Abstract
Purpose of Review
To review the postpartum management of hypertensive disorders of pregnancy.
Recent Findings
Hypertensive disorders are associated with an increased risk of future cardiovascular disease; however, there is a poor understanding of the underlying mechanisms and few recommendations to guide care in the postpartum period. Recent studies have shown high rates of masked hypertension and home blood pressure monitoring in the first year postpartum may be a promising opportunity to monitor health given evidence of high maternal adherence to this approach. In longer term, women with a history of a hypertensive disorder of pregnancy have higher blood pressures, increased risk of metabolic syndrome, and perhaps excess diastolic dysfunction. Triaging risk and improving handoff from the obstetrician to the primary care provider or subspecialist should be a priority in this population.
Summary
Hypertensive disorders of pregnancy remain an untapped opportunity to identify excess cardiovascular risk in affected women at a time when mitigating that risk during the reproductive years has the potential to improve future pregnancy health as well as improve women’s long-term cardiometabolic health.
Keywords: Preeclampsia, Hypertensive disorders of pregnancy, Postpartum management, Hypertension, Cardiovascular imaging, Prevention
Introduction
Hypertensive disorders of pregnancy, including gestational hypertension and preeclampsia, complicate 8–10% of pregnancies in the USA [1]. Gestational hypertension is the presence of new-onset hypertension (≥ 140 mmHg/90 mmHg) detected during pregnancy in a previously normotensive woman [1]. Preeclampsia is a heterogeneous syndrome characterized by new-onset hypertension and proteinuria and/or end-organ dysfunction. It is thought to result from uteroplacental insufficiency and leads to systemic endothelial dysfunction. In the severe form, in addition to higher blood pressures (≥ 160 mmHg/110 mmHg), end-organ dysfunction (such as eclampsia or acute renal or hepatic failure) develops, which often necessitates immediate delivery, and can lead to significant maternal and neonatal morbidity. Outside of delivery, care is otherwise supportive, with no effective treatments to prolong pregnancy or reverse the disease process. After delivery, women are often initiated on anti-hypertensive medication if blood pressures exceed 150 mmHg/100 mmHg and medication is down titrated as blood pressure normalizes in the first few weeks postpartum [1, 2]. Prevention of hypertensive disorders in future pregnancies is an active area of investigation. Currently, the only effective preventive strategy is low-dose aspirin starting in the late first trimester, which is recommended for women at high risk of preeclampsia, including women with pre-gestational diabetes, chronic hypertension, renal disease, a history of preeclampsia, or other well established risk factors [1]. Several newer modalities for prevention are under investigation, including statins and metformin [3, 4].
The association of preeclampsia with later-life cardiovascular disease (CVD) is well established and has been replicated in diverse populations across multiple studies [2, 5–7]. The biologic plausibility of this relationship is reinforced by the observation that the risk is amplified in women with recurrent preeclampsia, early-onset disease, or more severe forms [7, 8]. While many hypotheses have been proposed to explain this association, the underlying mechanisms are poorly understood. Proposed mechanisms for this increased risk include the presence of shared risk factors in preeclampsia and CVD, the possibility of common physiologic processes, or the distinct possibility that preeclampsia generates lasting endothelial dysfunction and causes the increased risk of CVD [9–11]. These mechanistic pathways are not mutually exclusive. In this article, we review the postpartum management of hypertensive disorders of pregnancy with a focus on identifying clinical opportunities and gaps in the evidence with the goal of improving subsequent pregnancy outcomes and long-term women’s cardiometabolic health.
Immediate Postpartum Management
After delivery, the primary management of hypertensive disorders of pregnancy is continued by the obstetrician in most cases. Immediately postpartum, the focus is on maternal stabilization and normalization of blood pressures with medication if indicated. The American College of Obstetricians and Gynecologists (ACOG) suggest maintaining blood pressures < 150/100 in the postpartum period, although initiation, titration of medication, and selection of the anti-hypertensive agent is based on clinical judgment, as there are no standardized management guidelines for specific anti-hypertensive agents or parameters for postpartum medication titration [12]. At present, in the USA, women are typically discharged from the hospital on postpartum day 2–4 and ACOG recommends a single blood pressure check between 3–10 days postpartum for women with a hypertensive disorder of pregnancy. Women with persistent hypertension or the need for titration of anti-hypertensive medications are typically seen more frequently in the postpartum period for medication management; however, this varies by institution [12]. Subsequently, women are typically seen at 4–6 weeks postpartum for a comprehensive postpartum visit and referred to their primary care physician (PCP) if there are additional needs for anti-hypertensive medication management [1].
One barrier to management in this period is the underutilization of postpartum care. There are limited data on the adherence to the 3–10 days postpartum blood pressure check, with one study showing that only 44% of eligible women were seen for this visit [13•]. Similarly, up to 40% of women are not seen for a traditional 4–6 weeks postpartum visit [14]. Attendance rates are even lower among populations with limited resources, which likely contribute to health disparities in these groups [14, 15]. ACOG and others have recommended implementation of innovative interventions to improve adherence to care in the immediate postpartum period, such as remote monitoring. Within the context of small randomized trials or research studies, text message-based home postpartum hypertension monitoring programs have been shown to increase patient engagement and improve compliance with blood pressure management follow-up [13•, 16, 17]. One such randomized trial compared a text-based remote monitoring protocol using home blood pressure monitoring for postpartum hypertension compared with usual care in a small cohort of women. They demonstrated increased compliance with having a single blood pressure value recorded in the first 10 days postpartum in the text messaging group compared with the standard care group [13•]. Remote hypertension monitoring may improve compliance with ACOG recommendations surrounding blood pressure assessment within the first 3–10 days postpartum and may identify women with otherwise unrecognized blood pressure elevations requiring medication in this period [1, 14]. However, whether broad implementation of remote monitoring programs improve maternal morbidity and mortality in this period or improve long-term outcomes has not been studied.
Management in the First Year Postpartum
Increasing evidence suggests high rates of progression to chronic hypertension or persistent hypertension in the first year following a hypertensive disorder of pregnancy [18, 19•, 20]. A recent study utilizing ambulatory and in-office BP monitoring found that 42% of women with preeclampsia with severe features had persistent hypertension at 1 year postpartum, with 17.5% of these cases only detected by APBM, suggesting that masked hypertension may contribute significantly to increased CVD risk in this population [19•]. Similarly, Smith et al. demonstrated that 56% of women with preeclampsia were hypertensive by 1-year postpartum [18]. Certain factors appear to amplify this risk, including overweight and obesity, Black race, and the severity of hypertensive disorder of pregnancy [15, 20].
