Abstract
This study was performed to investigate the relationship between patients’ activity and function levels and the incidence of preoperative deep venous thrombosis (DVT) prior to total hip arthroplasty (THA). We retrospectively reviewed 500 patients admitted for primary or revision THA from July 2014 to October 2018. The diagnosis of DVT was confirmed using Doppler ultrasonography 1 month before THA. The patients’ activity and hip function were evaluated using several clinical scores: the Harris Hip Score (HHS), Oxford Hip Score (OHS), University of California Los Angeles (UCLA) activity score, and visual analog scale (VAS) score. Those scores and the medical history were examined for correlations with preoperative DVT using univariate and multivariate models. Univariate regression analysis showed that older age, current steroid use, anticoagulant use, a history of DVT, collagen disease, a lower UCLA activity score, and a lower OHS were associated with an elevated risk of preoperative DVT. The multivariate analyses showed that a higher UCLA activity score (odds ratio (OR): 0.0049–0.012) and higher OHS (OR: 0.0012–0.0088) were associated with a lower risk of preoperative DVT in each model. Age (OR: 1.07 in both models), current steroid use (OR: 9.32–10.45), and a history of DVT (OR: 27.15–74.98) were associated with a higher risk of preoperative DVT in both models. Older age, current steroid use, a history of DVT, a lower UCLA activity score, and a lower OHS were risk factors for preoperative DVT before THA, even when controlling for potential confounders. Patients exhibiting low activity and low function levels were more likely to have DVT, even before surgery.
Keywords: deep venous thrombosis, total hip arthroplasty, University of California Los Angeles activity score, Oxford Hip Score, preoperative deep venous thrombosis
1. Introduction
Deep venous thromboembolism (DVT) is still a common complication after hip arthroplasty and can lead to pulmonary thromboembolism (PTE), although recent reviews have shown a significant decrease in the incidence of fatal PTE after total hip arthroplasty (THA) [1,2]. According to the American College of Chest Physicians’ evidence-based clinical practice guideline, hip surgery itself is a risk factor for DVT [3]. If patients undergoing THA have DVT before the surgery, the risk of extension of the thrombus and fatal PTE could be higher. Because patients with hip disability tend to be less active, physicians should be aware of the possibility that patients might already have DVT, even before the arthroplasty. Only a few studies have investigated the incidence of and risk factors for preoperative DVT [4,5], and these studies showed that the prevalence of DVT prior to THA ranged from 5% to 12%. To avoid critical complications associated with significant mortality, it is essential to determine the risk factors for DVT in patients with hip disability undergoing THA.
Several risk factors for DVT after THA have been reported, including age [6,7,8,9,10,11,12,13], female sex [7,8,9,10,11,13,14,15,16], a higher body mass index (BMI) [6,17], malignancy [6,7,11], and congestive heart disease [6,9,11,13]. Bed rest [18] and immobilization [19,20] are also potential risk factors for DVT; however, no studies have quantified the activity level and evaluated the effect of the activity level on the risk of DVT.
Several scoring tools are used to evaluate the activity of patients with hip disease. The Oxford Hip Score (OHS) is a patient-reported outcome measurement designed to evaluate hip function and pain. This score has been proven to be reliable and valid and has been used in many clinical trials since its introduction in 1996 [21,22]. The University of California Los Angeles (UCLA) activity score is another widely used clinical measurement tool. This score is derived from a 10-point activity scale that evaluates patient activity based on 10 descriptive activity levels, ranging from wholly inactive and dependent (level 1) to regular participation in impact sports, such as jogging or tennis (level 10) [23]. The UCLA activity score reportedly has the strongest correlation with other patient activity measurements, has the highest reliability, and is the most effective activity rating scale for patients undergoing total hip and knee arthroplasty [24]. The Harris Hip Score (HHS) is one of the most commonly used clinical-based outcome measures and consists of pain, function, and range-of-motion components. Because these measurements were designed for similar purposes, they show significant correlations (OHS and HHS [25,26], UCLA and HHS [24,27,28], and UCLA and OHS [24,29]).
This study was performed to determine whether clinical measurements of patient activity and function are associated with the risk of DVT prior to THA.
2. Patients and Methods
This was a retrospective study of primary and revision THAs performed from July 2014 to October 2018. During this period, patients undergoing primary or revision THA were subjected to preoperative ultrasound examination for the detection of DVT. All patients provided informed consent, and the study protocol was approved by the institutional review board of our hospital (#E884-2, approved on 19 May 2014). In total, 507 primary and revision THAs were performed at our institute during the study period. Exclusion criteria were acute trauma (fracture) and symptomatic DVT at the time of surgery. Three primary THAs performed for femoral neck fractures and four revision THAs performed for periprosthetic fractures were excluded from this study. There were no patients who had symptomatic DVT at the time of the index surgery.
Therefore, 500 THAs (425 primary THAs and 75 revision THAs) were included in the study. The reason for the index THA was secondary osteoarthritis (OA) due to developmental dysplasia in 249 cases, avascular necrosis of the femoral head in 82 cases, primary OA in 71 cases, rapidly destructive coxarthrosis in 12 cases, rheumatoid arthritis in 6 cases, post-traumatic OA in 4 cases, and OA secondary to infection in 2 cases. The reason for the index revision THA was aseptic loosening in 55 cases, infection in 9 cases, migration of the bipolar head into the acetabulum in 6 cases, and recurrent dislocation in 5 cases. Fifty-one patients were taking one or more anticoagulants for the treatment or prevention of myocardial infarction (13 cases), cerebral infarction (10 cases), atrial fibrillation (Af) (7 cases), arrhythmia other than Af (2 cases), valvular heart disease (4 cases), and unknown reasons (15 cases).
