Abstract
Antibody structure couples adaptive and innate immunity via Fab (antigen binding) and Fc (effector) domains that are connected by unique hinge regions. Because antibodies harbor two or more Fab domains, they are capable of cross-linking multi-determinant antigens, which is required for Fc-dependent functions through associative interactions with effector ligands including C1q and cell surface Fc receptors. The modular nature of antibodies, with distal ligand binding sites for antigen and Fc-ligands, is reminiscent of allosteric proteins, suggesting that allosteric interactions might contribute to Fc-mediated effector functions. This hypothesis has been pursued for over forty years and remains unresolved. Here we provide evidence that allosteric interactions between Fab and Fc triggered by antigen binding modulate binding of Fc to low-affinity Fc receptors (FcγR) for a human IgG1. This work opens the path to further dissection of the relative roles of allosteric and associative interactions in Fc-mediated effector functions.
Graphical Abstract
One Sentence Summary:
Antigen binding to Fab induces allosteric changes in the Fc region of a human IgG1 that modulate low affinity Fc-gamma receptor binding.
eToc blurb
Orlandi et.al. show that antigen binding to Fab increases binding of IgG1 Fc to low-affinity FcγR by conformational allostery. Leucine to alanine mutations at positions 234 and 235 in the lower hinge of IgG1 globally alter Fc structure and biological activity by configurational allostery.
Introduction
Antibody structure couples adaptive and innate immunity via Fab (antigen binding) and Fc (effector) domains that are connected by unique hinge regions. It is widely accepted that Fc-mediated effector functions require cooperative, multivalent cross-linking of antibodies by antigen for biological activity (reviewed in (Janda et al., 2016; Metzger, 1978; Yang et al., 2017)). These cooperative cross-linking interactions are collectively referred to as associative interactions (Janda et al., 2016; Yang et al., 2017) and can be valence-based (multiple associated epitopes), clustering based (assembly of multi-protein complexes of antigen, antibody, cell surface receptors), or template based (such as antigen-induced hexamerization of IgG at the cell surface through Fc-Fc interactions (Diebolder et al., 2014))(Yang et al., 2017)). There is also significant data indicating “configurational” allosteric interactions in Fc-mediated functions. As defined in (Yang et al., 2017), configurational allosteric interactions result from site specific covalent modifications that affect antibody conformation at distal sites. For example, a Y407E mutation in the CH3 E-Strand alters the CH3-CH3 interface resulting in dramatic changes in N297-glycan composition and antibody conformation (Rose et al., 2013). The Y407E mutation resulted in altered patterns of Hydrogen-Deuterium Exchange (HDX) as far away as residues in the CH2 domain that interact with N297-glycan a-1,6 mannose branch and residues at the CH2-CH3 interface. Configurational allosteric effects are likely responsible for well-known changes in Fab fine specificity by covalent changes in the CH1 and CL regions (reviewed in (Janda et al., 2016)). It is well accepted that various associative and configurational allosteric effects play a role in antibody structure in function (discussed in (Casadevall and Janda, 2012; Casadevall and Pirofski, 2012; Janda et al., 2016; Yang et al., 2017)).
By contrast, a role for a second form of allosteric cooperativity, conformational allosteric cooperativity, in antibody function is controversial (Yang et al., 2017). Allosteric cooperativity results in a conformational change being transmitted from one part of an immunoglobulin to another upon ligand binding. The modular nature of antibodies, with distal ligand binding sites for antigen and Fc-ligands, is reminiscent of allosteric proteins, raising the possibility that allosteric cooperativity might contribute to antibody function. A number of studies in the 1970s suggested that antigen binding by Fab induced allosteric changes in Fc that can be detected spectroscopically (Lancet et al., 1977; Lancet and Pecht, 1976; Pecht, 1976) or by cooperative activation of the complement cascade (Brown and Koshland, 1977; Hoffmann, 1976; Thompson and Hoffmann, 1971, 1974a, b). However, these studies were inconclusive (summarized in (Metzger, 1978)) and the prevailing thought emerged that multimeric antigen antibody complexes formed by associative interactions are largely responsible for ligand binding by Fc and biological activity and remains so today (Yang et al., 2017).
Against this backdrop, more recent studies using molecular dynamic analysis of antigen-Fab complex structures clearly indicate coherent intra-Fab allosteric changes after antigen binding (Corrada et al., 2013; Sela-Culang et al., 2012). Similar findings were reported over the years by HDX (Williams et al., 1996; Williams et al., 1997) and x-ray crystallography (Guddat et al., 1994; Guddat et al., 1995) analyses of Fab-antigen complexes. It is reasonably certain that conformational allosteric interactions occur across distal Fab residues after antigen binding, leaving the question of whether these interactions can also be communicated from Fab to Fc across the hinge upon antigen binding. This possibility is suggested indirectly by HDX changes in Fab consequent to binding of the neonatal Fc receptor (FcRn) at the CH2-CH3 interface (Jensen et al., 2015) and the binding to FcγRIIA (Yogo et al., 2019). Allosteric changes are supported further by alterations of protein-A and protein-G binding to this region after antigen binding (Oda et al., 2003). Most recently, molecular dynamics simulation of simple antigen binding to a human IgG1 mAb strongly supports this hypothesis (Zhao et al., 2018).
The MD simulations (Zhao et al., 2018) showed that monomeric binding of a small antigen to IgG1 results in a large re-orientation of the Fab domains into two dominant conformational clusters that are accompanied by open conformations of the CH2 domain that favor FcγR binding. Thus, antigen binding and FcγR binding are highly correlated phenomena. These studies suggest that antigen binding signals are mostly transmitted through the hinge via entropy driven interactions and that they can also propagate through the CL/CH1 domain. Both pathways are important for FcγR binding.
Below, we provide data supporting the MD simulations in (Zhao et al., 2018). We used a combination of antibody dependent cellular cytotoxicity (ADCC) assays, binding analyses, spectroscopic studies, HDX and x-ray crystallographic studies that monomeric antigen binding to human IgG1 mAb induces a conformational change in Fc that increases binding to low-affinity FcγRs. Additional data are presented that this effect must be accompanied by associative interactions for full biological activity.
Results
ADCC with wild-type mAbs and mAbs attenuated for FcγR binding
As shown in Figure 1, two human IgG1 mAbs, N5-i5 and C11, specific for adjacent sub-regions of Epitope Cluster A on HIV-1 gp120 (Gohain et al., 2015; Tolbert et al., 2017a; Tolbert et al., 2016), mediate potent ADCC with EC50s of approximately 3pM to 60pM (Guan et al., 2013). We also evaluated ADCC mediated by N5-i5-LALA and C11-LALA mutants harboring L to A mutations at residues 234 and 235 in the lower hinge region that are used commonly to abrogate binding to FcγR (Canfield and Morrison, 1991; Hezareh et al., 2001; Lund et al., 1992). To our surprise, ADCC by N5-i5-LALA and C11-LALA was not abrogated although it was attenuated significantly with EC50s for both mAbs of approximately 6nM and no plateau activity. We were puzzled by this observation because our preliminary ELISA studies showed that FcγR binding was abrogated for N5-i5-LALA and C11-LALA (Figure S1). Very similar observations were reported recently for LALA variants of an IgG3 anti-transferrin receptor mAb for cancer therapy (Leoh et al., 2015). Additionally, it was shown that IgG attenuated for FcγR binding by a D265A mutation or deglycosylation could still bind to FcγR when presented as a large immune complex (IC) (Lux et al., 2013). Based on these observations, we reasoned that N5-i5-LALA and C11-LALA might bind low-affinity FcγR as ICs but not as unliganded monomers.
Figure 1-. ADCC mediated by mAbs specific for Epitope Cluster A mAbs (Guan et. al., 2013) measured using the modified RFADCC assay (Gomez-Roman et al., 2006; Orlandi et al., 2016).
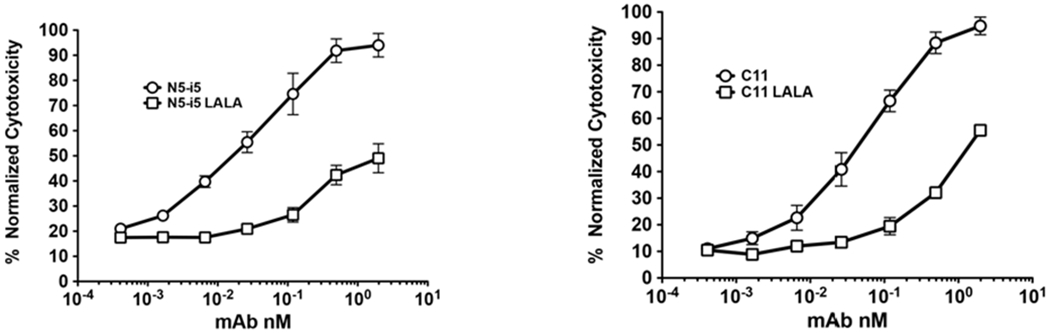
The left panel shows ADCC mediated by N5-i5 and N5-i5-LALA and the right panel shows ADCC mediated by C11 and C11-LALA. N5-i5 recognizes the A32 sub-region of Epitope Cluster A and C11 recognizes the adjacent C11 sub-region of epitope Cluster A (Guan et al., 2013; Tolbert et al., 2016; Tolbert et al. 2017). Means and +/− SD are shown for triplicate assay wells.
As shown in Figure S1, preliminary ELISA studies using FcγRIIA coated plates were consistent with this hypothesis. In the example shown, ELISA plates were coated with human soluble FcγRIIA and the plates probed with N5-i5-LALA or C11-LALA as unliganded monomers or as soluble ICs made with a single-chain gp120-CD4 fusion protein (FLSC) (Fouts et al., 2000) as antigen. Wild-type N5-i5 and C11 unliganded monomers were included as FcγR binding positive controls. FLSC expresses one copy each of the epitopes recognized by N5-i5 and C11 (Acharya et al., 2014; Gohain et al., 2016; Tolbert et al., 2017b; Tolbert et al., 2016), thus ICs made with single mAbs will have a 1:1 or 2:1 stoichiometry of antigen to antibody, which obviates the formation of larger antigen cross-linked multimers as illustrated in Figure S1. As expected, unliganded N5-i5 and C11 bound to FcγRIIA coated plates with maximal binding around 100nM but with no plateau. Also, as expected no apparent binding was observed in the case of N5-i5-LALA and C11-LALA as predicted by the LALA variant literature (Canfield and Morrison, 1991; Hessell et al., 2007; Hezareh et al., 2001; Lund et al., 1992; Morgan et al., 1995). By contrast, both N5-i5-LALA and C11-LALA ICs made with FLSC bound well to the FcγRIIA coated plate with half-maximal binding of approximately 6nM and 0.6nM, respectively. Plateau binding was clearly observed for C11 and apparent plateau binding was observed for N5-i5. This result confirms the previous observations that IgG1 abrogated for FcγR binding as a monomer can bind to FcγR when presented as immune complexes (Lux et al., 2013). This result potentially explains why residual ADCC activity for LALA variants was observed for IgG1 in our studies above and for IgG3 in (Leoh et al., 2015).
Binding of low-affinity FcγR to N5-i5 or C11 and their LALA variants as small immune complexes determined by fluorescence correlation spectroscopy (FCS)
Previously, we used FCS to quantify the binding of anti-Env IgG mAbs to HIV-1 virions in solution (Ray et al., 2014). This method is based on the change in diffusion of a fluorescently labeled mAb when it is complexed with larger structure, in that case an HIV-1 virion. FCS is carried out at low ligand concentrations to enable single molecule fluorescence where it provides an extremely sensitive window on protein-protein binding interactions. We adapted this system to determine whether fluorescently labeled soluble FcγRs bind differentially to N5-i5 or C11 and their LALA variants when complexed with FLSC. By employing FCS, we also gathered information about the IC-FcγR size at single molecule sensitivity (Acharya et al., 2014; Gohain et al., 2015b; Tolbert et al., 2017b; Tolbert et al., 2016) therefore were able to obviate the problems related to the ELISA based binding assays where the observed effect could be due to the formation of higher-ordered ICs on the ELISA plate surface. Labeling the smallest molecule in the system, FcγR, provided the most sensitive diffusion window to detect its differential binding to N5-i5 or C11 alone or in ICs formed using a 2:1 ratio of FLSC to IgG1. Further, the observed diffusion coefficients for complexes with ICs provided a test of whether multimeric ICs are formed (definitions in Fig. S2). The binding of N5-i5 and C11 and their LALA variants to FcγR in the absence and presence of FLSC is shown in Figure 2. There was a statistically significant increase in binding of the ICs to each of the low affinity FcγR over the binding observed for both wild-type and LALA variants of N5-i5 and C11 in the absence of antigen. The binding is consistent with either monovalent or bivalent occupancy of the Fabs of each IgG1. The average diffusion coefficient of the labeled FcγR in the absence of antigen was approximately of 45 um2/sec, which indicates a 1:1 binding stoichiometry. By contrast, average diffusion coefficient of the labeled FcγR for the N5-i5 and N5-i5-LALA ICs was reduced to approximately 34 um2/sec, which is consistent with the binding of FcγR to either monovalent or bivalent ICs of N5-i5 and C11. No higher ordered aggregates were observed consistent with the FcγR binding to ICs with a 1:1 or 2:1 ratio of antigen to mAb. This result strongly suggests that antigen binding favors the asymmetric “primed” Fc conformation required for low affinity FcγR binding that was identified by x-ray crystallography (Radaev et al., 2001; Sondermann et al., 2000) and NMR studies (Frank et al., 2014; Hanson and Barb, 2015; Subedi and Barb, 2015; Subedi et al., 2014). In addition, due to the single molecule sensitivity of FCS and its ability to detect low-affinity interactions, residual activity for the binding of LALA variants to the FcγR is apparent in most cases. This binding was not apparent using macroscopic methods such as ELISA (Figure S1) or SPR (not shown), suggesting that the conformation competent to bind FcγR is present in the LALA variant but it is a small component of the conformational ensemble as compared with wild-type IgG1.