Few interventions have been studied to improve blood pressure or prevent chronic hypertension in the first year postpartum following a hypertensive disorder of pregnancy. Small trials have examined the impact of various anti-hypertensive agents on acute control of hypertension either at the time of delivery or in the first few days postpartum [21, 22]. Similarly, ACOG guidelines address treatment of acute, severe hypertension immediately after delivery, but do not provide guidance on management in the weeks and months following delivery [23]. One small study showed that a lifestyle intervention designed to improve cardiometabolic risk factors following a complicated pregnancy in the first year postpartum was feasible and effective [24]. The recently published SNAP-HT trial demonstrated that systematic titration of antihypertensive medications in the postpartum period not only shortens the duration of anti-hypertensive therapy, but also may have a longer term benefit on blood pressure that persisted up to 6 months postpartum [25•]. The authors hypothesized that this beneficial impact is secondary to more favorable cardiovascular remodeling. These findings indicate that the first year postpartum may be critical to triage of risk and to facilitating identification of women with persistent or unresolved hypertension following a hypertensive disorder of pregnancy.
Barriers to Care in the Postpartum Period
Few healthcare providers carry out postpartum CVD risk counseling or screening, despite the fact that the postpartum period may be a time when women are motivated to modify their lifestyle [26]. While some providers are acutely aware of the risk of future CVD and attempt to educate patients in a timely manner, they encounter a variety of barriers to risk reduction. Identifying the appropriate timing and venue for this counseling as well as implementing recommended interventions for risk reduction is challenging. Much of this counseling occurs immediately after delivery, which is often a busy and emotional period for women. Amidst the birth of a newborn, many physical changes, pain, and sleep deprivation, it is not surprising that women do not retain all of the information that providers relay to them at the time of delivery [27]. While remote monitoring of blood pressure may be a promising method of improving adherence with care in the postpartum period, blood pressure monitoring is often not reimbursed by insurance, thus potentially disadvantaging women of lower socioeconomic status. Postpartum care after a hypertensive disorder of pregnancy is often fragmented, with no cohesive transition from the obstetrician to internist or cardiologist [26]. Further compounding this poor follow-up are the underlying socioeconomic issues that impact care in this period. The USA is one of the few developed countries without paid parental leave, which disparately impacts the most disadvantaged women. Similarly, access to care is a consideration, as many women with public insurance who were insured only during pregnancy lose insurance coverage within 60 days postpartum, significantly limiting their follow-up after a pregnancy complication, with specifics varying from state to state [14, 28]. Finally, emerging data suggest a significantly higher rate of posttraumatic stress disorder among women with a hypertensive disorder of pregnancy [29, 30]. These women may be less likely to return to the healthcare system for follow-up care after a prior traumatic delivery experience. Attending to the postpartum period within a trauma-informed care model may be beneficial but is to date untested [31].
Future Reproductive Health: Pre-Conception Care
Inter-pregnancy care focuses on care of women between pregnancies with the overall goal of improving outcomes for women and future infants. The Society for Maternal Fetal Medicine (SMFM) and ACOG recommend inter-pregnancy care for women with a history of hypertensive disorders of pregnancy [32]. In particular, they emphasize the importance of inter-pregnancy care to maximize women’s wellness not just between pregnancies and during subsequent pregnancies but along her entire life course. This inter-pregnancy care should actually start during the prenatal care period of the initial pregnancy, with SMFM recommending that during prenatal care, providers should discuss who will provide primary care after the immediate postpartum period, discuss contraception options, provide anticipatory guidance regarding breastfeeding and maternal health, and discuss the associations between pregnancy complications and long-term maternal health. At the 4–6 weeks comprehensive postpartum visit, providers should review complications of pregnancy and their implications for future maternal health and ensure that the patient has a primary medical home for ongoing care. Specifically, for women with a pregnancy complicated by preeclampsia or gestational hypertension, SMFM recommends that BP should be evaluated for resolution of hypertension and recommends that BP be maintained < 120/80 mmHg, with consideration for medication initiation or referral to a primary care physician if BP goals are not met. In addition, they recommend that women should achieve a normal BMI and that aspirin should be discussed for future pregnancies. In women with underlying chronic hypertension, in addition to the abovementioned recommendations, testing should be considered for ventricular hypertrophy, retinopathy, and renal disease in women with longstanding or uncontrolled hypertension [32].
Multidisciplinary Care and Transition of Care
Several centers throughout the USA and Canada have reported on multidisciplinary maternal health clinics for women with a hypertensive disorder of pregnancy [33, 34]. These clinics typically involve combinations of obstetricians, cardiologists, and primary care physicians with a goal of addressing care inequities for young women. A structured cardiovascular screening program ensures adequate follow-up after a hypertensive disorder of pregnancy and provides an opportunity for lifestyle counseling and interventions [26]. One such clinic, The Maternal Health Clinic in Ontario, Canada, identifies women with a pregnancy complication at the time of delivery and systematically refers them to the follow-up clinic [18]. Women undergo a complete assessment including screening history, physical examination, fasting bloodwork, and urinalysis at 6 months postpartum [35]. Similarly, Celi et al. report on successful implementation of a postpartum transition clinic after a hypertensive pregnancy in Boston. In their model, women are seen for multiple visits beginning in the immediate postpartum period. They demonstrate successful incorporation of home blood pressure monitoring, adjustment of anti-hypertensive medications, and implementation of preventative measures such as a nutritionist referral and follow-up with primary care physicians in a racially and socioeconomically diverse population. Follow-up in a dedicated postpartum preeclampsia clinic may help facilitate transition of care and provide a unique window for providers to focus on improving cardiometabolic health prior to a subsequent pregnancy, primary prevention of CVD, and counseling on risk factor modification.
Unfortunately, most women in the USA are not seen in such novel multidisciplinary clinics, and communication about future risk relies on relaying information from the obstetrician to both the patient and her primary care provider. In addition to improving this communication and counseling in the immediate postpartum period, consideration should be made for implementation of innovative interventions to improve this handoff. Examples include the utilization of standardized best practice alerts in the electronic medical record, which could more reliably incorporate pregnancy history into a patient’s history and facilitate risk identification. One recent study described a rule-based computerized algorithm to characterize preeclampsia by mining prenatal clinical notes within a large healthcare organization, which had a sensitivity of 88% and a specificity of 99% [36]. Until such innovative interventions can be implemented on a larger scale, focus should be on improving patient-provider education and communication surrounding the long-term implications of hypertensive disorders of pregnancy for women’s future CV health.
Management of Women with a History of Hypertensive Disorders of Pregnancy: Beyond the Postpartum Period
In the years after delivery, management of women with a history of hypertensive disorders of pregnancy becomes the responsibility of primary care physicians, family practitioners, and subspecialists such as cardiologists and nephrologists. It is often easy for these women to get lost to follow-up, as they are generally young and otherwise healthy. As part of the 2011 Guideline Update for the Prevention of CVD in Women, the American Heart Association (AHA) recommended that providers meeting female patients for the first time should take a detailed pregnancy history and ask specifically about pregnancy complications such as preeclampsia, gestational hypertension, preterm birth, and gestational diabetes [37]. These guidelines consider a history of preeclampsia or gestational hypertension to be a major risk factor for CVD. Thus, they recommend that women with a history of hypertensive disorders of pregnancy should have careful screening, monitoring, and control of other CVD risk factors such as hypertension, dyslipidemia, and diabetes. In the general population, it is recommended that CV risk factor screening be obtained every 4 to 6 years. In a higher risk population such as those with a history of hypertensive disorders of pregnancy, there are no specific data to guide our management, but we suggest that regular BP and cholesterol screening should be more frequent and should start as soon as 1 year postpartum.
