Abstract
The newly designed luminol structures of pyrazolopyridopyridazine diones and N-aminopyrazolopyrrolopyridine diones were synthesized from versatile 1,3-diaryfuropyrazolopyridine-6,8-diones, 1,3-diarylpyrazolopyrrolopyridine-6,8-diones, or 1,3-diaryl-7-methylpyrazolopyrrolopyridine-6,8-diones with hydrazine monohydrate. Photoluminescent and solvatofluorism properties containing UV–Vis absorption, emission spectra, and quantum yield (Φf) study of pyrazolopyridopyridazine diones and N-aminopyrazolopyrrolopyridine diones were also studied. Generally, most of pyrazolopyrrolopyridine-6,8-diones 6 exhibited the significant fluorescence intensity and the substituent effect when compared with N-aminopyrazolopyrrolopyridine diones, particularly for 6c and 6j with a m-chloro group. Additionally, the fluorescence intensity of 6j was significantly promoted due to the suitable conjugation conformation. Based on the quantum yield (Φf) study, the value of compound 6j (0.140) with planar structural skeletal was similar to that of standard luminol (1, 0.175).
Keywords: pyrazolopyridopyridazine dione, N-aminopyrazolopyrrolopyridine dione, luminol, photoluminescence
1. Introduction
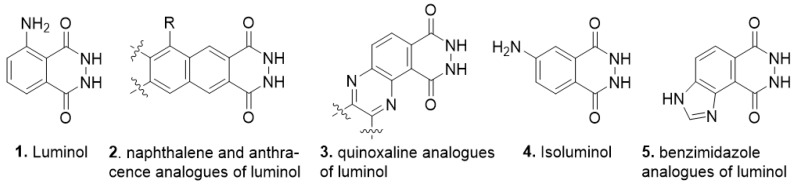
Sleep-disorders are one of the largest public health concerns in the whole world [1]. New functionalized pyrazolo [3,4-b]pyrrolo[3,4-d]pyridine derivatives were enthusiastically investigated to develop the increased potency and reduced side effects of novel sedative/hypnotic drug compounds for treatment of sleep-disorders [2,3]. On the other hand, pyrazolopyridopyridazine diones are well-known as the versatile precursors for synthesis of pyrazolopyridopyridazine phosphodiesterase type 5 (PDE5) inhibitors [4,5]. In recent years, chemiluminescent luminol derivatives have been an attractive detection technique in analytical applications such as presumptive test agents for latent blood detection [6,7,8,9], high-performance liquid chromatography (HPLC) [10,11], DNA, immunoassay, and cancer screening detection [12,13]. Since now, many newly designed luminol structures have been enthusiastically investigated to increase the chemiluminescence efficiency, intensity, sensitivity, quantum yield, or the recognition ability of the resulting chemiluminogens (Figure 1) [14,15,16,17,18,19].
Figure 1.
Luminol 1, naphthalene and anthracene 2, quinoxaline analogues of luminol 3, isoluminol 4, and benzimidazole analogues of luminol 5.
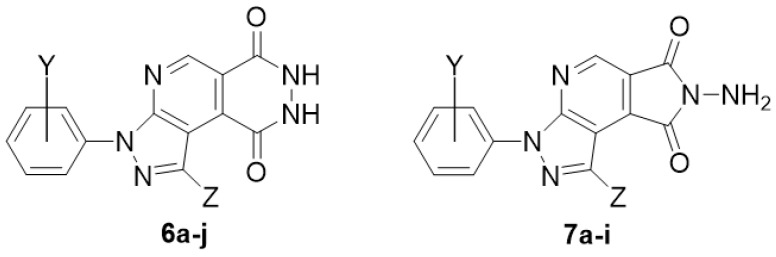
Furthermore, N-aminophthalimides were considered as phthalazine 1,4-dione tautomeric pairs [20,21]. N-Amino maleimides with pyridine heterocycle series also presented as a very important privileged substructure in organic synthesis for preparing diverse biologically active molecules [22]. Typically, the most important pharmacological effects that have been reported are potential antimicrobial [22] and anticancer activities [23]. Herein, we judiciously explore the insertion of pyridazinedione and N-Amino maleimide units into the pyrazolopyridine core ring for construction of the new designed luminol structures 6a–j and 7a–i from versatile 1,3-diarylpyrazolopyrrolopyridine-6,8-diones 11. Observably, we found that the series of pyridazinediones 6a–j would not only provide conjugation systems but also allow to modify the fluorescence intensity and biological activity (Figure 2).
Figure 2.
Pyrazolopyridopyridazine diones 6a–j and N-Aminopyrazolopyrrolopyridine diones 7a–i as luminol analogues.
2. Results and Discussion
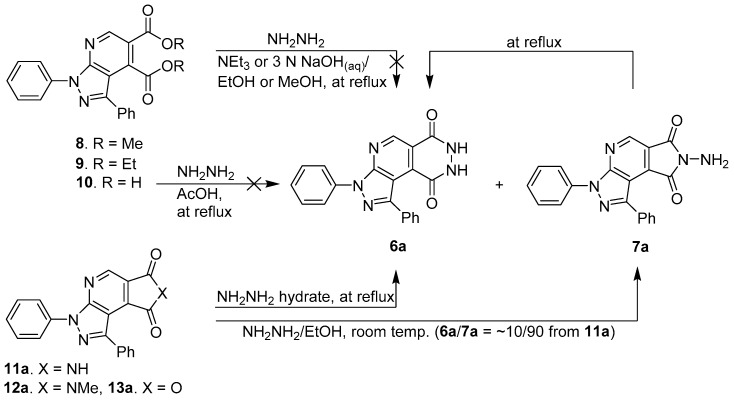
Initially, dimethyl 1,3-diphenyl-1H-pyrazolo[3,4-b]pyridine-4,5-dicarboxylate 8 and diethyl 1,3-diphenyl-1H-pyrazolo[3,4-b]pyridine-4,5-dicarboxylate 9 were prepared by following our previously reported literature [24] from N,N-diisopropylamidinyl pyrazolylimine and chosen as the model substrate for this investigation on the construction of pyrazolopyridopyridazine diones 6a (Scheme 1). Compounds 8 and 9 were reacted with hydrazine hydrate at reflux in methanol or ethanol solution under the basic condition for 24–36 h [25,26]. However, all the efforts for the predominant formation of 6a were unsuccessful. We also attempted to perform the hydrolysis of ester groups of compounds 8 and 9 under basic conditions to obtain 1,3-diphenyl-1H-pyrazolopyridine-4,5-dicarboxylic acid 10 [27,28]. Subsequently, pyrazolopyridine-4,5-dicarboxylic acid 10 was refluxed with hydrazine in acetic acid to carry out the cyclization for 8 h, but without success (Scheme 1).
Scheme 1.
Synthesis study of pyrazolopyridopyridazine dione 6a as luminol analogue.
In other attempts, we preliminarily tried to synthesize 1,3-phenylpyrazolopyrrolopyridine-6,8-dione 11a from N,N-diisopropylamidinyl pyrazolylimine with maleimide via our published InCl3/silica gel catalyzed hetero Diels-Alder reaction [29]. Subsequently, the resulting compound 11a was reacted with an excess of hydrazine hydrate in EtOH/H2O co-solution at room temperature for ~7 h [29,30]. The formation of the N-aminopyrazolopyrrolopyridine dione 7a was observed in 83% yield as the major product and accompanied with a trace amount of luminol-type pyrazolopyridopyridazine dione 6a (<10%, Scheme 1). Fortunately, compounds 6a and 7a can be successfully and selectively prepared via kinetic and thermodynamic control reactions [31,32].
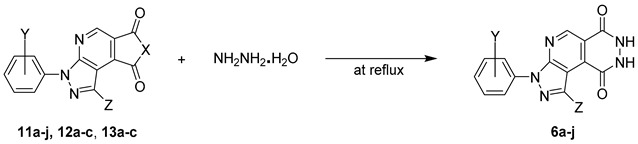
For further searching optimal conditions, we also prepared 7-methyl-1,3-phenylpyrazolopyrrolopyridine-6,8-dione 12a [29] and 1,3-diphenylfuropyrazolopyridine-6,8-dione 13a [33,34] as probes for monitoring cyclization tendency with hydrazine hydrate [29,30]. Most of the compounds 11a–13a were refluxed in neat hydrazine hydrate solution for ~5 h (Scheme 1 and Table 1). The reactions were monitored until the consumption of starting materials 11a–13a by TLC and produced the luminol-type pyrazolopyridopyridazine dione 6a. Compound 11a smoothly underwent the cyclization reaction to give luminol-type analogue 6a in better yield (84%, Entry 1, Table 1). However, compounds 12a–13a resulted in 32% and 18% low yields, respectively (Entries 2 and 3, Table 1). For further demonstration of reactivity efficiency, compounds 11b–c, 12b–c, and 13b–c bearing various substituents including o- and m-Cl in N-1-phenyl ring and phenyl at C-3 position of pyrazole moiety were synthesized and refluxed under the same condition (Entries 4–9, Table 1). Based on the experimental data of Table 1, the better yields of pyrazolopyridopyridazine dione products 6b–c were provided from 1,3-diarylpyrazolopyrrolopyridine-6,8-diones 11b–c (74% and 71%, Entries 4 and 7, Table 1). Unfortunately, 1,3-diaryl-7-methylpyrazolopyrrolopyridine-6,8-diones 12b–c and 1,3-diarylfuropyrazolopyridine-6,8-diones 13b–c showed poor reactivity for the formation of pyrazolopyridopyridazine diones 6b–c (Entries 5–6 and 8–9, Table 1).
Table 1.
