Abstract
Apoptosis in acute stroke is associated with a negative prognosis and is correlated with the severity of the neurological deficit. However, there is no evidence that indicates that, in the subacute phase of the stroke, the apoptosis process might activate neuroplasticity. Therefore, in this study, we investigated the effect of an extremely low frequency electromagnetic field (ELF-EMF) on the molecular mechanism of apoptosis, as used in the rehabilitation of post-stroke patients. Patients with moderate stroke severity (n = 48), 3–4 weeks after incident, were enrolled in the analysis and divided into ELF-EMF and non-ELF-EMF group. The rehabilitation program in both groups involves the following: kinesiotherapy—30 min; psychological therapy—15 min; and neurophysiological routines—60 min. Additionally, the ELF-EMF group was exposed to an ELF-EMF (40 Hz, 5 mT). In order to assess the apoptosis gene expression level, we measured the mRNA expression of BAX, BCL-2, CASP8, TNFα, and TP53. We found that ELF-EMF significantly increased the expression of BAX, CASP8, TNFα, and TP53, whereas the BCL-2 mRNA expression after ELF-EMF exposition remained at a comparable level in both groups. Thus, we suggest that increasing the expression of pro-apoptotic genes in post-stroke patients promotes the activation of signaling pathways involved in brain plasticity processes. However, further research is needed to clarify this process.
Keywords: extremely low frequency electromagnetic field, apoptosis, neuroplasticity, stroke
1. Introduction
The symptoms of brain damage caused by cerebral ischaemia are the consequence of massive cell death in the ischaemic area. Ischaemic cascades—successive biochemical changes leading to degradation of cell structures and membranes, and ultimately to brain cell death—activated after a few seconds of blockage of the cerebral blood flow [1]. Ischaemia causes cell death as a result of necrosis, apoptosis, and autophagy, the markers of which have been well documented [2,3,4,5].
There are many factors that can induce apoptosis of cells after ischaemia, such as inflammation, cytokine activation, cascade of free radicals, and induction of thrombin [6]. Neuronal apoptosis is regulated by various genes, such as BCL-2 (inhibitor of apoptosis) and BAX (activator of apoptosis) [7]. The BCL-2 protein (B cell lymphoma) is a product of the BCL-2 gene located on chromosome 18 at the 18q21.3 locus. Under normal conditions, the expression of BCL-2 is regulated by the p53 protein, and its main function is to protect the cell from apoptosis [8]. The p53 protein is called the ‘genome guard’, and plays an important role in the regulation of apoptosis as a transcription factor [8]. Increased expression of BCL-2 causes production of the BCL-2/BAX heterodimer and inhibition of cell apoptosis, whereas with an increased expression of BAX, the BAX/BAX homodimer is produced and cell apoptosis is activated. When BCL-2 is elevated and BAX is reduced, the BCL-2/BAX ratio increases and the cell might survive [9]. Caspases are evolutionary, conserved proteins, which play a key role in the mechanism of apoptosis and inflammation. The role of initiator caspases with a long pro-domain, including caspase 8, is in signal detection and activation of the cascade, leading to apoptosis [10].
Post-stroke recovery is associated with the compensatory plasticity of the brain, and is activated by rehabilitation. There are data showing that, in the subacute phase of stroke, proapoptotic pathways and mechanisms of neuroplasticity may be interrelated [11,12]. After a stroke, neuroplasticity begins immediately after the ischemic incident. Neuroplasticity involves synaptic potentiation and formation of a new synaptic junction. Although they remain in a weakened state, the connection between the brain centres is activated, and any damage to its function can be reintroduced partly or comprehensively, because the function of the damaged area has been assumed by other subcortical or cortical structures [13]. Neuroplasticity is associated with neurogenesis, in which fully functional nerve cells are produced. Nerve cells are generated essentially in the subgranular zone (SGZ) and the sub-ventricular zone (SVZ) [14]. Microenvironmental factors, growth factors, neurotrophins, hormones, and neurotransmitters regulate this process [15].
Post-stroke rehabilitation is a complex process, consisting of various therapeutic treatments. Correctly conducted rehabilitation is one of the most important forms of therapy after stroke. Low frequency electromagnetic field (ELF-EMF) therapy is one of the methods used in rehabilitation after stroke. This type of treatment is characterized by physical parameters describing ELF-EMF; that is, frequency (<50 Hz), magnetic induction (<10 mT), as well as the shape of the pulse (rectangular, trapezoidal, triangular, sinusoidal, unipolar, bipolar) [16]. ELF-EMF therapy has been noted for the minimal level of harm it causes, the extensive range of ways in which it can be used to treat various diseases, as well as the beneficial clinical results achieved with relatively small financial outlay. The nature of the ELF-EMF effect on the body allows deep tissue penetration, however, it is not a thermal method, which makes it possible to use it in the treatment of neurological diseases [17]. ELF-EMF affects the body at both the molecular and cellular level and causes the change in ionic channels. However, there is in fact no known mechanism in which calcium channels can be directly activated by ELF-EMF. This effect can be a consequence of mitochondrial and metabolic disturbances [18]. ELF-EMF therapy used in physical rehabilitation, including after strokes, increases muscle strength, reduces muscle spasticity as a consequence of pyramidal tracts damage, has an analgesic effect, as well as increases blood flow. Thereby, ELF-EMF affects regeneration of nerve tissue through improvements to its metabolism [19]. Despite the many substantiated beneficial effects of ELF-EMF, this form of therapy is not a routine method for post-stroke rehabilitation.