Women with a history of preeclampsia and gestational hypertension are at increased risk of hypertension in later life. In the 14 years after preeclampsia, women are 3.7 times more likely to develop hypertension than those with normotensive pregnancies [6]. Thirty-eight percent of women with a history of early-onset preeclampsia had developed hypertension by the 5th decade of life [38]. Starting at age 30, women with a history of hypertensive disorders of pregnancy are more likely to be treated for hypertension and on average, systolic blood pressure was ~ 10 mmHg higher in these women until age 55 [39•]. There are no clear guidelines on frequency of blood pressure monitoring in this population. However, in patients with elevated risk for hypertension, the United States Preventive Services Task Force (USPSTF) task force recommends at least yearly in office measurements [40]. Extrapolating from these recommendations, we also suggest yearly blood pressure measurements for the high-risk population of women with a history of hypertensive disorders of pregnancy. There is a growing body of evidence that suggests that we may be missing those with masked hypertension and that providers should consider obtaining home blood pressure measurements or ambulatory blood pressure measurements, especially in high-risk populations [41, 42]. Elevated home blood pressures and ambulatory blood pressures are associated with higher CVD risk and warrant treatment [43, 44]. According to the 2017 American College of Cardiology (ACC) and AHA Guidelines on Management of High Blood Pressure in Adults, stage 1 hypertension was redefined as systolic BP 130–139 mmHg or diastolic BP 80–89 mmHg [41]. Low-risk adults with stage 1 hypertension can be treated with nonpharmacologic therapy and repeat measurement in 3–6 months. However, those with stage 1 hypertension and high-ASCVD risk (> 10% 10-year ASCVD risk) should be treated with both nonpharmacologic therapy and anti-hypertensive medications. Without specific mention from the ACC/AHA on treatment of women with a history of hypertensive disorders of pregnancy, we recommend following guidelines for the general population.
The ACC and the AHA incorporate preeclampsia into the newest Cholesterol Management Guidelines published in 2018 [45•]. On first contact with a healthcare provider, young adults should have one screening cholesterol measurement and measured every 4 to 6 years thereafter. In their primary prevention section, preeclampsia is listed as a CVD “risk enhancer.” For women in the age range of 40 to 75 years old with an LDL between 70 and 190 mg/dL who do not have diabetes mellitus and who are at “borderline” (5 to < 7.5%) or “intermediate” (≥ 7.5 to 20%) 10-year ASCVD risk, the presence of a risk enhancer such as preeclampsia merits initiation of at least a moderate intensity statin. If decision on statin initiation is still uncertain, obtaining a coronary artery calcium (CAC) measurement can help risk stratify patients. The use of statins for primary prevention specifically among women with a history of hypertensive disorders of pregnancy has not been studied. Aspirin use may be beneficial for stroke prevention in this population, but evidence supported by randomized control trials is needed [46].
As for all women, those with a history of hypertensive disorders of pregnancy should be counseled on healthy lifestyle practices. This should include recommendation to follow diets such as the “DASH” diet, which are diets low in salt and animal fat and include a majority of fruits and vegetables and more plant-based protein. In addition, women should be advised to do moderate exercise for at least 30 min a day, 5 times per week [47]. Providers should discuss weight loss strategies with women who are overweight or obese. Women who smoke cigarettes should be counseled on smoking cessation. Online education modules and meeting with a lifestyle coach may be helpful motivators for women with a history of hypertensive disorders of pregnancy [48].
Although it is well known that preeclampsia is an independent risk factor for CVD including ischemic heart disease as well as heart failure [5, 6], hypertensive disorders of pregnancy have not yet been successfully incorporated into CVD risk prediction models. Traditional risk models, such as the Framingham or Modified Framingham Risk Score, tend to underestimate CV risk among younger women. Multiple studies have attempted to include a history of hypertensive disorders of pregnancy into 10-year CVD risk prediction models, but did not find an improvement in discrimination or reclassification [49, 50]. Women who are within 10 years postpartum are generally less than age 40 and thus, their overall CVD risk is still very low. In addition, women with hypertensive disorders of pregnancy also are more likely to have other traditional CVD risk factors and comorbidities that may mask contributions of hypertensive disorders of pregnancy to CVD risk estimation. In fact, those with a history of hypertensive disorders of pregnancy are more likely to have ASCVD risk scores greater than 7.5% [39•]. Using longer term models that evaluate 30 year or lifetime risk may be better at identifying a higher risk group [51]. Overall, development of risk models targeted at assessing longer term CV risk for younger women that include sex-specific risk factors such as hypertensive disorders of pregnancy is desperately needed.
Noninvasive Cardiovascular Imaging Tools
Beyond risk factor screening, the use of noninvasive cardiovascular imaging techniques may help identify women who may be at higher risk to develop clinical CVD. Around the time of delivery, women with preeclampsia are more likely to have structural cardiac changes assessed by transthoracic echocardiogram compared with women who are normotensive during pregnancy. These structural changes include more abnormal diastolic parameters such as an exaggerated reduction in E/A mitral inflow ratio and increased E/e’ ratio corresponding to increased left atrial and left ventricular pressures [52–54]. Additionally, women with gestational hypertension and women with preeclampsia are more likely to have an increase in the left ventricular (LV) mass, a finding associated with worse CV outcomes in the general adult population [52, 53, 55, 56]. Severe and preterm preeclampsia carries a poorer clinical prognosis and are associated with more pronounced peripartum structural changes on echocardiogram including increased right ventricular (RV) systolic pressure and abnormal right ventricular strain [57, 58].
A limited number of studies have evaluated echocardiographic changes in later life for women with a history of hypertensive disorders of pregnancy. For women 9 to 16 years after index pregnancy, preeclampsia history was associated with thicker LV walls and more abnormal diastolic function parameters such as an increased E/e’ ratio suggestive of increased left ventricular pressure [59•]. Other studies have shown an association of preeclampsia history with concentric LV remodeling and preclinical heart failure 4 to 10 years after delivery; however, these associations were attenuated after accounting for metabolic syndrome and hypertension [60, 61]. There are additional studies that did not find a significant difference in echocardiogram parameters in the 10-year follow-up time period for women with preeclampsia compared with normotensive controls [62]. This area warrants further study to identify characteristics associated with an increased risk for early structural cardiac changes and to evaluate the association with higher risk outcomes in this population. Intervention studies to determine if these structural changes and increased risk are reversible are also warranted.