For the evaluation of patient activity and hip function, preoperative clinical assessments were performed using the HHS, UCLA activity score, and OHS. The OHS was calculated according to the modified scoring system, in which each question was scored from 0 to 4, with 4 representing the best outcome or fewest symptoms. The overall score ranged from 0 to 48, with 48 being the best outcome. The patients rated the pain intensity of the affected hip joint using the VAS, which ranged from 0 (no pain) to 10 (worst imaginable pain). These clinical scores were obtained 1 month before THA. We considered the following patient-related covariates: age, sex, BMI, a history of major surgery, a history of major surgery within the last 12 months, a known history of DVT, current steroid use, administration of anticoagulants, smoking status, and the presence of comorbidities (congestive heart failure (CHF), Af, diabetes mellitus (DM), collagen disease, or malignancy). Major surgery was defined as surgery requiring anesthesia (general, orthopedic, neurologic, or gynecologic surgery), as reported in a previous study [30]. Before the arthroplasty, patients were asked by the surgeon and a nurse independently about their history of major surgery and the presence of comorbidities. All patients underwent ultrasonography approximately 1 month prior to THA. Experienced clinical technicians blind to the patients’ backgrounds and clinical measurements performed all examinations using Doppler ultrasonography (Aplio 300, 500, or i800; Canon Inc., Tokyo, Japan).
Statistical Analysis
Differences in proportions were calculated by the chi-square test. Differences in means were calculated by the Wilcoxon test to compare two groups. Probability values of <0.05 were considered significant. In the univariate regression, the dependent variable was preoperative DVT and the independent variables were age, sex, BMI, current steroid use, current anticoagulant use, smoking status, a history of DVT, a history of major surgery, a history of major surgery in the last 12 months, the presence of comorbidities (Af, CHF, DM, collagen disease, or malignancy), and preoperative clinical measurements (HHS, OHS, UCLA activity score, and VAS score). To examine the presence of potential multicollinearity in the following multivariate logistic analysis, the correlation among clinical scores was tested using the Spearman rank correlation coefficient.
In the multivariate logistic regression analyses, the dependent variable was preoperative DVT and the independent variables were demographic parameters and clinical measures reaching a significance level of p < 0.05 in the univariate analysis. To evaluate the presence of multicollinearity, the variance inflation factors for the selected variables were examined in each model. Receiver operating characteristic (ROC) curves were used to examine the relations between the true positive rate (sensitivity) and false positive rate (1 − specificity) and the areas under the ROC curve (AUC). The Youden index (sensitivity + specificity − 1) was used to determine the optimal predictive cutoffs for the clinical scores that showed significant relevance for the preoperative DVT risk.
All statistical analyses were performed using JMP Pro 14 software (SAS Institute, Cary, NC, USA).
3. Results
The patients’ demographic data are shown in Table 1. DVT was detected in 26 patients (5.2%) before the index THA, all of which were asymptomatic. Proximal DVT was found in nine cases (six in the femoral vein and three in the popliteal vein) and distal DVT (in calf veins) was found in 17 cases. When DVT was found, the surgeon consulted with cardiologists and the cardiologists decided whether an anticoagulant should be administered for each case. For the patients who had proximal DVT, an anticoagulant was newly started before the index surgery in four cases. No anticoagulant was used before surgery in two cases. The remaining three cases had been taking anticoagulants before the ultrasound screening and they continued the drug after the detection of DVT. For the patients with distal DVT, an anticoagulant was newly administered before THA in seven cases. No anticoagulant was used before THA in eight cases. The remaining two cases continued taking the anticoagulant they had been taking before the screening. The average age in the DVT group was significantly higher than that in the no-DVT group (70.5 vs. 64.5 years, p = 0.0083). The DVT and no-DVT groups contained 24 (92.3%) and 385 (81.2%) women, respectively (p = 0.12). The mean BMI was 23.6 ± 4.0 kg/m2 (range, 18.2–32.2 kg/m2) in the DVT group and 24.3 ± 4.2 kg/m2 (range, 12.2–41.8 kg/m2) in the no-DVT group (p = 0.63). Overall, 54 patients were currently using corticosteroids. The mean daily dose among these 54 patients was 7.5 ± 4.9 mg (range, 1–20 mg). Ten patients (38.5%) in the DVT group, but only 44 (9.3%) in the non-DVT group, were currently taking steroids; the proportion was significantly higher in the DVT group (p < 0.0001). Twenty patients (76.9%) in the DVT group and 327 (69.0%) in the no-DVT group had a history of major surgery (p = 0.22). When the history of major surgery was limited to 12 months prior to the index THA, the difference between the two groups was more marked (26.9% vs. 13.3%). More patients in the DVT group tended to have a history of major surgery within the last 12 months prior to the index THA, although the difference was not significant (p = 0.053). Significantly more patients had a history of DVT in the DVT group than in the no-DVT group (23.1% vs. 1.1%, p < 0.0001). Among the comorbidities, collagen disease was present in more patients in the DVT group than in the no-DVT group (p = 0.023).