The results of pyrazolopyridopyridazine diones 6a–j from reactants 11a–j, 12a–c, or 13a–c with hydrazine hydrate.
| Entry | S.M. | X | Y | Z | Reaction Time | Products | Yields (%) |
|---|---|---|---|---|---|---|---|
| 1 | 11a | NH | Ph | Ph | 5 | 6a | 84 |
| 2 | 12a | NMe | Ph | Ph | 5 | 6a | 32 |
| 3 | 13a | O | Ph | Ph | 5 | 6a | 18 |
| 4 | 11b | NH | o-Cl-Ph | Ph | 5 | 6b | 74 |
| 5 | 12b | NMe | o-Cl-Ph | Ph | 5 | 6b | 24 |
| 6 | 13b | O | o-Cl-Ph | Ph | 5 | 6b | 13 |
| 7 | 11c | NH | m-Cl-Ph | Ph | 5 | 6c | 71 |
| 8 | 12c | NMe | m-Cl-Ph | Ph | 5 | 6c | 38 |
| 9 | 13c | O | m-Cl-Ph | Ph | 5 | 6c | 11 |
| 10 | 11d | NH | p-Cl-Ph | Ph | 5 | 6d | 81 |
| 11 | 11e | NH | p-Br-Ph | Ph | 5 | 6e | 77 |
| 12 | 11f | NH | p-Me-Ph | Ph | 5 | 6f | 84 |
| 13 | 11g | NH | p-OMe-Ph | Ph | 5 | 6g | 81 |
| 14 | 11h | NH | p-CN-Ph | Ph | 5 | 6h | 73 |
| 15 | 11i | NH | p-NO2-Ph | Ph | 5 | 6i | 69 |
| 16 | 11j | NH | m-Cl-Ph | H | 5 | 6j | 71 |
Furthermore, we applied this reliable procedure to reactants 11d–j bearing p-Cl-Ph, p-Br-Ph, p-Me-Ph, p-OMe-Ph, p-CN-Ph, p-NO2-Ph, and m-Cl-Ph at the N-1 position and phenyl and H at C-3 position of pyrazolic ring. Various substituted reactants 11d–j were demonstrated to proceed smoothly. Both electron-donating and electron-withdrawing substituents were all well-tolerated in good yields (69–84%, Entries 10–16, Table 1). All of 1,3-diarylpyrazolopyrrolopyridine-6,8-diones 6a–j were fully characterized by spectroscopic methods. For example, compound 6a presented one singlet at δ 9.41 ppm for pyrazolopyridine ring N=CH–C=C in 1H-NMR and two peaks at δ 153.1 and 155.7 ppm for pyridazine dione carbon O=C–NH in 13C-NMR spectrum. Its IR absorptions showed peaks at 3161 cm−1 for stretching of the –NH group and at 1014 cm−1 for stretching of the N–N group.
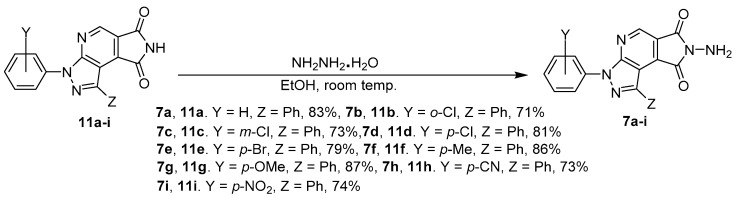
For the further controlled experiment for photoluminescence study, we also tried to prepare a series of N-aminopyrazolopyrrolopyridine diones 7a–i as the comparison cases (Scheme 2). Treatment of pyrazolopyrrolopyridine-6,8-diones 11a–i with 5.0 equivalents of hydrazine hydrate in EtOH/H2O co-solution was performed in an ice-bath to room temperature for 48 h. The corresponding N-aminopyrazolopyrrolopyridine diones 7a–i were obtained in 71–87% yields and characterized by spectroscopic methods. For example, compound 7a presented one singlet peak at δ 8.83 ppm for pyrazolopyridine ring N=CH–C=C in 1H-NMR and two peaks at δ 164.1 and 164.4 ppm for phthalimide moiety carbon O=C–NH in 13C-NMR spectrum. Its IR absorptions showed peaks at 3172 and 3276 cm−1 for stretching of the –NH2 group and at 1014 cm−1 for stretching of the N–N group. Due to the structural skeletons being very similar between 6 and 7, the identify method should be our next future evaluation.
Scheme 2.
The results of N-aminopyrazolopyrrolopyridine diones 7a–i from pyrazolopyrrolopyridine-6,8-diones 11a–i with hydrazine hydrate.
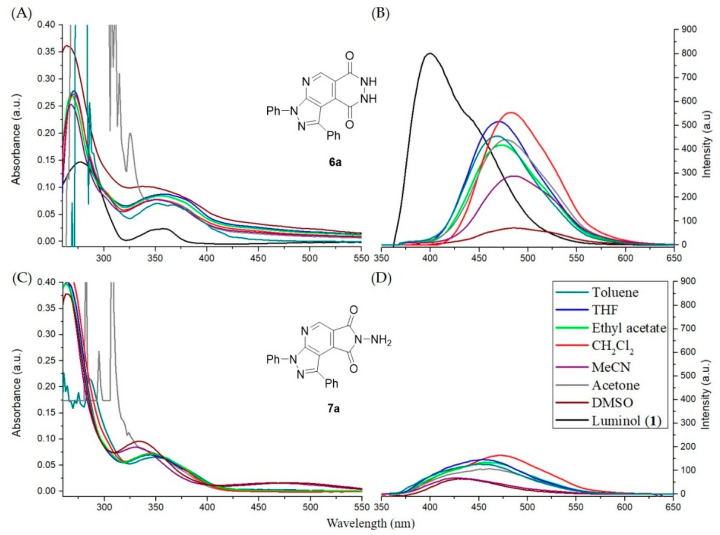
Luminol (1), compounds 6a and 7a were dissolved in DMSO to prepare a stock solution (1 × 10−3 M). Then the stock solutions of compounds 6a and 7a were individually diluted to a concentration of 10 μM in the presence of various solvents such as toluene, THF, ethyl acetate (EA), CH2Cl2, MeCN, acetone, and DMSO. The standard stock solution of luminol (10 μM) was diluted in DMSO solution as the standard sample. The UV–Vis absorption and fluorescence emission spectra of the pyrazolopyridopyridazine dione 6a and N-aminopyrazolopyrrolopyridine dione 7a compounds in the above-mentioned solution of varying polarities were reported in Table 2. The pyrazolopyridopyridazine dione 6a has better solubility in polar organic solvents, such as DMSO > THF > acetone, but N-aminopyrazolopyrrolopyridine dione 7a has the solubility only in highly polar solvents like DMSO. Luminol (1) was also measured and used as the standard sample. The UV–Vis absorption spectra of the compounds 6a and 7a in all the studied solvents were almost nearly the same; their absorption property is independent of the solvent polarity (Figure 3 and Table 2). All these compounds exhibit two highly intense absorption maxima peaks. Among these two, the first one was a high energy absorption between 253 nm and 286 nm for 6a and 7a probably due to the π−π* transition of the aryl core [35] while the low energy band between 329 nm and 366 nm is attributed to the intramolecular charge transfer transition (ICT). However, the rigidity in the structure of compounds 6a and 7a exhibited the stronger blue-shifted absorption (~15 nm) than luminol (1) in DMSO solution, as shown in Figure 3 and Table 2. In comparison with 6a and 7a, they demonstrated a similar absorption intensity, and compound 6a has obvious red-shift ~20 nm with respect to 7a.
Table 2.
UV-Vis absorption maximum and fluorescence emission peak wavelength of luminol (1), pyrazolopyridopyridazine dione 6a and N-aminopyrazolopyrrolopyridine dione 7a in the different solvents.
| Compound | Solvent | λmax/nm of UV-Vis | λmax/nm of PL |
|---|---|---|---|
| 6a | Toluene | - 1,366 | 469 |
| 6a | THF | 271,358 | 471 |
| 6a | Ethyl acetate | 268, 356 | 473 |
| 6a | CH2Cl2 | 271, 350 | 483 |
| 6a | MeCN | 268, 351 | 488 |
| 6a | Acetone | - 1, 353 | 477 |
| 6a | DMSO | 264, 338 | 486 |
| 7a | Toluene | 286, 1 ,348 | 452 |
| 7a | THF | 264, 344 | 454 |
| 7a | Ethyl acetate | 262, 343 | 459 |
| 7a | CH2Cl2 | 264, 344 | 471 |
| 7a | MeCN | 261, 329 | 425, 461 |
| 7a | Acetone | - 1, 338 | 452 |
| 7a | DMSO | 264, 335 | 429, 478 |
| Luminol (1) | DMSO | 350 | 392 |
1 It was overlapped with solvent absorption band.
Figure 3.
Photoluminescence spectra of luminol (1), pyrazolopyridopyridazine dione 6a and N-aminopyrazolopyrrolopyridine dione 7a in the different solvents. (A) Absorption and (B) emission spectra of luminol (1) and compound 6a. (C) Absorption and (D) emission spectra of compound 7a.