Cardiac MRI is an alternative noninvasive imaging technique that can provide more accurate and reproducible measures of cardiac chamber sizes as well as ventricular and atrial function. Gadolinium contrast during cardiac MRI also allows the assessment of myocardial infiltrative processes and late gadolinium enhancement (LGE) has been shown to be associated with adverse outcomes and arrhythmias. In patients with hypertension, cardiac MRI is a useful tool to differentiate etiology of left ventricular hypertrophy and provides a more accurate assessment of myocardial ischemia, especially small vessel disease [63]. Cardiac MRI has also helped evaluate medication treatment benefits on reversal of left ventricular remodeling with use of medications such as angiotensin receptor blockers among patients with hypertension or metformin among patients with diabetes [64, 65]. Given the association of left ventricular remodeling with worse CVoutcomes, reversal of remodeling has potential implications for CVD risk modification [66]. For women with a history of preeclampsia, cardiac MRI may be a way to evaluate those who have evidence of structural cardiac changes following a preeclamptic pregnancy. However, there are very limited published data on cardiac MRI among patients with a history of pregnancy complications to guide our practice patterns [67].
At delivery and at 1 year postpartum, women with a history of preeclampsia have evidence of subclinical vascular changes that include abnormalities in their carotid intima media thickness with a thicker intima/media ratio [68]. Differences in CIMT among women with a history of preeclampsia tend to persist in the 10 years after delivery [69–71]. Considering coronary artery calcium, in one study with women ~ 3 decades after pregnancy, women with preeclampsia were found to be more likely to have a higher CAC score compared with those who were normotensive during pregnancy, a finding that persisted even after adjustment for traditional risk factors [72]. A history of pregnancy complications including preeclampsia has been shown to be associated with nonobstructive and obstructive coronary artery disease on coronary CT angiogram in asymptomatic women [73, 74]. It is possible that optimizing CV health during and shortly after pregnancy may improve subclinical measures such as CIMT or coronary artery calcification, but randomized control trials are needed to confirm these potential benefits [75].
Leveraging noninvasive CV imaging tools may help providers identify subclinical vascular and structural cardiac changes which could guide targeted therapies for those who may be at higher risk to develop clinical CVD. Differentiating women at lower risk from those at moderate to high risk via imaging modalities will allow providers to triage patients for more aggressive treatment in the higher risk group. To date, however, there are large gaps in the data regarding use of CV imaging among women with hypertensive disorders of pregnancy and provide an opportunity for further study.
Conclusions
While the evidence linking hypertensive disorders of pregnancy with future CVD is robust and compelling, there remain many unanswered questions on management both in the immediate postpartum period as well as longer term. First, in the USA, care in the postpartum period is fragmented and poorly attended by patients. This results in an inconsistent transition of care from the obstetrician to the primary care physician and communication about future risk. One limitation to research on effective interventions and assistance in guideline development is the time interval between a pregnancy complication and a cardiovascular event. The authors propose consideration of two-tiered endpoints when evaluating screening, interventions or risk score calculators. As hypertension contributes to more cardiovascular events in women compared with men, its clinical importance as a primary endpoint should not be overlooked [76]. Finally, we propose modeling clinical care following a hypertensive disorder of pregnancy after the guidelines for management after a pregnancy complicated by gestational diabetes (GDM). Following GDM, the American Diabetes Association recommends screening for type 2 diabetes with an oral glucose tolerance test at 4–8 weeks postpartum and again every 1 to 3 years thereafter [77]. While this approach is untested following hypertensive disorders of pregnancy, future studies could provide evidence about its feasibility, efficacy, and cost-effectiveness.
Hypertensive disorders of pregnancy are still an untapped opportunity to identify excess CVD risk in affected women at a time when mitigating that risk during the reproductive years has the potential to improve future pregnancy health as well as improve women’s long-term cardiometabolic health. We have summarized the evidence and gaps in the postpartum care of women with hypertensive disorders of pregnancy (Fig. 1). We also propose opportunities that can be tested to leverage the postpartum years as a unique sex-specific time to improve women’s health.
Fig. 1.
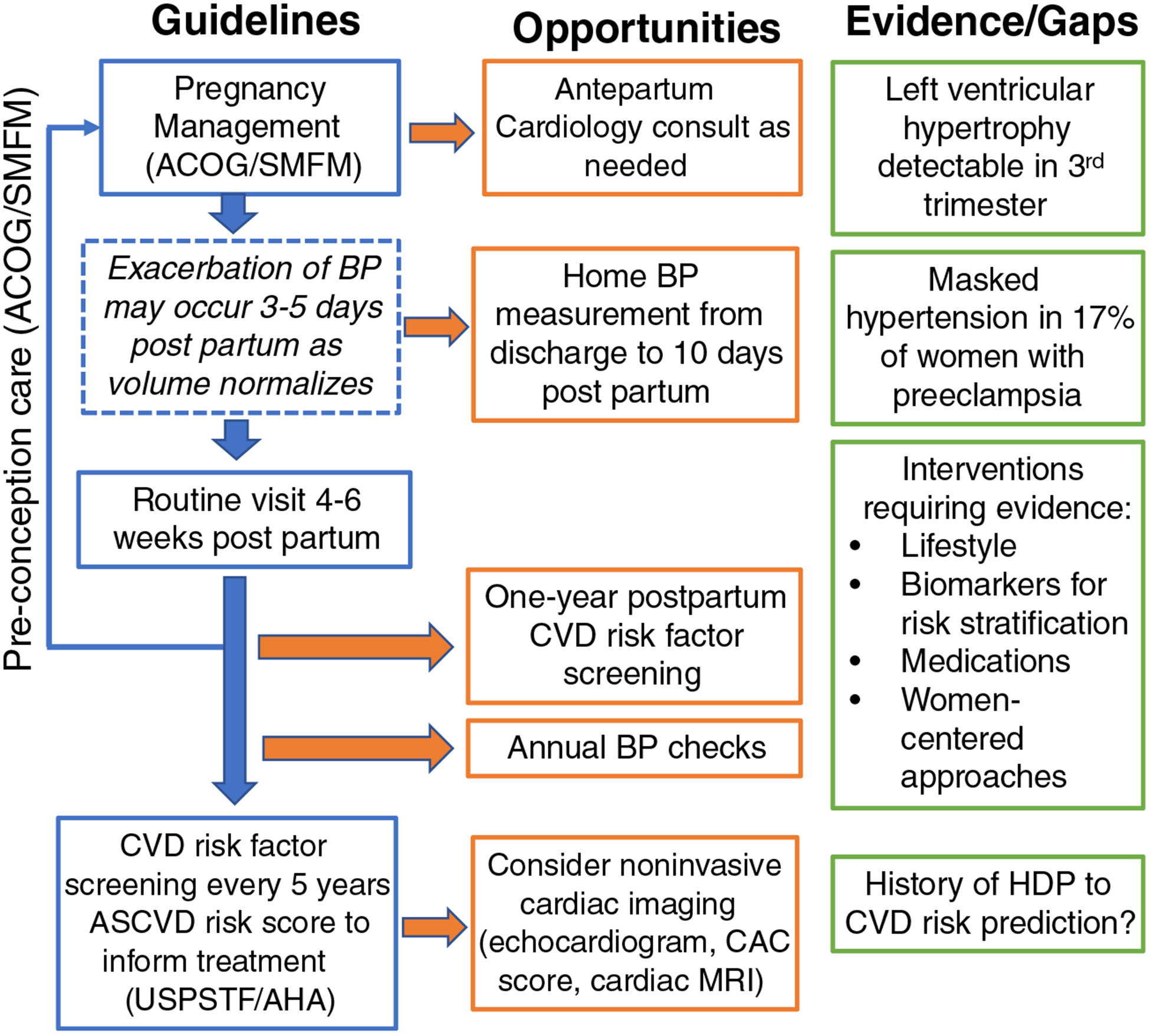
Guidelines, opportunities, evidence, and gaps regarding the improvement of postpartum health among women with hypertensive disorders of pregnancy
Footnotes
This article is part of the Topical Collection on Preeclampsia
Conflict of Interest The authors declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
- 1.ACOG Practice Bulletin No. 202: Gestational Hypertension and Preeclampsia. Obstet Gynecol [Internet]. 2019. [cited 2018 Dec 26];133:e1–25. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30575675. [DOI] [PubMed] [Google Scholar]
- 2.Hauspurg A, Ying W, Hubel CA, Michos ED, Ouyang P. Adverse pregnancy outcomes and future maternal cardiovascular disease. Clin Cardiol [Internet]. 2018. [cited 2018 Feb 25];41:239–46. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29446836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brownfoot FC, Hastie R, Hannan NJ, Cannon P, Tuohey L, Parry LJ, et al. Metformin as a prevention and treatment for preeclampsia: effects on soluble fms-like tyrosine kinase 1 and soluble endoglin secretion and endothelial dysfunction. Am J Obstet Gynecol [Internet]. 2016. [cited 2018 Mar 12];214:356.e1–356.e15. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26721779. [DOI] [PubMed] [Google Scholar]