Apoptosis in stroke patients is an ambiguous process. On the one hand, increased apoptosis in the acute phase of a stroke is correlated with larger neurological deficits. On the other hand, the process of programmatic cell death is one of the physiological mechanisms for eliminating damaged cells [20]. Our previous research confirms the significant effect of ELF-EMF on the enhancement of neuroplasticity and biochemical parameters correlated with the improvement of physical and motor condition according to clinical scales (Mini-Mental State Examination—MMSE, Activities of Daily Living—ADL, Geriatric Depression Scale—GDS) [21,22,23,24,25]. In this work, we undertook assessment of the effect of ELF-EMF on the crucial mechanisms of apoptosis in order to search for the molecular basis of ELF-EMF activity.
2. Materials and Methods
2.1. Subjects Presentation and Blood Collection
Forty-eight patients with moderate stroke severity, 3–4 weeks after incident, were enrolled for the analysis. Twenty-six patients were recruited into the ELF-EMF study group and twenty two patients were recruited into the non-ELF-EMF control group. The NIHSS scores were 4.9 ± 3.1 and 5.4 ± 2.9 for the study group and control group, respectively; mean age was 48.8 ± 7.7 and 44.8 ± 8.0, respectively. Exclusion criteria included the following: chronic or significant acute inflammatory factors, dementia, haemorrhagic stroke, neurological illness other than stroke, and/or decreased consciousness in their medical pre-stroke history. Patients with metal and/or electronic implants were excluded from the study group. Neurorehabilitation with neurological and internal examination was conducted for four weeks at the Neurorehabilitation Ward of Dr K. Jonscher Municipal Medical Center in Lodz, Poland. This consisted of the following: kinesiotherapy—30 min; psychological therapy—15 min; and neurophysiological routines—60 min; as well as ELF-EMF treatment.
Blood samples were collected into citrate, phosphate, dextrose, adenine (CDPA1) containing tubes, before and after ten sessions of therapy, at the same time of day (between 07:00 and 09:00), under fasting conditions. They were frozen at −80 °C immediately upon collection. All blood samples were collected and stored using the same protocol. The study was conducted in accordance with the principles of the Helsinki Declaration. The Bioethics Committee of the Faculty of Biology and Environmental Protection of the University of Lodz, Poland, confirmed this study with Resolution No. 13/KBBN-UŁ/II/2016.
2.2. Setting and Treatment of ELF-EMF
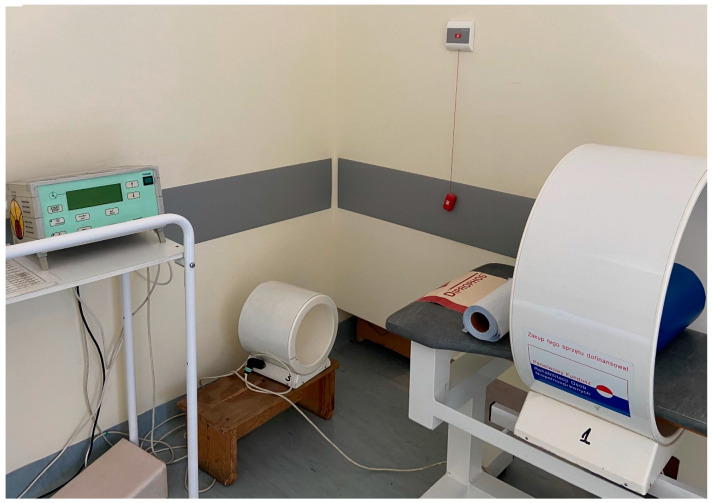
Magnetronic MF10 generator (EiE Elektronika i Elektromedycyna, Otwock, Poland) was used to generate ELF-EMF with the following parameters: magnetic induction—5 mT, frequency—40 Hz, wave forms—rectangular, bipolar, time of session—30 min, and exposed area—pelvic girdle (Figure 1). In this research, a coil applicator with five layers of 187 turns of 1.45 mm twin-parallel wires was used with the following parameters: length—270 mm and diameter—550 mm. ELF-EMF intensity inside the solenoid was nonequivalent. The induction coils of the generator were assembled horizontally, while apportionment of ELF-EMF was vertical. The established value of magnetic induction of 5 mT was located in the applicator geometrical center, whereas, with the proximity of the coil surface, the change of ELF-EMF intensity increased by around plus 1.4. The computer system controlled ELF-EMF itself. Post-stroke patients were exposed in 10 standard sessions, five times per week for two weeks. All subjects were placed in applicator, but only patients in the ELF-EMF group were exposed to ELF-EMF, and patients in the non-ELF-EMF group were given sham exposures. The sham exposure was obtained by setting, but not turning on the device.
Figure 1.
Device to generate extremely low frequency electromagnetic field (ELF-EMF)—Magnetronic MF10 (EiE Elektronika i Elektromedycyna, Otwock, Poland).
2.3. Isolation of RNA
Reagent® (Sigma-Aldrich, Saint Louis, MO, USA) was used for lysis of frozen samples, following which phase separation was effectuated. InviTrap Spin Universal RNA Mini Kit (Stratec Biomedical Systems, Birkenfeld, Germany) was used for purification of the RNA-containing aqueous phase. A Synergy HTX Multi-Mode Microplate Reader, equipped with a Take3 Micro-Volume Plate (BioTek Instruments, Inc., Winooski, VT, USA), was used to estimate, purify, and quantify the RNA.
2.4. Reverse Transcription
Diluted RNA samples (to 20 ng/µL) were transcribed onto cDNA using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems™, Waltham, MA, USA). All procedures were conducted in accordance with the producents’ protocol.
2.5. Real-Time PCR
Levels of expression of the investigated genes (BAX, BCL-2, CASP8, TNFα, and TP53) were obtained using TaqMan probes: Hs00180269_m1 for human BAX gene; Hs00608023_m1 for human BCL-2 gene; Hs01018151_m1 for human CASP8 gene; Hs00174128_m1 for human TNFα gene; Hs01034249_m1 for human TP53 gene, and Hs99999905_m1 for human GAPDH gene (endogenous control) (Life Technologies, Carlsbad, CA, USA). A TaqMan Universal Master Mix II (Life Technologies, Carlsbad, CA, USA) was used to perform the real-time PCRs, which were executed in a CFX96 real-time PCR system (BioRad Laboratories, Hercules, CA, USA). All steps were conducted according to the producers’ recommendations. The equation 2-ΔCt (ΔCt = Ctstudied gene - CtGAPDH) was used to calculate the relative expressions of the analyzed genes.