Table 1.
Demographics of each group.
| Variables | Overall | DVT | No DVT | p Value # | |
|---|---|---|---|---|---|
| n | 500 | 26 | 474 | ||
| Sex | Male | 91 | 2 | 89 | 0.12 |
| Female | 409 | 24 | 385 | ||
| Age, years | 64.79 ± 12.7 (22–89) | 70.0 ± 14.6 (35–87) | 64.5 ± 12.5 (22–89) | 0.0083 | |
| BMI, kg/m2 | 24.0 ± 4.0 (12.2–41.7) | 23.6 ± 3.9 (18.2–32.2) | 24.3 ± 4.2 (12.2–41.8) | 0.63 | |
| Current steroid use | 54 | 10/26 | 44/474 | <0.0001 | |
| Previous DVT history | 11/500 | 6/26 | 5/474 | <0.0001 | |
| Current anticoagulant use | 51/500 | 6/26 | 45/474 | 0.026 | |
| Atrial fibrillation | 15/500 | 1/26 | 14/474 | 0.80 | |
| Congestive heart failure | 16/500 | 1/26 | 15/474 | 0.85 | |
| Major surgery | 347/500 | 20/26 | 327/474 | 0.22 | |
| Major surgery within last 12 months | 70/500 | 7/26 | 63/474 | 0.053 | |
| Diabetes mellitus | 54/500 | 3/26 | 51/474 | 0.9 | |
| Collagen disease | 55/500 | 7/26 | 48/474 | 0.023 | |
| Malignancy | 78/500 | 3/26 | 75/474 | 0.55 | |
| Current smoking | 36/500 | 1/26 | 35/474/ | 0.50 |
Data are shown as the number of patients or the mean ± standard deviation (range). DVT: deep venous thrombosis and BMI: body mass index. # Comparison of the presence and absence of preoperative DVT.
The breakdown of collagen diseases in each group is shown in Table 2. Significantly more patients were under the administration of anticoagulants in the DVT group than in the no-DVT group (23.1% vs. 9.5%). There was no difference in the incidence of DM, Af, CHF, or malignant disease between the DVT and no-DVT groups. The percentage of current smokers was not significantly different between the DVT and no-DVT group.
Table 2.
Breakdown of collagen disease in each group.
| Type of Collagen Disease | Overall (n = 500) |
DVT (n = 26) |
No DVT (n = 474) |
|---|---|---|---|
| Rheumatoid arthritis | 23 | 2 | 21 |
| Systemic lupus erythematosus | 15 | 2 | 13 |
| Dermatomyositis | 8 | 1 | 7 |
| Systemic sclerosis | 3 | 3 | 0 |
| Sjögren syndrome | 3 | 0 | 3 |
| Polymyalgia rheumatica | 2 | 0 | 2 |
| Polyarteritis nodosa | 1 | 0 | 1 |
| Adult Still’s disease | 1 | 0 | 1 |
| Behçet’s disease | 1 | 0 | 1 |
| Eosinophilic granulomatosis with polyangiitis | 1 | 0 | 1 |
DVT: deep venous thrombosis.
The clinical scores are shown in Table 3. Hereafter, the score and scale are given as the mean ± standard deviation (range). The mean UCLA activity score was 2.64 ± 0.91 (2–5) in the DVT group and 3.75 ± 1.33 (1–8) in the no-DVT group (p < 0.0001). The mean OHS was 22.50 ± 12.32 (1–45) in the DVT group and 29.73 ± 9.97 (1–48) in the no-DVT group (p = 0.006). The VAS score was higher in the DVT group (6.88 ± 2.50 (0–10)) than in the no-DVT group (6.04 ± 2.82 (0–10)); however, the difference was not significant (p = 0.21). The difference in the HHS between the DVT group (50.9 ± 17.79 (17.0–84.1)) and no-DVT group (52.66 ± 16.04 (5.7–99.6)) was also not statistically significant (p = 0.55).
Table 3.
Clinical measurement scores for each group.
| Variables | Overall | DVT | No DVT | p Value # |
|---|---|---|---|---|
| Oxford Hip Score | 29.4 ± 10.2 | 22.5 ± 12.3 | 29.7 ± 10.0 | 0.0060 |
| Harris Hip Score | 52.6 ± 16.1 | 50.9 ± 17.8 | 52.7 ± 16.0 | 0.55 |
| UCLA activity score | 3.69 ± 1.34 | 2.64 ± 0.91 | 3.75 ± 1.33 | <0.0001 |
| VAS | 6.08 ± 2.81 | 6.88 ± 2.50 | 6.04 ± 2.82 | 0.21 |
DVT: deep venous thrombosis, UCLA: University of California Los Angeles, and VAS: visual analog scale. # Comparison of the presence and absence of preoperative DVT.
Because hip clinical scores reportedly have strong correlations with one another [24,25,26,27,28,29], the correlation between each combination of the four clinical measurements (UCLA activity score, OHS, HHS, and VAS score) was tested using Spearman’s rank correlation coefficient. The p-value and Spearman’s rank correlation coefficient for each combination are shown in Table 4 and Table 5, respectively. All six combinations of the four clinical measurements (UCLA activity score, OHS, HHS, and VAS score) had a statistically significant correlation. All combinations except the UCLA activity score and VAS score (coefficient = −0.14) had an absolute coefficient value of 0.3 to 0.6, which is considered a moderate correlation.