Figure 2-. Single molecule analysis of FcγR-mAb interactions by fluorescence correlation spectroscopy.
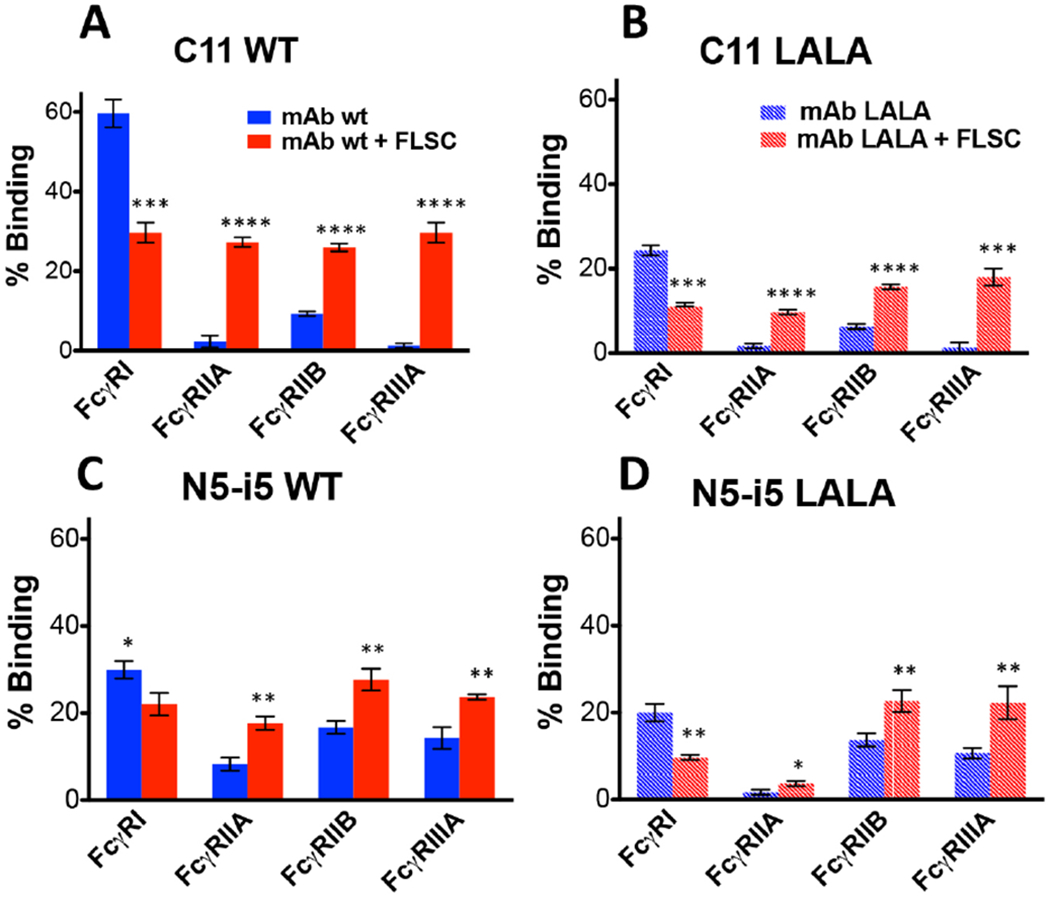
Fraction of FcγRs bound to monomeric mAbs alone or previously engaged with their specific antigens. FcγRs were labeled with Alexa-Fluor 647 and their coefficient of diffusion were first determined. Then the receptors were incubated with mAbs wt or LALA (respectively C11 Panels A-B, N5-i5 Panels C-D) alone (Blue bars with a diffusion coefficient of 45 um2/sec) or previously engaged in the binding to their specific antigens (FLSC) (Red bars with a diffusion coefficient of 34 um2/sec). The experiments were conducted with limiting concentrations of monomeric mAbs, FLSC and labeled FcγRs in solution (Ray K. et al., 2014). The data shown are the results of the average of 2 independent experiments analyzed with an unpaired two-tailed t-test, * P < 0.1, ** P <0.01, ***P <0.001; ****P <0.0001. See also Figure S1.
It should be noted that in contrast to the antigen-induced increased binding to low-affinity FcγR, our FCS data also revealed decreased binding to high-affinity FcγRI that was similarly antigen-dependent. We do not understand the basis for this differential effect of antigen binding on the interaction between Fc and low-affinity vs. high-affinity FcγR. We presume that the allosteric effect is involved in both cases. We have carried out extensive literature searches to identify data suggesting that the high-affinity FcγRI binds immune complexes any differently (greater or less) than free IgG1 but to no avail. It is likely that there are sociological relationships among low high-affinity and low-affinity FcγR on the same cell but they are ill defined currently. We are constructing new cell lines to ferret out these interactions, but, at this point, we have only the empirical data presented in this manuscript that the two FcγR receptor types behave differently to non-crosslinked immune complexes.
Collectively, our ELISA, FCS, and ITC studies show consistently that antigen binding increased the binding of wild-type and LALA variants to low-affinity FcγR. These results suggest that antigen binding allosterically induces a conformational change in Fc that favors the binding to low affinity FcγR for at least two independent epitopes. Results with the LALA variants also suggest that IC binding to low-affinity FcγR is not sufficient for potent ADCC and that these mutations affect IgG1 structure more broadly than just impinging FcγR binding.
Hydrogen-Deuterium Exchange reveals antigen-induced allosteric changes in Fc region of N5-i5 IgG1
Further testing of the allosteric hypothesis was carried out using HDX-MS for N5-i5 in the presence and absence of FLSC. We chose N5-i5 because we have determined the atomic structures of its free Fab (Guan et al., 2013) and its Fab complexed with gp120 core proteins (Acharya et al., 2014; Tolbert et al., 2017b) permitting detailed structural interpretation of antigen-induced changes in HDX. No structure is available yet for C11 bound to its cognate epitope. We established protease digestion conditions for N5-i5 that provided sequence coverages of 91% and 88% with redundancies of 2.74 and 1.93 for the heavy and light chains respectively. HDX-MS was carried out for free N5-i5 and N5-i5 bound to FLSC (2:1 molar FLSC/N5i5 ratio) and delta plots were constructed and shown in Figure 3A, Upper (heavy chain) and lower (light chain) panels. FLSC binding to N5-i5 resulted in substantially increased protection for heavy chain residues within the variable region of Fab: A23-T31 (CDRH1), W47-F58 (CDRH2), and L96-D101 (CDRH3) as well as light chain residues I18-F32 (CDRL1) (numbering of N5-i5 Fab is from PDB:4H8W ((Acharya et al., 2014)). These regions overlap with the antigen binding site of N5-i5 Fab defined by x-ray crystallography of N5-i5 Fab-gp120 core-CD4 complex (Acharya et al., 2014). This magnitude of protection is expected because the binding of N5-i5 Fab to its cognate epitope is very tight with the surface area buried at the complex interface of approximately 1,890 Å2 (Acharya et al., 2014). There was also increased exchange for light chain residues y87-y91 (CDRL3) and K205-P209 (CL), although increased protection dominated the light chain changes. The increased exchange for K205-P209 in the C-terminus of the light chain also consistent with earlier HDX data (Williams et al., 1996; Williams et al., 1997) and molecular dynamics simulations showing that allosteric changes are transmitted within a Fab consequent to antigen binding (Corrada et al., 2013; Sela-Culang et al., 2012).
Figure 3-. Legend- HDX-MS of N5i5 bound to FLSC compared with unliganded N5i5.
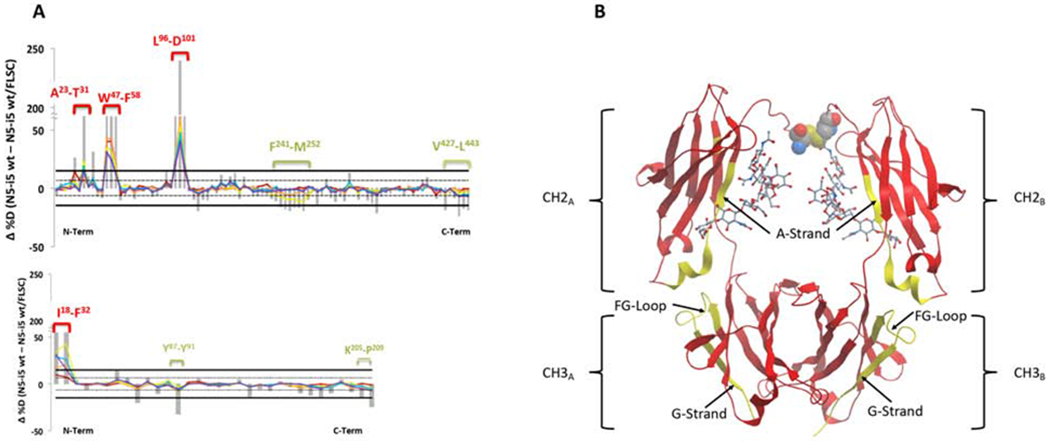
Panel A shows the percent difference plots (unliganded – FLSC bound) in deuterium uptake for the heavy chain (top) and the light chain (bottom). Individual peptidic fragments are plotted on the x-axis going from N to C terminus based on the sequence number of the first residue of the peptide. Color coded traces represent the percent deuterium uptake difference for a given peptide at a given deuterium incubation time point (10 sec red, 1 min orange, 10 min yellow, 1 hr blue, 2 hr purple). The grey bars represent the sum of the percent deuterium uptake differences integrated over all time points and is used only for statistical purposes. 98% confidence intervals are plotted as horizontal lines with solid lines for the sum of differences and hashed lines for individual time points. Regions displaying significant differences are indicated as brackets with sequences indicated. Panel B shows the significant regions mapped onto cartoon representation of onto IgG1 Fc from PDB:1E4K. Rendered in yellow are the regions displaying significant changes in deuterium uptake, here increased uptake upon FLSC binding. L234-L235 are rendered as CPK in the lower hinge and the glycans are rendered as sticks. See also Figure S4.
Interestingly, increased exchange was observed in the Fc region for residues F241-M252 and V427-L443 in the CH2 and CH3 domains (using Eu numbering for IgG1 Fc residues), respectively (Figure 3A and B). These increases are smaller in magnitude than the protection seen in Fab but they are statistically significant and in the range of exchange for regions not involved in forming a protein-protein interface. The residues of F241-M252 are on the CH2 A-Strand (Figure 3B). The first ten residues of V427-L443 are on the CH3 FG-Loop and remaining six residues are on the CH3 G-Strand that includes the heavy chain C-terminus. These residues include structural elements that are key determinants of affinity for Fc to FcγR, although they do not directly contact FcγR. Residues F241 and F243 interact with the GlcNAc2 and GlcNAc5′ via CH-π interactions on the α-1,6 branch of the N297 linked glycan where they contribute to Fc stability (Jassal et al., 2001; Lund et al., 1996; Stewart et al., 2011; Voynov et al., 2009), increased FcγR binding (Lund et al., 1996; Stewart et al., 2011; Yu et al., 2013), and Fc-mediated effector functions (Lund et al., 1996; Stewart et al., 2011; Yu et al., 2013). The interaction between N297-linked glycans with CH2 buries approximately 500 Å2 of each CH2 surface and is conformationally dynamic (Barb, 2015; Barb et al., 2012; Barb and Prestegard, 2011; Frank et al., 2014; Subedi et al., 2014). Our data suggests that antigen-binding alters the dynamic interactions in this region of CH2 favoring binding to low-affinity FcγR. Our data also suggests that antigen binding affects the CH2-CH3 interface, which is also a major non-contact contributor to low-affinity FcγR binding (Frank et al., 2014; Subedi and Barb, 2015; Subedi et al., 2014). The CH2-CH3 interface is stabilized by two hydrogen bonds between K248-E380 and K338-E430 as well as by a “ball in socket” joint where I251 (CH2 domain) is the “ball” and the “socket” is comprised CH3 residues M428, E430, and H435. Increased HDX after antigen binding was observed at all residues for the ball in socket joint at the CH2-CH3 interface as well as in K248, the H-bond donor of K248-E380, and in E430, the H-bond donor in K338-E430 of the interface. These data are consistent with the CH2-CH3 interface adopting a conformation primed for binding to low-affinity FcγR after antigen binding as proposed in (Frank et al., 2014; Subedi and Barb, 2015; Subedi et al., 2014).
