Abstract
Korean army dogs are raised for special purposes and have contributed much to society. However, several diseases occur in dogs. Canine hip dysplasia (CHD) is a musculoskeletal disorder that occurs frequently in Korean army dogs and interferes with their activities. If we could control CHD, this would have a positive effect on their performance. This study performed a genome-wide association study (GWAS) in 69 Korean army dogs to find significant loci for CHD using 170K single nucleotide polymorphisms (SNPs). CHD was classified according to the Norberg angle criterion. The control group comprised 62 dogs classified as relatively normal, and 7 dogs with severe CHD formed the case group. From the GWAS analysis, we concluded that SNPs present on chromosome 4 might have a significant impact on the overall expression of canine hip dysplasia.
Keywords: Single nucleotide polymorphism (SNP), Canine hip dysplasia (CHD), Genome-wide association study (GWAS)
INTRODUCTION
As travel and trade among countries increases, more substances that threaten national security, such as drugs and illegal agricultural products, are imported. Therefore, dogs that can detect and identify these threats are becoming more important. These dogs are trained for special purposes, such as detection dogs to detect explosives, quarantine-detection dogs to detect illegal agricultural products, drug-detection dogs to detect drug trafficking, and life-saving dogs. Training regular dogs as special-purpose dogs is costly due to their low efficiency. Therefore, special-purpose dogs that have greater ability are being developed using advanced somatic cell technology. Although we produce dogs that perform special tasks by replicating excellent breeds, musculoskeletal disorders such as hip dysplasia (HD) can be a major obstacle to producing these dogs. Therefore, a technology capable of diagnosing these musculoskeletal diseases in advance will be very useful for the production of dogs that perform special tasks.
Hip dysplasia is a very common genetic disorder with very negative effects on animal welfare. HD is a musculoskeletal disorder involving an unstable connection between the femoral head and acetabulum and is accompanied by severe pain. HD is very common, affecting more than 75% of dogs in American households [1]. Currently, treatment for HD is limited to relieving symptoms with mass therapy. HD, which was first reported to result from inbreeding 70 years ago, is a polygenic disease [2]. Therefore, the most effective way to eliminate HD is to exclude individuals with a genetic predisposition to HD through artificial selection [3]. To breed dogs selectively to eliminate HD, it is necessary to record information on the disease phenotype and lineage. However, disease records and lineage information is very limited. Bartolomé, Segarra [4] reported a predictive model that can detect genetic loci associated with HD using a genome-wide association study (GWAS). Lavrijsen [5] explored the susceptibility loci associated with HD in Labrador Retrievers and proposed a predictive model. The present study performed a GWAS analysis through a case–control study of HD and explored various genetic loci associated with HD.
MATERIALS AND METHODS
Phenotype assignment for case–control study
Blood samples were collected from dogs in accordance with animal welfare guidelines in cooperation with the National Institute of Animal Science (NIAS) and Department of Defense Military Observer Corps, Korea, to search for susceptibility loci associated with HD. Blood samples were obtained from Shepherd (n = 57) and Malinois (n = 12) breeds that were being trained for special purposes at the Department of Defense Military Observer Corps. Collected all samples followed The Institutional Animal Care and Use Committee (IACUC) of National Institute of Animal Science (NIAS, RDA) protocol approval by ‘Development of early diagnosis technology for degenerative muscular skeleton system in special purpose dog’ project (No. 2016-177). Using the Norberg angle, the phenotypes associated with HD were classified into seven intervals, as shown in Table 1, and divided into the normal range (> 80°) and the case (< 79°) groups. For case–control analysis, scores of 1 to 6 were used as controls, and scores of 7 as cases. We also distinguished left hip dysplasia (LHD), right hip dysplasia (RHD), and total hip dysplasia (THD) phenotypes based on these criteria. This enabled different hypotheses to identify the differences based on how the case group was divided.