- 4.Costantine MM, Cleary K, Eunice Kennedy Shriver National Institute of Child Health and Human Development Obstetric- Fetal Pharmacology Research Units Network. Pravastatin for the prevention of preeclampsia in high-risk pregnant women. Obstet Gynecol [Internet]. 2013. [cited 2019 mar 9];121:349–53. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23344286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu P, Haththotuwa R, Kwok CS, Babu A, Kotronias RA, Rushton C, et al. Preeclampsia and future cardiovascular health: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2017;10. [DOI] [PubMed] [Google Scholar]
- 6.Bellamy L, Casas J-P, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. Bmj [Internet] 2007;335:974–974. Available from: 10.1136/bmj.39335.385301.BE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDonald SD, Malinowski A, Zhou Q, Yusuf S, Devereaux PJ. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am Heart J. 2008;156:918–30. [DOI] [PubMed] [Google Scholar]
- 8.Auger N, Fraser WD, Schnitzer M, Leduc L, Healy-Profitós J, Paradis G. Recurrent pre-eclampsia and subsequent cardiovascular risk. Heart [Internet]. 2017. [cited 2017 Nov 15];103:235–43. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27530133. [DOI] [PubMed] [Google Scholar]
- 9.Romundstad PR, Magnussen EB, Smith GD, Vatten LJ. Hypertension in pregnancy and later cardiovascular risk: common antecedents? Circulation [Internet]. 2010. [cited 2018 Jun 25];122: 579–84. Available from: http://circ.ahajournals.org/cgi/doi/10.1161/CIRCULATIONAHA.110.943407 [DOI] [PubMed] [Google Scholar]
- 10.Powe CE, Levine RJ, Karumanchi SA. Preeclampsia, a disease of the maternal endothelium: the role of antiangiogenic factors and implications for later cardiovascular disease. Circulation [Internet]. 2011. [cited 2017 Nov 20];123:2856–69. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21690502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haug EB, Horn J, Markovitz AR, Fraser A, Klykken B, Dalen H, et al. Association of conventional cardiovascular risk factors with cardiovascular disease after hypertensive disorders of pregnancy: analysis of the Nord-Trøndelag Health Study. JAMA Cardiol [Internet]. 2019. [cited 2019 Sep 6];4:628–35. Available from: 10.1001/jamacardio.2019.1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cairns AE, Pealing L, Duffy JMN, Roberts N, Tucker KL, Leeson P, et al. Postpartum management of hypertensive disorders of pregnancy: a systematic review. BMJ Open [Internet]. 2017. [cited 2019 Jun 29];7:e018696 Available from: 10.1136/bmjopen-2017-018696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.•.Hirshberg A, Downes K, Srinivas S. Comparing standard office-based follow-up with text-based remote monitoring in the management of postpartum hypertension: a randomised clinical trial. BMJ Qual Saf [Internet]. 2018. [cited 2019 Jan 3];27(11):871–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29703800. [DOI] [PubMed] [Google Scholar]; Summary: Small randomized control trial comparing a text-based remote monitoring protocol for postpartum hypertension compared to usual care in a small cohort of women. They demonstrate increased compliance with having a single blood pressure value recorded in the first ten days postpartum in the text messaging group. Remote hypertension monitoring may improve adherence, improve access to care and may identify women with otherwise unrecognized blood pressure elevations requiring medication in this period, although future study is needed to determine if this improves outcomes in the immediate postpartum period.
- 14.ACOG Committee Opinion No. 736 Optimizing Postpartum Care. Obstet Gynecol [Internet]. 2018. [cited 2018 Apr 25];131:e140–50. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29683911. [DOI] [PubMed] [Google Scholar]
- 15.Levine LD, Nkonde-Price C, Limaye M, Srinivas SK. Factors associated with postpartum follow-up and persistent hypertension among women with severe preeclampsia. J Perinatol. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoppe KK, Williams M, Thomas N, Zella JB, Drewry A, Kim K, et al. Telehealth with remote blood pressure monitoring for postpartum hypertension: a prospective single-cohort feasibility study. Pregnancy Hypertens [Internet]. 2019. [cited 2019 Apr 10];15: 171–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30825917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hauspurg A, Lemon L, Quinn B, Binstock A, Larkin J. Beigi R, et al. A postpartum remote hypertension monitoring protocol implemented at a hospital level: Obs Gynecol (In Press; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith G, Pudwell J, Saade G. Impact of the New American Hypertension Guidelines on the Prevalence of Postpartum Hypertension. Am J Perinatol [Internet]. 2019. [cited 2018 Sep6];36:440–2. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30170330. [DOI] [PubMed] [Google Scholar]
- 19.•.Benschop L, Duvekot JJ, Versmissen J, van Broekhoven V, Steegers EAP, Roeters van Lennep JE. Blood pressure profile 1 year after severe preeclampsia. Hypertension [Internet]. 2018. [cited 2018 Feb 24];71(3):HYPERTENSIONAHA.117.10338. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29437895 [DOI] [PubMed] [Google Scholar]; Prospective cohort study of women with severe preeclampsia followed one-year postpartum with office and ambulatory blood pressure monitoring. The authors demonstrate that over 40% of women with preeclampsia with severe features had persistent hypertension at one-year postpartum, with 18% of these cases only detected by ambulatory blood pressure monitoring, suggesting that masked hypertension may contribute to increased CV risk in this population.