Table 4.
Probability (p) value for the correlation of each combination of clinical measurements.
| UCLA | OHS | HHS | VAS | |
|---|---|---|---|---|
| UCLA | <0.0001 | <0.0001 | <0.0001 | 0.0039 |
| OHS | <0.0001 | <0.0001 | <0.0001 | |
| HHS | <0.0001 | 0.0032 | ||
| VAS | <0.0001 |
OHS: Oxford Hip Score, HHS: Harris Hip Score, UCLA: University of California Los Angeles, and VAS: visual analog scale.
Table 5.
Spearman rank correlation coefficient for each combination of clinical measurements.
| UCLA | OHS | HHS | VAS | |
|---|---|---|---|---|
| UCLA | 1.0 | 0.34 | 0.43 | −0.14 |
| OHS | 1.0 | 0.42 | −0.60 | |
| HHS | 1.0 | −0.31 | ||
| VAS | 1.0 |
OHS: Oxford Hip Score, HHS: Harris Hip Score, UCLA: University of California Los Angeles, and VAS: visual analog scale.
A multivariate logistic regression analysis was performed with two different models: regression with parameters reaching a significance of p < 0.05 in the univariate analysis (i) with the OHS as the representative clinical measurement and (ii) with the UCLA activity score as the representative clinical measurement. This was performed to avoid potential multicollinearity between the OHS and UCLA activity score, which was implied in Table 4 and Table 5 and shown in previous literature [24], demonstrating a significant correlation between the OHS and UCLA activity score (r = 0.48, p < 0.0001).
In Model 1, age, a history of DVT, current steroid use, and the OHS were significantly correlated with preoperative DVT (Table 6). In Model 2, age, a history of DVT, current steroid use, and the UCLA activity score were significant risk factors for preoperative DVT (Table 7). Age, a history of DVT, and current steroid use were significant risk factors for preoperative DVT in both models, and the UCLA activity score and OHS were significantly correlated with preoperative DVT in each model when involved as an independent variable.
Table 6.
Multivariate logistic regression analysis using two parameters reaching p < 0.05 in univariate testing with OHS as a representative clinical measurement.
| Variables | Odds Ratio | 95% CI | p Value | VIF |
|---|---|---|---|---|
| OHS | 0.95 | 0.91–0.99 | 0.031 | 1.02 |
| Age | 1.07 | 1.03–1.12 | 0.00056 | 1.12 |
| Previous history of DVT | 27.15 | 3.31–222.53 | 0.0021 | 1.18 |
| Current anticoagulant use | 0.48 | 0.09–2.63 | 0.39 | 1.20 |
| Collagen disease | 0.93 | 0.19–4.64 | 0.93 | 1.60 |
| Current steroid use | 10.45 | 2.31–47.34 | 0.0023 | 1.67 |
OHS: Oxford Hip Score, DVT: deep venous thrombosis, CI: confidence interval, and VIF: variance inflation factor.
Table 7.
Multivariate logistic regression analysis using two parameters reaching p < 0.05 in univariate testing with the UCLA activity score as a representative clinical measurement.
| Variables | Odds Ratio | 95% CI | p Value | VIF |
|---|---|---|---|---|
| UCLA activity score | 0.49 | 0.28–0.84 | 0.0099 | 1.16 |
| Age | 1.07 | 1.02–1.12 | 0.0027 | 1.27 |
| Previous history of DVT | 74.98 | 8.54–658.18 | <0.0001 | 1.17 |
| Current anticoagulant use | 0.34 | 0.056–2.06 | 0.24 | 1.20 |
| Collagen disease | 1.03 | 0.23–4.68 | 0.97 | 1.55 |
| Current steroid use | 9.32 | 2.14–40.68 | 0.0030 | 1.69 |
UCLA: University of California Los Angeles, DVT: deep venous thrombosis, CI: confidence interval, and VIF: variance inflation factor.
The mean UCLA activity score in patients with proximal DVT was 2.5 ± 0.5, whereas that in patients with distal DVT was 2.7 ± 1.0. The difference was not significant (p = 0.69). There was no significant difference between the proximal DVT group and distal DVT group in HHS (46.8 ± 12.7 vs. 53.5 ± 20.2, p = 0.35), or in OHS (21.9 ± 9.0 vs. 22.9 ± 14.2, p = 0.78).
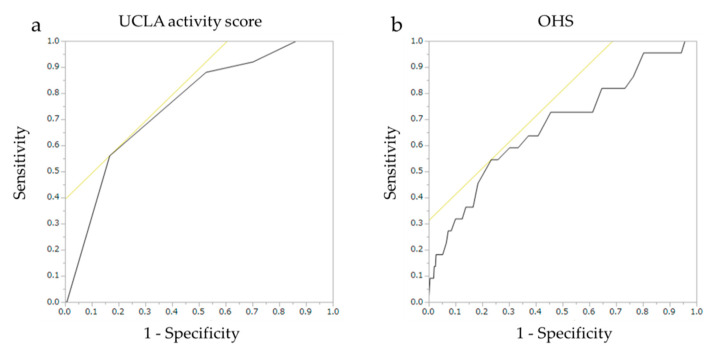
The ROC curves are shown in Figure 1. The AUCs (95% confidence interval) for the UCLA activity score and OHS were 0.755 (0.645–0.840) and 0.673 (0.536–0.786), respectively. The optimal predictive cutoff for the UCLA activity score was 2 (sensitivity, 56.0%; specificity, 83.3%). Its positive (PPV) and negative (NPV) predictive values were 15.7% and 94.7%, respectively. Its Youden Index was 0.393. The optimal predictive cutoff for OHS was 22 (sensitivity, 54.5%; specificity, 76.6%; PPV, 10.4%; NPV, 97.1%). Its Youden Index was 0.312 (Table 8).