Table 1. Phenotypes of hip dysplasia in Korean army dogs using Norberg angle.
| Score | 1 (Normal) | 2 | 3 | 4 | 5 | 6 | 7 (Disease) |
|---|---|---|---|---|---|---|---|
| Norberg angle | 105 ↑ | 100–104 | 95–99 | 90–94 | 85–89 | 80–84 | 79 ↓ |
gDNA extraction, genotyping, and quality control
Genomic DNA was extracted from blood collected from 69 army dogs using standard protocols [6]. Two breeds were genotyped using an Illumina CanineHD BeadChip (Illumina, San Diego, CA, USA) array, which contains 165,929 genetic variants and genotypes single nucleotide polymorphisms (SNPs) in the dog genome. The standardization level of genetic variants determined using the Illumina CanineHD BeadChip was managed using PLINK1.9 software [7] The Korean army dogs consisted of Shepherd and Malinois groups. When genetic variation was controlled for, almost all of the mutations were removed, so missing genotypes were limited to 10%. Finally, the minor allele frequency was set at less than 0.05 110,356 SNPs remaining after quality control were used for analysis.
Genome-wide association study
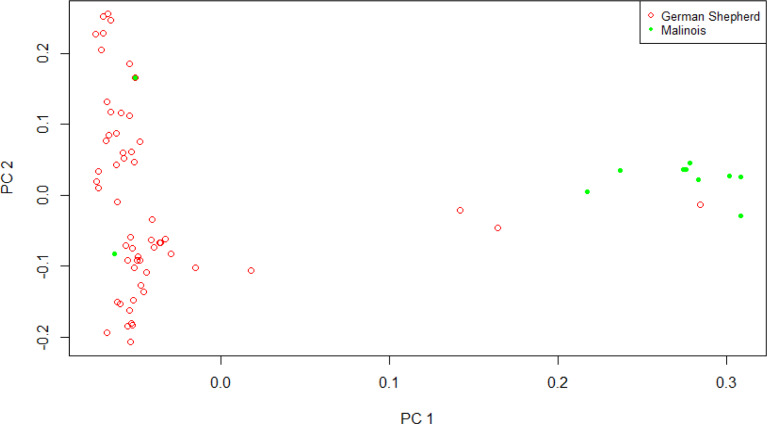
Case–control association analysis was performed for the GWAS for HD in dogs. Using the phenotype information for Shepherds and Malinois, principal component analysis was performed to understand population stratification (Fig. 1). We used GWAS statistical model following below
Fig. 1. Principal component analysis (PCA) result between Korean army dog populations.
Where, Y is phenotype, X and Z are design matrices, β is a vector of fixed effects. u is additive genetic effect for each marker. and e is vector of residual.
In this analysis, the eigenvalue of the principal component was set to the covariance to correct for population stratification. We assumed that there are no environmental effects on the phenotype, and a correlation analysis between genotype and phenotype was performed using the association analysis option, which has a case–control function on Plink v 1.9 [7]. To test for statistical significance, the chi-square test was performed, and the p-value obtained was corrected using the Bonferroni correction. The risk allele (minor allele) was used to calculate the odds ratio for predicting disease. We used the -assoc option of Plink v1.9 to perform the GWAS, converted the p-value to -log p, and made a Manhattan plot using R-software.
RESULTS AND DISCUSSION
Hip dysplasia phenotype based on the Norberg angle
Studies of HD have suggested that SNPs present on many chromosomes have a polygenic effect on the expression of HD [8]. HD may be the most common musculoskeletal disorder caused by a genetic effect, but it is also strongly influenced by non-genetic factors [9]. For example, it is more likely to occur in large dogs than small dogs and is more frequent in old dogs than in young dogs because of bone degeneration. In this study, 69 Korean army dogs [Shepherd (n = 57) and Malinois (n = 12)] were classified according to the Norberg angle standards shown in Table 1. As shown in Table 2, the control group was classified as a relatively normal group with scores of 1 to 6, and the case group was classified as the disease group with a score of 7. In fact, setting the criteria for controls and cases was difficult and involved a subjective decision regarding whether only a 7 would be considered a case or whether scores of 5 and 6 could also be classified as cases. We assigned only scores of 7 to the case group because the phenotype information was based on the evaluation process for selection before military service. Ultimately, there were 62 controls and 7 cases for LHD or RHD, whereas the THD group comprised 58 controls and 11 cases. Interestingly, all of the cases were Shepherds, perhaps due to the small sample of Malinois.