2.6. Data Analysis
All assays were conducted twice and calculated as mean values. For all participants, the values of the expression before treatment were used as the output value (100%). Data from the assays executed on these same participants after treatment were formulated as a percentage of the output value. Data obtained in this way were expressed as mean ± SD.
Stats Direct statistical software v.2.7.2 was used for all statistical analyses. Paired Student’s t-tests (for normal distribution) and Wilcoxon tests (for distribution deviating from normal) were used to analyze significant differences between the data obtained for subjects before and after treatments. Changes in parameters after appropriate treatments for comparison of differences between the ELF-EMF group and the non-ELF-EMF group were calculated. For this analysis, unpaired Student’s t-tests or Mann–Whitney U tests were used. Additionally, a correlation analysis between the changes in experimental and clinical parameters was executed using a Spearman’s rank correlation. A level of p < 0.05 was accepted as statistically significant for all analyses.
3. Results
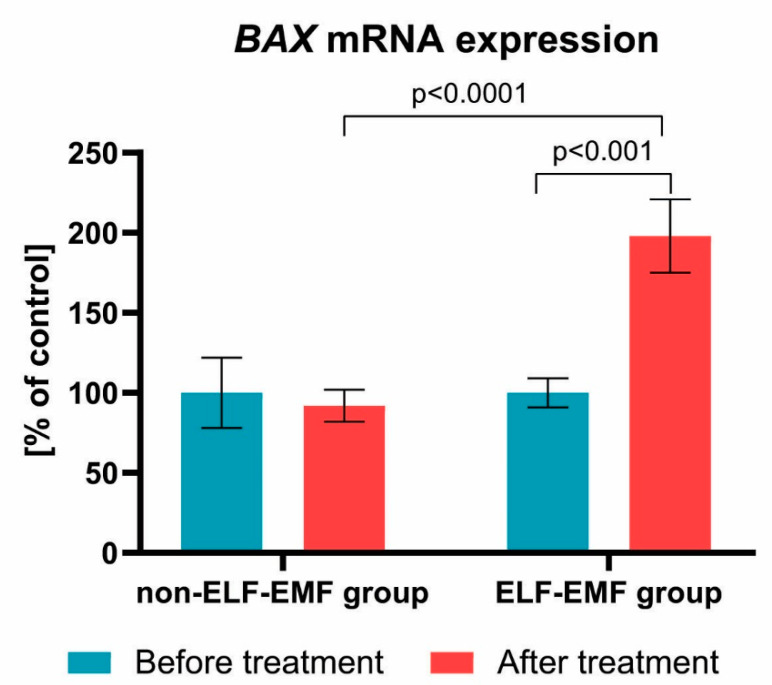
Our comparative analysis shows the effect of ELF-EMF therapy on the expression level of various genes involved in apoptosis. As presented in Figure 2, the expression of the BAX gene in the ELF-EMF group after 10 sessions of rehabilitation was significantly higher as compared with the non-ELF-EMF group (p < 0.001). The increase of the BAX mRNA gene expression level in the ELF-EMF group was about 100% (p < 0.001), while in the non-ELF-EMF group, it did not change (p > 0.05) (Figure 2).
Figure 2.
Comparison of the BAX mRNA expression acquired from the study group vs. control. Results formulated as parameter changes before and after therapy (100% expressed for the level of BAX mRNA expression in each patient sample before treatment). Statistical significance between ELF-EMF and non-ELF-EMF groups after 10 sessions amount to p < 0.0001.
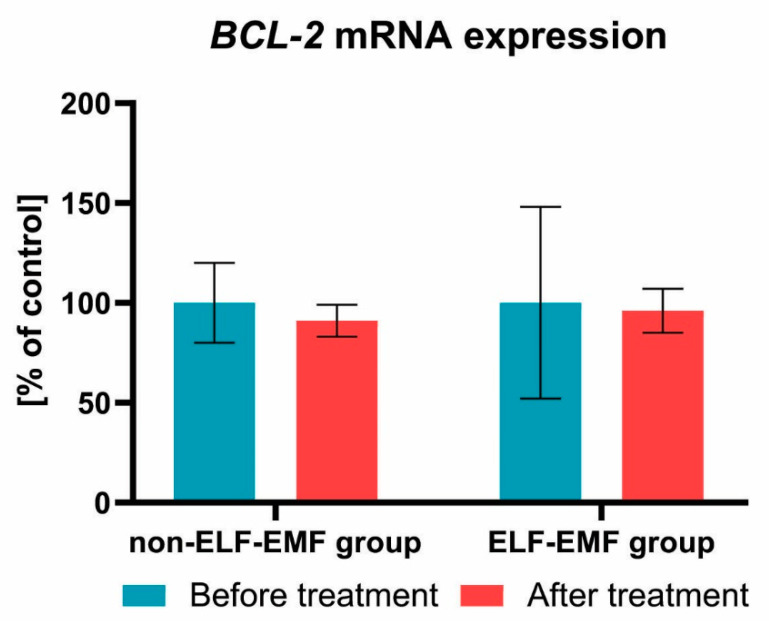