LALA mutations globally impact N5-i5 conformation distally from the lower hinge
We also carried out HDX-MS studies of the unliganded N5-i5-LALA variant compared with unliganded N5-i5 and after antigen binding to the two mAbs. As shown in Figure 4A, the HDX pattern of the N5-i5-LALA heavy chain was strikingly different from the wild-type N5-i5 in the absence of antigen. These results identify a previously unknown configurational allosteric network (Yang et al., 2017) and show that the LALA mutations affect IgG1 conformation beyond the contact residues for FcγR. For example, increases in deuteration were observed across almost the entire molecule including residues V5-S17 and S59-F67, in the VH domain, residues A114-T151, W154-T160, and V181-T193 in the CH1 domain. Increased deuteration was also observed F241-C261, T307-E357, I377-L398, and L406-S444 in the CH2-CH3 domains of Fc. There was also a significant decrease in deuteration in residues S161-L170 in the CH1 domain, located at the CH1-CL interface. These results, summarized structurally for the Fc region in Figure 4B, show that the L to A mutations cause a global change in N5-i5-LALA dynamics extending distally across the hinge into the Fab region and to distal regions of the CH2 and CH3 domains. Thus, the LALA mutations alter the conformation of residues in the CH2 and CH3 domains that control glycan interactions as well as the CH2-CH3 and CH3-CH3 interfaces. Although increased deuteration is also found in the Fab region it did not extend to contact residues and there is no difference in affinity or thermodynamic variables for FLSC binding between N5-i5 and N5-i5-LALA as determined by isothermal calorimetry (Figure S3). This result is consistent with earlier studies showing that the LALA mutation does not alter the binding of mAb b12 to gp120 or its ability to neutralize HIV-1 (Hessell et al., 2007; Hezareh et al., 2001). It remains possible that the increased Fab deuteration involves non-contact residues that play a role in the propagation of antigen induced allosteric changes across the hinge into the Fc region though testing this hypothesis is beyond the scope of this report.
Figure 4-. HDX-MS of N5i5 wild type heavy chain vs N5i5 LALA mutant heavy chain.
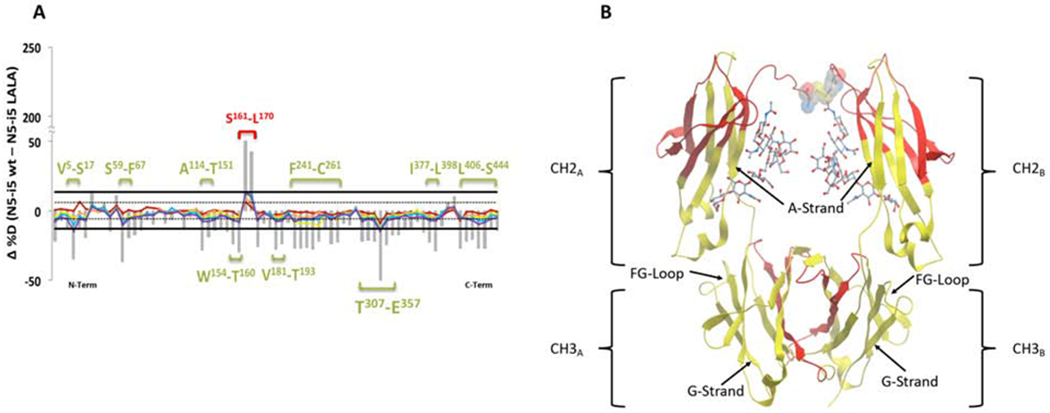
Panel A shows the percent difference plots (WT – LALA) in deuterium uptake for the heavy chain. Individual peptidic fragments are plotted on the x-axis going from N to C terminus based on the sequence number of the first residue of the peptide. Color coded traces represent the percent deuterium uptake difference for a given peptide at a given deuterium incubation time point (10 sec red, 1 min orange, 10 min yellow, 1 hr blue, 2 hr purple). The grey bars represent the sum of the percent deuterium uptake differences integrated over all time points and is used only for statistical purposes. 98% confidence intervals are plotted as horizontal lines with solid lines for the sum of differences and hashed lines for individual time points. Regions displaying significant differences are indicated as brackets with sequences indicated. Panel B shows the significant regions mapped onto cartoon representation of onto IgG1 Fc from PDB:1E4K. Rendered in yellow are the regions displaying significant changes in deuterium uptake, here increased uptake in the LALA mutant. L234-L235 are rendered as CPK in the lower hinge and the glycans are rendered as sticks. See also Figure S3.
Antigen binding induces similar conformational changes in N5-i5-LALA AND N5-i5
HDX-MS was also carried out for FLSC bound to N5-i5-LALA and the results are shown in Figure 5. As expected, decreased deuteration was observed in CDRH1 (A23-T31), CDRH2 (W47-D62), and CDRH3 (L96-L114) of antigen-bound N5-i5-LALA (Figure 5A), which was also observed for antigen-bound N5-i5 (Figure 3A), although there were slight differences in CDRH2 and CDRH3. There was increased deuteration in CH2 residues F241-M253 and S424-G446 in antigen-bound N5-i5-LALA, which were also seen for antigen-bound N5-i5. It should be noted that the CH residues showing increased exchange were slightly different between N5-i5-LALA (S424-G446) and N5-i5 (V427-L443) bound to antigen. Taken together, antigen binding induces very similar conformational changes in both N5-i5-LALA and N5-i5, which supports further that the changes in CH2 and CH3 are associated with a conformation competent to bind FcγR. Although the HDX patterns for N5-i5 or N5-i5-LALA bound to FLSC are very similar, there are two major changes in N5-i5-LALA that are not observed in N5-i5.
Figure 5-. HDX-MS of N5i5-LALA bound to FLSC compared with unliganded N5i5.
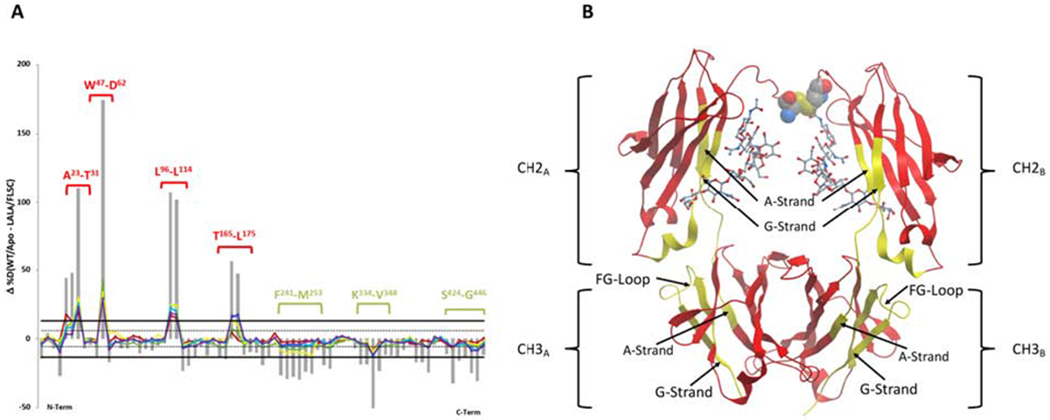
Panel A shows the percent difference plots (unliganded – FLSC bound LALA N5i5) in deuterium uptake for the heavy chain. Individual peptidic fragments are plotted on the x-axis going from N to C terminus based on the sequence number of the first residue of the peptide. Color coded traces represent the percent deuterium uptake difference for a given peptide at a given deuterium incubation time point (10 sec red, 1 min orange, 10 min yellow, 1 hr blue, 2 hr purple). The grey bars represent the sum of the percent deuterium uptake differences integrated over all time points and is used only for statistical purposes. 98% confidence intervals are plotted as horizontal lines with solid lines for the sum of differences and hashed lines for individual time points. Regions displaying significant differences are indicated as brackets with sequences indicated. Panel B shows the significant regions mapped onto cartoon representation of onto IgG1 Fc from PDB:1E4K. Rendered in yellow are the regions displaying significant changes in deuterium uptake, here increased uptake. L234-L235 are rendered as CPK in the lower hinge and the glycans are rendered as sticks. See also Figure S4.
First, less exchange was observed for CH1 residues T165-L175 of N5-i5-LALA bound to FLSC. Residues T165-V169 are on the CH1 D-Strand and residues L170-L175 are on the CH1 DE-Turn. This region includes residues that determine association between CH1 and CL in the Fab region. For example, F166 contacts A175 and S176 on the CL E-Strand, P167 and A168 contact T163 on the CL D-Strand, and V169 contacts both T163 and E161 on the CL D-Strand. These residues stabilize the interior of the CH1-CL interface and the reduced Deuterium exchange suggests that the conformation of this interface differs between FLSC bound N5-i5 and N5-i5-LALA, providing additional support for the occurrence of allosteric communication between Fab and Fc. This is consistent with MD simulations (Zhao et al., 2018) suggesting that allosteric communication propagates largely through the hinge region but also through the CH1-CL domains as we see above.
Interestingly, the largest differences in the Deuterium exchange were observed for residues of the Fc region when the antigen bound N5-i5-LALA was compared to the unliganded N5-i5 (Figure 5). For example, CH2 residues K334-V348 are more deuterated comparing FLSC bound to -LALA vs N5-i5 (Figure 5A). This region was unaffected when FLSC was bound to N5-i5 (Figure 3, panels A and B). Residues K334-K340 are on the CH2 G-Strand, residues G341-R344 form a connector between the CH2 and CH3 domains, and residues E345-V348 are on the CH3 A-Strand. In the discussion, we propose that these changes alter associative interactions as both N5-i5-LALA and N5-i5 can bind low affinity FcγR when presented as an IC.
X-ray crystallography shows that LALA mutations significantly alter the conformation of IgG1 Fc
The above studies strongly suggest that the LALA mutations significantly alter Fc conformation beyond FcγR contact residues. We explored this hypothesis by solving three independent structures of IgG1-LALA Fcs by x-ray crystallography. The IgG1-LALA Fcs were obtained by papain digestion of LALA mutants of N5-i5, C11, and N12-i2 IgG1 (a mAb specific for the gp120 co-receptor binding site as described in (Guan et al., 2013)) and x-ray crystallography performed as described in Methods. Although N5-i5-LALA, C11-LALA and N12-i2-LALA Fcs originated from the same LALA mutated IgG1 backbone they crystalized with slightly different unit cell parameters of the same P212121 space group (Table S1). Figure 6 shows the structures superimposed on the CH3A-CH3B domains in which the alanine residues introduced for Leu234 and Leu235 are visible along with the N297-glycan core, which is fucosylated. As shown in Figure 6A, there was significant diversity in the CH2 conformations while the CH3 conformations are less heterogeneous, which is consistent with literature (Frank et al., 2014; Hanson and Barb, 2015; Subedi and Barb, 2015; Subedi et al., 2014). In addition, there are clear differences in the relative orientations of CH2 and CH3 domains of chain A and B among the LALA-Fc structures. N5-i5-LALA Fc has the smallest CH2-CH3 angle for chain A and B (defined as the three-point angle between the Ca carbons of residues Y300, M428, and Q362 of each chain) of 92.9° and 85.7°, respectively. CH2-CH3 angles of chain A/B of C11- and N12-i2-LALA Fc are 99.3°/86.5° and 98.5°/86.4°, respectively. Further, alignments of LALA-Fc structures to structures of wild-type IgG1 (Figure 6b, only the fucosylated variants of IgG1 Fcs available in Protein Data Bank were selected for comparisons) indicate significant differences in the distances between CH2 domains. The CH2 domains of the LALA variants are more distal as compared with wild-type IgG1 Fcs with the distance between the Ca carbons of residues P238 on CH2A and CH2B domains ranging between 30.0 Å and 35.9 Å as compared to 16.7 Å to 19.5 Å for wild-type IgG1 Fcs. Collectively, these data support the hypothesis that the three LALA variant IgG1 Fcs are conformationally less constrained and sample more conformations in their crystal structures than their wild-type counterparts. Thus, x-ray crystallography shows that the LALA mutations in the lower hinge increase the conformational range of the CH2 domains as suggested by our solution phase studies. In this context, it should be noted that the L234A L235A mutations map into the asymmetric docking site on Fc for both FcγR and C1q (Canfield and Morrison, 1991; Hezareh et al., 2001; Lund e tal., 1992; Morgan et al., 1995). Thus, the effect of the LALA mutations is to widen the inter-Fc distance of residues in the FcγR docking site of Fc, which very likely contributes to the loss of affinity of FcγR for monomeric IgG1.
Figure 6-. Crystal structures of Fc domain of LALA mutant of mAbs N5-i5, N12-i2 and C11.