Table 2. Classification of Korean army dogs according to Norberg angle.
| Group | Number of animal | Number of control | Number of case |
|---|---|---|---|
| Left dysplasia | 69 | 62 | 7 |
| Malinois | 12 | 12 | 0 |
| Shepherd | 57 | 50 | 7 |
| Right dysplasia | 69 | 62 | 7 |
| Malinois | 12 | 12 | 0 |
| Shepherd | 57 | 50 | 7 |
| Total dysplasia | 69 | 58 | 11 |
| Malinois | 12 | 12 | 0 |
| Shepherd | 57 | 46 | 11 |
Genome-wide association study of CHD
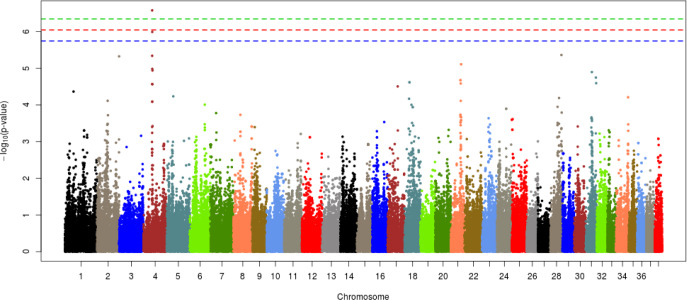
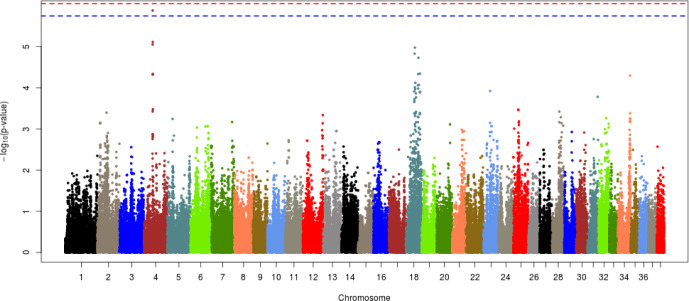
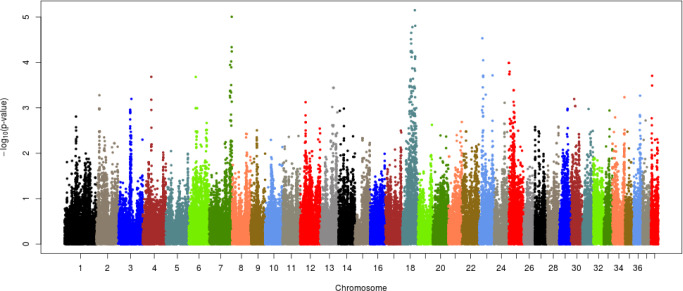
Each of the given phenotypes was scored as 1 (control) or 2 (case), and GWAS analyses were performed for left or right hip and for both hips. Manhattan plots for LHD, RHD, and THD suggested SNP effects based on the Bonferroni correction criterion (0.05/number of SNPs tested). The results, presented in Figs. 2–4, revealed a single candidate SNP on chromosome 4 in the LHD group, whereas there were no significant SNPs for RHD or THD. The BICF2S2352810 (4:34585340) SNP was significantly detected in LHD. This SNP is located in the multimerin 2 (MMRN2) gene, but it is unclear whether this gene affects HD directly. The MMRN2 gene is associated with gonadotropin-releasing hormone (GnRH) expression via the integrin pathway. GnRH regulates a variety of reproductive activities and affects the secretion of growth hormone (GH); activating GnRH inhibits the release of GH [10–12]. GH secretion may adversely affect bone development and may thereby ultimately contribute to HD expression. In addition, the ENSCAFG00000016100 gene (4:34756078–34763159) located near the BICF2S2352810 SNP controls calcium excretion and might be associated with this gene in a larger sample. Among the pathways between these genes, there is arginine biosynthesis, which acts as a stimulator of GH. Therefore, this function estimated that it can be involved in bone growth and development. In addition, two suggestive SNPs, one of BICF2P650805 (7:79852044) and one of BICF2G630691124 (18:45206432), were identified for RHD traits, and one suggestive SNP of BICF2S2352810 (4:34585340) was identified for THD traits (Figs. 3 and 4, Table 3). These SNPs were located in the cap-binding complex-dependent translation initiation factor (CTIF), CD151 molecule (CD151), tetraspanin 4 (TSPAN4), and MMRN2 genes. However, these genes are not considered direct factors in the development of HD. For the RHD trait, the DYM (7:79286614–79629043) and ALX4 (18:44922150–44967550) genes are located adjacent to the BICF2P650805 and BICF2G630691124 SNPs, respectively, and their functions are related to bone and muscle development. These functions related to candidate genes need to be confirmed as causal mutations using a larger sample size. In addition, it is necessary to consider the different aspects of LHD, RHD, and THD. We anticipate that this may be the result of problems such as ambiguity in the criteria for dividing phenotypes and small sample sizes. Similar patterns may be identified if more clear phenotypes or sufficient sample sizes can be obtained through the further studies.
