. In a computer-generated randomised order, each participant wearing PPE (N95 mask respirator, disposable gown, face shield, double gloves) attempted to intubate the trachea. A cuffed tracheal tube of 7.0 mm internal diameter was used; a stylet formed in the shape of each blade was used for the McGrath® and Macintosh laryngoscopes, but not for the other videolaryngoscopes. Attending staff removed the stylet (if used) and connected the breathing system to the tracheal tube. For the Airtraq®, the Airwayscope®, and the Kingvison®, a heat and moisture exchanger was already attached to the tracheal tube.
Time to intubate the trachea (defined as time from placing the tip of the blade between the upper and lower incisors to confirming successful lung ventilation) was measured for each attempt. The attempt was judged to have failed if tracheal intubation required >120 s. Friedman's two-way analysis of variance was used to compare intubation time, and if P>0.05, 95% confidence intervals (CIs) for paired median difference between a videolaryngoscope and a Macintosh laryngoscope were calculated. A sample size of 8 was calculated based on an expectation that intubation time is faster for the Airwayscope® than for the Macintosh laryngoscope on 90% of occasions (as a cross-over design), with a power of 0.8, and P=0.05. Statistical analysis was performed by using SPSS version 24 (IBM Corp., Armonk, NY, USA), with manual calculations for the 95% CIs for the median differences.8
Tracheal intubation was successful except for one attempt with the Airtraq®. Intubation time was shorter for the Airwayscope® than for the Macintosh laryngoscope (median difference [95% CI for paired median difference]: –8 [–13, –3] s), and shorter for the McGrath® than for the Macintosh laryngoscope (–7 [–12, –3] s) (Table 1 ).
Table 1.
Time to intubate the trachea and success rate. Times are expressed as median [IQR] (range) in seconds. ∗P<0.05 compared with the Macintosh laryngoscope.
| Time to intubate the trachea | Success rate (%) | Median difference [95% CI for median difference] | |
|---|---|---|---|
| Macintosh laryngoscope | 27 [25, 31] (24–34) | 100 | - |
| Airwayscope® s-100 | 19 [18, 22] (15–39) ∗ | 100 | -8 [-13, -3] |
| Airtraq® AVANT | 30 [25, 41] (19–120) | 85.7 | 3 [-5, 16] |
| Kingvision® | 24 [21, 29] (14–40) | 100 | -3 [-11, 12] |
| McGrath® | 20 [19, 22] (18–26) ∗ | 100 | -7 [-12, -3] |
Using simulating of tracheal intubation in a patient with COVID-19 disease, the Airwayscope® and McGrath® laryngoscopes were more effective than the other laryngoscopes. Although the Airtraq® is appealing as a single-use device, wearing goggles and a face shield and using the box made it difficult to see the glottis through the eyepiece of the device. Therefore, when this device is to be used a camera monitor needs to be attached to the eyepiece. The Airwayscope®, which has been shown to be effective with a difficult airway,9 can be disinfected after use by immersing the whole device into disinfectant solution. The blade of the Kingvison® is disposable, but its display cannot be immersed in liquid and thus can only be disinfected by an alcohol wipe.
In conclusion, our simulation study indicates that different videolaryngoscopes perform differently depending on the circumstances. Despite the small numbers, the Airwayscope® provided shorter intubation times compared with other laryngoscopes for tracheal intubation in simulation of patients with COVID-19.
Declarations of interest
TA is an editor of the British Journal of Anaesthesia; the other authors have no conflict of interest.
Acknowledgements
We thank the staff of the Department of Anesthesiology, Dokkyo Medical University Saitama Medical Centre, for their participation.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2020.06.002.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Peng P.W.H., Ho P.-L., Hota S.S. Outbreak of a new coronavirus: what anaesthetists should know. Br J Anaesth. 2020;124:497–501. doi: 10.1016/j.bja.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yao W., Wang T., Jiang B. Emergency tracheal intubation in 202 patients with COVID-19 in Wuhan, China: lessons learnt and international expert recommendations. Br J Anaesth. 2020 doi: 10.1016/j.bja.2020.03.026. Advance Access published on April 10, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lai HY. Aerosol Box. Protects healthcare providers during endotracheal intubation. Available from: https://sites.google.com/view/aerosolbox/home (accessed 18 June, 2020).
- 4.Canelli R., Connor C.W., Gonzalez M., Nozari A., Ortega R. Barrier enclosure during endotracheal intubation. N Engl J Med. 2020 doi: 10.1056/NEJMc2007589. Advanced Access published on April 3, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cook T.M., El-Boghdadly K., McGuire B., McNarry A.F., Patel A., Higgs A. Consensus guidelines for managing the airway in patients with COVID-19. Anaesthesia. 2020 doi: 10.1111/anae.15054. Advanced Access published on March 27, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kleine-Brueggeney M., Greif R., Schoettker P., Savoldelli G.L., Nabecker S., Theiler L.G. Evaluation of six videolaryngoscopes in 720 patients with a simulated difficult airway: a multicentre randomized controlled trial. Br J Anaesth. 2016;116:670–679. doi: 10.1093/bja/aew058. [DOI] [PubMed] [Google Scholar]
- 7.Asai T. Videolaryngoscopes: do they truly have roles in difficult airways? Anesthesiology. 2012;116:515–517. doi: 10.1097/ALN.0b013e318246e866. [DOI] [PubMed] [Google Scholar]
- 8.Campell M., Garder M.J. Medians and their differences. In: Altman D.G., Machin D., Bryant T., Gardner M.J., editors. Statistics with confidence. 2nd edn. BMJ Books; Bristol: 1989. pp. 36–44. [Google Scholar]
- 9.Asai T., Liu E.H., Matsumoto S. Use of the Pentax-AWS in 293 patients with difficult airways. Anesthesiology. 2009;110:898–904. doi: 10.1097/ALN.0b013e31819c45e5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.