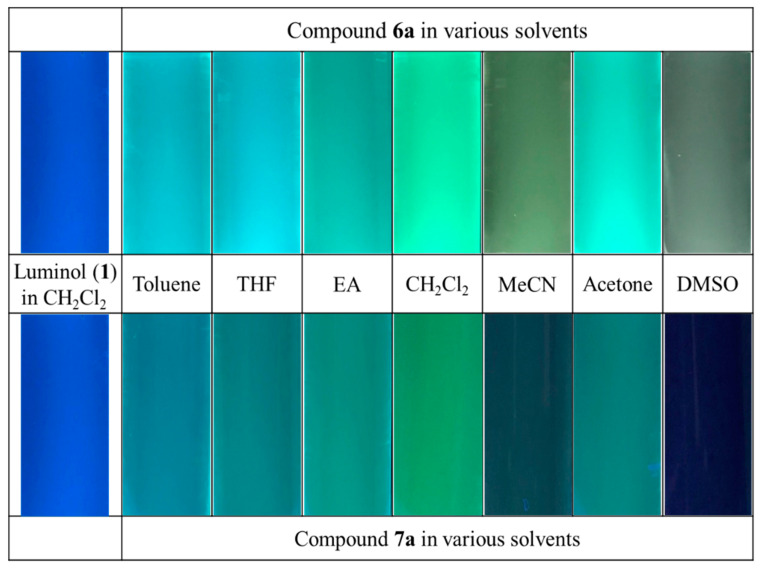
Consequently, we investigated the photoluminescence properties of the compounds 6a and 7a with luminol (1). For the fluorescence spectra, as shown in Figure 3 and Table 2, both the fluorescence intensity and the maximal position slightly varied depending on the solvent. Compound 6a displayed a characteristic emission band of the excitation wavelengths between 400 and 600 nm, and the λmaxs of PL was ~480 nm with the intense greenish-blue fluorescence in Figure 3 and Figure 4. For compound 7a, it’s emission spectrum was between 350 and 550 nm, and the λmaxs of PL was ~450 nm with the intense bluish-green fluorescence in Figure 3 and Figure 4. Compounds 6a and 7a exhibited a red-shift ~80 nm or ~60 nm as compared to luminol (1). Therefore, new luminol analogues 6a and 7a were efficiently conjugate and connect two chromophores (pyrazole and pyridine) to lead to an increase of aromaticity and provide the greenish-blue or bluish-green fluorescent materials (Table 2 and Figure 4) [36]. Particularly, the best positive solvatofluorism phenomenon was presented in CH2Cl2 solution. It was also beneficial for the visibility of the naked eye due to the bathochromic (red-shift) phenomenon from blue color to green (Figure 4).
Figure 4.
Color pictures of the fluorescence of compounds 6a and 7a in various solvents under excitation at 365 nm.
Moreover, the maximum of fluorescence wavelength and intensity, as shown in Figure 3, significantly vary with the diluted solvent. Further, we surprisingly observed the more significant solvent effect on compound 6a when compared with compound 7a. As shown in Figure 3, similar fluorescence spectra but a significant difference in intensity (∼6 times) were observed in varying solvents. Of note, it was interesting that toluene, THF, EA, and CH2Cl2 had differences in their polarity (toluene: 0.099, THF: 0.207, EA: 0.228, CH2Cl2: 0.309, with respect to the reference polarity of DMSO: 0.444) [37,38]. However, for the above solvents, we observed a strong intensity, in comparison to that for protic or/and polar solvent (DMSO). The intensities of fluorescence bands were reversed in protic or/and polar solvents. Therefore, the solvent polarity modulation of fluorescence was quite interesting. It was well studied that amide tautomer of pyrazolopyridopyridazine dione 6a was efficiently produced in toluene, THF, EA, and CH2Cl2 solvents [39,40,41,42,43,44]. In alcoholic (protic) and DMSO solvent, there exists competition between intermolecular bonding of the nearest hydrogen with the hydroxyimine tautomer of 6-hydroxypyrazolopyridopyridazin-9-one 6a. Therefore, different intensities of behavior were observed in different polarity solutions. On the other hand, the different fluorescence intensity between structural isomers 6a and 7a was also observed [45]. The aromaticity of compound 6a possessed the bathochromic shift of fluorescence maximum λmax by 12 nm and ∼4 times significant intensity in CH2Cl2 solution when compared with compound 7a (Table 2 and Figure 3) [46]. However, the intramolecular and intermolecular hydrogen bondings between the amino and carbonyl groups of N-aminopyrazolopyrrolopyridine dione 7a were formed to lead to the poor intensity in solution.
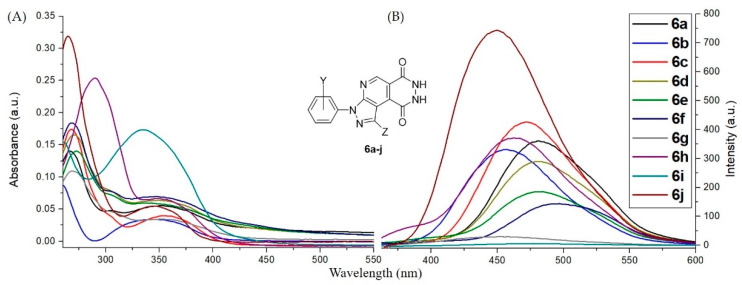
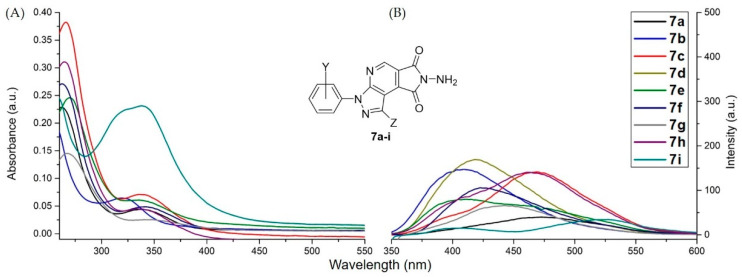
For further investigation of substituent efficiency of compounds 6 and 7 in photoluminescence properties, we synthesized a series of pyrazolopyridopyridazine diones 6a–j and N-aminopyrazolopyrrolopyridine diones 7a–i bearing various substituents including o-, m- and p-Cl, p-Br, p-Me, p-OMe, p-CN, and p-NO2 groups in N1-phenyl ring of pyrazole moiety. Generally, most of the substituents such as o-, m- and p-Cl, p-Br, p-Me, and p-CN in N1-phenyl of pyrazolic ring of compounds 6 possessed the blue-shift phenomenon range ~10 to 30 nm with significant fluorescence intensity when compared with compound 6a, particularly for 6c with meta-chloro group (Figure 5). For compounds 6g and 6i with the strong electron-donating (p-OMe) or electron-withdrawing groups (p-NO2), they exhibited negative photoluminescence properties (Figure 5). While we modified the skeletal structure of pyrazolopyridopyridazine dione 6j, in which Ph-group was replaced to H atom on C-3 position of pyrazolic ring, the blue-shift phenomenon was remarkably observed in photoluminescence spectra. Additionally, the fluorescence intensity of 6j was significantly promoted about 2.3 times in comparison with compound 6a (Figure 5). Based on the result of the substituent study, we conceived that compound 6j was an effective substrate that possessed suitable conjugation conformation without the torsion effect to facilitate the photoluminescence properties [26]. For compounds 7a–i bearing the above various substituents, they provided the weak fluorescence intensity [45] and possessed the blue-shift phenomenon when compared with 7a, except for 7c with m-chloro group and 7h with p-CN group (Figure 6). Generally, compounds 7a–i were the inappropriate photoluminescent substrates [45].
Figure 5.
Photoluminescence spectra of pyrazolopyridopyridazine diones 6a–j dissolved in DMSO to prepare a stock solution (1.0 mM). Then the stock solutions were diluted with CH2Cl2 to a concentration of 10 μM. (A) Absorption and (B) emission spectra of compounds 6a–j.
Figure 6.
Photoluminescence spectra of N-aminopyrazolopyrrolopyridine diones 7a–i dissolved in DMSO to prepare a stock solution (1.0 mM). Then the stock solutions were diluted with CH2Cl2 to a concentration of 10 μM. (A) Absorption and (B) emission spectra of compounds 7a–i.
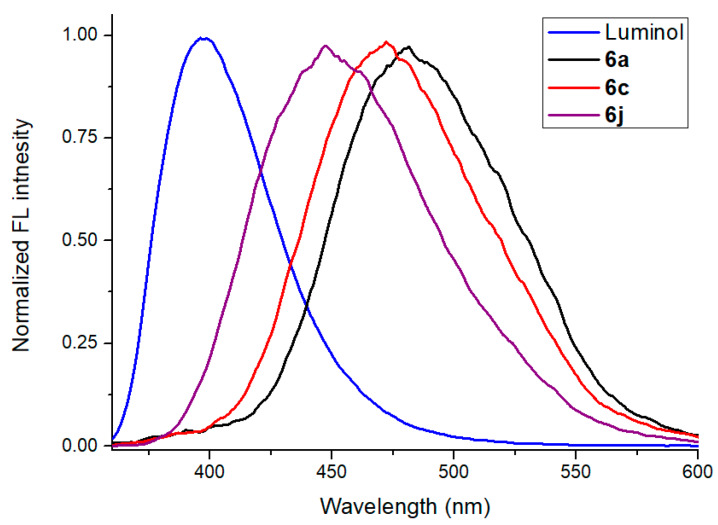
The quantum yields (Φf) of luminol (1) and pyrazolopyridopyridazine diones 6a, 6c, and 6j were measured in the CH2Cl2 solution using quinine sulfate in 0.05M H2SO4 (Φf = 0.60) as the standard (excitation wavelength 350 nm) [47,48]. The quantum yields (Φf) values of luminol (1) and pyrazolopyridopyridazine diones 6a, 6c, and 6j were estimated as 0.175, 0.056, 0.067, and 0.140 in CH2Cl2 solution, respectively, indicating that the Φf value of 6j was similar to that of luminol (1, Table 3). Moreover, we also investigated the quantum yields of 6j in various solvents by using the same condition. The estimated values order trendy was as 0.218 (THF) > 0.209 (Toluene) > 0.140 (CH2Cl2) > 0.083 (acetone) > 0.049 (EA), indicating THF provided the largest Φf value among them (Table 3). On the other hand, most of the quantum yields (Φf) pyrazolopyridopyridazine diones 6a–i in CH2Cl2 solution were predicted to be an almost identical value (ca. 0.05–0.06). Interestingly, the high Φf value of 6j was obtained and possibly caused by a particular improvement in the planar skeletal conformation (Table 3 and Figure 7).
Table 3.