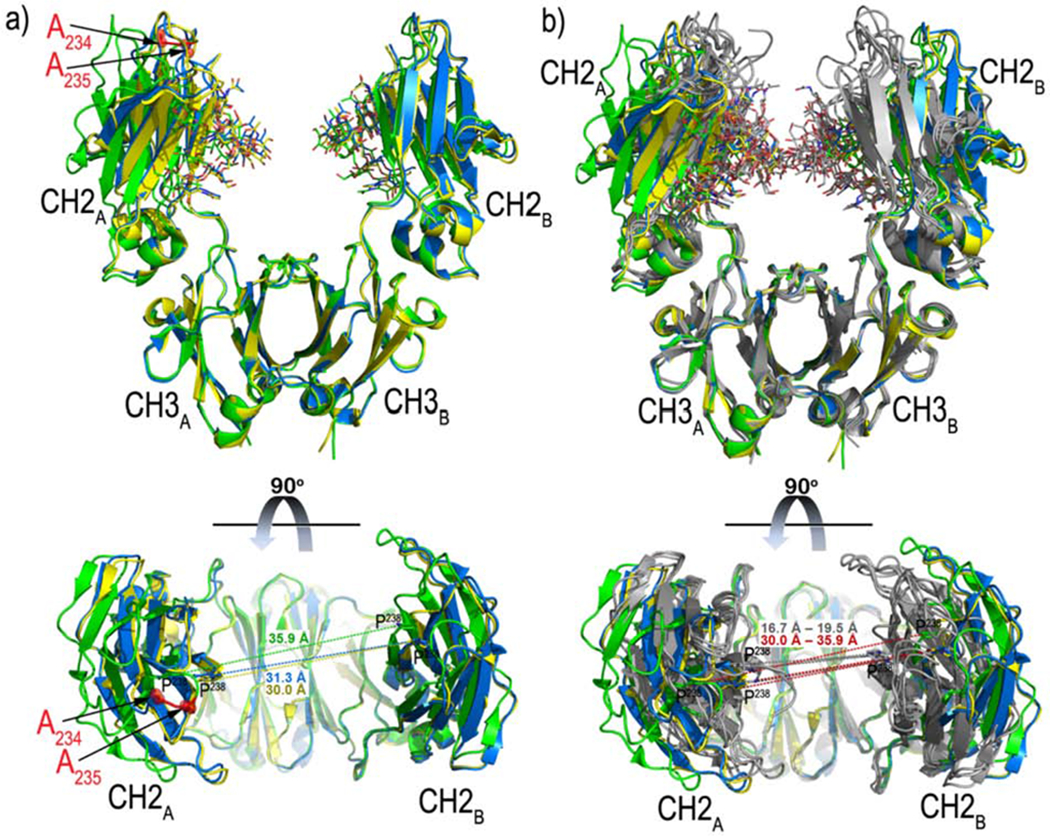
Panel A shows the structures of N5-i5 LALA Fc (green), C11 LALA Fc (blue) and N12-i2 LALA Fc (yellow) are superimposed based on the CH3A-CH3B domains and shown as ribbon diagrams (top). The alanine residues introduced at Leu234 and Leu235 (as seen in the N5-i5 LALA Fc structure) are shown in red and sugars attached to Asn297 are shown as sticks. Superimpositions of the individual CH2 and CH3 domains resulted in root-mean-square deviations (RMSD) in the range of 0.37 - 0.66 Å and 0.30 – 0.50 Å, respectively. Chains A and B (CH2A-CH3A and CH2B-CH3B) could be superimposed with RMSD values in the range of 0.80-1.21 Å for N5-i5 and C11, 0.90-1.37 Å for N5-i5 and N12-i2, r and 0.41-1.34 Å for C11and N12-i2. Finally, the whole Fc molecules could be superimposed with the RMSD values of 1.90 Å and 2.00 Å for N5-i5/C11 and N5-i5/N12-i2 respectively and 0.45 Å for C11 and N12-i2 r. A 90° view shows a distribution of the distances between Pro238 of the CH2A and CH2B domains. Panel B compares the Fc structures of LALA mutants with IgG1. The structures of N5-i5 LALA Fc, C11 LALA Fc and N12-i2 LALA Fc (colored as in panel a) are superimposed based on the CH3A-CH3B domains to structures of the IgG1 Fc (from PDB codes: 3ave (Matsumiya et al., 2007), 4dz8 (Strop et al., 2012), 1h3y (Krapp et al., 2003), 1h3v (Krapp et al., 2003), shown in grey) (top panel). A 90° view shows the distribution of the distances between Pro238 of the CH2A and CH2B domains. Individual CH2 and CH3 domains of the LALA mutants and wild-type IgG1 resulted in RMSDs in the range of 0.30 - 1.90 Å and 0.20 – 0.77 Å, respectively. Superimpositions of chains A and B resulted in RMSDs in the range of 0.80 – 2.46 Å.
Discussion
The studies described above suggest a new two-step model for the integration of allosteric and associative interactions in effector functions mediated by low-affinity FcγR. In the first step, antigen binding to Fab generates a cooperative allosteric change in Fc that increases IgG binding to low-affinity FcγR on effector cell surfaces. This step was predicted in recent MD simulations of the interaction between antigen-bound IgG1 and FcγRI (Zhao et al., 2018). Our studies provide the first laboratory-based data consistent with this hypothesis. In the second step, these complexes enter into associative interactions among themselves, with other cell surface ligands, or as mixtures of both, to generate higher-ordered complexes that are required for optimal effector function. This model is based on two previously unknown allosteric changes in Fc that modulate FcγR binding.
First, we uncovered an antigen-induced conformational allosteric change in Fc that increases binding to low-affinity FcγR in solution phase binding assays. These changes are observed for both the wild type and the LALA variant of N5-i5. Interestingly, although the significantly increased Deuterium exchange spanning residues of almost entire molecule was observed for unliganded LALA-N5-i5 as compared to wild-type, antigen binding by N5-i5-LALA resulted in a Deuterium exchange pattern nearly identical to that observed for antigen bound wild-type N5-i5. This pattern is characterized by increased deuteration of a portion of the CH2 N297-glycan interactive in residues that modulate the affinity of Fc for low-affinity FcγR via protein-glycan interaction (Frank et al., 2014; Subedi and Barb, 2015; Subedi et al., 2014). It is also characterized by increased deuteration of residues at the CH2-CH3 interface that also affect Fc affinity for low affinity FcγR (Frank et al., 2014; Subedi and Barb, 2015) as well as ligand binding at the CH2-CH3 interface. We believe that these data provide the most compelling case to date for conformational allosteric cooperativity in Fc consequent to antigen binding by Fab.
Second, we found an unexpected example of configurational allosteric cooperativity for N5-i5-LALA. This example is characterized by global alterations of HDX that extends well into Fab and across the Fc in residues distal to the LALA mutations in the lower hinge, although these mutations do not affect affinity for antigen (Hessell et al., 2007; Hezareh et al., 2001). The N5-i5-LALA variant is also capable of allosteric conformational cooperativity as shown by antigen-dependent increased FcγR binding with an HDX pattern is remarkably similar to that of antigen bound wild-type N5-i5. However, because the antigen engagement by N5-i5-LALA and C11-LALA increases their affinities for low-affinity FcγRs but do not rescue their full ADCC activities (which are attenuated though not abrogated), we propose that the LALA mutations affect the ability of Fc to engage in associative interactions, which are required for effective receptor triggering and Fc-mediated effector functions. This is supported by the nearly identical increased deuteration patterns of antigen bound N5-i5 or N5-i5-LALA in the CH2 N297-glycan interactive surface and the CH2-CH3 interface, both of which affect affinity of Fc for low-affinity FcγR. On the other hand, the increased deuteration of K334-V348, observed only in the N5-i5-LALA IC, suggests that the LALA mutation also affects CH2-CH3 communication. This region includes seven residues of the CH2 domain C-terminus, the four residues of the CH2-CH3 connector, and four residues of the CH3 domain N-terminus. These residues contribute to a network of interactions that participate in the carbohydrate binding interface, the CH2-CH3 “ball in socket” interface, as well as CH3 domain stabilization. Thus, our data suggests the LALA mutations alter the conformation of key residues for associative interactions even after antigen-dependent allosteric cooperative changes in Fc that affect affinity for low-affinity FcγR.
We propose the following to explain antigen-dependent conformational shifts between Fc conformations that are unsuitable or primed for FcγR binding (Subedi and Barb, 2015; Subedi et al., 2014). Studies by Adam Barb’s group showed the primed conformation is characterized by an optimal CH2-CH3 orientation, dictated by the lower hinge and CH2-CH3 interface (Subedi et al., 2014), and allosteric stabilization of the C’/E loop via glycan interactions with the CH2 glycan interactive surface (Subedi and Barb, 2015). The primed conformation is dominant in solution as determined by NMR, requiring only small changes to assume the FcγR-bound conformation, which accounts for the ability of free IgG at high concentrations to bind FcγR (Subedi and Barb, 2015). The unsuitable conformations are sampled on a microsecond scale, defined by solvent exposure of the α-1,6 glycan terminus whose predominant state in the primed conformation is coordinated with the CH2 A-Strand surface (Barb et al., 2010; Barb et al., 2012; Barb and Prestegard, 2011; Frank et al., 2014). The α-1,3 glycan terminus is much less constrained and typically solvent exposed in both the primed and unprimed Fc conformations (Barb, 2015; Barb et al., 2010; Barb et al., 2012; Barb and Prestegard, 2011; Frank et al., 2014; Hanson and Barb, 2015; Subedi and Barb, 2015; Subedi et al., 2014; Subedi et al., 2015). These observations are also consistent with the recent MD studies implicating the same regions in antigen promoted FcγR binding(Zhao et al., 2018). Taken together, these observations support a model in which binding to low-affinity FcγR is modulated via residues outside the Fc contact site that involve stabilization of the C’/E loop by glycan interactions with the CH2 A-Strand and residues in the lower hinge along with the CH2-CH3 interface. Our data extends the Subedi-Barb model showing that antigen binding also influences the same residues in the CH2 N297-glycan binding surface and the CH2-CH3 interface that characterize the primed conformation. Our data is also consistent with MD predictions of antigen promoted FcγR binding (Emsley and Cowtan, 2004).
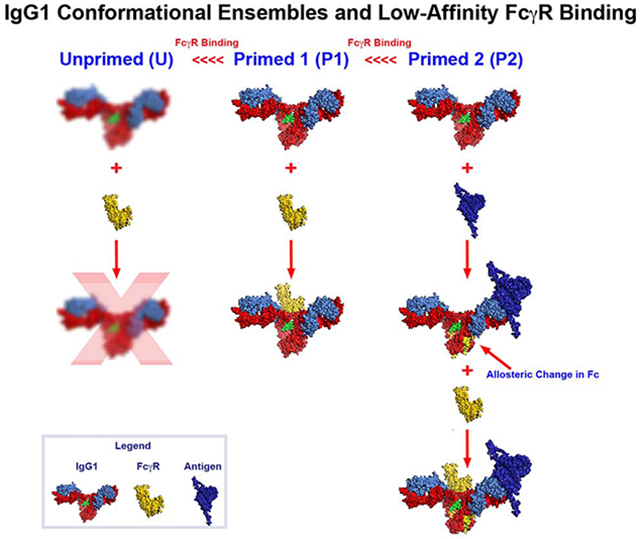
Because antigen-binding results in moderately increased binding of IgG1 to low-affinity FcγR, we propose that the primed state is actually a conformational ensemble whose members have slightly different but favorable pathways to the FcγR-bound state as predicted in (Zhao et al., 2018). Hereafter we will refer to the primed state as the P Ensemble (for primed ensemble) and the two conformations represented by free IgG and antigen-bound IgG as P1 and P2, respectively. We will also refer to the conformations unsuitable to bind FcγR as the U Ensemble. Two conformational signatures were identified by the Barb group that discriminate between Conformational Ensembles P and U. First, the C’/E loop is stabilized in Ensemble P through glycan interactions with the polypeptide chain largely through CH-π interactions between F241 and F243 with GlcNAc2 and GlcNAc5’ of the N297-glycan, respectively (Subedi and Barb, 2015; Subedi et al., 2014). Second, as stated in (Subedi and Barb, 2015) the CH2 domain is thought to “twist” relative to the CH3 domain into the conformation receptive for low-affinity FcγR binding (Subedi and Barb, 2015; Subedi et al., 2014). Our HDX studies show that antigen binding by Fab results in specific conformational changes in these same CH2-CH3 regions that discriminate Ensembles P and U. How are these two signatures affected by antigen binding to assume the P2 conformation that exhibits increased binding to low-affinity FcγR?
Figure S4 lists CH2 N297-glycan interactive surface residues in order of decreasing contact area and CH2 strand location (determined using PDB:1E4K). Residues of the CH2 A-Strand account for 48.69% of the CH2 contact glycan interactive surface and are the only residues of the CH2 N297-glycan interactive surface that undergo conformational change upon antigen binding to N5-i5 (Figure S4).These residues include F241 and F243 that account for 35.07% of the contact area for the CH2 N297-glycan interactive surface and that provide the critical CHp interactions with GlcNAc2 and GlcNAc5’ glycans that stabilize the C’/E loop in the P1 conformation (Subedi et al., 2014). Thus, our studies suggest that the A-Strand contact residues of the CH2 N297-glycan interactive site are sensitive to the occupancy of Fab by antigen via allosteric interactions that favor the P2 conformation and increased low-affinity FcγR binding.