Figure 1.
The ROC curves of the (a) UCLA activity score and (b) OHS. ROC, receiver operating characteristic; UCLA, University of California Los Angeles; OHS, Oxford Hip Score.
Table 8.
The diagnostic value of the UCLA activity score and OHS.
| Variables | AUC | Youden Index | Predictive Cutoff | Sensitivity | Specificity | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|
| UCLA activity score | 0.755 | 0.393 | 2 | 56.0 | 83.3 | 15.7 | 94.7 |
| OHS | 0.673 | 0.312 | 22 | 54.5 | 76.6 | 10.4 | 97.1 |
UCLA: University of California Los Angeles, OHS: Oxford Hip Score, AUC: area under the curve, PPV: positive predictive value, and NPV: negative predictive value.
4. Discussion
In the present study, 5.2% of the patients who were scheduled for THA had DVT, even before the surgery. This proportion is in good accordance with those of previous studies (5.8%–12.3%) [4,5]. A few reports have focused on preoperative DVT before other types of surgery. Akeda et al. [31] reported that preoperative DVT was identified using a Doppler ultrasound in nine (4.3%) of 209 patients undergoing spinal surgery. In another study, preoperative DVT was identified using a Doppler ultrasound in 25 (3.8%) of 654 patients undergoing surgery for gynecologic malignancy [32].
Older age is a predictor of preoperative DVT after THA [6,7,8,9,10,11,12,13] and before THA [5]. Our results also demonstrated that older patients were significantly more likely to have DVT before THA.
Although a high BMI of >30 kg/m2 has been associated with a higher risk of DVT after THA [6,17] and PTE after THA [33], we found no correlation between BMI and preoperative DVT. Likewise, two other studies also showed no significant correlation between a high BMI and preoperative DVT [4,5]. Because the average BMI in the present study and the two above-mentioned studies was relatively low (23–24 kg/m2), a possible explanation is that the studies contained too few patients with obesity to examine the effect of excess body weight on the incidence of DVT. A larger number of patients with obesity should be evaluated to more fully examine the effect of a high BMI.
Corticosteroid use is reportedly associated with DVT after surgery [34,35,36]. In the present study, corticosteroid use significantly increased the risk of DVT, even before surgery.
The effect of DM on the risk for DVT after THA has been discussed in several studies. In their meta-analysis of five studies, Zhang et al. [37] showed no correlation between DM and postoperative DVT (odds ratio (OR): 1.02, p = 0.88). We also found no significant correlation between a history of DM and the risk of DVT before THA.
Some authors have referred to malignancy as a significant risk factor for postoperative DVT after THA [6,7,11], whereas others have shown no significant difference between malignancy and DVT, providing an OR of 1.00 (0.72–1.39) [10] and 1.00 (0.32–3.11) [16]. In the present study, a history of malignancy was not associated with preoperative DVT; however, we could not divide patients with active and inactive malignancies because it was difficult to determine the exact stage of the tumor or the recurrence only from the medical records in our institute.
Patients with a history of DVT have a higher risk of postoperative DVT after THA [6,9,10]. The present study also indicated that a history of DVT was significantly correlated with an increased risk of preoperative DVT before THA. Although no previous studies have demonstrated an increased risk of preoperative DVT prior to THA for patients with a known history of DVT, a history of DVT would understandably be a predictor of preoperative DVT.
Some studies have shown that CHF was associated with an increased risk of postoperative DVT after arthroplasty [9,11,13], whereas Beksac et al. [6] reported no correlation between CHF and postoperative DVT. In the present study, CHF was not a predictor of preoperative DVT. However, whether Af is associated with the risk of postoperative DVT has not been rigorously studied. Previous studies that did not focus on the postoperative period demonstrated that Af was associated with an increased risk of DVT [38,39]. Our results showed no association between Af and preoperative DVT. However, only 16 patients had CHF and 15 had Af. It would be difficult to draw a conclusion regarding CHF and Af as risk factors for preoperative DVT in this cohort.
Conclusions regarding the effect of anticoagulants on the risk of preoperative DVT would also be difficult to draw in this study. While anticoagulant use was associated with an increased risk of DVT in the univariate model, the association was not significant in the multivariate models. This was unexpected because anticoagulants themselves are expected to prevent DVT. Most patients with a history of DVT might have been taking anticoagulants to prevent the recurrence or expansion of DVT, which could have strongly affected the association between anticoagulant use and DVT risk in this study.
A history of major surgery is also a reported risk factor for DVT [4,30]. In this study, we included a history of major surgery itself and major surgery within the last 12 months, because many patients had undergone major surgeries a decade previously or could not recall exactly when the previous major surgery was performed. As a result, a history of major surgery itself was not a risk factor, but major surgery within the last 12 months tended to increase the risk of preoperative DVT (p = 0.053).