- 20.Hauspurg A, Countouris ME, Jeyabalan A, Hubel CA, Roberts JM, Schwarz EB, et al. Risk of hypertension and abnormal biomarkers in the first year postpartum associated with hypertensive disorders of pregnancy among overweight and obese women. Pregnancy Hypertens [Internet]. 2019. [cited 2019 Mar 9];15:1–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30825904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veena P, Perivela L, Raghavan SS. Furosemide in postpartum management of severe preeclampsia: a randomized controlled trial. Hypertens Pregnancy Taylor and Francis Ltd. 2017;36:84–9. [DOI] [PubMed] [Google Scholar]
- 22.Sharma KJ, Greene N, Kilpatrick SJ. Oral labetalol compared to oral nifedipine for postpartum hypertension: a randomized controlled trial. Hypertens pregnancy [Internet]. 2017. [cited 2019 Aug 26];36:44–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27786578. [DOI] [PubMed] [Google Scholar]
- 23.ACOG Committee Opinion No. 767 Summary: Emergent Therapy for Acute-Onset, Severe Hypertension During Pregnancy and the Postpartum Period. Obstet Gynecol. NLM (Medline); 2019;133: 409–12. [DOI] [PubMed] [Google Scholar]
- 24.Berks D, Hoedjes M, Raat H, Franx A, Looman CWN, Van Oostwaard MF, et al. Feasibility and effectiveness of a lifestyle intervention after complicated pregnancies to improve risk factors for future cardiometabolic disease. Pregnancy Hypertens [Internet]. 2019. [cited 2019 Aug 26];15:98–107. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30825935. [DOI] [PubMed] [Google Scholar]
- 25.•.Cairns AE, Tucker KL, Leeson P, Mackillop LH, Santos M, Velardo C, et al. Self-management of postnatal hypertension: the SNAP-HT trial. Hypertens (Dallas, Tex 1979) [Internet]. 2018. [cited 2018 Nov 1];72(2): 425–432. Available from: 10.1161/HYPERTENSIONAHA.118.10911 [DOI] [PubMed] [Google Scholar]; This small trial randomized women to systematic titration of antihypertensive medications in the postpartum period and demonstrated that this not only shortens the duration of antihypertensive therapy, but also may have a longer-term benefit on blood pressure that persisted up to 6 months postpartum. Currently, there are few guidelines on blood pressure management in this period and if confirmed in larger studies, these findings suggest that blood pressure management in this period may improve longer-term CV outcomes.
- 26.Spaan J, Peeters L, Spaanderman M, Brown M. Cardiovascular Risk management after a hypertensive disorder of pregnancy. Hypertension [Internet]. 2012. [cited 2017 Nov 27];60:1368–73. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23071130. [DOI] [PubMed] [Google Scholar]
- 27.Swan HD, Borshoff DC. Informed consent-recall of risk information following epidural analgesia in labour. Anaesth Intensive Care [Internet]. 1994. [cited 2018 Nov 1];22:139–41. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8210014. [DOI] [PubMed] [Google Scholar]
- 28.Brindis CD, Freund KM. The ramifications of recent health policy actions for cardiovascular care of women: progress, threats, and opportunities. Clin Cardiol [Internet]. 2018. [cited 2019 Aug 26];41:173–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29485710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Porcel J, Feigal C, Poye L, Postma IR, Zeeman GG, Olowoyeye A, et al. Hypertensive disorders of pregnancy and risk of screening positive for posttraumatic stress disorder: a cross-sectional study. Pregnancy Hypertens. Elsevier B.V; 2013;3:254–60. [DOI] [PubMed] [Google Scholar]
- 30.Gaugler-Senden IPM, Duivenvoorden HJ, Filius A, De Groot CJM, Steegers EAP, Passchier J. Maternal psychosocial outcome after early onset preeclampsia and preterm birth. J Matern Neonatal Med. 2012;25:272–6. [DOI] [PubMed] [Google Scholar]
- 31.Sperlich M, Seng JS, Li Y, Taylor J, Bradbury-Jones C. Integrating trauma-informed care into maternity care practice: conceptual and practical issues. J Midwifery Womens Health [Internet]. 2017. [cited 2019 Sep 4];62:661–72. Available from: 10.1111/jmwh.12674 [DOI] [PubMed] [Google Scholar]
- 32.American College of Nurse-Midwives and the National Association of Nurse Practitioners in Women’s Health, American College of Obstetricians and Gynecologists and the Society for Maternal–Fetal Medicine, Louis JM, Bryant A, Ramos, Stuebe, et al. Interpregnancy care. Am J Obstet Gynecol [Internet]. 2019. [cited 2019 Aug 21];220:B2–18. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30579872. [Google Scholar]
- 33.Celi AC, Seely EW, Wang P, Thomas AM, Wilkins-Haug LE. Caring for women after hypertensive pregnancies and beyond: implementation and integration of a postpartum transition clinic. Matern Child Health J. Springer New York LLC; 2019; [DOI] [PubMed] [Google Scholar]
- 34.Smith GN. The Maternal Health Clinic: improving women’s cardiovascular health. Semin Perinatol [Internet]. 2015. [cited 2017 Nov 27];39:316–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26077232. [DOI] [PubMed] [Google Scholar]
- 35.Cusimano MC, Pudwell J, Roddy M, Cho C-KJ, Smith GN. The maternal health clinic: an initiative for cardiovascular risk identification in women with pregnancy-related complications. Am J Obstet Gynecol [Internet]. 2014. [cited 2017 Nov 27];210:438.e1–438.e9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24316270. [DOI] [PubMed] [Google Scholar]
- 36.Xie F, Im T, Getahun D. A computerized algorithm to capture patient’s past preeclampsia and eclampsia history from prenatal clinical notes. Health Informatics J [Internet]. 2018. [cited 2019 Sep 4];146045821775424. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29388496. [DOI] [PubMed] [Google Scholar]
- 37.Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd-Jones DM, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women–2011 update: a guideline from the American Heart Association. Circulation [Internet]. 2011. [cited 2017 Nov 15];123:1243–62. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21325087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bokslag A, Teunissen PW, Franssen C, van Kesteren F, Kamp O, Ganzevoort W, et al. Effect of early-onset preeclampsia on cardiovascular risk in the fifth decade of life. Am J Obs Gynecol. 2017;216:523.e1–7. [DOI] [PubMed] [Google Scholar]
- 39.•.Groenhof TKJ, Zoet GA, Franx A, Gansevoort RT, Bots ML, Groen H, et al. Trajectory of cardiovascular risk factors after hypertensive disorders of pregnancy. Hypertension. 2019;73:171–8 [DOI] [PubMed] [Google Scholar]; Prospective cohort study investigating association of hypertensive disorders of pregnancy and CV risk factor trajectory. Women with a history of hypertensive disorders of pregnancy have higher BP, more antihypertensive medication prescription, and elevated calculated ASCVD risk almost immediately after delivery (age 30s–40s). Some of this risk is attenuated as women approach menopause.