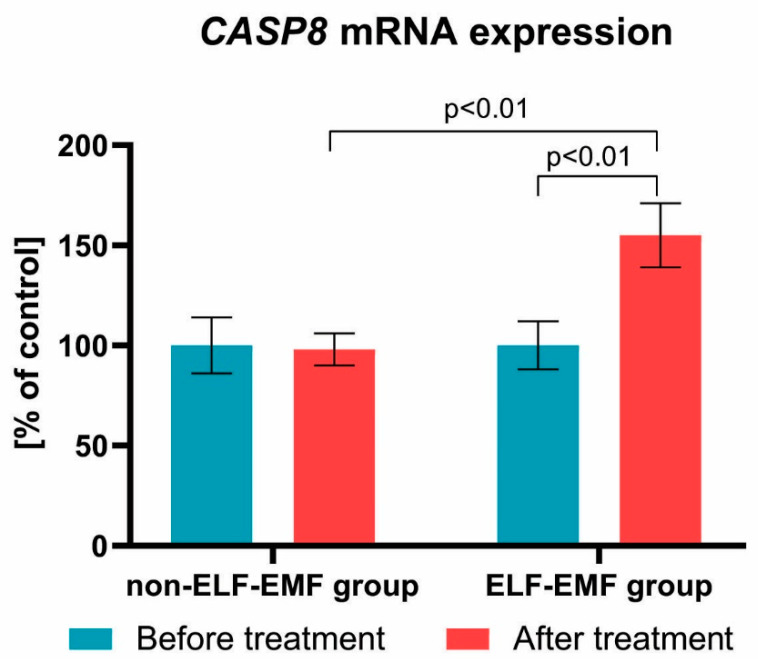
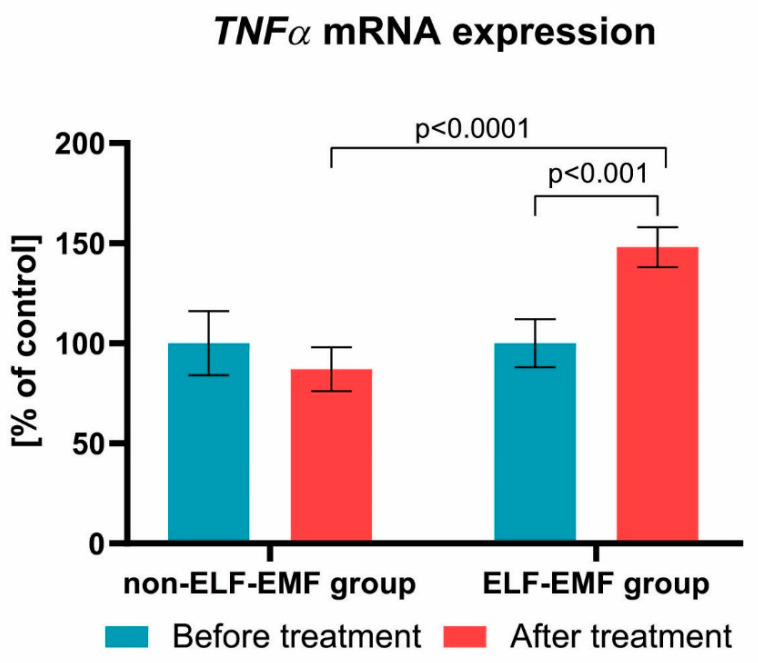
We also found that the expression of the BCL-2 gene after a series of 10 physical treatments remained at a comparable level in both groups (Figure 3). Subsequently, we estimated the impact of the ELF-EMF on CASP8 gene expressions in the whole blood samples. We showed that, after ELF-EMF therapy, the expression of CASP8 mRNA in the ELF-EMF group increased by about 50% (p < 0.01), but in the non-ELF-EMF group, it remained at the same level (p > 0.05) (Figure 4). Similarly, as shown in Figure 5, the expression level of TNFα was increased in the ELF-EMF group by about 50% (p < 0.001), whereas in the non-ELF-EMF group, it remained at the same level (p > 0.05).
Figure 3.
Comparison of the BCL-2 mRNA expression acquired from the study group vs. control. Results formulated as parameter changes before and after therapy (100% expressed for the level of BCL-2 mRNA expression in each patient sample before treatment).
Figure 4.
Comparison of the CASP8 mRNA expression acquired from the study group vs. control group. Results formulated as parameter changes before and after therapy (100% expressed for the level of CASP8 mRNA expression in each patient sample before treatment). Statistical significance between ELF-EMF and non-ELF-EMF groups after 10 sessions amount to p < 0.01.
Figure 5.
Comparison of the TNFα mRNA expression acquired from the study group vs. control group. Results formulated as parameter changes before and after therapy (100% expressed for the level of TNFα mRNA expression in each patient sample before treatment). Statistical significance between ELF-EMF and non-ELF-EMF groups after 10 sessions amount to p < 0.0001.
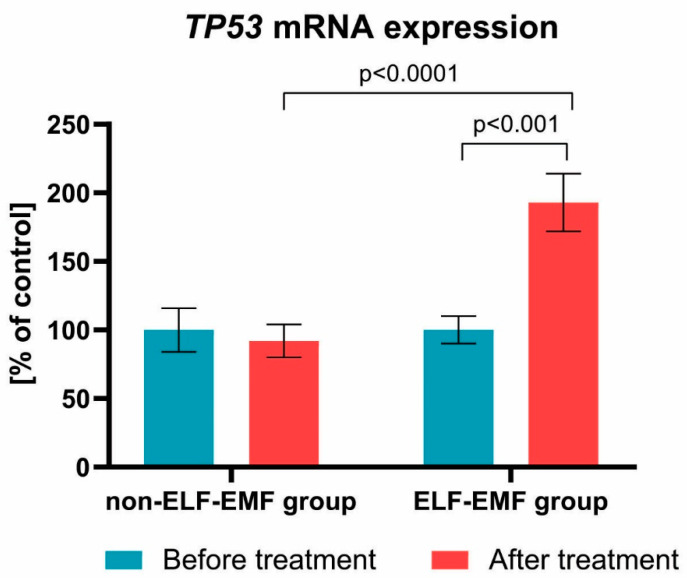
Additionally, we examined the TP53 mRNA expression level. We demonstrated that, after rehabilitation, the mRNA expression of TP53 gene in the study group increased by about 100% (p < 0.001), while in the control group, it remained unchanged (p > 0.05) (Figure 6).