Hip clinical scores reportedly have strong correlations with one another [24,25,26,27,28,29]. Naal et al. [24] showed a significant correlation between the UCLA activity score and OHS (r = 0.48, p < 0.0001) and between the UCLA activity score and HHS (r = 0.56, p < 0.0001) among patients undergoing THA. Beaulé et al. [27] found a strong correlation between the UCLA activity score and HHS with a Pearson correlation coefficient of 0.57. Sechriest et al. [28] found that the UCLA score was positively correlated with the HHS (r = 0.52, p = 0.001). Parsons et al. [26] reported that the OHS was correlated with the HHS in their hip resurfacing series. Taking these correlations into account, we considered that if the clinical scores were employed in the same multivariate regression, there must be a significant risk for multicollinearity that should be avoided to strictly examine the effect of each hip score on the risk of preoperative DVT. Therefore, we performed separate multivariate logistic regression analyses using either the UCLA activity score or OHS as the representative clinical score, whereas in the univariate analysis, both the UCLA activity score and OHS showed a significant correlation with the incidence of preoperative DVT. The effect of clinical scores on the location of the thrombosis (proximal or distal) was also examined; however, there was no association between those scores (UCLA activity score, OHS, or HHS) and the location of thrombosis.
Collagen disease and current steroid use might also be confounding factors that are significantly correlated with each other. However, because their variance inflation factors were <2, both of these parameters were included in each multivariate model. While steroid use was significantly associated with the presence of preoperative DVT, collagen disease was not an independent risk factor for preoperative DVT.
This study had some limitations. First, the number of patients with preoperative DVT was relatively small. If more patients had preoperative DVT, various other potential confounders could be included in the analysis. Second, this cohort included various types of collagen diseases. Although each disease might have had a different effect on the tendency to cause DVT, these diseases were handled as a single disease group because of the small number of patients.
In conclusion, older age, current corticosteroid use, a history of DVT, a lower UCLA activity score, and a lower OHS were significant risk factors for preoperative DVT in patients scheduled for THA. The commonly used activity and hip function scales could help predict the risk of preoperative DVT.
Author Contributions
T.K. designed the study and wrote the initial draft of the manuscript. K.G., Y.K., and S.M. contributed to the analysis and interpretation of data and critical review of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
All authors declare that there are no conflicts of interest associated with this manuscript.
References
- 1.Samama C.M. Fast-Track Procedures in Major Orthopaedic Surgery: Is Venous Thromboembolism Prophylaxis Still Mandatory? Thromb. Haemost. 2019;119:3–5. doi: 10.1055/s-0038-1676613. [DOI] [PubMed] [Google Scholar]
- 2.Xu K., Chan N.C., Ibrahim Q., Kruger P., Sinha S., Bhagirath V., Ginsberg J., Bangdiwala S., Guyatt G., Eikelboom J., et al. Reduction in Mortality following Elective Major Hip and Knee Surgery: A Systematic Review and Meta-Analysis. Thromb. Haemost. 2019;119:668–674. doi: 10.1055/s-0039-1677732. [DOI] [PubMed] [Google Scholar]
- 3.Falck-Ytter Y., Francis C.W., Johanson N.A., Curley C., Dahl O.E., Schulman S., Ortel T.L., Pauker S.G., Colwell C.W. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(Suppl. 2):e278S–e325S. doi: 10.1378/chest.11-2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wakabayashi H., Hasegawa M., Niimi R., Sudo A. Clinical analysis of preoperative deep vein thrombosis risk factors in patients undergoing total hip arthroplasty. Thromb. Res. 2015;136:855–858. doi: 10.1016/j.thromres.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 5.Imai N., Miyasaka D., Shimada H., Suda K., Ito T., Endo N. Usefulness of a novel method for the screening of deep vein thrombosis by using a combined D-dimer- and age-based index before total hip arthroplasty. PLoS ONE. 2017;12:e0172849. doi: 10.1371/journal.pone.0172849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beksac B., Gonzalez Della Valle A., Salvati E.A. Thromboembolic disease after total hip arthroplasty: Who is at risk? Clin. Orthop. Relat. Res. 2006;453:211–224. doi: 10.1097/01.blo.0000238848.41670.41. [DOI] [PubMed] [Google Scholar]
- 7.Baser O., Supina D., Sengupta N., Wang L., Kwong L. Impact of postoperative venous thromboembolism on Medicare recipients undergoing total hip replacement or total knee replacement surgery. Am. J. Health Syst. Pharm. 2010;67:1438–1445. doi: 10.2146/ajhp090572. [DOI] [PubMed] [Google Scholar]