Further, the C-terminal residue of the CH2 A-Strand, L251, is also affected conformationally by antigen binding and it is the “ball” of the “ball in socket” CH2-CH3 interface that is also a key control point for the primed conformations. Antigen binding also affects the conformation of the “socket” residues M428, E430, and H435 located on the CH3 FG-Loop that is the key CH2-CH3 interaction surface. Thus, antigen binding increases the binding to low-affinity FcγR, which is associated with a shift to the P2 conformation and conformational changes in the CH2 N297-glycan interactive surface and the CH2-CH3 interface. Although antigen binding clearly increases the binding of IgG1 to low-affinity FcγR, this alone cannot account for ADCC potency, which is in the pM range for N5-i5 and C11 (Guan et al., 2013). Thus, associative interactions must play a major role in ADCC potency. Fortuitously, our data suggests that the LALA variants provide a new tool to differentiate the relative roles of allosteric and associative interactions in Fc-mediated effector function.
The attenuating LALA mutations in the lower hinge were identified empirically in studies mapping the binding of FcγR and C1q to IgG (Canfield and Morrison, 1991; Hezareh et al., 2001; Lund et al., 1992; Morgan et al., 1995). X-ray crystallography showed subsequently that L235 is part of the asymmetrical Fc-FcγR contact surface (Radaev et al., 2001; Sondermann et al., 2000), which is consistent with the loss of FcγR binding when it is mutated to alanine. While the LALA mutations are used widely to attenuate FcγR binding and biological activity, they can retain residual activity depending on the system (Figure 1 and (Leoh et al., 2015)). Our studies yielded new insights into the impact of the LALA mutations on IgG structure and function. First, N5-i5-IgG1-LALA and C11-IgG1-LALA variants can bind to low-affinity FcγR in solution when they are presented as monovalent/bivalent ICs although their binding is greatly attenuated as free IgG1 molecules. This unexpected observation shows that the LALA mutations do not abrogate the low-affinity FcγR binding site on Fc when it is conformationally constrained by antigen binding. By contrast, free IgG1-LALA binding to FcγR is only detectable in our hands by FCS indicating that it is largely in the U conformational ensemble, suggesting large impact on IgG structure than simply altering a contact residue for FcγR, which was confirmed by HDX analyses of both free N5-i5-IgG1-LALA and N5-i5-IgG1-LALA bound to FLSC.
HDX studies showed markedly increased exchange in regions of the N5-i5-IgG1-LALA heavy chain that are distal to the LALA mutations in the lower hinge as compared with wild type N5-i5-IgG1. Increased deuteration of N5-i5-LALA as compared with N5-i5 was seen widely across heavy chain regions FR1, FR2, CH1, CH2, and CH3. Notably, there were no differences in exchange between the contact residues of N5-i5-LALA and N5-i5 for FLSC, which is consistent with the lack of affinity differences between the two mAbs by ITC and studies comparing antigen binding affinities between wild-type and LALA variant IgGs (c.f., (Hessell et al., 2007; Hezareh et al., 2001)). Thus, the LALA mutations result in a significant increase in the conformational sampling of IgG1 that extend far beyond the lower hinge region. This conclusion is supported by the significantly increased deuterium exchange that spans residues of almost entire Fc molecule of the antigen unbound LALA IgG variant (Figure 6). Additionally, the three LALA Fc structures obtained from crystals of the same space group reveal significant alterations of how the CH2-CH3 monomers are assembled into the dimer. The LALA Fcs show increased (as compared to the wild-type) and variable distances between the Cα carbons of P238 on the CH2A and CH2B and heterogeneity of the CH2-CH3 angles. Notably, these distances are in the range of 30 Å to 35 Å for the LALA variants as opposed to 16.7 Å to 19.5 Å for wild-type IgG1 Fcs. Collectively, these results suggest the increased conformational flexibility of the LALA Fc backbone and point toward the possibility that the LALA mutations affect much more than just the contact residues for FcγR in the lower hinge region, raising the question of whether they also affect other Fc functions. Antigen binding shifts both wild-type and LALA variants of N5-i5 and C11 to the P2 conformation, which is competent for soluble FcγR binding. Hence, the LALA mutations do not abrogate the FcγR binding site. Rather, they shift the predominant conformation of free IgG1-LALA to the U conformational ensemble. Our studies show also that the LALA mutations result in significant conformational alterations regions of the CH2 and CH3 domains that are responsible for associative interactions.
For example, the CH2-CH3 interface has been long known to be a “consensus” binding site that specifically binds multiple natural ligands with different protein folds (DeLano et al., 2000). These ligands include Protein-A (Deisenhofer, 1981), Protein-G (Sauer-Eriksson et al., 1995), rheumatoid factor (RF) (Sohi et al., 1996), and the neonatal Fc receptor (FcRn) (Burmeister et al., 1994). Additionally, the CH2-CH3 consensus site also binds peptides isolated by phage display (DeLano et al., 2000) and it overlaps the IgG hexamerization site that is involved in the antigen-dependent formation of surface complexes of antigen, IgG, and C1q that initiate the complement cascade (Diebolder et al., 2014; Lindorfer et al., 2016; Ugurlar et al., 2018; Wang et al., 2016). Unlike FcγR and C1q, consensus site ligands bind symmetrically at the CH2-CH3 interface with a 2:1 stoichiometry. It is of particular note that antigen-binding to hexameric IgG strongly promotes complement activation showing that paratope occupancy has downstream effects on Fc function, consistent with the observations presented above for ADCC (Wang et al., 2016).
In summary, we propose the following two-step model for the differential roles of antigen dependent allosteric interactions and associative interactions in FcγR -mediated effector function. In this model, antigen dependent allosteric interactions shift Fc to the P2 conformation that is primed for binding a single low-affinity FcγR. We propose that this binding differentiates target cell bound IgGs to a CH2-CH3 conformation that more favorably engages in associative interactions than free IgG. Since free IgG is expected to be in great excess over target cell surface bound IgG in vivo, this conformational shift of antigen bound IgG prevents free IgG from competing its engagement of associative interactions mediated by the CH2-CH3 consensus site. When presented as ICs on target cell surfaces, LALA variants retain the ability to bind low-affinity FcγR on effector cells but they do not efficiently enter into associative interactions that are required for FcγR -mediated effector function. This model provides a new experimental framework for the dissection of allosteric and associative interactions in effector functions mediated by low-affinity FcγR. In this regard, we have observed additional specificity-dependent effects on FcγR binding that suggest both positive and negative allosteric effects upon antigen binding by a panel of structurally defined mAbs specific for epitopes on HIV-1 gp120, which are the subject of future research.
STAR★Methods
Lead Contact and Materials Availability
Further information and requests for resources should be directed to and will be fulfilled by the Lead Contact, George K. Lewis (glewis@ihv.umaryland.edu). All unique/stable reagents generated in this study are available from the Lead Contact without restriction.
Experimental model and subject details
RFADCC assay was performed using EGFP-CEM-NKr-CCR5-SNAP cell line (Orlandi et al., 2016), originally derived from the T lymphoblastic cells isolated from a 4-years old female CCRF-CEM cells (ATCC CCL-119). EGFP-CEM-NKr-CCR5-SNAP cells were grown in RPMI 1640 medium with glutamine and supplemented with 10% fetal bovine serum (FBS), HEPES buffer (Sigma) 10 mM, sodium pyruvate (Sigma) 500 μM, β-Mercaptoethanol (GIBCO) 50 μM and gentimicin (GIBCO) 50 μg/ml and with 1.5 mg/ml of Neomycin G418 (Gemini, Bio-Products) at 37C; and PBMC isolated from healthy donors (purchased from SeraCare) were used as effector cells.
All mAbs were produced by transient transfection in HEK-293Fs cells, maintained in FreeStyle 293 Expression Medium. HEK-293Fs cells derived from the 293 cell line (ATCC CRL-1573), which is a permanent line established from primary female embryonal human kidney transformed with sheared human adenovirus type 5 DNA.
Methods details
Monoclonal antibodies (mAbs) and RFADCC Assay
Monoclonal antibodies N5-i5 and C11 have been described in our previous publications (Acharya et al., 2014; Gohain et al., 2015; Guan et al., 2013; Tolbert et al., 2017b; Tolbert et al., 2016). The LALA mutants of C11 and N5-i5 were created by L to A substitutions at residues 234 and 235 in the lower hinge region by site directed mutagenesis. These mutations have been used widely to abrogate binding to FcγR (Hessell et al., 2007; Hezareh et al., 2001). All mAbs were purified using protein-A chromatography as described previously by our group (Acharya et al., 2014; Gohain et al., 2015; Guan et al., 2013; Tolbert et al., 2017a; Tolbert et al., 2016).
ADCC was assessed using the rapid fluorescence antibody-dependent cellular cytotoxicity assay (RFADCC) (Gomez-Roman et al., 2006) as modified in our laboratory for high-throughput assays (Orlandi et al., 2016). Briefly, EGFP-CEM-NKr-CCR5SNAP cells stained with SNAP-Surface Alexa Fluor 647 (New England BioLabs) and sensitized with recombinant HIV-1 Ba-L gp120 were used as targets and PBMC were utilized as effectors. All the antibodies activity was analyzed with four-fold serial dilutions starting from a concentration of 0.3 ug/ml. At the end of the reaction with the mAbs (2 hours) the fixed samples were collected (approximately 35,000 events/sample) on a BD Fortessa Special Order (BD) instrument (BD Biosciences) and analyzed using FlowJo software (Tree Star, Ashland, OR). ADCC activity (= % cytotoxicity) is defined as the percentage of EGFP-CEM-NKr-CCR5-SNAP target cells that lose EGFP staining but retain the CCR5-SNAP tag dye.
Fluorescence correlation spectroscopy
FCS measurements were performed to assess the in vitro binding of human FcγR to mAbs (C11 and N5-i5) in solution using a confocal microscope (MicroTime 200; PicoQuant, Berlin, Germany). PicoQuant Symphotime software was used to generate the autocorrelation curves and analyses. An aliquot (11 ul) of a given reaction mixture was loaded onto a glass coverslip sample chamber and sealed for spectroscopic measurements. FCS measurements and analyses were performed as previously reported (Ray et al., 2014). For this report, human recombinant His-tagged FcγR receptors I, IIA/B and IIIA (R&D systems) were labeled with Alexa Fluor 647. Purified Alexa-647 labeled FcγR were quantified by a UV-visible (UV-vis) spectrometer (Nanodrop 2000; Thermo-Scientific, Wilmington, DE). Dye-to-protein ratios were determined by measuring absorbance at 280 nm (protein) versus 650 nm (dye). Ratios varied from 1 to 1.6 depending on the FcγR. We chose to label FcγR for these studies because they are smallest in size (~22-32kDa) of the three proteins, mAb, FLSC, and FcγR, used in our assays permitting the greatest dynamic range of response, otherwise the reagents and mathematical analyses were the same as described in our earlier reports (Mengistu et al., 2015; Ray et al., 2014; Tolbert et al., 2017b).
Enzyme-linked immunosorbent assays (ELISA)
ELISA assays were carried out as described in our previous publications (Guan et al., 2013) with the following modifications. For binding of IC to FcγR coated ELISA plates, Nickel conjugated plates (Pierce-Aldrich, catalog number 15442) were coated overnight at room temperature with recombinant human His tagged-FcγRs (I, IIA, IIB, IIIA from R&D Systems) diluted to 0.2ug/ml in PBS. The next day, the plates were blocked for 1h at room temperature with 25nM TBS, 0.5% BSA, 0.05% Tween-20 and subsequently washed once with 0.05% Tween-20 in PBS. To test the ability of mAbs or ICs to bind to FcγRs, the antibodies passed down a 1:4 dilution series starting from 20ug/ml to 0. 062 ng/ml and incubated with FLSC (for ICs) or PBS (monomeric mAbs) for 1 hour at room temperature before plating on the FcγR coated plates for 2 hours at room temperature. The plates were washed twice with the above buffer and binding detected using horseradish peroxidase conjugated Fab’2 goat anti-human IgG (H+L) (KPL cat n. 214-1006) added for 1h at RT. After washing, 100 ul/well TMB substrate (Southern Biotech) was added to each well and reactions were stopped by adding 50 ul/well 2N H2SO4. The absorbance was measured at 450 nm with optical correction at 540nm or 570nm, using a SpectraMax M2 (Molecular Devices).
Crystallography and Protein purification
N5-i5 LALA monoclonal antibody (mAb) was purified by HiTrap protein A column (GE Healthcare) chromatography from 293T supernatants prepared by transfecting plasmids encoding the heavy- and light-chain genes of the mAb. The Fc of the mAb was prepared from the purified IgG (10 mg/ml) by proteolytic digestion with immobilized papain (Pierce, Rockford, IL) and purified using a protein A column to remove Fab (GE Healthcare, Piscataway,NJ) followed by gel filtration chromatography on a Superdex 200 16/60 column (GE Healthcare, Piscataway, NJ) equilibrated with 25 mM Tris-HCl buffer pH 7.2 with 0.35 M NaCl. The elution peak of the Fc corresponded to a molecular weight of approximately 50 kDa and was collected and concentrated to 6mg/ml for use in the crystallization trials.