- 40.Siu AL. Screening for high blood pressure in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;163:778–86. [DOI] [PubMed] [Google Scholar]
- 41.Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: a report of the American College of Cardiology/American Heart Association Task F. Hypertension [Internet]. 2018;71:1269–324. Available from: https://www.ncbi.nlm.nih.gov/pubmed/29133354. [DOI] [PubMed] [Google Scholar]
- 42.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Hear J [Internet]. 2018;39:3021–104 Available from: https://www.ncbi.nlm.nih.gov/pubmed/30165516. [DOI] [PubMed] [Google Scholar]
- 43.Banegas JR, Ruilope LM, de la Sierra A, Vinyoles E, Gorostidi M, de la Cruz JJ, et al. Relationship between clinic and ambulatory blood-pressure measurements and mortality. N Engl J Med [Internet]. 2018. [cited 2018 Apr 24];378:1509–20. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29669232. [DOI] [PubMed] [Google Scholar]
- 44.Yang W-Y, Melgarejo JD, Thijs L, Zhang Z-Y, Boggia J, Wei F-F, et al. Association of Office and Ambulatory Blood Pressure With Mortality and Cardiovascular Outcomes Association of Office and Ambulatory Blood Pressure With Mortality and CV Outcomes Association of Office and Ambulatory Blood Pressure With Mortality and CV Outcomes. JAMA [Internet]. 2019;322:409–420. Available from: 10.1001/jama.2019.9811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.•.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation [Internet]. 2019;139: e1082–e1143. Available from: https://www.ncbi.nlm.nih.gov/pubmed/30586774 [DOI] [PMC free article] [PubMed] [Google Scholar]; The updated ACC/AHA guidelines on management of blood cholesterol include preeclampsia as a risk factor for CVD and a risk enhancer in decision tree for initiation of a statin for primary prevention in patients at intermediate or high risk to development of CVD.
- 46.Miller EC, Boehme AK, Chung NT, Wang SS, Lacey JV Jr, Lakshminarayan K, et al. Aspirin reduces long-term stroke risk in women with prior hypertensive disorders of pregnancy. Neurology [Internet]. 2019;92:e305–16 Available from: https://www.ncbi.nlm.nih.gov/pubmed/30587515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, et al. The Physical Activity Guidelines for Americans. JAMA [Internet]. 2018;320:2020–8 Available from: https://www.ncbi.nlm.nih.gov/pubmed/30418471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rich-Edwards JW, Stuart JJ, Skurnik G, Roche AT, Tsigas E, Fitzmaurice GM, et al. Randomized Trial to Reduce Cardiovascular Risk in Women with Recent Preeclampsia. J Womens Heal [Internet]. 2019/06/20 2019; Available from: https://www.ncbi.nlm.nih.gov/pubmed/31215837. [DOI] [PubMed] [Google Scholar]
- 49.Stuart JJ, Tanz LJ, Cook NR, Spiegelman D, Missmer SA, Rimm EB, et al. Hypertensive disorders of pregnancy and 10-year cardiovascular risk prediction. J Am Coll Cardiol [Internet]. 2018;72: 1252–63 Available from: https://www.ncbi.nlm.nih.gov/pubmed/30190003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Markovitz AR, Stuart JJ, Horn J, Williams PL, Rimm EB, Missmer SA, et al. Does pregnancy complication history improve cardiovascular disease risk prediction? Findings from the HUNT study in Norway. Eur Hear J [Internet]. 2019;40:1113–20 Available from: https://www.ncbi.nlm.nih.gov/pubmed/30596987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gladstone RA, Pudwell J, Nerenberg KA, Grover SA, Smith GN. Cardiovascular risk assessment and follow-up of women after hypertensive disorders of pregnancy: a prospective cohort study. J Obs Gynaecol can [Internet]. 2019/01/19 2019;41:1157–1167 e1.Available from: https://www.ncbi.nlm.nih.gov/pubmed/30655227. [DOI] [PubMed] [Google Scholar]
- 52.Castleman JS, Ganapathy R, Taki F, Lip GY, Steeds RP, Kotecha D. Echocardiographic structure and function in hypertensive disorders of pregnancy: a systematic review. Circ Cardiovasc imaging [Internet]. 2016/09/10 2016;9 Available from: https://www.ncbi.nlm.nih.gov/pubmed/27609819. [DOI] [PubMed] [Google Scholar]
- 53.Vlahovic-Stipac A, Stankic V, Popovic ZB, Putnikovic B, Neskovic AN. Left ventricular function in gestational hypertension: serial echocardiographic study. Am J Hypertens [Internet]. 2010;23:85–91 Available from: https://www.ncbi.nlm.nih.gov/pubmed/19763118. [DOI] [PubMed] [Google Scholar]
- 54.Rafik Hamad R, Larsson A, Pernow J, Bremme K, Eriksson MJ. Assessment of left ventricular structure and function in preeclampsia by echocardiography and cardiovascular biomarkers. J Hypertens [Internet]. 2009;27:2257–64 Available from: http://www.ncbi.nlm.nih.gov/pubmed/19809366. [DOI] [PubMed] [Google Scholar]
- 55.Stewart MH, Lavie CJ, Shah S, Englert J, Gilliland Y, Qamruddin S, et al. Prognostic implications of left ventricular hypertrophy. Prog Cardiovasc Dis [Internet]. 2018. [cited 2019 Sep 6];61:446–55. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30408469. [DOI] [PubMed] [Google Scholar]
- 56.Desai CS, Bartz TM, Gottdiener JS, Lloyd-Jones DM, Gardin JM. Usefulness of left ventricular mass and geometry for determining 10-year prediction of cardiovascular disease in adults aged >65 years (from the Cardiovascular Health Study). Am J Cardiol [Internet]. 2016. [cited 2019 Sep 6];118:684–90. Available fromhttp://www.ncbi.nlm.nih.gov/pubmed/27457431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Melchiorre K, Sutherland GR, Watt-Coote I, Liberati M, Thilaganathan B. Severe myocardial impairment and chamber dysfunction in preterm preeclampsia. Hypertens Pregnancy [Internet]. 2012;31:454–71 Available from: https://www.ncbi.nlm.nih.gov/pubmed/23030711. [DOI] [PubMed] [Google Scholar]
- 58.Vaught AJ, Kovell LC, Szymanski LM, Mayer SA, Seifert SM, Vaidya D, et al. Acute cardiac effects of severe pre-eclampsia. J Am Coll Cardiol [Internet]. 2018. [cited 2018 Oct 11];72:1–11. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29957219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.•.Bokslag A, Franssen C, Alma LJ, Kovacevic I, Kesteren FV, Teunissen PW, et al. Early-onset preeclampsia predisposes to preclinical diastolic left ventricular dysfunction in the fifth decade of life: an observational study. PLoS One [Internet]. 2018;13: e0198908 Available from: https://www.ncbi.nlm.nih.gov/pubmed/29894501 [DOI] [PMC free article] [PubMed] [Google Scholar]; A small observational study showing that a history of preeclampsia is associated with diastolic dysfunction measured by ehcocardiogram parameters in later life.