- 8.Pedersen A.B., Mehnert F., Sorensen H.T., Emmeluth C., Overgaard S., Johnsen S.P. The risk of venous thromboembolism, myocardial infarction, stroke, major bleeding and death in patients undergoing total hip and knee replacement: A 15-year retrospective cohort study of routine clinical practice. Bone Jt. J. 2014;96:479–485. doi: 10.1302/0301-620X.96B4.33209. [DOI] [PubMed] [Google Scholar]
- 9.Wu P.-K., Chen C.-F., Chung L.-H., Liu C.-L., Chen W.-M. Population-based epidemiology of postoperative venous thromboembolism in Taiwanese patients receiving hip or knee arthroplasty without pharmacological thromboprophylaxis. Thromb. Res. 2014;133:719–724. doi: 10.1016/j.thromres.2014.01.039. [DOI] [PubMed] [Google Scholar]
- 10.Pedersen A.B., Mehnert F., Johnsen S.P., Husted S., Sorensen H.T. Venous thromboembolism in patients having knee replacement and receiving thromboprophylaxis: A Danish population-based follow-up study. J. Bone Jt. Surg. Am. 2011;93:1281–1287. doi: 10.2106/JBJS.J.00676. [DOI] [PubMed] [Google Scholar]
- 11.Guijarro R., Montes J., Roman C.S., Arcelus J.I., Barillari G., Granero X., Monreal M. Venous thromboembolism and bleeding after total knee and hip arthroplasty. Findings from the Spanish National Discharge Database. Thromb. Haemost. 2011;105:610–615. doi: 10.1160/TH10-10-0645. [DOI] [PubMed] [Google Scholar]
- 12.Cohen A., Eriksson B.I., Puskas D., Shi M., Bocanegra T., Weitz J., Raskob G.E. Oral direct factor Xa inhibition with edoxaban for thromboprophylaxis after elective total hip replacement. A randomised double-blind dose-response study. Thromb. Haemost. 2010;104:642–649. doi: 10.1160/TH10-02-0142. [DOI] [PubMed] [Google Scholar]
- 13.Kapoor A., Labonte A.J., Winter M.R., Segal J.B., Silliman R.A., Katz J.N., Losina E., Berlowitz D. Risk of venous thromboembolism after total hip and knee replacement in older adults with comorbidity and co-occurring comorbidities in the Nationwide Inpatient Sample (2003–2006) BMC Geriatr. 2010;10:63. doi: 10.1186/1471-2318-10-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joseph J.E., Low J., Courtenay B., Neil M.J., McGrath M., Ma D. A single-centre prospective study of clinical and haemostatic risk factors for venous thromboembolism following lower limb arthroplasty. Br. J. Haematol. 2005;129:87–92. doi: 10.1111/j.1365-2141.2005.05419.x. [DOI] [PubMed] [Google Scholar]
- 15.Kang B.J., Lee Y.-K., Kim H.J., Ha Y.-C., Koo K.-H. Deep venous thrombosis and pulmonary embolism are uncommon in East Asian patients after total hip arthroplasty. Clin. Orthop. Relat. Res. 2011;469:3423–3428. doi: 10.1007/s11999-011-1979-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baser O., Supina D., Sengupta N., Wang L., Kwong L. Clinical and cost outcomes of venous thromboembolism in Medicare patients undergoing total hip replacement or total knee replacement surgery. Curr. Med. Res. Opin. 2011;27:423–429. doi: 10.1185/03007995.2010.545940. [DOI] [PubMed] [Google Scholar]
- 17.Friedman R.J., Hess S., Berkowitz S.D., Homering M. Complication rates after hip or knee arthroplasty in morbidly obese patients. Clin. Orthop. Relat. Res. 2013;471:3358–3366. doi: 10.1007/s11999-013-3049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gibbs N.M. Venous thrombosis of the lower limbs with particular reference to bed-rest. Br. J. Surg. 1957;45:209–236. doi: 10.1002/bjs.18004519102. [DOI] [PubMed] [Google Scholar]
- 19.Jørgensen P.S., Warming T., Hansen K., Paltved C., Berg H.V., Jensen R., Kirchhoff-Jensen R., Kjaer L., Kerbouche N., Leth-Espensen P., et al. Low molecular weight heparin (Innohep) as thromboprophylaxis in outpatients with a plaster cast: A venografic controlled study. Thromb. Res. 2002;105:477–480. doi: 10.1016/S0049-3848(02)00059-2. [DOI] [PubMed] [Google Scholar]
- 20.Lassen M.R., Borris L.C., Nakov R.L. Use of the low-molecular-weight heparin reviparin to prevent deep-vein thrombosis after leg injury requiring immobilization. N. Engl. J. Med. 2002;347:726–730. doi: 10.1056/NEJMoa011327. [DOI] [PubMed] [Google Scholar]
- 21.Murray D.W., Fitzpatrick R., Rogers K., Pandit H.G., Beard D.J., Carr A.J., Dawson J. The use of the Oxford hip and knee scores. J. Bone Jt. Surg. Br. 2007;89:1010–1014. doi: 10.1302/0301-620X.89B8.19424. [DOI] [PubMed] [Google Scholar]
- 22.Field R.E., Cronin M.D., Singh P.J. The Oxford hip scores for primary and revision hip replacement. J. Bone Jt. Surg. Br. 2005;87:618–622. doi: 10.1302/0301-620X.87B5.15390. [DOI] [PubMed] [Google Scholar]
- 23.Amstutz H.C., Thomas B.J., Jinnah R., Kim W., Grogan T., Yale C. Treatment of primary osteoarthritis of the hip. A comparison of total joint and surface replacement arthroplasty. J. Bone Jt. Surg. Am. 1984;66:228–241. doi: 10.2106/00004623-198466020-00010. [DOI] [PubMed] [Google Scholar]