Quantum yields of fluorescence of luminol (1) and pyrazolopyridopyridazine diones 6a, 6c, and 6j.
| Compound | Solvent | λfl 1/nm | Φf 2 |
|---|---|---|---|
| 6a | CH2Cl2 | 481 | 0.056 |
| 6c | CH2Cl2 | 472 | 0.067 |
| 6j | CH2Cl2 | 450 | 0.140 |
| 6j | THF | 435 | 0.218 |
| 6j | Toluene | 438 | 0.209 |
| 6j | Acetone | 437 | 0.083 |
| 6j | Ethyl acetate | 437 | 0.049 |
| Luminol (1) | CH2Cl2 | 399 | 0.175 |
1 Fluorescence maximum wavelength (λfl). 2 Φf: Fluorescence quantum efficiency, relative to quinine sulfate (Φf = 0.60).
Figure 7.
Normalized fluorescence spectra of luminol and pyrazolopyridopyridazine diones 6a, 6c, and 6j in the CH2Cl2 solution (excitation wavelength 350 nm).
3. Experimental Section
3.1. General Information
All reagents were used as obtained commercially. All reactions were carried out under argon or nitrogen atmosphere and monitored by thin-layer chromatography (TLC). Flash column chromatography was carried out on silica gel (230–400 mesh). Analytical thin-layer chromatography was performed using pre-coated plates (silica gel 60 F-254) purchased from Merck Inc. Flash column chromatography purification was carried out by gradient elution using n-hexane in ethyl acetate (EtOAc) unless otherwise stated. 1H-NMR was recorded at 400, 500, or 600 MHz and 13C-NMR recorded at 100, 125, or 150 MHz, respectively, in DMSO-d6 as the solvent. The standard abbreviations s, d, t, q, and m refer to the singlet, doublet, triplet, quartet, and multiplet, respectively. Coupling constant (J), whenever discernible, have been reported in Hz. Infrared spectra (IR) were recorded as neat solutions or solids; mass spectra were recorded using electron impact or electrospray ionization techniques. The wavenumbers reported are referenced to the polystyrene 1601 cm−1 absorption. ESI-MS analyses were performed on an Applied Biosystems API 300 mass spectrometer. High-resolution mass spectra (HRMS) were recorded on a JEOL JMS-HX110 mass spectrometer with an electron ionization (EI) source The UV-visible absorption and emission spectra were performed on a Perkin-Elmer Lambda 265 and Perkin-Elmer LS50B, a fused quartz cuvette (10 mm × 10 mm) at room temperature, respectively. Quantum yields were obtained by using quinine sulfate (0.60 in 0.05 M H2SO4) as a reference. Stock solutions (1 × 10−3 M) of luminol (1), compounds of 6a–j and 7a–i were prepared in dimethyl sulfoxide (DMSO).
3.2. Standard Procedure for Synthesis of Pyrazolopyridopyridazine Diones 6a–j
The reliable procedure involved the treatment of 1,3-diarylpyrazolopyrrolopyridine-6,8-diones (11a–j), 1,3-diaryl-7-methylpyrazolopyrrolopyridine-6,8-diones (12a–c) 1,3-diarylfuropyrazolopyridine-6,8-diones (13a–c, 1.0 equiv.) with hydrazine monohydrate (~40 equiv.) in neat solution at reflux for 5 h. When the reaction was completed, the reaction mixture was added to water (10 mL) for precipitation. The precipitate was filtered, washed with cold water (10 mL), and n-hexane/EA (1/2, 15 mL) to give the corresponding crude pyrazolopyridopyridazine diones 6a–j. The crude desired products 6a–j were recrystallized in acetone/THF (1/4) solution to obtain the pure pyrazolopyridopyridazine diones 6a–j in 11–84% yields. The low solubility of the compounds 6a–j made the 13C-NMR characterization of quaternary and carbonyl carbons of these substrates unclear [25,26].
1,3-Diphenyl-7,8-dihydro-3H-pyrazolo[4′,3′:5,6]pyrido[3,4-d]pyridazine-6,9-dione (6a), Light yellow solid; yield: 84%; mp 292–295 °C. 1H-NMR (DMSO-d6, 600 MHz) δ 7.43–7.47 (m, 4H, ArH), 7.60–7.64 (m, 4H, ArH), 8.20 (d, J = 7.9 Hz, 2H, ArH), 9.43 (s, 1H, ArH), 10.20 (br, 1H, NH); 13C{1H} NMR (DMSO-d6, 150 MHz) δ 109.55, 122.46 (2 × CH), 126.67, 127.16, 127.78, 129.26 (2 × CH + 2 × CH), 130.21 (2 × CH + CH), 134.99, 138.16, 147.54, 149.49, 151.35, 153.28, 155.21; FT-IR (KBr) v: 3161, 3033, 2907, 1662, 1584, 1499, 1414, 1356, 1306, 906 cm−1; MS (EI) m/z (relative intensity): 356 (24), 355 (M+, 100), 354 (27), 270 (24), 269 (12), 268 (12), 77 (39); HRMS (EI) m/z: [M]+ Calcd for C20H13N5O2: 355.1069; found: 355.1065.
3-(2-Chlorophenyl)-1-phenyl-7,8-dihydro-3H-pyrazolo[4′,3′:5,6]pyrido[3,4-d]pyridazine-6,9-dione (6b), Yellow-brown solid; yield: 74%; mp 332–335 °C; 1H-NMR (DMSO-d6, 500 MHz) δ 7.40 (br, 3H, ArH), 7.60–7.61 (m, 2H, ArH), 7.63 (d, J = 7.5 Hz, 1H, ArH), 7.67 (t, J = 7.5 Hz, 1H, ArH), 7.79–7.80 (m, 2H, ArH), 9.35 (s, 1H, ArH); 13C{1H} NMR (DMSO-d6, 125 MHz) δ 109.33, 124.53, 126.66, 127.77, 128.01, 128.18, 128.45, 130.23 (2 × CH), 130.30, 130.47, 131.33, 131.36, 134.73, 134.94, 147.84, 149.81, 152.65, 155.49, 156.94; FT-IR (KBr) v: 3427, 3281, 3060, 2921, 1621, 1561, 1508, 1430, 1351, 905 cm−1; MS (EI) m/z (relative intensity): 391 (M+ + 2, 29), 390 (22), 389 (M+, 100), 355 (12), 354 (52), 304 (15), 268 (17), 111 (13), 77 (44); HRMS (EI) m/z: [M]+ Calcd for C20H12ClN5O2: 389.0680; found: 389.0678.
3-(3-Chlorophenyl)-1-phenyl-7,8-dihydro-3H-pyrazolo[4′,3′:5,6]pyrido[3,4-d]pyridazine-6,9-dione (6c), Yellow solid; yield: 71%; mp 228–229 °C; 1H-NMR (DMSO-d6, 500 MHz) δ 7.42–7.43 (m, 3H, ArH), 7.50 (d, J = 6.7 Hz, 1H, ArH), 7.60 (d, J = 5.2 Hz, 2H, ArH), 7.64 (t, J = 8.0 Hz, 1H, ArH), 8.26 (d, J = 8.0 Hz, 1H, ArH), 8.34 (s, 1H, ArH), 9.44 (s, 1H, ArH), 12.06 (br, 1H, NH); 13C{1H} NMR (DMSO-d6, 125 MHz) δ 107.59, 119.56, 120.40, 121.43, 126.72 (3 × CH), 127.93, 130.14 (2 × CH + 1 × C), 130.99, 133.49, 134.67, 139.34, 148.03, 149.66, 151.53, 155.16, 157.57; FT-IR (KBr) v: 3453, 3344, 3296, 1651, 1595, 1483 cm−1; MS (EI) m/z (relative intensity): 391 (M+ + 2, 33), 390 (27), 389 (M+, 100), 388 (14), 304 (14), 111 (11), 77 (17); HRMS (EI) m/z: [M]+ Calcd for C20H12ClN5O2: 389.0680; found: 389.0686.
3-(4-Chlorophenyl)-1-phenyl-7,8-dihydro-3H-pyrazolo[4′,3′:5,6]pyrido[3,4-d]pyridazine-6,9-dione (6d), Light yellow solid; yield: 81%; mp 339–341 °C; 1H-NMR (DMSO-d6, 500 MHz) δ 7.40–7.41 (m, 3H, ArH), 7.58–7.60 (m, 2H, ArH), 7.68 (d, J = 8.9 Hz, 2H, ArH), 8.29 (d, J = 8.9 Hz, 2H, ArH), 9.41 (s, 1H, ArH); 13C{1H} NMR (DMSO-d6, 125 MHz) δ 107.56, 123.52 (2 × CH), 126.60 (2 × CH), 126.64, 127.77, 127.82, 129.23 (2 × CH), 130.18 (2 × CH), 131.06, 134.89, 137.12, 147.88, 149.80, 151.35, 156.76, 157.30; FT-IR (KBr) v: 3345, 3206, 1656, 1494, 1446, 1307, 1094, 902 cm−1; MS (EI) m/z (relative intensity): 391 (M+ + 2, 36), 390 (31), 389 (M+, 100), 388 (20), 354 (12), 304 (19), 268 (11), 111 (15), 77 (24); HRMS (EI) m/z: [M]+ Calcd for C20H12ClN5O2: 389.0680; found: 389.0687.
3-(4-Bromophenyl)-1-phenyl-7,8-dihydro-3H-pyrazolo[4′,3′:5,6]pyrido[3,4-d]pyridazine-6,9-dione (6e), Light yellow solid; yield: 77%;mp 337–339 °C; 1H-NMR (DMSO-d6, 500 MHz) δ 7.40–7.41 (m, 3H, ArH), 7.59 (d, J = 5.5 Hz, 2H, ArH), 7.82 (d, J = 9.0 Hz, 2H, ArH), 8.25 (d, J = 9.0 Hz, 2H, ArH), 9.42 (s, 1H, ArH); 13C{1H} NMR (DMSO-d6, 125 MHz) δ 107.67, 119.42, 123.81 (2 × CH), 126.64 (2 × CH), 127.82, 130.16 (2 × CH + C), 132.16 (2 × CH + C), 134.88, 137.56, 147.92, 149.78, 151.36, 157.59, 159.25; FT-IR (KBr) v: 3435, 3345, 3266, 1655, 1536, 1492, 1443, 1307, 1094, 916, 902 cm−1; MS (EI) m/z (relative intensity): 436 (24), 435 (M+ + 2, 98), 434 (39), 433 (M+, 100), 432 (14), 354 (11), 350 (11), 348 (12), 268 (14), 77 (26); HRMS (EI) m/z: [M]+ Calcd for C20H12BrN5O2: 433.0174; found: 433.0171.