Figure 6.
Comparison of the TP53 mRNA expression acquired from the ELF-EMF group vs. the non-ELF-EMF group. Results formulated as parameter changes before and after therapy (100% expressed for the level of TP53 mRNA expression in each patient sample before treatment). Statistical significance between ELF-EMF and non-ELF-EMF groups after 10 sessions amount to p < 0.0001.
4. Discussion
An increased apoptotic process in acute phase of stroke is proven, and associated with the severity of neurological deficit [26,27]. There is still little information about apoptosis in the subacute/chronic phase of stroke. Within several minutes after ischaemia, neuronal cells in the infract core died [28], however, in penumbra, neuronal cells died slowly within a couple of hours after the incident [29]. Deng et al. studied the dynamics of apoptotic processes in mice that had experienced an induced stroke. They showed that markers of apoptosis had grown 2–5 h after ischemic, peaking after 5 h. At that time, the stroke volume and neurological deficit expended slowly, implying that apoptosis activation might inhibit ampliation of the ischeamic core and promote neuronal survival. Moreover, after this time, apoptosis was normalized [30].
Apoptosis, as programmed cell death, aims to eliminate damaged cells and, therefore, also those resulting from hypoxia/reperfusion. Magavi et al. investigated the neurogenesis of the adult cerebral cortex. They observed that induced apoptosis promoted the formation of new neurons [12]. Similarly, Chen et al. found the activation of neurogenesis in mice with induced apoptosis in corticospinal motor neurons [11].
Our innovative research concerned ELF-EMF activity in vivo in post-stroke patients. Most of the available studies were conducted on cell lines or animal models. The results obtained from research carried out using other research models cannot be precisely related to human rehabilitation. Thus, we relate current results only to our previous research that showed that ELF-EMF therapy improved neuroregeneration processes at the molecular level. In our previous papers, we found that rehabilitation treatments with the use of ELF-EMF intensification processes of neuroplasticity in post-acute stroke patients [21,22,23]. We found that, after exposition to ELF-EMF, the brain-derived neurotrophic factor (BDNF) expression at both the mRNA and protein level was increased, as well as plasma cytokine levels—hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF), stem cell factor (SCF), and interleukin 1β (IL-1β)—were elevated [22,25]. Moreover, we observed that ELF-EMF affected the synthesis of nitric oxide (NO), which participates in plasticity processes [23].
In this study, we demonstrated that ELF-EMF increased BAX mRNA expression in vivo in post-acute stroke patients (Figure 2). Our results concur with the research shown by Wang et al. [31]. They observed that 3 mT EMF promotes osteoclast apoptosis by up-regulation of RANK, NFATc1, TRAP, CTSK, BAX, and BAX/BCL-2 expression, thereby enhancing osteoclast formation and maturation [31]. On the other hand, Tenuzzo et al. [32] assessed the effect of exposition of 6 mT EMF on expression of BAX, p53, HSP70, and BCL-2 in human lymphocytes. They found that, after exposition, the BAX and p53 gene expression rose, while the hsp70 and BCL-2 gene expression decreased.
We observed that, after ELF-EMF rehabilitation, the mRNA expression of BCL-2 remained at a comparable level in both groups. Our results concur with a study shown by Ding et al. [33]. They estimated the effect of ELF-EMF on H2O2-induced apoptosis and necrosis in human leukaemia HL-60 cells. They observed that the level of BCL-2 in H2O2-treated cells was comparable to its level in cells treated with both H2O2 and ELF-EMF. They also suggested that EFF-EMF itself cannot induce apoptosis and necrosis [33].
We demonstrated that the CASP8 and TNFα mRNA expression were elevated in the ELF-EMF group (Figure 4; Figure 5). Xie et al. estimated the impact of EMF therapy on chondrocyte morphology and apoptosis, and the expression of apoptosis-related proteins in rabbits with anterior cruciate ligament transection. They found after, exposition on ELF-EMF, the expression level of CASP8 was higher in comparison with the healthy group, but that there was an insignificant difference in comparison with the untreated group with anterior cruciate ligament transection. The EMF group was also characterized by better clinical parameters [34].
In our current study, we found that expression of TP53 mRNA increased by about 100% (Figure 6) in the ELF-EMF group. Vincenzi et al. [35] evaluated the impact of pulsed electromagnetic fields (PEMF) on p53 activation and the stimulation of A3 adenosine receptors in NF-kB, cytotoxicity, apoptosis, and cell proliferation. They found that PEMF treatment alone was not enough to affect regulation of p53 expression. However, the coinstantaneous treatment of tumour cells with PEMF and agonist of A(3) adenosine receptors (A3AR)—2-chloro-N 6-(3-iodobenzyl)adenosine-5′-N-methyl-uronamide (Cl-IB-MECA)—caused a further significant elevation of protein levels of p53, compared with Cl-IB-MECA alone [35]. Similarly, Ma et al. [36] evaluated the impact of ELF-EMF on the proliferation and differentiation of neural stem cells. They observed that, after exposure to ELF-EMF, cell cycle analyses do not significantly change in the studied group compared with the control. Additionally, they found no significant difference in the expression level of the p53 gene [36].
The potential mechanism of intensification of apoptosis processes by ELF-ELM could be related to its effect on calcium channels. The effect of ELF-EMF on Ca2+ flux is well documented [37,38,39,40]. The increase of Ca2+ ions is an alarm signal that may present differently according to the physiological details. It has been shown that apoptosis can be dependent on an increase in the level of mitochondrial calcium, which in turn induces the release of mitochondrial cytochrome c and activation of proteases [41]. In addition to intrinsic and extrinsic pathways of apoptosis, there are also reticular pathways. The reticulum pathway is caused by disturbance of the ion balance (especially Ca2+ ions), and the accumulation of improperly folded or modified proteins in the cell. In the caspase-independent pathway, calpain plays an important role in apoptosis. Calpain is a cysteine protease activated by Ca2+ ions. After stimulation, calcium is released from the endoplasmic reticulum and is bound with many factors, including with calpain [42].