Crystallization
Initial crystal screens were done in robotic vapor-diffusion sitting drop trials using commercially available sparse matrix crystallization screens from Emerald BioSystems (Precipitant Wizard Screen) and Index Screen from Hamptons Research. The screens were monitored periodically for protein crystals. Conditions that produced micro crystals were then reproduced and optimized using the hanging-drop, vapor diffusion method with drops of 0.5 μl protein and 0.5 μl precipitant solution equilibrated against 700 μl of reservoir. Plates were incubated at room temperature (22°C). Optimized crystals of N5-i5, N12-i2, and C11 LALA-Fc were grown from two different well solutions containing either 25% PEG 3350 and 0.1 M Bis Tris pH 6.5 or 20% w/v PEG MME 5000 and 0.1 M Bis Tris pH 6.5. Crystals were frozen after a brief soak in well solution supplemented with 20% MPD prior to being frozen in liquid nitrogen and used for data collection.
Hydrogen/Deuterium Exchange Mass spectrometry (HDX-MS) measurements
N5-i5 and LALA mutant were exchanged into PBS using Amicon l filters to achieve a final enzyme concentration of >100 μM. The peptide coverage maps for N5-i5 and LALA mutant were obtained from undeuterated controls as follows: 0.75 μL of N5-i5 or LALA mutant in PBS was diluted with 4.25 μL of PBS buffer, pH 7.4 at room temperature followed with the addition of 55 μL of ice cold quench (50 mM Glycine buffer, 7.04 M Guanidine-HCl, 0.5 M TCEP pH 2.4). The samples were incubated for 5 min on ice with occasional pulse vortexing followed by the addition of 190 μL of buffer A (H2O, 0.1% Formic acid) and then immediately injected into a Waters HDX nanoAcquity UPLC (Waters, Milford, MA) with in-line pepsin digestion (Waters Enzymate BEH pepsin column). Peptic fragments were trapped on an Acquity UPLC BEH C18 peptide trap and separated on an Acquity UPLC BEH C18 column. A 7 min, 5 to 35% Acetonitrile (0.1% Formic acid) gradient was used to elute peptides directly into a Waters Synapt G2 mass spectrometer (Waters, Milford, MA). MSE data were acquired with a 20 to 30 V ramp trap CE for high energy acquisition of product ions along with continuous lock mass (Leu-Enk) for mass accuracy correction. Peptides were identified using the ProteinLynx Global Server 2.5.1 (PLGS) from Waters. Further filtering constraints were applied in DynamX with 0.3 fragments per residues. Optima (LC/MS) grade water and acetonitrile were obtained from Fisher Scientific and formic acid was obtained from Sigma-Aldrich. D2O, DCl and NaOD were obtained from Cambridge Isotopes.
Unliganded and antigen bound HD exchange reactions were performed as follows: 0.75 μL of antibody (166 μM N5-i5 or 200 μM N5-i5-LALA) or antibody/antigen complex (200 μM respective antibody with 400 μM FLSC) in PBS buffer was incubated in 4.25 μL of PBS buffer, 99.99% D2O, pH 7.4. All reactions were performed at 25°C. Prior to injection, deuteration reactions were quenched at various times (10 sec, 1 min, 10 min, 1 hr and 2 hr) with 55 μL of 50 mM Glycine buffer, 7.04 M Guanidine-HCl, 0.5 M TCEP, pH 2.4 and further diluted using buffer A as indicated above. Back exchange correction was performed against fully deuterated controls acquired by incubating 0.75 μL of antibody in 4.25 μL of PBS buffer, 99.99 D2O, pD 7.4 containing 7 M deuterated Guanidine DCl, 25mM DTT for 2 hrs at 25°C prior to quenching, dilution and injection. All deuteration time points and controls were acquired in triplicates.
The Deuterium uptake by the identified peptic fragments through increasing deuteration time and for the fully deuterated control was determined using Water’s DynamX 3.0 software. The normalized percentage of deuterium uptake (%D) at an incubation time t for a given peptide was calculated as follows: %D = 100·(mt − m0)/(mf − m0)
With mt the centroid mass at incubation time t, m0 the centroid mass of the undeuterated control and mf the centroid mass of the fully deuterated control. The percent deuteration difference plots Δ%D (Apo-Antigen bound) were generated using the percent deuteration calculated. Confidence intervals for the Δ%D were determined using the method outlined in (Houde et al., 2009) adjusted to percent deuteration using the fully deuterated controls. This approach involves the use of a stringent two-criteria condition for determining the statistical significance of deuterium uptake differences observed for any given peptide: 1/ a difference in deuterium uptake at any single incubation time point experimentally probed which is superior to the 98% confidence interval as determined using the overall standard deviation from the entire data set (all peptides, all time points, all states). 2/ a summed difference in deuterium uptake integrated over all time points probed (here 5 time points) which is superior to its respective 98% confidence interval as determined using the overall standard deviation propagated to the number of time point. The 98% confidence interval of +/− 6.62%D for any single time point and +/− 14.8%D for the sum of Δ%D over all time point were determined for wild type N5-i5. For N5-i5-LALA, a 98% confidence interval of +/− 5.2%D for any single time point and +/− 11.7%D for the sum of Δ%D over all time point were determined. Confidence intervals (98%) were plotted on the Δ%D plots as horizontal dashed lines.
Isothermal titration microcalorimetry (ITC)
ITC experiments were performed to characterize first the interaction of N5-i5 wild type and LALA to their antigen FLSC and then to FcγRIIA using an iTC200 instrument (GE Healthcare). The ICs composed of N5-i5 wild type or LALA and FLSC were formed incubating an equal molar ratio of mAb-Ag for 30 min at RT and then fractionated and collected into different-size components using Superdex200 column 10/300 and 5 mM Tris HCl, 150 mM NaCl at pH 7.2 as elution buffer. The mAbs, FLSC and FcγRIIA were co-dialyzed against 1× phosphate-buffered saline (pH 7.4; Cellgro Mediatech, Herndon, VA) to standardize the buffer conditions and reduce the heat of mixing. The sample cell was maintained at 250 and filled with mAb solution of N5-i5 wt/LALA or ICs at a concentration of 11 μM. The injection syringe was filled with FLSC or FcγRIIA at a concentration of 111 μM. During the titration experiments, the sample solution was stirred at 450 rpm. After the establishment of the baseline stability, a succession of approximately 15 microinjections of constant volume was made. The isothermal titration curves were recorded and analyzed with ORIGIN (version 5.0) software (Microcal Inc.). Thermodynamic parameters were calculated from the Gibbs free energy equation: ΔG = ΔH – TΔS, where ΔG, ΔH and ΔS are the changes in free energy, enthalpy, and entropy of binding, respectively. T is the absolute temperature, and R = 1.98 cal mol−1 K−1. The one set of sites binding model was used to calculate the thermodynamic parameters.
Quantification and Statistical Analysis
Data collection and structure solution and refinement
Diffraction data for N5-i5 LALA-Fc, N12--2 LALA-Fc, and C11 LALA Fc were collected at the Stanford Synchrotron Radiation Light Source (SSRL) at the beam line BL 12-2 equipped with ADSC Quantum 315 area detectors. The crystals belong to a space group P212121 with unitcell parameters of a = 48.9, b = 73.8, c = 136.9 Å (N5-i5 LALA-Fc), a = 49.0, b = 74.8, c = 145.8 Å (N12-i2 LALA-Fc), or a = 49.0, b = 75.2, c = 148.3 Å (C11 LALA-Fc) and α = β = γ = 90°, and one Fc dimer present in the asymmetric unit (ASU). Data were indexed and integrated with HKL2000 (Otwinowski et al., 1997). The structures were solved by molecular replacement with Phaser (McCoy, 2007) from the CCP4 suite (N., 1994) based on the coordinates of PDB ID 3AVE. Refinement was carried out with Refmac (Murshudov et al., 1997) and/or Phenix (Adams et al., 2002). Refinement was coupled with manual refitting and rebuilding in COOT (Emsley and Cowtan, 2004). The final models to resolutions of 2.4 Å (N5-i5 LALA-Fc), 2.44 Å (N12-i2 LALA-Fc), or 2.6 Å (C11 LALA-Fc) were refined to an R-factor of 0.216 and Rfree of 0.273 (N5-i5 LALA-Fc, PDB ID 6CHF), 0.216 and 0.264 (N12-i2 LALA-Fc, PDB ID 6CJX), and 0.193 and 0.241 (C11 LALA-Fc, PDB ID 6CJC). Data collection and refinement statistics are shown in (Table S1).
Structure validation and analysis
The quality of the final refined model was monitored using the program MolProbity. Structural alignments were performed using the Dali server (Holm, 2019) and the program lsqkab (Kabsch et al., 1976) from the CCP4 suite to allow comparisons between structures. The PISA (Krissinel and Henrick, 2007) and PIC (Tina et al., 2007) webservers were used to determine contact surfaces and residues of the N5-i5 Fab-gp120 interface, the Fc-Fcγ receptor interface, or the Fc-FcRn receptor interface to aid in the analysis of HD exchange data. All illustrations were prepared with the PyMol Molecular Graphic suite (http://pymol.org) (DeLano Scientific, San Carlos, CA, USA). Structure validation and analysis are shown in (Figure 6).
Analysis of Antibody Binding by FCS
The data shown is the mean of triplicate measurements. Error bars represent the standard deviation. independent experiments analyzed with unpaired two-tailed t-test. The experiment was repeated with similar results. Statistical significance was evaluated using unpaired two-tailed student t test (GraphPad Prism), * P < 0.1, ** P <0.01, ***P <0.001; ****P <0.0001. Statistical analysis of FCS data is shown in (Figure 2).
Data and Code Availability
The atomic coordinates generated in this study are freely available at the RCSB (https://www.rcsb.org) with the following PDB accession codes: PDB: 6CHF (N5-i5 Fc-LALA), PDB: 6CJC (C11 Fc-LALA), PDB: 6CJX (N12-i2 Fc-LALA). The human mAbs N5-i5 and C11 are freely available at the HIV Database (https://www.hiv.lanl.gov) with the following record numbers: 2971 (mAb N5-i5) and 707 (mAb C11).