1-Phenyl-3-(p-tolyl)-7,8-dihydro-3H-pyrazolo[4′,3′:5,6]pyrido[3,4-d]pyridazine-6,9-dione (6f), Light yellow solid; yield: 84%; mp 346–348 °C; 1H-NMR (DMSO-d6, 500 MHz) δ 2.40 (s, 3H, CH3), 7.39–7.42 (m, 5H, ArH), 7.58–7.60 (m, 2H, ArH), 8.07 (d, J = 8.4 Hz, 2H, ArH), 9.39 (s, 1H, ArH); 13C{1H} NMR (DMSO-d6, 125 MHz) δ 20.61, 106.97, 118.99, 122.32 (2 × CH), 126.60 (2 × CH), 127.68, 128.86, 129.60 (2 × CH), 130.21 (2 × CH), 135.09, 135.83, 136.55, 147.26, 149.35, 151.18, 152.87, 155.65; FT-IR (KBr) v: 3436, 3345, 3206, 2919, 1656, 1534, 1514, 1480, 1453, 1310, 1096, 903 cm−1; MS (EI) m/z (relative intensity): 370 (25), 369 (M+, 100), 368 (16), 354 (14), 284 (18), 91 (15), 77 (19); HRMS (EI) m/z: [M]+ Calcd for C21H15N5O2: 369.1226; found: 369.1216.
3-(4-Methoxyphenyl)-1-phenyl-7,8-dihydro-3H-pyrazolo[4′,3′:5,6]pyrido[3,4-d]pyridazine-6,9-dione (6g), Deep yellow solid; yield: 81%; mp 311–313 °C; 1H-NMR (DMSO-d6, 500 MHz) δ 3.85 (s, 3H, OCH3), 7.17 (d, J = 11.2 Hz, 2H, ArH), 7.43 (s, 3H, ArH), 7.58–7.60 (m, 2H, ArH), 8.03 (d, J = 11.2 Hz, 2H, ArH), 9.38 (s, 1H, ArH); 13C{1H} NMR (DMSO-d6, 125 MHz) δ 55.49, 106.64, 114.38 (2 × CH), 115.59, 124.31 (2 × CH), 124.60, 126.71 (2 × CH), 127.77, 130.23 (2 × CH), 131.18, 135.03, 147.01, 149.17, 151.12, 152.63, 156.80, 158.33; FT-IR (KBr) v: 3435, 3226, 3065, 2886, 1650, 1590, 1535, 1516, 1441, 1362, 1252, 1170, 905 cm−1; MS (EI) m/z (relative intensity): 386 (24), 385 (M+, 100), 370 (13), 77 (18); HRMS (EI) m/z: [M]+ Calcd for C21H15N5O3: 385.1175; found: 385.1180.
3-(4-Cyanophenyl)-1-phenyl-7,8-dihydro-3H-pyrazolo[4′,3′:5,6]pyrido[3,4-d]pyridazine-6,9-dione (6h), Yellow solid; yield: 73%; mp 344–347 °C; 1H-NMR (DMSO-d6, 500 MHz) δ 7.43–7.44 (m, 3H, ArH), 7.60–7.61 (m, 2H, ArH), 8.08 (d, J = 6.6 Hz, 2H, ArH), 8.56 (d, J = 6.6 Hz, 2H, ArH), 9.45 (s, 1H, ArH); 13C{1H} NMR (DMSO-d6, 125 MHz) δ 108.84, 118.51, 121.39, 121.70 (2 × CH), 126.74 (2 × CH), 128.07, 128.63, 130.08 (2 × CH), 130.15, 133.62 (2 × CH), 134.51, 141.67, 148.75, 149.75, 151.87, 155.15, 156.71; FT-IR (KBr) v: 3397, 3284, 3056, 2228, 1606, 1569, 1516, 1430, 1400, 1317, 905 cm−1; MS (EI) m/z (relative intensity): 381 (26), 380 (M+, 100), 379 (23), 295 (17), 102 (13), 77(29); HRMS (EI) m/z: [M]+ Calcd for C21H12N6O2: 380.1022; found: 380.1030.
3-(4-Nitrophenyl)-1-phenyl-7,8-dihydro-3H-pyrazolo[4′,3′:5,6]pyrido[3,4-d]pyridazine-6,9-dione (6i), Yellow solid; yield: 69%; mp 340–342 °C; 1H-NMR (DMSO-d6, 500 MHz) δ 7.41–7.44 (m, 3H, ArH), 7.61 (d, J = 6.3 Hz, 2H, ArH), 8.48 (d, J = 8.3 Hz, 2H, ArH), 8.67 (d, J = 8.3 Hz, 2H, ArH), 9.47 (s, 1H, ArH), 12.11 (br, 1H, NH); 13C{1H} NMR (DMSO-d6, 125 MHz) δ 113.65, 121.61 (2 × CH), 124.52, 125.13 (2 × CH), 126.85 (2 × CH), 128.22, 130.12 (2 × CH), 130.27, 134.47, 143.27, 145.04, 149.17, 149.92, 152.09, 154.07, 158.18; FT-IR (KBr) v: 3435, 1637, 1596, 1522, 1341, 1112, 905 cm−1; MS (EI) m/z(relative intensity): 401 (23), 400 (M+, 100), 370 (22), 315 (11), 77(19); HRMS (EI) m/z: [M]+ Calcd for For C20H12N6O4: 400.0920; found: 400.0919.
3-(3-Chlorophenyl)-7,8-dihydro-3H-pyrazolo[4′,3′:5,6]pyrido[3,4-d]pyridazine-6,9-dione (6j), Light yellow solid; yield: 71%; mp 351–352 °C; 1H-NMR (DMSO-d6, 500 MHz) δ 7.51 (d, J = 8.03 Hz, 1H, ArH), 7.66 (t, J = 8.0 Hz, 1H, ArH), 8.28 (d, J = 8.0 Hz, 1H, ArH), 8.39 (s, 1H, ArH), 8.88 (s, 1H, ArH), 9.40 (s, 1H, ArH), 10.20 (br, 1H, NH); 13C{1H} NMR (DMSO-d6, 125 MHz) δ 109.53, 119.80, 120.84, 126.68 (2 × C), 131.14 (CH + C), 133.57, 136.04, 139.62, 149.36, 150.78, 152.47, 155.87; FT-IR (KBr) v: 3433, 3294, 3168, 2974, 1639, 1594, 1568, 1487, 1448, 1274, 1218, 1125 cm−1; MS (EI) m/z (relative intensity): 315 (M+ + 2, 35), 314 (28), 313 (M+, 100), 278 (12), 255 (13), 227 (21), 111(12), 75 (11); HRMS (EI) m/z: [M]+ Calcd for C14H8ClN5O2: 313.0367; found: 313.0367.
3.3. Standard Procedure for Synthesis of N-Aminopyrazolopyrrolopyridine Diones (7a–i)
The reliable procedure involved the treatment of 1,3-diarylpyrazolopyrrolopyridine-6,8-diones (11a–i, 1.0 equiv.) with hydrazine monohydrate (~5.0 equiv.) in EtOH/H2O (2.0 mL/2.0 mL) in ice-bath to room temperature within 48 h. When the reaction was completed, the reaction mixture was added to water (10 mL) for precipitation. The precipitate was filtered, washed with cold water (10 mL) and n-hexane/EA (1/2, 15 mL) to give the corresponding crude N-aminophthalimides 7a–i. The crude desired products 7a–i were recrystallized in acetone/THF (1/4) solution to obtain the pure N-aminophthalimides 7a–i in 71–87 % yields [31,32].
7-Amino-1,3-diphenylpyrazolo[3,4-b]pyrrolo[3,4-d]pyridine-6,8-(3H,7H)-dione (7a), White solid; yield: 83%; mp 217–219 °C; 1H-NMR (DMSO-d6, 400 MHz) δ 4.55 (br, 2H, NH2), 7.42 (t, J = 7.5 Hz, 1H, ArH), 7.49–7.55 (m, 3H, ArH), 7.60–7.65 (m, 4H, ArH), 8.25 (d, J = 8.1 Hz, 2H, ArH), 8.82 (s, 1 H, ArH); 13C{1H} NMR (DMSO-d6, 100 MHz) δ 111.65, 121.43 (2 × CH), 123.37, 126.80, 128.27 (2 × CH), 128.58 (2 × CH), 128.96, 129.41 (2 × CH), 132.04, 138.44, 139.23, 146.02, 148.82, 150.61, 164.18, 164.49; FT-IR (KBr) v: 3275, 3208, 3172, 3035, 1633.0, 1572, 1518.7, 1501 cm−1; MS (EI) m/z (relative intensity): 356 (20), 355 (M+, 100), 354 (18), 270 (13), 77.0(18); HRMS (EI) m/z: [M]+ Calcd for C20H13N5O2: 355.1069; found: 355.1060.