Morabito et al. [43] evaluated the impact of ELF-EMF on morphology, proliferation, and differentiation in pheochromocytoma cells (PC12), as well as on the induction of oxidative stress dependent on Ca2+. Oxidative stress causes overproduction of reactive oxygen species (ROS), which leads to apoptotic cell death. Cell growth and viability were examined after ELF-EMF exposure (50 Hz, 01,1 mT) using morphological and colorimetric analysis. Assay immediately after exposure demonstrated an increased level of ROS and a decreased catalase (CAT) activity, without affecting the Ca2+ level. On the other hand, seven days’ exposure caused an increased CAT activity, which may suggest the cell adaptation on ELF-EMF. Furthermore, long-term exposure caused an intracellular Ca2 level. Calcium activates voltage-gated (L-type) Ca2 channels, which, via cell pathways (extracellular signal-regulated kinases, c-Jun N-terminal protein kinase/stress-activated protein kinase, and p38), could lead to the activation of gene expression regulating apoptosis, cell survival, and differentiation [43]. Our previous research is compatible with Morabito studies. We observed that, two weeks post-stroke, rehabilitation with ELF-EMF exposition caused an increase in antioxidant enzymes activity: CAT and superoxide dismutase (SOD) [24], as well as elevation of the expression of antioxidant enzymes genes: CAT, SOD (SOD1 and SOD2), and glutathione peroxidase (GPx1 and GPx4) [21]. Moreover, we demonstrated that 10 sessions of ELF-EMF therapy decreased the level of parameters of oxidative stress in patients after stroke [24].
What is particularly important in our previous works is that we reported that, after ELF-EMF treatment, the improvement of biochemical markers was accompanied by the enhancement of clinical parameters. ELF-EMF improves motor condition expressed in the ADL scale, as well as mental efficiency assessed in the MMSE and GDS scales [21,22,23,24,25]. Our research to date clearly shows that ELF-EMF significantly boosts the effectiveness of rehabilitation. In our current study, we established that ELF-EMF additionally increases the expression of pro-apoptotic genes. Enhancement of apoptosis in post-stroke rehabilitated patients can significantly contribute to improving repair processes and increasing neuroplasticity owing to the removal of redundant or damaged cells.
On the basis of all of our research, we suggest that increasing the expression of these genes in actively rehabilitated post-stroke patients promotes the induction and/or intensification of signaling pathways involved in brain plasticity. However, subsequent study is needed to elucidate the exact mechanism of this process, which can include a simultaneous action of a variety of repair systems. Nevertheless, we recommended that the inclusion of ELF-EMF therapy could intensify the efficacy of the treatment after stroke.
Author Contributions
Conceptualization, N.C., M.B., and J.S.-B.; Data curation, N.C.; methodology, N.C., E.M., J.S.-B., and M.B.; formal analysis, N.C., M.C., E.S., E.M., J.S.-B., and M.B.; investigation, N.C. and E.S.; resources, N.C. and E.S.; writing—original draft preparation, N.C. and J.S.-B.; writing—review and editing, M.C., E.M., T.S., M.B., and J.S.-B.; visualization, N.C. and M.B.; supervision, M.B. and J.S.-B.; project administration, M.B. and J.S.-B.; funding acquisition, T.S. and J.S.-B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. This study was approved by the Bioethics Committee of the University of Lodz, Poland with Resolution No. 13/KBBN-UŁ/II/2016. All participants gave their written informed consent prior to participation. The study was executed according to the principles of the Helsinki Declaration.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Werner C., Engelhard K. Pathophysiology of traumatic brain injury. Br. J. Anaesth. 2007;99:4–9. doi: 10.1093/bja/aem131. [DOI] [PubMed] [Google Scholar]
- 2.Galluzzi L., Vitale I., Aaronson S.A., Abrams J.M., Adam D., Agostinis P., Alnemri E.S., Altucci L., Amelio I., Andrews D.W., et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25:486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Novgorodov S.A., Voltin J.R., Gooz M.A., Li L., Lemasters J.J., Gudz T.I. Acid sphingomyelinase promotes mitochondrial dysfunction due to glutamate-induced regulated necrosis. J. Lipid Res. 2018;59:312–329. doi: 10.1194/jlr.M080374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Z., Zhou F., Dou Y., Tian X., Liu C., Li H., Shen H., Chen G. Melatonin alleviates intracerebral hemorrhage-induced secondary brain injury in rats via suppressing apoptosis, inflammation, oxidative stress, DNA damage, and mitochondria injury. Transl. Stroke Res. 2018;9:74–91. doi: 10.1007/s12975-017-0559-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu W.W., Zhang Y.Y., Su J., Liu A.F., Wang K., Li C., Liu Y.E., Zhang Y.Q., Lv J., Jiang W.J. Ischemia reperfusion injury after gradual versus rapid flow restoration for middle cerebral artery occlusion rats. Sci. Rep. 2018;8:1638. doi: 10.1038/s41598-018-20095-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang J., Gan Y., Han P., Yin J., Liu Q., Ghanian S., Gao F., Gong G., Tang Z. Ischemia-induced Neuronal Cell Death Is Mediated by Chemokine Receptor CX3CR1. Sci. Rep. 2018;8:556. doi: 10.1038/s41598-017-18774-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neto-Neves E.M., Frump A.L., Vayl A., Kline J.A., Lahm T. Isolated heart model demonstrates evidence of contractile and diastolic dysfunction in right ventricles from rats with sugen/hypoxia-induced pulmonary hypertension. Physiol. Rep. 2017;5 doi: 10.14814/phy2.13438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wafa A., Moassass F., Liehr T., Bhatt S., Aljapawe A., Al Achkar W. A high complex karyotype involving eleven chromosomes including three novel chromosomal aberrations and monoallelic loss of. Mol. Cytogenet. 