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies and Recombinant Proteins | ||
| Human mAb N5-i5 | Isolated from patient (Guan et al., 2013) | HIV Database: record number 2971 |
| Human mAb C11 | Lewis lab (Guan et al., 2013) | HIV Database: record number 707 |
| Human mAb N5-i5 LALA | Lewis lab | PDB:4H8W;PDB:6CHF |
| Human mAb C11 LALA | Lewis lab | PDB:4FZ8;PDB:6CJC |
| Human mAb N12-i2 LALA | Lewis lab | PDB:3QEG; 6CJX |
| Human mAb Synagis (Palivizumab) | Medimmune | 60574-4114-1 |
| Human Recombinant FcγR receptor I/CD64 | R&D systems | Cat# 1257-FC-050 |
| Human Recombinant FcγR receptor IIA/CD32a | R&D systems | Cat# 1330-CD-050 |
| Human Recombinant FcγR receptor IIB/CD32b | R&D systems | Cat# 1875-CD-050 |
| Human Recombinant FcγR receptor IIIA/CD16a | R&D systems | Cat# 4325-FC-050 |
| Horseradish peroxidase conjugated Fab’2 goat anti-human IgG (H+L) | KPL | Cat# 214-1006 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| RPMI 1640 medium | ThermoFisher | Cat# 11875085 |
| fetal bovine serum (FBS) | Gibco | Cat# 16140-071 |
| HEPES buffer | Sigma | Cat# H0887 |
| Sodium Pyruvate | Sigma | Cat# S8636 |
| β-Mercaptoethanol | Gibco | Cat# 21985-023 |
| Gentimicin | Gibco | Cat# 15750-060 |
| G418 Sulfate Solution | Gemini Bio - Products | Cat# 400-113 |
| FreeStyle 293 Expression Medium | ThermoFisher | Cat# 12338018 |
| HIV-1 Ba-L gp120 | Lewis lab (Guan et al., 2013) | N/A |
| Full length single chain protein | (Fouts et al., 2000) | FLSC |
| Amicon Pro Affinity Concentration Kit Ni-NTA | EMD Millipore | Cat# ACK5010NT |
| Amicon ultra 0.5ML - 10KDa cutoff | EMD Millipore | Cat# UFC501024 |
| Pierce™ Nickel Coated Plates, Clear, 96-Well | Thermo Fisher Scientific | Cat # 15442 |
| TMB substrate | Southern Biotech | Cat# 0410-01L |
| HiTrap protein A column | GE Healthcare | Cat# GE17-0402-01 |
| Immobilized papain | Pierce | Cat# 20341 |
| Protein A column | GE Healthcare | Cat# GE17-0402-01 |
| Superdex 200 16/60 column | GE Healthcare | Cat# 28989335 |
| Superdex 200 10/300 column | GE Healthcare | Cat# 17517501 |
| Waters Enzymate BEH pepsin column | Waters | Cat # 186007233 |
| Acquity UPLC BEH C18 peptide trap | Waters | Cat # 186003975 |
| Acquity UPLC BEH C18 column | Waters | Cat # 186002346 |
| Critical Commercial Assays | ||
| Alexa Fluor 647 Antibody Labeling Kit | Thermo Fisher Scientific | Cat# A20186 |
| SNAP-Surface Alexa Fluor 647 | New England BioLabs | Cat# S9136S |
| Deposited Data | ||
| N5-i5 Fab | (Acharya et al., 2014) | Protein Data Bank ID 4H8W |
| IGG1 FC FRAGMENT-FC-GAMMA RECEPTOR III COMPLEX | (Sondermann et al., 2000) | Protein Data Bank ID 1E4K |
| Fucosylated Fc Fragment from Human Immunoglobulin G1 | (Matsumiya et al. 2007) | Protein Data Bank ID 3AVE |
| human IgG1 Fc fragment Heterodimer | (Strop et al. 2012) | Protein Data Bank ID 4DZ8 |
| human IgG1 Fc-fragment,high salt condition | (Krapp et al. 2003) | Protein Data Bank ID 1H3Y |
| HUMAN IGG1 FC-FRAGMENT,GLYCOFORM (G2F)2,SG P212121 | (Krapp et al. 2003) | Protein Data Bank ID 1H3V |
| N5-i5 Fc-LALA structure | This work | Protein Data Bank ID 6CHF |
| C11 Fc-LALA structure | This work | Protein Data Bank ID 6CJC |
| N12-i2 Fc-LALA structure | This work | Protein Data Bank ID 6CJX |
| Experimental Models: Cell Lines | ||
| EGFP-CEM-NKr-CCR5-SNAP cells | (Orlandi et al., 2016) | N/A |
| HEK-293Fs | Thermo Fisher Scientific | Cat # R79007 |
| Recombinant DNA | ||
| N5-i5 LALA Heavy Chain expression plasmid | Lewis lab | N/A |
| C11 LALA Heavy Chain expression plasmid | Lewis lab | N/A |
| N12-i2 LALA Heavy Chain expression plasmid | Lewis lab | N/A |
| Software and Algorithms | ||
| FlowJo software | Tree Star, Inc., San Carlos, Calif. | https://www.flowjo.com |
| Symphotime software | PicoQuant | https://www.picoquant.com/ |
| ORIGIN 5.0 software | Microcal Inc. | https://www.originlab.com |
| PyMol Molecular Graphic suite | DeLano Scientific, San Carlos, CA, USA | https://pymol.org |
| MolProbity | (Chen et al., 2010) | http://molprobity.biochem.duke.edu/ |
| ProteinLynx 649 Global Server 2.5.1 (PLGS) | Waters | www.waters.com |
| DynamX | Waters | www.waters.com |
| GraphPad Prism | GraphPad Software, San Diego, CA, USA | https://www.graphpad.com |
| HKL2000 | (Otwinowski et al., 1997) | https://hkl-xray.com |
| Phaser | (McCoy, 2007) | https://www.ccp4.ac.uk/ |
| CCP4 suite | No authors | https://www.ccp4.ac.uk/ |
| Refmac | (Murshudov et al., 1997) | https://www.ccp4.ac.uk/ |
| Phenix | (Adams et al., 2002) | https://www.phenix-online.org/ |
| COOT | (Emsley and Cowtan, 2004) | https://www2.mrc-lmb.cam.ac.uk |
| Dali server | (Holm, 2019) | http://ekhidna.biocenter.helsinki.fi/dali_server |
| lsqkab | (Kabsch et al., 1976) | https://www.ccp4.ac.uk/ |
| PISA | (Krissinel and Henrick, 2007) | https://www.ebi.ac.uk/pdbe/pisa/ |
| PIC | (Tina et al., 2007) | http://pic.mbu.iisc.ernet.in/ |
Highlights.
A conformational allosteric network connects antigen binding with Fc function.
Point mutations in Fc perturb Fc structure by configurational allostery.
Both conformational and configurational allostery affect Fc effector functions.
Acknowledgments-
The authors thank our colleagues in the Division of Vaccine Research, Institute of Human Virology for helpful comments and criticism of the work. In addition, the authors thank Dr. Daniel Bonsor of Institute of Human Virology of University of Maryland for his technical help with the ITC measurements and analysis.
Research Support
The research was supported grant OPP1033109, Bill and Melinda Gates Foundation (GKL) as well as NIH grants R01AI087181 (GKL), 5P01AI120756 (ALD, GKL, G Tomaras PI), P01AI124912 (ALD, GKL, R Gallo PI), R01AI116274 (MP), R01AI129769 (MP) and R01AI150447 (KR). Research was supported in part by the National Institutes of Health (NIH)-funded Research Resource for Biomedical Glycomics (NIH grant P41GM10349010). In addition, the research was supported by internal funds of the Institute of Human Virology as well as University of Maryland Baltimore, School of Pharmacy Mass Spectrometry Center (SOP1841-IQB2014).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests- The authors declare no competing interests
Literature Cited-
- Acharya P, Tolbert WD, Gohain N, Wu X, Yu L, Liu T, Huang W, Huang CC, Kwon YD, Louder RK, et al. (2014). Structural definition of an antibody-dependent cellular cytotoxicity response implicated in reduced risk for HIV-1 infection. J Virol 88, 12895–12906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams PD, Grosse-Kunstleve RW, Hung LW, Ioerger TR, McCoy AJ, Moriarty NW, Read RJ, Sacchettini JC, Sauter NK, and Terwilliger TC (2002). PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr D Biol Crystallogr 58 1948–1954. [DOI] [PubMed] [Google Scholar]
- Barb AW (2015). Intramolecular N-glycan/polypeptide interactions observed at multiple N-glycan remodeling steps through [(13)C,(15)N]-N-acetylglucosamine labeling of immunoglobulin G1. Biochemistry 54 313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barb AW, Borgert AJ, Liu M, Barany G, and Live D (2010). Intramolecular glycan-protein interactions in glycoproteins. Methods Enzymol 478 365–388. [DOI] [PubMed] [Google Scholar]
- Barb AW, Meng L, Gao Z, Johnson RW, Moremen KW, and Prestegard JH (2012). NMR characterization of immunoglobulin G Fc glycan motion on enzymatic sialylation. Biochemistry 51 4618–4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barb AW, and Prestegard JH (2011). NMR analysis demonstrates immunoglobulin G N-glycans are accessible and dynamic. Nat Chem Biol 7, 147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JC, and Koshland ME (1977). Evidence for a long-range conformational change induced by antigen binding to IgM antibody. Proc Natl Acad Sci U S A 74 5682–5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmeister WP, Huber AH, and Bjorkman PJ (1994). Crystal structure of the complex of rat neonatal Fc receptor with Fc. Nature 372 379–383. [DOI] [PubMed] [Google Scholar]
- Canfield SM, and Morrison SL (1991). The binding affinity of human IgG for its high affinity Fc receptor is determined by multiple amino acids in the CH2 domain and is modulated by the hinge region. J Exp Med 173 1483–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadevall A, and Janda A (2012). Immunoglobulin isotype influences affinity and specificity. Proc Natl Acad Sci U S A 109 12272–12273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadevall A, and Pirofski LA (2012). A new synthesis for antibody-mediated immunity. Nat Immunol 13 21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen VB, Arendall WB 3rd, Headd JJ, Keedy DA, Immormino RM, Kapral GJ, Murray LW, Richardson JS, and Richardson DC (2010). MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr D Biol Crystallogr 66, 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrada D, Morra G, and Colombo G (2013). Investigating allostery in molecular recognition: insights from a computational study of multiple antibody-antigen complexes. J Phys Chem B 117 535–552. [DOI] [PubMed] [Google Scholar]
- Deisenhofer J (1981). Crystallographic refinement and atomic models of a human Fc fragment and its complex with fragment B of protein A from Staphylococcus aureus at 2.9- and 2.8-A resolution. Biochemistry 20 2361–2370. [PubMed] [Google Scholar]
- DeLano WL, Ultsch MH, de Vos AM, and Wells JA (2000). Convergent solutions to binding at a protein-protein interface. Science 287 1279–1283. [DOI] [PubMed] [Google Scholar]
- Diebolder CA, Beurskens FJ, de Jong RN, Koning RI, Strumane K, Lindorfer MA, Voorhorst M, Ugurlar D, Rosati S, Heck AJ et al. (2014). Complement is activated by IgG hexamers assembled at the cell surface. Science 343 1260–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley P, and Cowtan K (2004). Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr 60 2126–2132. [DOI] [PubMed] [Google Scholar]
- Fouts TR, Tuskan R, Godfrey K, Reitz M, Hone D, Lewis GK, and DeVico AL (2000). Expression and characterization of a single-chain polypeptide analogue of the human immunodeficiency virus type 1 gp120-CD4 receptor complex. J Virol 74 11427–11436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank M, Walker RC, Lanzilotta WN, Prestegard JH, and Barb AW (2014). Immunoglobulin G1 Fc domain motions: implications for Fc engineering. J Mol Biol 426 1799–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohain N, Tolbert WD, Acharya P, Yu L, Liu T, Zhao P, Orlandi C, Visciano ML, Kamin-Lewis R, Sajadi MM, et al. (2015). Cocrystal Structures of Antibody N60-i3 and Antibody JR4 in Complex with gp120 Define More Cluster A Epitopes Involved in Effective Antibody-Dependent Effector Function against HIV-1. J Virol 89 8840–8854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohain N, Tolbert WD, Orlandi C, Richard J, Ding S, Chen X, Bonsor DA, Sundberg EJ, Lu W, Ray K, et al. (2016). Molecular basis for epitope recognition by non-neutralizing anti-gp41 antibody F240. Sci Rep 6, 36685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Roman VR, Florese RH, Patterson LJ, Peng B, Venzon D, Aldrich K, and Robert-Guroff M (2006). A simplified method for the rapid fluorometric assessment of antibody-dependent cell-mediated cytotoxicity. Journal of immunological methods 308 53–67. [DOI] [PubMed] [Google Scholar]
- Guan Y, Pazgier M, Sajadi MM, Kamin-Lewis R, Al-Darmarki S, Flinko R, Lovo E, Wu X, Robinson JE, Seaman MS, et al. (2013). Diverse specificity and effector function among human antibodies to HIV-1 envelope glycoprotein epitopes exposed by CD4 binding. Proceedings of the National Academy of Sciences of the United States of America 110 E69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guddat LW, Shan L, Anchin JM, Linthicum DS, and Edmundson AB (1994). Local and transmitted conformational changes on complexation of an anti-sweetener Fab. J Mol Biol 236, 247–274. [DOI] [PubMed] [Google Scholar]
- Guddat LW, Shan L, Fan ZC, Andersen KN, Rosauer R, Linthicum DS, and Edmundson AB (1995). Intramolecular signaling upon complexation. FASEB J 9, 101–106. [DOI] [PubMed] [Google Scholar]
- Hanson QM, and Barb AW (2015). A perspective on the structure and receptor binding properties of immunoglobulin G Fc. Biochemistry 54 2931–2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessell AJ, Hangartner L, Hunter M, Havenith CE, Beurskens FJ, Bakker JM, Lanigan CM, Landucci G, Forthal DN, Parren PW, et al. (2007). Fc receptor but not complement binding is important in antibody protection against HIV. Nature 449 101–104. [DOI] [PubMed] [Google Scholar]
- Hezareh M, Hessell AJ, Jensen RC, van de Winkel JG, and Parren PW (2001). Effector function activities of a panel of mutants of a broadly neutralizing antibody against human immunodeficiency virus type 1. J Virol 75 12161–12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann LG (1976). Antibodies as allosteric proteins--I. A hypothesis. Immunochemistry 13 725–730. [DOI] [PubMed] [Google Scholar]