7-Amino-3-(2-chlorophenyl)-1-phenylpyrazolo[3,4-b]pyrrolo[3,4-d]pyridine-6,8-(3H,7H)-dione (7b), Yellow solid; yield: 71%; mp 165–166 °C; 1H-NMR (DMSO-d6, 500 MHz) δ 4.52 (br, 2H, NH2), 7.48 (d, J = 7.0 Hz, 1H, ArH), 7.52 (d, J = 7.1 Hz, 2H, ArH), 7.60–7.68 (m, 4H, ArH), 7.74 (d, J = 7.7 Hz, 1H, ArH), 7.79 (d, J =7.7 Hz, 1H, ArH), 8.71 (s, 1H, ArH); 13C{1H} NMR (DMSO-d6, 125 MHz) δ 108.50, 122.16, 122.88, 123.33, 127.72 (2 × CH), 129.27 (3 × CH), 129.72 (2 × CH), 131.26, 131.46, 136.60, 136.98, 143.86, 145.72, 153.20, 166.81, 168.47; FT-IR (KBr) v: 3337, 3296, 2952, 2920, 1778, 1740, 1498, 1375, 1315, 1014 cm−1; MS (EI) m/z (relative intensity): 391 (M+ + 2, 32), 390 (22), 389 (M+, 100), 355 (18), 354 (90), 304 (12), 268 (11), 77 (18); HRMS (EI) m/z: [M]+ Calcd for C20H12ClN5O2: 389.0680; found: 389.0672.
7-Amino-3-(3-chlorophenyl)-1-phenylpyrazolo[3,4-b]pyrrolo[3,4-d]pyridine-6,8-(3H,7H)-dione (7c), Yellow solid; yield: 73%; mp 173–175 °C; 1H-NMR (DMSO-d6, 400 MHz) δ 4.55 (br, 2H, NH2), 7.47–7.54 (m, 4H, ArH), 7.64–7.67 (m, 3H, ArH), 8.31 (d, J = 8.9 Hz, 1H, ArH), 8.42 (s, 1H, ArH), 8.86 (s, 1H, ArH); 13C{1H} NMR (DMSO-d6, 100 MHz) δ 112.05, 119.33, 120.34, 123.72, 126.32, 128.28 (2 × CH), 128.57 (2 × CH), 129.11, 131.21, 131.73, 133.65, 139.41, 139.64, 146.61, 148.98, 150.72, 163.96, 164.33; FT-IR (KBr) v: 3264, 3168, 3034, 1649, 1614, 1595, 1488, 1431, 1300, 803 cm−1; MS (EI) m/z (relative intensity): 391 (M+ + 2, 33), 390 (24), 389 (M+, 100), 374 (13), 304 (11), 77 (14); HRMS (EI) m/z: [M]+ Calcd for C20H12ClN5O2: 389.0680; found: 389.0688.
7-Amino-3-(4-chlorophenyl)-1-phenylpyrazolo[3,4-b]pyrrolo[3,4-d]pyridine-6,8-(3H,7H)-dione (7d), Light yellow solid; yield: 81%; mp 226–227 °C; 1H-NMR (DMSO-d6, 500 MHz) δ 4.55 (br, 2H, NH2), 7.48–7.55 (m, 3H, ArH), 7.64 (d, J = 7.6 Hz, 2H, ArH), 7.69 (d, J = 8.4 Hz, 2H, ArH), 8.35 (d, J = 8.4 Hz, 2H, ArH), 8.84 (s, 1 H, ArH); 13C{1H} NMR (DMSO-d6, 125 MHz) δ 111.85, 122.54 (2 × CH), 123.55, 128.24 (2 × CH), 128.53 (2 × CH), 129.03, 129.36 (2 × CH), 130.67, 131.81, 137.31, 139.34, 146.35, 148.88, 150.57, 163.99, 164.34; FT-IR (KBr) v: 3275, 3207, 3170, 1633, 1499, 828 cm−1; MS (EI) m/z (relative intensity): 391 (M+ + 2, 34), 390 (28), 389 (M+, 100), 388 (15), 304 (12), 77 (13); HRMS (EI) m/z: [M]+ Calcd for C20H12ClN5O2: 389.0680; found: 389.0686.
7-Amino-3-(4-bromophenyl)-1-phenylpyrazolo[3,4-b]pyrrolo[3,4-d]pyridine-6,8-(3H,7H)-dione (7e), Yellow solid; yield: 79%; mp 235–239 °C; 1H-NMR (DMSO-d6, 500 MHz) δ 4.55 (br, 2H, NH2), 7.49–7.55 (m, 3H, ArH), 7.64 (d, J = 6.9 Hz, 2H, ArH), 7.82 (d, J = 8.7 Hz, 2H, ArH), 8.29 (d, J = 8.7 Hz, 2H, ArH), 8.84 (s, 1 H, ArH); 13C{1H} NMR (DMSO-d6, 125 MHz) δ 111.91, 118.95, 122.81 (2 × CH), 123.55, 128.25 (2 × CH), 128.54 (2 × CH), 129.04, 131.80, 132.28 (2 × CH), 137.75, 139.33, 146.40, 148.90, 150.59, 164.00, 164.35; FT-IR (KBr) v: 3275, 3208, 3170, 3071, 3037, 1632, 1495, 826 cm−1; MS (EI) m/z (relative intensity): 436 (22), 435 (M+ + 2, 100), 354 (33), 433 (M+, 99), 432 (12), 420 (19), 419 (11), 418 (19), 354 (11), 350 (10), 348 (11), 268 (17), 77 (26); HRMS (EI) m/z: [M]+ Calcd for C20H12BrN5O2: 433.0174; found: 433.0171.
7-Amino-1-phenyl-3-(p-tolyl)pyrazolo[3,4-b]pyrrolo[3,4-d]pyridine-6,8-(3H,7H)-dione (7f), Yellow solid; yield: 86%; mp 232–233 °C; 1H-NMR (DMSO-d6, 500 MHz) δ 2.40 (s, 3H, CH3), 4.55 (br, 2H, NH2), 7.42 (d, J = 8.0 Hz, 2H, ArH), 7.49 (d, J = 7.3 Hz, 1H, ArH), 7.52 (t, J = 7.3 Hz, 2H, ArH), 7.64 (d, J = 7.3 Hz, 2H, ArH), 8.12 (d, J = 8.0 Hz, 2H ArH), 8.81 (s, 1H, ArH); 13C{1H} NMR (DMSO-d6, 125 MHz) δ 20.63, 111.44, 121.35(2 × CH), 123.18, 128.21 (2 × CH), 128.54 (2 × CH), 128.85, 129.73 (2 × CH), 132.09, 136.06, 136.18, 139.13, 145.69, 148.72, 150.44, 164.20, 164.50; FT-IR (KBr) v: 3276, 3209, 3171, 3032, 1634, 1517 cm−1; MS (EI) m/z (relative intensity): 370 (25), 369 (M+, 100), 368 (13), 354 (21), 284 (12), 207 (10), 91.1(11), 77.1(13); HRMS (EI) m/z: [M]+ Calcd for C21H15N5O2: 369.1226; found: 369.1231.
7-Amino-3-(4-methoxyphenyl)-1-phenylpyrazolo[3,4-b]pyrrolo[3,4-d]pyridine-6,8-(3H,7H)-dione (7g), Yellow solid; yield: 87%; mp 331–332 °C; 1H-NMR (DMSO-d6, 500 MHz) δ 3.83 (s, 3H, OCH3), 4.53 (br, 2H, NH2), 7.17 (d, J = 8.7 Hz, 2H, ArH), 7.48–7.53 (m, 3H, ArH), 7.62 (d, J = 7.1 Hz, 2H, ArH), 8.06 (d, J = 8.7 Hz, 2H, ArH), 8.78 (s, 1H, ArH); 13C{1H} NMR (DMSO-d6, 125 MHz) δ 55.64, 111.24, 114.62 (2 × CH), 123.14, 123.51 (2 × CH), 128.37 (2 × CH), 128.65 (2 × CH), 128.97, 131.60, 132.25, 139.14, 145.54, 148.83, 150.44, 158.16, 164.43, 164.73; FT-IR (KBr) v: 3215, 3066, 3004, 2963, 2935, 2837, 1639, 1577, 1516, 1462, 1443, 1252 cm−1; MS (EI) m/z (relative intensity): 386 (21), 385 (M+, 100), 370 (14), 77.0(10); HRMS (EI) m/z: [M]+ Calcd for C21H15N5O3: 385.1175; found: 385.1182.
7-Amino-3-(4-cyanophenyl)-1-phenylpyrazolo[3,4-b]pyrrolo[3,4-d]pyridine-6,8-(3H,7H)-dione (7h), Yellow solid; yield: 73%; mp 333–335 °C; 1H-NMR (DMSO-d6, 500 MHz) δ 4.55 (br, 2H, NH2), 7.50–7.55 (m, 3H, ArH), 7.65 (d, J = 7.1 Hz, 2H, ArH), 8.09 (d, J = 8.5 Hz, 2H, ArH), 8.61 (d, J = 8.5 Hz, 2H, ArH), 8.87 (s, 1 H, ArH); 13C{1H} NMR (DMSO-d6, 125 MHz) δ 108.41, 112.58, 118.68, 120.75 (2 × CH), 124.12, 128.35 (2 × CH), 128.59 (2 × CH), 129.33, 131.55, 133.84 (2 × CH), 139.53, 141.99, 147.47, 149.11, 151.12, 163.83, 164.27; FT-IR (KBr) v: 3330, 3274, 2227, 1665, 1635, 1607, 1518, 1409, 1255, 844 cm−1; MS (EI) m/z (relative intensity): 381 (22), 380 (M+, 100), 379 (18), 295 (13), 77(14); HRMS (EI) m/z: [M]+ Calcd for C21H12N6O2: 380.1022; found: 380.1023.