2016;9:91. doi: 10.1186/s13039-016-0300-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walensky L.D. BCL-2 in the crosshairs: Tipping the balance of life and death. Cell Death Differ. 2006;13:1339–1350. doi: 10.1038/sj.cdd.4401992. [DOI] [PubMed] [Google Scholar]
- 10.Kruidering M., Evan G.I. Caspase-8 in apoptosis: The beginning of “the end”? IUBMB Life. 2000;50:85–90. doi: 10.1080/713803693. [DOI] [PubMed] [Google Scholar]
- 11.Chen J., Magavi S.S., Macklis J.D. Neurogenesis of corticospinal motor neurons extending spinal projections in adult mice. Proc. Natl. Acad. Sci. USA. 2004;101:16357–16362. doi: 10.1073/pnas.0406795101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Magavi S.S., Leavitt B.R., Macklis J.D. Induction of neurogenesis in the neocortex of adult mice. Nature. 2000;405:951–955. doi: 10.1038/35016083. [DOI] [PubMed] [Google Scholar]
- 13.Balbinot G., Schuch C.P. Compensatory Relearning Following Stroke: Cellular and Plasticity Mechanisms in Rodents. Front. Neurosci. 2018;12:1023. doi: 10.3389/fnins.2018.01023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zelentsova-Levytskyi K., Talmi Z., Abboud-Jarrous G., Capucha T., Sapir T., Burstyn-Cohen T. Protein S Negatively Regulates Neural Stem Cell Self-Renewal through Bmi-1 Signaling. Front. Mol. Neurosci. 2017;10:124. doi: 10.3389/fnmol.2017.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aimone J.B., Li Y., Lee S.W., Clemenson G.D., Deng W., Gage F.H. Regulation and function of adult neurogenesis: From genes to cognition. Physiol. Rev. 2014;94:991–1026. doi: 10.1152/physrev.00004.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patruno A., Ferrone A., Costantini E., Franceschelli S., Pesce M., Speranza L., Amerio P., D’Angelo C., Felaco M., Grilli A., et al. Extremely low-frequency electromagnetic fields accelerates wound healing modulating MMP-9 and inflammatory cytokines. Cell Prolif. 2018;51:e12432. doi: 10.1111/cpr.12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar S., Dey S., Jain S. Extremely low-frequency electromagnetic fields: A possible non-invasive therapeutic tool for spinal cord injury rehabilitation. Electromagn. Biol. Med. 2017;36:88–101. doi: 10.1080/15368378.2016.1194290. [DOI] [PubMed] [Google Scholar]
- 18.Cichon N., Olejnik A., Miller E., Saluk J. The multipotent action of magnetic fields. Biologia. 2016;71:1103–1110. doi: 10.1515/biolog-2016-0142. [DOI] [Google Scholar]
- 19.Mattsson M.O., Simkó M. Emerging medical applications based on non-ionizing electromagnetic fields from 0 Hz to 10 THz. Med. Devices. 2019;12:347–368. doi: 10.2147/MDER.S214152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radak D., Katsiki N., Resanovic I., Jovanovic A., Sudar-Milovanovic E., Zafirovic S., Mousad S.A., Isenovic E.R. Apoptosis and acute brain ischemia in ischemic stroke. Curr. Vasc. Pharmacol. 2017;15:115–122. doi: 10.2174/1570161115666161104095522. [DOI] [PubMed] [Google Scholar]
- 21.Cichon N., Bijak M., Synowiec E., Miller E., Sliwinski T., Saluk-Bijak J. Modulation of antioxidant enzyme gene expression by extremely low frequency electromagnetic field in post-stroke patients. Scand. J. Clin. Lab. Investig. 2018;78:626–631. doi: 10.1080/00365513.2018.1542540. [DOI] [PubMed] [Google Scholar]
- 22.Cichon N., Saluk-Bijak J., Miller E., Sliwinski T., Synowiec E., Wigner P., Bijak M. Evaluation of the effects of extremely low frequency electromagnetic field on the levels of some inflammatory cytokines in post-stroke patients. J. Rehabil. Med. 2019;51:854–860. doi: 10.2340/16501977-2623. [DOI] [PubMed] [Google Scholar]
- 23.Cichoń N., Czarny P., Bijak M., Miller E., Śliwiński T., Szemraj J., Saluk-Bijak J. Benign effect of extremely low-frequency electromagnetic field on brain plasticity assessed by nitric oxide metabolism during poststroke rehabilitation. Oxid. Med. Cell. Longev. 2017;2017:2181942. doi: 10.1155/2017/2181942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cichoń N., Bijak M., Miller E., Saluk J. Extremely low frequency electromagnetic field (ELF-EMF) reduces oxidative stress and improves functional and psychological status in ischemic stroke patients. Bioelectromagnetics. 2017;38:386–396. doi: 10.1002/bem.22055. [DOI] [PubMed] [Google Scholar]
