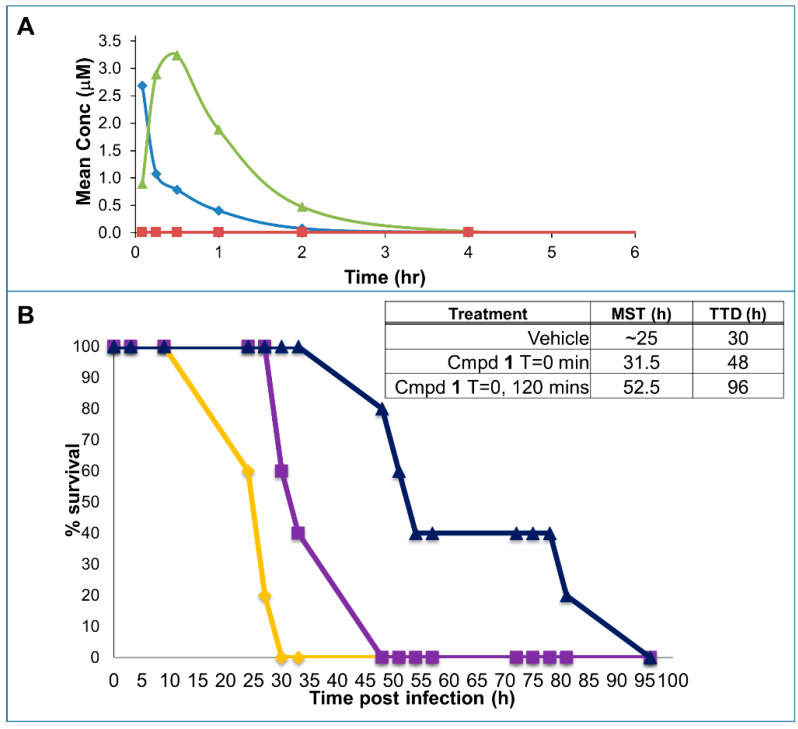
Figure 3.
In vivo pharmacology and efficacy of compound 1. (A) Preliminary pharmacokinetics (PK) studies in mice with compound 1. Groups of 3 mice each received compound 1 via the tail veil (I.V.) (1 mg/kg, blue), intraperitoneally (I.P.) (3 mg/kg, green), or orally (P.O.) (30 mg/kg, not shown as values were too low). Blood was collected at times 5 min, 15 min, 30 min, 60 min, 2 h, 4 h, 8 h. Mean AUC I.V. = 870 h × ng/mL; Mean AUC I.P. = 2600 h × ng/mL (%F I.P./I.V. ~100%). Mean AUC P.O. = 26 h × ng/mL (%F P.O./I.V. < 1%). As a reference, levels of drug necessary to attain 50% furin inhibition is also indicated (red). (B) Efficacy of compound 1 in the anthrax toxemia model. With cohorts of 5 mice per group, all received 100 µg LF and 100 µg PA I.V. at time 0. Furin inhibitor compound 1 (3 mg/kg) was administered I.P. either 15 min prior to the toxin (purple curve) or 15 min before and 1.75 h after the toxin (dark blue curve) and compared to vehicle control (orange curve).