7-Amino-3-(4-nitrophenyl)-1-phenylpyrazolo[3,4-b]pyrrolo[3,4-d]pyridine-6,8-(3H,7H)-dione (7i), Yellow solid; yield: 74%; mp 281–282 °C; 1H-NMR (DMSO-d6, 500 MHz) δ 7.53–7.57 (m, 3H, ArH), 7.93 (d, J = 6.5 Hz, 2H, ArH), 8.48 (d, J = 9.2 Hz, 2H, ArH), 8.67 (d, J = 9.2 Hz, 2H, ArH), 9.19 (s, 1H, ArH); 13C{1H} NMR (DMSO-d6, 125 MHz) δ 109.57, 121.41 (2 × CH), 123.52, 125.21 (2 × CH), 127.91 (2 × CH), 129.73, 129.90 (2 × CH), 131.21, 136.89, 143.24, 144.26, 145.12, 147.11, 153.90, 166.71, 168.43; FT-IR (KBr) v: 3190, 3120, 3064, 1595, 1500, 1341, 1112, 857 cm−1; MS (EI) m/z (relative intensity): 400 (M+, 4), 386 (26), 385 (100), 338 (13), 236 (10), 77 (13); HRMS (EI) m/z: [M]+ Calcd for C20H12N6O4: 400.0920; found: 400.0925.
3.4. Determination of the Fluorescence Quantum Yield
The fluorescence quantum yield Φx was determined through the comparative method. The quinine sulfate (Φst = 0.60, λex= 350 nm) in H2SO4 0.05 M was used as the standard, and it was calculated by following equation [48]:
| Φx/Φst = [Ast/Ax] [nx2/nst2] [Dx/Dst], | (1) |
where st: standard; x: sample; Φ: quantum yield; A: absorbance at the excitation wavelength; D: area under the fluorescence spectra on an energy scale; n: the refractive index of the solution. In the process of detection, the absorbance should be controlled and lower than 0.1.
4. Conclusions
Pyrazolopyridopyridazine diones 6 and N-aminopyrazolopyrrolopyridine diones 7 can be prepared in three synthesis methods from 1,3-diarylpyrazolopyrrolopyridine-6,8-diones, 1,3-diaryl-7-methylpyrazolopyrrolopyridine-6,8-diones, or 1,3-diarylfuropyrazolopyridine-6,8-diones with hydrazine monohydrate. Based on the experimental results, 1,3-diarylpyrazolopyrrolopyridine-6,8-diones were conceived as the best reactive starting materials. Furthermore, compounds 6 and 7 were also selectively synthesized under kinetic and thermodynamic control reactions. For the further photoluminescence, solvatofluorism, and quantum yield (Φf) studies, pyrazolopyridopyridazine diones 6 generally exhibited the stronger fluorescence intensity and possessed the significant substituent effect, particularly for 6c with a m-chloro group. On the other hand, the best Φf value of 6j was obtained (Φf = 0.140) and similar to luminol (1, Φf = 0.175), possibly caused by the planar skeletal conformation. Based on the above photoluminescence studies, we also found that the efficient introduction of the pyrazole and pyridine chromophores led to an increase in the conjugation and aromaticity of compounds 6 and 7 when compared with the standard luminol.
Acknowledgments
We are grateful to the Tsuzuki Institute for Traditional Medicine and the China Medical University, Taiwan (CMU108-N-04) for financial support.
Supplementary Materials
The following are available online, copies of 1H and 13C-NMR spectra of compounds 6a–6j and 7a–7i.
Author Contributions
F.F.W. conceived and designed the experiments; C.-C.T., C.-Y.C., and S.-E.T. performed the experiments; H.T., N.U., C.-Y.L., C.C.T., C.-Y.C., and S.-E.T. analyzed the data; F.F.W., H.T., N.U., C.-Y.L., contributed reagents/materials/analysis tools; F.F.W., C.-Y.L., C.C.T., and S.-E.T. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Tsuzuki Institute for Traditional Medicine and the China Medical University, Taiwan (CMU108-N-04)
Conflicts of Interest
The authors declare no conflicts of interest.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References
- 1.Boivin D.B., Tremblay G.M., James F.O. Working on Atypical Schedules. Sleep Med. 2007;8:578–589. doi: 10.1016/j.sleep.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 2.Menegatti R., Silva G.M., Zapata-Sudo G., Raimundo J.M., Sudo R.T., Barreiro E.J., Fraga C.A.M. Design, Synthesis, and Pharmacological Evaluation of New Neuroactive Pyrazolo[3,4-b]pyrrolo[3,4-d]pyridine Derivatives with in vivo Hypnotic and Analgesic Profile. Bioorg. Med. Chem. 2006;14:632–640. doi: 10.1016/j.bmc.2005.08.042. [DOI] [PubMed] [Google Scholar]
- 3.Nascimento-Junior N.M., Mendes T.C.F., Leal D.M., Correa C.M.N., Sudo R.T., Zapata-Sudo G., Barreiro E.J., Fraga C.A.M. Microwave-assisted Synthesis and Structure–activity Relationships of Neuroactive pyrazolo[3,4-b]pyrrolo[3,4-d]pyridine Derivatives. Bioorg. Med. Chem. Lett. 2010;20:74–77. doi: 10.1016/j.bmcl.2009.11.038. [DOI] [PubMed] [Google Scholar]
- 4.Yu G., Macor J.E., Chung H.-J., Humora M., Katipally K., Wang Y., Kim S. Fused Pyridopyridazine Inhibitors of cGMP Phosphodiesterase. No. 6,316,438. U.S. Patent. 2001 Nov 13;
- 5.Yu G., Mason H., Wu X., Wang J., Chong S., Beyer B., Henwood A., Pongrac R., Seliger L., He B., et al. Substituted Pyrazolopyridopyridazines as Orally Bioavailable Potent and Selective PDE5 Inhibitors: Potential Agents for Treatment of Erectile Dysfunction. J. Med. Chem. 2003;46:457–460. doi: 10.1021/jm0256068. [DOI] [PubMed] [Google Scholar]
- 6.Li Y., Zhu H., Trush M.A. Detection of Mitochondria-derived Reactive Oxygen Species Production by the Chemilumigenic Probes Lucigenin and Luminol. Biochim. Biophys. Acta. 1999;1428:1–12. doi: 10.1016/S0304-4165(99)00040-9. [DOI] [PubMed] [Google Scholar]
- 7.García-Campaña A.M., Baeyens W.R.G. Chemiluminescence in Chemical Analysis. Marcel Dekker; New York, NY, USA: 2001. [Google Scholar]
- 8.Roda A. Chemiluminescence and Bioluminescence: Past, Present, and Future. RSC; Cambridge, UK: 2011. [Google Scholar]
- 9.Barni F., Lewis S.W., Berti A., Miskelly G.M., Lago G. Forensic Application of the Luminol Reaction as a Presumptive Test for Latent Blood Detection. Talanta. 2007;72:896–913. doi: 10.1016/j.talanta.2006.12.045. [DOI] [PubMed] [Google Scholar]
- 10.Zhou H., Yue H., Zhou Y., Wang L., Fu Z. A Novel Disposable Immunosensor based on Quenching of Electrochemiluminescence Emission of Ru(bpy)32+ by Amorphous Carbon Nanoparticles. Sens. Actuators B. 2015;209:744–750. doi: 10.1016/j.snb.2014.12.051. [DOI] [Google Scholar]
- 11.Yoshida H., Ureshino K., Ishida J., Nohta H., Yamaguchi M. Chemiluminescent Properties of some Luminol related Compounds (II) Dyes Pigm. 1999;41:177–182. doi: 10.1016/S0143-7208(98)00067-9. [DOI] [Google Scholar]
- 12.Gu W., Deng X., Gu X., Jia X., Lou B., Zhang X., Li J., Wang E. Stabilized, Superparamagnetic Functionalized Graphene/Fe3O4@Au Nanocomposites for a Magnetically-controlled Solid-state Electrochemiluminescence. Biosensing Application. Anal. Chem. 2015;87:1876–1881. doi: 10.1021/ac503966u. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y.-Z., Hao N., Feng Q.-M., Shi H.-W., Xu J.-J., Chen H.-Y. A Ratiometric Electrochemiluminescence Detection for Cancer Cells using g-C3N4 Nanosheets and Ag–PAMAM–luminol nanocomposites. Biosens. Bioelectron. 2016;77:76–82. doi: 10.1016/j.bios.2015.08.057. [DOI] [PubMed] [Google Scholar]
- 14.Chan C.M. An improved Synthesis of a Chemiluminescent Cyclic Hydrazide: N-(7-Aminobutyl)-N-ethyl-naphthalene-1,2-dicarboxylic hydrazide. Synth. Commun. 1989;19:1981–1985. doi: 10.1080/00397918908052590. [DOI] [Google Scholar]
- 15.Ishida J., Yamaguchi M., Nakahara T., Nakamura M. 4,5-Diaminophthalhy drazide as a highly Sensitive Chemiluminescence Reagent for α-Keto Acids in Liquid Chromatography. Anal. Chim. Acta. 1990;231:1–6. doi: 10.1016/S0003-2670(00)86389-8. [DOI] [Google Scholar]
- 16.Sasamoto K., Ohkura Y. A New Chemiluminogenic Substrate for N-Acetyl-β-D-glucosaminidase, 4′-(6′-Diethylaminobenzofuranyl)phthalylhydrazido-N-acetyl-β-D-glucosaminide. Chem. Pharm. Bull. 1991;39:411–416. doi: 10.1248/cpb.39.411. [DOI] [Google Scholar]
- 17.Ishida J., Takada M., Yakabe T., Yamaguchi M. Chemiluminescent Properties of some Luminol related Compounds. Dyes Pigm. 1995;27:1–7. doi: 10.1016/0143-7208(94)00030-6. [DOI] [Google Scholar]