- 60.Ghossein-Doha C, van Neer J, Wissink B, Breetveld NM, de Windt LJ, van Dijk AP, et al. Pre-eclampsia: an important risk factor for asymptomatic heart failure. Ultrasound Obs Gynecol [Internet]. 2017;49:143–9 Available from: https://www.ncbi.nlm.nih.gov/pubmed/27804179. [DOI] [PubMed] [Google Scholar]
- 61.Breetveld NM, Ghossein-Doha C, van Neer J, Sengers M, Geerts L, van Kuijk SMJ, et al. Decreased endothelial function and increased subclinical heart failure in women several years after pre-eclampsia. Ultrasound Obs Gynecol [Internet]. 2018;52:196–204 Available from: https://www.ncbi.nlm.nih.gov/pubmed/28557250. [DOI] [PubMed] [Google Scholar]
- 62.Al-Nashi M, Eriksson MJ, Ostlund E, Bremme K, Kahan T. Cardiac structure and function, and ventricular-arterial interaction 11 years following a pregnancy with preeclampsia. J Am Soc Hypertens [Internet]. 2016;10:297–306 Available from: https://www.ncbi.nlm.nih.gov/pubmed/26852291. [DOI] [PubMed] [Google Scholar]
- 63.Mavrogeni S, Katsi V, Vartela V, Noutsias M, Markousis-Mavrogenis G, Kolovou G, et al. The emerging role of Cardiovascular Magnetic Resonance in the evaluation of hypertensive heart disease. BMC Cardiovasc Disord. 2017;17:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schmieder RE, Wagner F, Mayr M, Delles C, Ott C, Keicher C, et al. The effect of sacubitril/valsartan compared to olmesartan on cardiovascular remodelling in subjects with essential hypertension: the results of a randomized, double-blind, active-controlled study. Eur Hear J. 2017;38:3308–17. [DOI] [PubMed] [Google Scholar]
- 65.Mohan M, Al-Talabany S, McKinnie A, Mordi IR, Singh JSS, Gandy SJ, et al. A randomized controlled trial of metformin on left ventricular hypertrophy in patients with coronary artery disease without diabetes: the MET-REMODEL trial. Eur Hear J. 2019/04/18 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Drazner MH. The progression of hypertensive heart disease. Circulation [Internet]. 2011/01/26 2011;123:327–34. Available from: https://www.ncbi.nlm.nih.gov/pubmed/21263005. [DOI] [PubMed] [Google Scholar]
- 67.Ersboll AS, Bojer AS, Hauge MG, Johansen M, Damm P, Gustafsson F, et al. Long-term cardiac function after peripartum cardiomyopathy and Preeclampsia: a Danish Nationwide, clinical follow-up study using maximal exercise testing and cardiac magnetic resonance imaging. J Am Hear Assoc [Internet]. 2018/10/30 2018;7:e008991 Available from: https://www.ncbi.nlm.nih.gov/pubmed/30371259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Akhter T, Wikstrom AK, Larsson M, Naessen T. Individual common carotid artery wall layer dimensions, but not carotid intimamedia thickness, indicate increased cardiovascular risk in women with preeclampsia: an investigation using noninvasive highfrequency ultrasound. Circ Cardiovasc Imaging [Internet]. 2013/07/03 2013;6:762–8. Available from: https://www.ncbi.nlm.nih.gov/pubmed/23811751. [DOI] [PubMed] [Google Scholar]
- 69.Milic NM, Milin-Lazovic J, Weissgerber TL, Trajkovic G, White WM, Garovic VD. Preclinical atherosclerosis at the time of preeclamptic pregnancy and up to 10 years postpartum: systematic review and meta-analysis. Ultrasound Obstet Gynecol [Internet]. 2017. [cited 2019 Oct 10];49:110–5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27859887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cortés YI, Catov JM, Brooks M, El Khoudary SR, Thurston RC, Matthews KA, et al. Pregnancy-related events associated with subclinical cardiovascular disease burden in late midlife: SWAN. Atherosclerosis [Internet]. 2019. [cited 2019 Oct 10];289:27–35. Available from: http://www.ncbi.nlm.nih.gov/pubmed/31446211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McDonald SD, Ray J, Teo K, Jung H, Salehian O, Yusuf S, et al. Measures of cardiovascular risk and subclinical atherosclerosis in a cohort of women with a remote history of preeclampsia. Atherosclerosis [Internet]. 2013;229:234–9 Available from: https://www.ncbi.nlm.nih.gov/pubmed/23664201. [DOI] [PubMed] [Google Scholar]
- 72.White WM, Mielke MM, Araoz PA, Lahr BD, Bailey KR, Jayachandran M, et al. A history of preeclampsia is associated with a risk for coronary artery calcification 3 decades later. Am J Obs Gynecol [Internet]. 2016;214:519 e1–8 Available from: https://www.ncbi.nlm.nih.gov/pubmed/26874301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zoet GA, Benschop L, Boersma E, Budde RPJ, Fauser BCJM, van der Graaf Y, et al. Prevalence of subclinical coronary artery disease assessed by coronary computed tomography angiography in 45-to 55-year-old women with a history of preeclampsia. Circulation [Internet]. 2018. [cited 2019 Oct 10];137:877–9. Available from: https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.117.032695 [DOI] [PubMed] [Google Scholar]
- 74.Wichmann JL, Takx RAP, Nunez JH, Vliegenthart R, Otani K, Litwin SE, et al. Relationship between pregnancy complications and subsequent coronary artery disease assessed by coronary computed tomographic angiography in Black women. Circ Cardiovasc Imaging. 2019; [DOI] [PubMed] [Google Scholar]
- 75.Benschop L, Schalekamp-Timmermans S, Schelling SJC, Steegers EAP, Roeters van Lennep JE. Early pregnancy cardiovascular health and subclinical atherosclerosis. J Am Hear Assoc [Internet]. 2019;8:e011394 Available from: https://www.ncbi.nlm.nih.gov/pubmed/31331213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, et al. Heart Disease and Stroke Statistics-2018 Update: a report from the American Heart Association. Circulation [Internet]. 2018. [cited 2019 Sep 2];137:e67–492. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29386200. [DOI] [PubMed] [Google Scholar]
- 77.Management of diabetes in pregnancy. Diabetes Care. 2017;40: S114–9. [DOI] [PubMed] [Google Scholar]