Fig. 2. Manhattan plot of GWAS for left hip dysplasia in Korean army dogs, the significant threshold for green line is 0.05/number of SNPs and suggestive threshold for red line is 0.1/number of SNPs, blue line is 0.2/number of SNPs.
GWAS, genome-wide association study; SNPs, single nucleotide polymorphisms.
Fig. 4. Manhattan plot of GWAS for total hip dysplasia in Korean army dogs, there were no significant SNPs, the suggestive threshold for red line is 0.1/number of SNPs, blue line is 0.2/number of SNPs.
GWAS, genome-wide association study; SNPs, single nucleotide polymorphisms.
Fig. 3. Manhattan plot of GWAS for right hip dysplasia in Korean army dogs, there was no significant and suggestive relationship SNPs.
GWAS, genome-wide association study; SNPs, single nucleotide polymorphisms.
Table 3. Candidate markers associated with hip dysplasia in Korean army dogs.
| Type | Marker | CHR : Position | MAF | Candidate gene | p-value | Odds ratio | CHISQ | Function |
|---|---|---|---|---|---|---|---|---|
| LHD | BICF2S2352810 | 4 : 34585340 | 0.07246 | MMRN2 | 2.64727e-07 | 22.5 | 29.4 | Protein binding |
| RHD | BICF2P650805 | 7 : 79852044 | 0.07971 | CTIF | 9.88738e-06 | 10.93 | 16.35 | Protein binding |
| BICF2G630691124 | 18 : 45206432 | 0.1397 | CD151, TSPAN4 | 7.10262e-06 | 9.167 | 16.86 | Integrin binding | |
| THD | BICF2S2352810 | 4 : 34585340 | 0.07246 | MMRN2 | 1.33445e-06 | 17.58 | 23.51 | Protein binding |
CHR, canine chromosome; MAF, minor allele frequency; CHISQ, chi-square value; LHD, left hip dysplasia; RHD, right hip dysplasia; THD, total hip dysplasia.
Several studies based on various approaches have examined HD in dogs. Huang, Hayward [8] performed a GWAS of HD in 921 dogs of various breeds and found that the CTBP2 gene associated with the BICF2G630265083 SNP on chromosome 28 had a major effect on HD compared with other genes. In the GWAS results for HD in Korean army dogs, only one SNP on chromosome 4 had a significant effect. In addition, the BICF2S23731107 (28:37925846) SNP on chromosome 28 was found to have a suggestive effect; it is approximately 3 Mb from the CTBP2 gene (28:34260856–34415696) but has potential effects due to linkage disequilibrium relationships. However, there is still much ambiguity regarding HD expression, especially with our results and with the explanation of HD from previous results and variation in the CTBP2 gene and BICF2S23731107 (28:37925846) SNP presented in the current study. The possibility of additional causal mutations between the two cannot be ruled out. If causal mutations were found, it would facilitate our understanding of HD expression.
Recently, it was suggested that GWASs that assume polygenic traits not only have difficulty identifying specific causes and treatments for diseases but also are limited for understanding the population genetic structure of the population with a particular disease [13]. An alternative approach is the next-generation sequencing (NGS) method, in which rare variants at very low frequencies are associated with disease, and the effects of the rare variants can be combined with the common variant. Therefore, more insight can be achieved by combining various approaches, such as NGS and other methods, in future research [14].
CONCLUSION
This study used a GWAS to identify genes related to HD, a disease directly implicated in the ability of Korean army dogs to perform their missions. We identified the BICF2S2352810 SNP; however, because HD is the result of polygenic effects, it is difficult to explain HD expression using only a single SNP. Therefore, although the GWAS results did not show any direct significance, we speculate that the influence of SNPs on chromosomes 7 and 18 is derives from genes involved in bone growth, such as DYM and ALX4. The use of NGS in future studies to identify rare variants associated with HD expression might suggest better solutions for the prevention and treatment of HD.
Acknowledgements
This work was carried out with the support of Research Grants from Chungnam National University, Korea.
Competing interests
No potential conflict of interest relevant to this article was reported.
Funding sources
“Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ012833022019)” Rural Development Administration, Korea and Research Grants from Chungnam National University, Korea.