- 24.Naal F.D., Impellizzeri F.M., Leunig M. Which is the best activity rating scale for patients undergoing total joint arthroplasty? Clin. Orthop. Relat. Res. 2009;467:958–965. doi: 10.1007/s11999-008-0358-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weel H., Lindeboom R., Kuipers S.E., Vervest T.M.J.S. Comparison between the Harris- and Oxford Hip Score to evaluate outcomes one-year after total hip arthroplasty. Acta Orthop. Belg. 2017;83:98–109. [PubMed] [Google Scholar]
- 26.Parsons N.R., De Souza R.-M., Oni T., Achten J., Krikler S.J., Costa M.L. A comparison of Harris and Oxford hip scores for assessing outcome after resurfacing arthroplasty of the hip: Can the patient tell us everything we need to know. HIP Int. 2010;20:453–459. doi: 10.1177/112070001002000407. [DOI] [PubMed] [Google Scholar]
- 27.Beaule P.E., Dorey F.J., Hoke R., Le Duff M., Amstutz H.C. The value of patient activity level in the outcome of total hip arthroplasty. J. Arthroplast. 2006;21:547–552. doi: 10.1016/j.arth.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Sechriest V.F., 2nd, Kyle R.F., Marek D.J., Spates J.D., Saleh K.J., Kuskowski M. Activity level in young patients with primary total hip arthroplasty: A 5-year minimum follow-up. J. Arthroplast. 2007;22:39–47. doi: 10.1016/j.arth.2006.02.083. [DOI] [PubMed] [Google Scholar]
- 29.Kawai T., Kataoka M., Goto K., Kuroda Y., So K., Matsuda S. Patient- and Surgery-Related Factors that Affect Patient-Reported Outcomes after Total Hip Arthroplasty. J. Clin. Med. 2018;7:358. doi: 10.3390/jcm7100358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heit J.A., Silverstein M.D., Mohr D.N., Petterson T.M., O’Fallon W.M., Melton L.J., 3rd Risk factors for deep vein thrombosis and pulmonary embolism: A population-based case-control study. Arch. Intern. Med. 2000;160:809–815. doi: 10.1001/archinte.160.6.809. [DOI] [PubMed] [Google Scholar]
- 31.Akeda K., Matsunaga H., Imanishi T., Hasegawa M., Sakakibara T., Kasai Y., Sudo A. Prevalence and countermeasures for venous thromboembolic diseases associated with spinal surgery: A follow-up study of an institutional protocol in 209 patients. Spine (Phila Pa 1976) 2014;39:791–797. doi: 10.1097/BRS.0000000000000295. [DOI] [PubMed] [Google Scholar]
- 32.Shiozaki T., Tabata T., Motohashi T., Kondo E., Tanida K., Okugawa T., Ikeda T. Preoperative management of patients with gynecologic malignancy complicated by existing venous thromboembolism. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012;164:85–88. doi: 10.1016/j.ejogrb.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 33.Sloan M., Sheth N., Lee G.-C. Is Obesity Associated with Increased Risk of Deep Vein Thrombosis or Pulmonary Embolism After Hip and Knee Arthroplasty? A Large Database Study. Clin. Orthop. Relat. Res. 2019;477:523–532. doi: 10.1097/CORR.0000000000000615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lieber B., Han J., Appelboom G., Taylor B.E., Han B., Agarwal N., Connolly E.S. Association of Steroid Use with Deep Venous Thrombosis and Pulmonary Embolism in Neurosurgical Patients: A National Database Analysis. World Neurosurg. 2016;89:126–132. doi: 10.1016/j.wneu.2016.01.033. [DOI] [PubMed] [Google Scholar]
- 35.Buchanan I.A., Lin M., Donoho D.A., Ding L., Giannotta S.L., Attenello F., Mack W.J., Liu J.C. Venous Thromboembolism After Degenerative Spine Surgery: A Nationwide Readmissions Database Analysis. World Neurosurg. 2019;125:e165–e174. doi: 10.1016/j.wneu.2019.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shen M., Cutrera N.J., Dodd A.C., Wallace C., Avilucea F.R., Melbourne C., Jahangir A.A., Mir H.H., Obremskey W.T., Sethi M.K. The risk of deep vein thrombosis in total joint patients compared to orthopaedic trauma patients: Need for new prevention guidelines. J. Clin. Orthop. Trauma. 2017;8(Suppl. 2):S52–S56. doi: 10.1016/j.jcot.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang J., Chen Z., Zheng J., Breusch S.J., Tian J. Risk factors for venous thromboembolism after total hip and total knee arthroplasty: A meta-analysis. Arch. Orthop. Trauma Surg. 2015;135:759–772. doi: 10.1007/s00402-015-2208-8. [DOI] [PubMed] [Google Scholar]
- 38.Enga K.F., Rye-Holmboe I., Hald E.M., Mathiesen E.B., Njølstad I., Wilsgaard T., Braekkan S.K., Løchen M.-L., Hansen J.-B. Atrial fibrillation and future risk of venous thromboembolism:the Tromso study. J. Thromb. Haemost. 2015;13:10–16. doi: 10.1111/jth.12762. [DOI] [PubMed] [Google Scholar]
- 39.Noel P., Gregoire F., Capon A., Lehert P. Atrial fibrillation as a risk factor for deep venous thrombosis and pulmonary emboli in stroke patients. Stroke. 1991;22:760–762. doi: 10.1161/01.STR.22.6.760. [DOI] [PubMed] [Google Scholar]