- Holm L (2019). Benchmarking fold detection by DaliLite v.5. Bioinformatics 35, 5326–5327. [DOI] [PubMed] [Google Scholar]
- Houde D, Arndt J, Domeier W, Berkowitz S, and Engen JR (2009). Characterization of IgG1 Conformation and Conformational Dynamics by Hydrogen/Deuterium Exchange Mass Spectrometry. Anal Chem 81 5966. [DOI] [PubMed] [Google Scholar]
- Janda A, Bowen A, Greenspan NS, and Casadevall A (2016). Ig Constant Region Effects on Variable Region Structure and Function. Front Microbiol 7, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jassal R, Jenkins N, Charlwood J, Camilleri P, Jefferis R, and Lund J (2001). Sialylation of human IgG-Fc carbohydrate by transfected rat alpha2,6-sialyltransferase. Biochem Biophys Res Commun 286, 243–249. [DOI] [PubMed] [Google Scholar]
- Jensen PF, Larraillet V, Schlothauer T, Kettenberger H, Hilger M, and Rand KD (2015). Investigating the interaction between the neonatal Fc receptor and monoclonal antibody variants by hydrogen/deuterium exchange mass spectrometry. Mol Cell Proteomics 14 148–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabsch W, Kabsch H, and Eisenberg D (1976). Packing in a new crystalline form of glutamine synthetase from Escherichia coli. J Mol Biol 100 283–291. [DOI] [PubMed] [Google Scholar]
- Krapp S, Mimura Y, Jefferis R, Huber R, and Sondermann P (2003). Structural Analysis of Human IgG-Fc Glycoforms Reveals a Correlation Between Glycosylation and Structural Integrity. Journal of Molecular Biology 325, 979–989. [DOI] [PubMed] [Google Scholar]
- Krissinel E, and Henrick K (2007). Inference of macromolecular assemblies from crystalline state. J Mol Biol 372, 774–797. [DOI] [PubMed] [Google Scholar]
- Lancet D, Licht A, Schechter I, and Pecht I (1977). Hapten-induced allosteric transition in the light chain dimer of an immunoglobulin. Nature 269, 827–829. [DOI] [PubMed] [Google Scholar]
- Lancet D, and Pecht I (1976). Kinetic evidence for hapten-induced conformational transition in immunoglobin MOPC 460. Proc Natl Acad Sci U S A 73, 3549–3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leoh LS, Daniels-Wells TR, Martinez-Maza O, and Penichet ML (2015). Insights into the effector functions of human IgG3 in the context of an antibody targeting transferrin receptor 1. Mol Immunol 67, 407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindorfer MA, Cook EM, Tupitza JC, Zent CS, Burack R, de Jong RN, Beurskens FJ, Schuurman J, Parren PW, and Taylor RP (2016). Real-time analysis of the detailed sequence of cellular events in mAb-mediated complement-dependent cytotoxicity of B-cell lines and of chronic lymphocytic leukemia B-cells. Mol Immunol 70 13–23. [DOI] [PubMed] [Google Scholar]
- Lund J, Pound JD, Jones PT, Duncan AR, Bentley T, Goodall M, Levine BA, Jefferis R, and Winter G (1992). Multiple binding sites on the CH2 domain of IgG for mouse Fc gamma R11. Mol Immunol 29 53–59. [DOI] [PubMed] [Google Scholar]
- Lund J, Takahashi N, Pound JD, Goodall M, and Jefferis R (1996). Multiple interactions of IgG with its core oligosaccharide can modulate recognition by complement and human Fc gamma receptor I and influence the synthesis of its oligosaccharide chains. J Immunol 157 4963–4969. [PubMed] [Google Scholar]
- Lux A, Yu X, Scanlan CN, and Nimmerjahn F (2013). Impact of immune complex size and glycosylation on IgG binding to human FcgammaRs. J Immunol 190 4315–4323. [DOI] [PubMed] [Google Scholar]
- Matsumiya S, Yamaguchi Y, Saito J, Nagano M, Sasakawa H, Otaki S, Satoh M, Shitara K, and Kato K (2007). Structural comparison of fucosylated and nonfucosylated Fc fragments of human immunoglobulin G1. J Mol Biol 368 767–779. [DOI] [PubMed] [Google Scholar]
- McCoy AJ (2007). Solving structures of protein complexes by molecular replacement with Phaser. Acta Crystallogr D Biol Crystallogr 63, 32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengistu M, Ray K, Lewis GK, and DeVico AL (2015). Antigenic properties of the human immunodeficiency virus envelope glycoprotein gp120 on virions bound to target cells. PLoS Pathog 11, e1004772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger H (1978). The effect of antigen on antibodies: recent studies. Contemp Top Mol Immunol 7, 119–152. [DOI] [PubMed] [Google Scholar]
- Morgan A, Jones ND, Nesbitt AM, Chaplin L, Bodmer MW, and Emtage JS (1995). The N-terminal end of the CH2 domain of chimeric human IgG1 anti-HLA-DR is necessary for C1q, Fc gamma RI and Fc gamma RIII binding. Immunology 86, 319–324. [PMC free article] [PubMed] [Google Scholar]
- Murshudov GN, Vagin AA, and Dodson EJ (1997). Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D Biol Crystallogr 53, 240–255. [DOI] [PubMed] [Google Scholar]
- N., C.C.P. (1994). The CCP4 suite: programs for protein crystallography. Acta Crystallogr D Biol Crystallogr 50, 760–763. [DOI] [PubMed] [Google Scholar]
- Oda M, Kozono H, Morii H, and Azuma T (2003). Evidence of allosteric conformational changes in the antibody constant region upon antigen binding. Int Immunol 15 417–426. [DOI] [PubMed] [Google Scholar]
- Orlandi C, Flinko R, and Lewis GK (2016). A new cell line for high throughput HIV-specific antibody-dependent cellular cytotoxicity (ADCC) and cell-to-cell virus transmission studies. J Immunol Methods 433 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otwinowski Z, Minor W, and Charles W Carter Jr. (1997). Processing of X-ray diffraction data collected in oscillation mode. In Methods in Enzymology (Academic Press; ), pp. 307–326. [DOI] [PubMed] [Google Scholar]
- Pecht I (1976). Proceedings: Recognition and allostery in the function of immunoglobulins. Hoppe Seylers Z Physiol Chem 357, 615–616. [PubMed] [Google Scholar]
- Radaev S, Motyka S, Fridman WH, Sautes-Fridman C, and Sun PD (2001). The structure of a human type III Fcgamma receptor in complex with Fc. J Biol Chem 276 16469–16477. [DOI] [PubMed] [Google Scholar]
- Ray K, Mengistu M, Yu L, Lewis GK, Lakowicz JR, and DeVico AL (2014). Antigenic properties of the HIV envelope on virions in solution. J Virol 88, 1795–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose RJ, van Berkel PH, van den Bremer ET, Labrijn AF, Vink T, Schuurman J, Heck AJ, and Parren PW (2013). Mutation of Y407 in the CH3 domain dramatically alters glycosylation and structure of human IgG. MAbs 5, 219–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer-Eriksson AE, Kleywegt GJ, Uhlen M, and Jones TA (1995). Crystal structure of the C2 fragment of streptococcal protein G in complex with the Fc domain of human IgG. Structure 3, 265–278. [DOI] [PubMed] [Google Scholar]
- Sela-Culang I, Alon S, and Ofran Y (2012). A systematic comparison of free and bound antibodies reveals binding-related conformational changes. J Immunol 189 4890–4899. [DOI] [PubMed] [Google Scholar]
- Sohi MK, Corper AL, Wan T, Steinitz M, Jefferis R, Beale D, He M, Feinstein A, Sutton BJ, and Taussig MJ (1996). Crystallization of a complex between the Fab fragment of a human immunoglobulin M (IgM) rheumatoid factor (RF-AN) and the Fc fragment of human IgG4. Immunology 88, 636–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondermann P, Huber R, Oosthuizen V, and Jacob U (2000). The 3.2-A crystal structure of the human IgG1 Fc fragment-Fc gammaRIII complex. Nature 406 267–273. [DOI] [PubMed] [Google Scholar]
- Stewart R, Thom G, Levens M, Guler-Gane G, Holgate R, Rudd PM, Webster C, Jermutus L, and Lund J (2011). A variant human IgG1-Fc mediates improved ADCC. Protein Eng Des Sel 24 671–678. [DOI] [PubMed] [Google Scholar]
- Strop P, Ho WH, Boustany LM, Abdiche YN, Lindquist KC, Farias SE, Rickert M, Appah CT, Pascua E, Radcliffe T, et al. (2012). Generating bispecific human IgG1 and IgG2 antibodies from any antibody pair. J Mol Biol 420 204–219. [DOI] [PubMed] [Google Scholar]
- Subedi GP, and Barb AW (2015). The Structural Role of Antibody N-Glycosylation in Receptor Interactions. Structure 23, 1573–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subedi GP, Hanson QM, and Barb AW (2014). Restricted motion of the conserved immunoglobulin G1 N-glycan is essential for efficient FcgammaRIIIa binding. Structure 22, 1478–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subedi GP, Johnson RW, Moniz HA, Moremen KW, and Barb A (2015). High Yield Expression of Recombinant Human Proteins with the Transient Transfection of HEK293 Cells in Suspension. J Vis Exp, e53568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JJ, and Hoffmann LG (1971). Homotropic cooperative binding of the first component of guinea pig complement to rabbit IgG-erythrocyte complexes: a possible allosteric effect. Proc Natl Acad Sci U S A 68 2730–2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JJ, and Hoffmann LG (1974a). Cooperative binding of a complement component to antigen-antibody complexes--I: complexes containing rabbit IgG antibody. Immunochemistry 11, 431–445. [DOI] [PubMed] [Google Scholar]
- Thompson JJ, and Hoffmann LG (1974b). Cooperative binding of a complement component to antigen-antibody complexes. II. Effect of variations in temperature and ionic strength. Immunochemistry 11 537–541. [DOI] [PubMed] [Google Scholar]
- Tina KG, Bhadra R, and Srinivasan N (2007). PIC: Protein Interactions Calculator. Nucleic Acids Res 35, W473–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert WD, Gohain N, Alsahafi N, Van V, Orlandi C, Ding S, Martin L, Finzi A, Lewis GK, Ray K, et al. (2017a). Targeting the Late Stage of HIV-1 Entry for Antibody-Dependent Cellular Cytotoxicity: Structural Basis for Env Epitopes in the C11 Region. Structure. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert WD, Gohain N, Alsahafi N, Van V, Orlandi C, Ding S, Martin L, Finzi A, Lewis GK, Ray K, et al. (2017b). Targeting the Late Stage of HIV-1 Entry for Antibody-Dependent Cellular Cytotoxicity: Structural Basis for Env Epitopes in the C11 Region. Structure 25, 1719–1731 e1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert WD, Gohain N, Veillette M, Chapleau JP, Orlandi C, Visciano ML, Ebadi M, DeVico AL, Fouts TR, Finzi A, et al. (2016). Paring Down HIV Env: Design and Crystal Structure of a Stabilized Inner Domain of HIV-1 gp120 Displaying a Major ADCC Target of the A32 Region. Structure 24 697–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugurlar D, Howes SC, de Kreuk BJ, Koning RI, de Jong RN, Beurskens FJ, Schuurman J, Koster AJ, Sharp TH, Parren P, et al. (2018). Structures of C1-IgG1 provide insights into how danger pattern recognition activates complement. Science 359, 794–797. [DOI] [PubMed] [Google Scholar]
- Voynov V, Chennamsetty N, Kayser V, Helk B, Forrer K, Zhang H, Fritsch C, Heine H, and Trout BL (2009). Dynamic fluctuations of protein-carbohydrate interactions promote protein aggregation. PLoS One 4, e8425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, de Jong RN, van den Bremer ET, Beurskens FJ, Labrijn AF, Ugurlar D, Gros P, Schuurman J, Parren PW, and Heck AJ (2016). Molecular Basis of Assembly and Activation of Complement Component C1 in Complex with Immunoglobulin G1 and Antigen. Mol Cell 63 135–145. [DOI] [PubMed] [Google Scholar]
- Williams DC Jr., Benjamin DC, Poljak RJ, and Rule GS (1996). Global changes in amide hydrogen exchange rates for a protein antigen in complex with three different antibodies. J Mol Biol 257 866–876. [DOI] [PubMed] [Google Scholar]
- Williams DC Jr., Rule GS, Poljak RJ, and Benjamin DC (1997). Reduction in the amide hydrogen exchange rates of an anti-lysozyme Fv fragment due to formation of the Fv-lysozyme complex. J Mol Biol 270 751–762. [DOI] [PubMed] [Google Scholar]
- Yang D, Kroe-Barrett R, Singh S, Roberts CJ, and Laue TM (2017). IgG cooperativity - Is there allostery? Implications for antibody functions and therapeutic antibody development. MAbs, 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogo R, Yamaguchi Y, Watanabe H, Yagi H, Satoh T, Nakanishi M, Onitsuka M, Omasa T, Shimada M, Maruno T, et al. (2019). The Fab portion of immunoglobulin G contributes to its binding to Fcgamma receptor III. Scientific reports 9, 11957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Baruah K, Harvey DJ, Vasiljevic S, Alonzi DS, Song BD, Higgins MK, Bowden TA, Scanlan CN, and Crispin M (2013). Engineering hydrophobic protein-carbohydrate interactions to fine-tune monoclonal antibodies. J Am Chem Soc 135 9723–9732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Nussinov R, and Ma B (2018). Antigen binding allosterically promotes Fc receptor recognition. mAbs, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The atomic coordinates generated in this study are freely available at the RCSB (https://www.rcsb.org) with the following PDB accession codes: PDB: 6CHF (N5-i5 Fc-LALA), PDB: 6CJC (C11 Fc-LALA), PDB: 6CJX (N12-i2 Fc-LALA). The human mAbs N5-i5 and C11 are freely available at the HIV Database (https://www.hiv.lanl.gov) with the following record numbers: 2971 (mAb N5-i5) and 707 (mAb C11).