Availability of data and material
Upon reasonable request, the datasets of this study can be available from the corresponding author.
Authors’ contributions
Conceptualization: Choi BH, Lee SH.
Data curation: Lee DH, Kim YK.
Formal analysis: Kang JM, Lee SH.
Methodology: Choi BH, Lee SH.
Software: Kang JM, Lee DH, Kim YK.
Validation: Seo D, Lee SH.
Investigation: Kang JM, Seo D.
Writing - original draft: Kang JM, Seo D.
Writing - review & editing: Kang JM, Seo D, Lee SH, Lee DH, Kim YK, Choi BH, Lee SH.
Ethics approval and consent to participate
The Institutional Animal Care and Use Committee (IACUC) of National Institute of Animal Science (NIAS, RDA) protocol approval by ‘Development of early diagnosis technology for degenerative muscular skeleton system in special purpose dog’ project (No. 2016-177).
REFERENCES
- 1.Zhou Z, Sheng X, Zhang Z, Zhao K, Zhu L, Guo G, et al. Differential genetic regulation of canine hip dysplasia and osteoarthritis. PLOS ONE. 2010;5:e13219. doi: 10.1371/journal.pone.0013219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schnelle G. Some new diseases in the dog. Am Kennel Gaz. 1935;52:25–6. [Google Scholar]
- 3.Spady TC, Ostrander EA. Canine behavioral genetics: pointing out the phenotypes and herding up the genes. Am J Hum Genet. 2008;82:10–8. doi: 10.1016/j.ajhg.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartolom N, Segarra S, Artieda M, Francino O, Sánchez E, Szczypiorska M, et al. A genetic predictive model for canine hip dysplasia: integration of Genome Wide Association Study (GWAS) and candidate gene approaches. PLOS ONE. 2015;10:e0122558. doi: 10.1371/journal.pone.0122558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lavrijsen ICM, Leegwater PAJ, Martin AJ, Harris SJ, Tryfonidou MA, Heuven HCM, et al. Genome Wide Analysis Indicates genes for basement membrane and cartilage matrix proteins as candidates for hip dysplasia in labrador retrievers. PLOS ONE. 2014;9:e87735. doi: 10.1371/journal.pone.0087735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meurs KM, Fox PR, Magnon AL, Liu SK, Towbin JA. Molecular screening by polymerase chain reaction detects panleukopenia virus DNA in formalin-fixed hearts from cats with idiopathic cardiomyopathy and myocarditis. Cardiovasc Pathol. 2000;9:119–26. doi: 10.1016/S1054-8807(00)00031-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–75. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang M, Hayward JJ, Corey E, Garrison SJ, Wagner GR, Krotscheck U, et al. A novel iterative mixed model to remap three complex orthopedic traits in dogs. PLOS ONE. 2017;12:e0176932. doi: 10.1371/journal.pone.0176932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson BJ, Nicholas FW, James JW, Wade CM, Tammen I, Raadsma HW, et al. Heritability and phenotypic variation of canine hip dysplasia radiographic traits in a cohort of Australian German Shepherd Dogs. PLOS ONE. 2012;7:e39620. doi: 10.1371/journal.pone.0039620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Favier RP, Mol JA, Kooistra HS, Rijnberk A. Large body size in the dog is associated with transient GH excess at a young age. J Endocrinol. 2001;170:479–84. doi: 10.1677/joe.0.1700479. [DOI] [PubMed] [Google Scholar]
- 11.Antoniazzi F, Zamboni G, Bertoldo F, Lauriola S, Tat L. Bone development during GH and GnRH analog treatment. Eur J Endocrinol. 2004;151:S47–54. doi: 10.1530/eje.0.151s047. [DOI] [PubMed] [Google Scholar]
- 12.Fernandes J, Vieira AS, Kramer-Soares JC, Da Silva EA, Lee KS, Lopes-Cendes I, et al. Hippocampal microRNA-mRNA regulatory network is affected by physical exercise. Biochim Biophys Acta Gen Subj. 2018;1862:1711–20. doi: 10.1016/j.bbagen.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Bodmer W, Bonilla C. Common and rare variants in multifactorial susceptibility to common diseases. Nat Genet. 2008;40:695–701. doi: 10.1038/ng.f.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahn SM. Phenotype-genotype association study: from GWAS to NGS. Endocrinol Metab. 2011;26:187–92. doi: 10.3803/EnM.2011.26.3.187. [DOI] [Google Scholar]