- 25.Cichoń N., Bijak M., Czarny P., Miller E., Synowiec E., Sliwinski T., Saluk-Bijak J. Increase in blood levels of growth factors involved in the neuroplasticity process by using an extremely low frequency electromagnetic field in post-stroke patients. Front. Aging Neurosci. 2018;10:294. doi: 10.3389/fnagi.2018.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lorberboym M., Blankenberg F.G., Sadeh M., Lampl Y. In vivo imaging of apoptosis in patients with acute stroke: Correlation with blood-brain barrier permeability. Brain Res. 2006;1103:13–19. doi: 10.1016/j.brainres.2006.05.073. [DOI] [PubMed] [Google Scholar]
- 27.Lorente L., Martín M.M., Pérez-Cejas A., González-Rivero A.F., Sabatel R., Ramos L., Argueso M., Solé-Violán J., Cáceres J.J., Jiménez A., et al. Non-Survivor Ischemic Stroke Patients Maintain High Serum Caspase-Cleaved Cytokeratin-18 Levels. Brain Sci. 2020;10:132. doi: 10.3390/brainsci10030132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olsen T.S., Larsen B., Herning M., Skriver E.B., Lassen N.A. Blood flow and vascular reactivity in collaterally perfused brain tissue. Evidence of an ischemic penumbra in patients with acute stroke. Stroke. 1983;14:332–341. doi: 10.1161/01.STR.14.3.332. [DOI] [PubMed] [Google Scholar]
- 29.Balduini W., Carloni S., Buonocore G. Autophagy in hypoxia-ischemia induced brain injury. J. Matern. Fetal Neonatal Med. 2012;25(Suppl. 1):30–34. doi: 10.3109/14767058.2012.663176. [DOI] [PubMed] [Google Scholar]
- 30.Deng Y.H., He H.Y., Yang L.Q., Zhang P.Y. Dynamic changes in neuronal autophagy and apoptosis in the ischemic penumbra following permanent ischemic stroke. Neural. Regen. Res. 2016;11:1108–1114. doi: 10.4103/1673-5374.187045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang P., Liu J., Yang Y., Zhai M., Shao X., Yan Z., Zhang X., Wu Y., Cao L., Sui B., et al. Differential intensity-dependent effects of pulsed electromagnetic fields on RANKL-induced osteoclast formation, apoptosis, and bone resorbing ability in RAW264.7 cells. Bioelectromagnetics. 2017;38:602–612. doi: 10.1002/bem.22070. [DOI] [PubMed] [Google Scholar]
- 32.Tenuzzo B., Vergallo C., Dini L. Effect of 6mT static magnetic field on the bcl-2, bax, p53 and hsp70 expression in freshly isolated and in vitro aged human lymphocytes. Tissue Cell. 2009;41:169–179. doi: 10.1016/j.tice.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 33.Ding G.R., Nakahara T., Hirose H., Koyama S., Takashima Y., Miyakoshi J. Extremely low frequency magnetic fields and the promotion of H2O2-induced cell death in HL-60 cells. Int. J. Radiat. Biol. 2004;80:317–324. doi: 10.1080/09553000410001679802. [DOI] [PubMed] [Google Scholar]
- 34.Xie W., Zhou J., Luo Q.L., Liu H.F., He C.Q. Pulsed electromagnetic field therapy inhibits chondrocyte apoptosis in rabbits with osteoarthritis. Sichuan Da Xue Xue Bao Yi Xue Ban. 2014;45:107–110. [PubMed] [Google Scholar]
- 35.Vincenzi F., Targa M., Corciulo C., Gessi S., Merighi S., Setti S., Cadossi R., Borea P.A., Varani K. The anti-tumor effect of A3 adenosine receptors is potentiated by pulsed electromagnetic fields in cultured neural cancer cells. PLoS ONE. 2012;7:e39317. doi: 10.1371/journal.pone.0039317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma Q., Deng P., Zhu G., Liu C., Zhang L., Zhou Z., Luo X., Li M., Zhong M., Yu Z., et al. Extremely low-frequency electromagnetic fields affect transcript levels of neuronal differentiation-related genes in embryonic neural stem cells. PLoS ONE. 2014;9:e90041. doi: 10.1371/journal.pone.0090041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grassi C., D’Ascenzo M., Torsello A., Martinotti G., Wolf F., Cittadini A., Azzena G.B. Effects of 50 Hz electromagnetic fields on voltage-gated Ca2+ channels and their role in modulation of neuroendocrine cell proliferation and death. Cell Calcium. 2004;35:307–315. doi: 10.1016/j.ceca.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 38.Morgado-Valle C., Verdugo-Díaz L., García D.E., Morales-Orozco C., Drucker-Colín R. The role of voltage-gated Ca2+ channels in neurite growth of cultured chromaffin cells induced by extremely low frequency (ELF) magnetic field stimulation. Cell Tissue Res. 1998;291:217–230. doi: 10.1007/s004410050992. [DOI] [PubMed] [Google Scholar]
- 39.Pall M.L. Electromagnetic fields act via activation of voltage-gated calcium channels to produce beneficial or adverse effects. J. Cell. Mol. Med. 2013;17:958–965. doi: 10.1111/jcmm.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun Z.C., Ge J.L., Guo B., Guo J., Hao M., Wu Y.C., Lin Y.A., La T., Yao P.T., Mei Y.A., et al. Extremely low frequency electromagnetic fields facilitate vesicle endocytosis by increasing presynaptic calcium channel expression at a central synapse. Sci. Rep. 2016;6:21774. doi: 10.1038/srep21774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martinou J.C., Green D.R. Breaking the mitochondrial barrier. Nat. Rev. Mol. Cell Biol. 2001;2:63–67. doi: 10.1038/35048069. [DOI] [PubMed] [Google Scholar]
- 42.Harwood S.M., Yaqoob M.M., Allen D.A. Caspase and calpain function in cell death: Bridging the gap between apoptosis and necrosis. Ann. Clin. Biochem. 2005;42:415–431. doi: 10.1258/000456305774538238. [DOI] [PubMed] [Google Scholar]
- 43.Morabito C., Guarnieri S., Fanò G., Mariggiò M.A. Effects of acute and chronic low frequency electromagnetic field exposure on PC12 cells during neuronal differentiation. Cell. Physiol. Biochem. 2010;26:947–958. doi: 10.1159/000324003. [DOI] [PubMed] [Google Scholar]