- 18.Ishida J., Takada M., Hara S., Sasamoto K., Kina K., Yamaguchi M. Development of a Novel Chemiluminescent Probe, 4-(5′,6′-dimethoxybenzothiazolyl)phthalhydrazide. Anal. Chim. Acta. 1995;309:211–219. doi: 10.1016/0003-2670(95)00082-B. [DOI] [Google Scholar]
- 19.Yoshida H., Nakao R., Nohta H., Yamaguchi M. Chemiluminescent Properties of some Luminol-related compounds—Part 3. Dyes Pigm. 2000;47:239–245. doi: 10.1016/S0143-7208(00)00080-2. [DOI] [Google Scholar]
- 20.Smith M.B., March J. March’s Advanced Organic Chemistry: Reactions, Mechanisms, and Structure. 6th ed. John Wiley & Sons; Hoboken, NJ, USA: 2007. [Google Scholar]
- 21.Antonov L. Tautomerism: Methods and Theories. 1st ed. Wiley-VCH; Weinheim, Germany: 2013. [Google Scholar]
- 22.Ali Ahmed H.E., Abdel-Salam H.A., Shaker M.A. Synthesis, Characterization, Molecular Modeling, and Potential Antimicrobial and Anticancer Activities of Novel 2-Aminoisoindoline-1,3-dione derivatives. Bioorg. Chem. 2016;66:1–11. doi: 10.1016/j.bioorg.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Conchon E., Anizon F., Aboab B., Golsteyn R.M., Léonce S., Pfeiffer B., Prudhomme M. Synthesis, in Vitro Antiproliferative Activities, and Chk1 Inhibitory Properties of Pyrrolo[3,4-a]carbazole-1,3-diones, Pyrrolo[3,4-c]carbazole-1,3-diones, and 2-Aminopyridazino[3,4-a]pyrrolo[3,4-c]carbazole-1,3,4,7-tetraone. Eur. J. Med. Chem. 2008;43:282–292. doi: 10.1016/j.ejmech.2007.03.026. [DOI] [PubMed] [Google Scholar]
- 24.Tseng C.-C., Yen W.-P., Tsai A.-E., Hu Y.-T., Takayama H., Kuo Y.-H., Wong F.F. ZnCl2-Catalyzed Aza-Diels–Alder Reaction for the Synthesis of 1H-Pyrazolo[3,4-b]pyridine-4,5-dicarboxylate Derivatives. Eur. J. Org. Chem. 2018;2018:1567–1571. [Google Scholar]
- 25.Deshmukh M.S., Sekar N. Chemiluminescence properties of luminol related quinoxaline analogs: Experimental and DFT based approach to photophysical properties. Dyes Pigm. 2015;117:49–60. doi: 10.1016/j.dyepig.2015.02.006. [DOI] [Google Scholar]
- 26.Deshmukh M.S., Sekar N. Chemiluminescence Properties of Luminol related o-Hydroxybenzimidazole analogues: Experimental and DFT based Approach to Photophysical Properties. Dyes Pigm. 2015;113:189–199. doi: 10.1016/j.dyepig.2014.08.009. [DOI] [Google Scholar]
- 27.Peryasami G., Martelo L., Baleizã C., Berberan-Santos M.N. Strong Green Chemiluminescence from Naphthalene analogues of Luminol. New J. Chem. 2014;38:2258–2261. doi: 10.1039/c4nj00364k. [DOI] [Google Scholar]
- 28.Spurlin S.R., Cooper M.M. A Chemiluminescent Precolumn Labelling Reagent for High-Performance Liquid Chromatography of Amino Acids. Anal. Lett. 1986;19:2277–2283. doi: 10.1080/00032718608064553. [DOI] [Google Scholar]
- 29.Yen W.-P., Liu P.-L., Uramaru N., Wong F.F. Indium(III) chloride/silica gel catalyzed synthesis of pyrazolo[3,4-b]pyrrolo[3,4-d]pyridines. Tetrahedron. 2015;71:8798–8803. doi: 10.1016/j.tet.2015.09.042. [DOI] [Google Scholar]
- 30.Neumann H., Klaus S., Klawona M., Strűbina D., Hűbner S., Gördes D., von Wangelin A.J., Lalk M., Beller M. A New Efficient Synthesis of Substituted Luminols using Multicomponent Reactions. Z. Naturforsch. 2004;59b:431–438. doi: 10.1515/znb-2004-0411. [DOI] [Google Scholar]
- 31.Flitsch W., Krämer U., Zimmerman H. Cyclische Verbindungen mit Heterobrückenatomen, V. Zur Chemie der 1-Amino-pyrrole. Chem. Ber. 1969;102:3268–3276. doi: 10.1002/cber.19691021005. [DOI] [Google Scholar]
- 32.Dey S.K., Lightner D.A. 1,1′-Bipyrroles: Synthesis and Stereochemistry. J. Org. Chem. 2007;72:9395–9397. doi: 10.1021/jo7016789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gompper R., Sobotta R. Neue Elektronenreiche Butadiene. Tetrahedron Lett. 1979;11:921–924. doi: 10.1016/S0040-4039(01)86051-2. [DOI] [Google Scholar]
- 34.Fang J.M., Yang C.C., Wang Y.W. Use of α-Anilino Dienenitriles as Nucleophiles in Cycloadditions. J. Org. Chem. 1989;54:477–481. doi: 10.1021/jo00263a040. [DOI] [Google Scholar]
- 35.Tyagi P., Venkateswararao A., Thomas K.R.J. Solution Processable Indoloquinoxaline derivatives containing Bulky Polyaromatic Hydrocarbons: Synthesis, Optical Spectra, and Electroluminescence. J. Org. Chem. 2011;76:4571–4581. doi: 10.1021/jo2004764. [DOI] [PubMed] [Google Scholar]
- 36.Martelo L., Periyasami G., Fedorov A.A., Baleizão C., Berberan-Santos M.N. Chemiluminescence of Naphthalene analogues of Luminol in Solution and Micellar Media. Dyes Pigm. 2019;168:341–346. doi: 10.1016/j.dyepig.2019.05.005. [DOI] [Google Scholar]
- 37.Reichardt C. Solvatochromic Dyes as Solvent Polarity Indicators. Chem. Rev. 1994;94:2319–2358. doi: 10.1021/cr00032a005. [DOI] [Google Scholar]
- 38.Dutkiewlcz M. Classification of Organic Solvents based on Correlation between Dielectric β Parameter and Empirical Solvent Polarity Parameter ENT. J. Chem. Soc. Faraday Trans. 1990;86:2237–2241. doi: 10.1039/ft9908602237. [DOI] [Google Scholar]
- 39.Behera S.K., Karak A., Krishnamoorthy G. Photophysics of 2-(4′-Amino-2′-hydroxyphenyl)-1H-imidazo-[4,5-c]pyridine and its analogues: Intramolecular Proton Transfer Versus Intramolecular Charge Transfer. J. Phys. Chem. B. 2015;119:2330–2344. doi: 10.1021/jp5064808. [DOI] [PubMed] [Google Scholar]
- 40.Behera S.K., Murkherjee A., Sadhuragiri G., Elumalai P., Sathiyendiran M., Kumar M., Mandal B.B., Krishnamoorthy G. Aggregation induced enhanced and Exclusively Highly Stokes Shifted Emission from an Excited State Intramolecular Proton Transfer Exhibiting Molecule. Faraday Discuss. 2017;196:71–90. doi: 10.1039/C6FD00171H. [DOI] [PubMed] [Google Scholar]
- 41.Chen Y.-T., Wu P.-J., Peng C.-Y., Shen J.-Y., Tsai C.-C., Hu W.-P., Chou P.-T. A study of the Competitive Multiple Hydrogen Bonding Effect and its associated Excited-state Proton Transfer Tautomerism. Phys. Chem. Chem. Phys. 2017;19:28641–28646. doi: 10.1039/C7CP05002J. [DOI] [PubMed] [Google Scholar]
- 42.Wang Q., Niu Y., Wang R., Wu H., Zhang Y. Acid-induced Shift of Intramolecular Hydrogen Bonding Responsible for Excited-state Intramolecular Proton Transfer. Chem. Asian J. 2018;13:1735–1743. doi: 10.1002/asia.201800457. [DOI] [PubMed] [Google Scholar]
- 43.Tang Z., Lu M., Liu K., Zhao Y., Qi Y., Wang Y., Zhang P., Zhou P. Solvation Effect on the ESIPT Mechanism of 2-(4′-Amino-2′-hydroxyphenyl)-1H-imidazo-[4,5-c]pyridine. J. Photochem. Photobiol. A. 2018;367:261–269. doi: 10.1016/j.jphotochem.2018.08.028. [DOI] [Google Scholar]
- 44.Satapathy A.K., Behera S.K., Yadav A., Laxmi Mahour L.N., Yelamaggad C.V., Sandhya K.L., Sahoo B. Tuning the Fluorescence Behavior of Liquid Crystal Molecules containing Schiff-base: Effect of Solvent Polarity. J. Lumin. 2019;210:371–375. doi: 10.1016/j.jlumin.2019.02.056. [DOI] [Google Scholar]
- 45.Skripnikova T.A., Lysova S.S., Zevatskii Y.E., Myznikov L.V., Vorona S.V., Artamonva T.V. Physico-chemical Properties of Isomeric Forms of Luminol in Aqueous Solutions. J. Mol. Struct. 2018;1154:59–63. doi: 10.1016/j.molstruc.2017.10.004. [DOI] [Google Scholar]
- 46.Deepa S., Reddy S.R., Rajendrakumar K. Green Chemiluminescence of Highly Fluorescent Symmetrical Azo-based Luminol derivative. Orient. J. Chem. 2018;34:894–905. doi: 10.13005/ojc/340238. [DOI] [Google Scholar]
- 47.Brouwer A.M. Standards for Photoluminescence Quantum Yield Measurements in Solution (IUPAC Technical Report) Pure Appl. Chem. 2011;83:2213–2228. doi: 10.1351/PAC-REP-10-09-31. [DOI] [Google Scholar]
- 48.Kiyama M., Iwano S., Otsuka S., Lu S.W., Obata R., Miyawaki A., Hirano T., Maki S.A. Quantum Yield Improvement of Red-light-emitting Firefly Luciferin Analogues for in vivo Bioluminescence Imaging. Tetrahedron. 2018;74:652–660. doi: 10.1016/j.tet.2017.11.051. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.