Abstract
Circadian rhythmicity is an approximately 24h cell-autonomous period driven by transcription–translation feedback loops of specific genes, which are referred to as ‘circadian clock genes’. In mammals, the central circadian pacemaker, which is located in the hypothalamic suprachiasmatic nucleus, controls peripheral circadian clocks. The circadian system regulates virtually all physiological processes, which are further modulated by changes in external environment, such as light exposure and timing of food intake. Chronic circadian disruption caused by shift work, travel across time zones or irregular sleep-wake cycles has long-term consequences on our health and is an important lifestyle factor that contributes to the risk of obesity, type 2 diabetes mellitus and cancer. Although the hypothalamic–pituitary–thyroid axis is under the control of the circadian clock via the suprachiasmatic nucleus pacemaker, daily TSH secretion profiles are disrupted in some patients with hypothyroidism and hyperthyroidism. Disruption of circadian rhythms has been recognized as a perturbation of the endocrine system and of cell cycle progression. Expression profiles of circadian clock genes are abnormal in well-differentiated thyroid cancer but not in the benign nodules or a healthy thyroid. Therefore, the characterization of the thyroid clock machinery might improve the preoperative diagnosis of thyroid cancer.
Introduction
Thyroid hormones (precursor thyroxine T4 and active T3) are iodine containing compounds (iodothyronines) that are important for metabolism, heat production, proper development and differentiation of cells, and growth. Thyroid hormone synthesis and secretion are primarily regulated by TSH, which is derived from thyrotrophs located in the pars distalis in the anterior pituitary gland. TSH production and secretion are regulated by hypothalamic thyrotropin-releasing hormone (TRH). Circulating thyroid hormones control the synthesis and release of TRH and TSH from pars distalis as part of a classic negative-feedback loop called the ‘hypothalamic–pituitary–thyroid (HPT) axis’1,2(FIG. 1).
Figure 1. Hypothalamic–pituitary–thyroid axis.
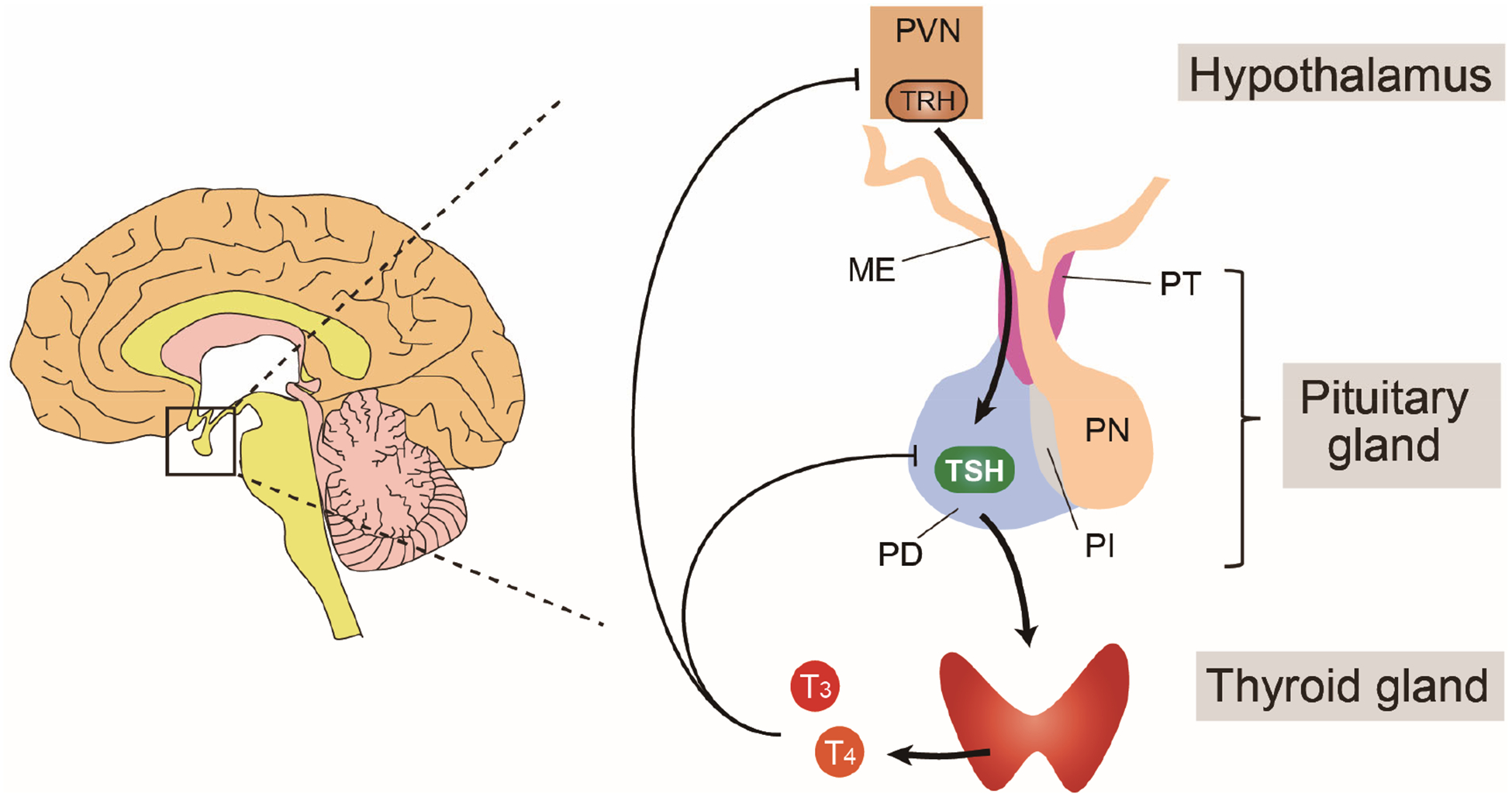
Thyrotropin-releasing hormone (TRH) produced in the paraventricular nucleus (PVN) is secreted from the median eminence (ME) and transported to the pituitary via the hypothalamus–hypophyseal portal system. TRH stimulates thyrotrophs to synthesize TSH in the pars distalis (PD) of the pituitary gland by upregulating mRNA levels of TSHA and TSHB. TSH stimulates the thyroid gland to produce thyroid hormones (predominantly the prohormone T4) via the TSH receptor on the thyroid follicle cell membrane. TSH induces most of the essential events in thyroid hormone production. T3 derived from circulating T4 regulates synthesis and release of TRH and pars distalis TSH as part of a negative feedback loop mediated by the thyroid hormone receptor. This regulatory pathway is called the ‘hypothalamic–pituitary–thyroid axis’1,2. PT, pars tuberalis; PN, pars intermediate; PI, pars nervosa.
Organisms are exposed to various rhythmic events, such as daily and seasonal cycles. To better adapt to these cyclic environmental changes, organisms have evolved biological clocks. An approximately 24-h cell-autonomous circadian clock is present in virtually all cells of the body, and this circadian system tightly regulates physiological functions and endocrine rhythms3.
Several lines of evidence indicate that chronic circadian disruption, which can be caused by shift work or travel across time zones, has long-term consequences on human health and increases the risk of weight gain, type 2 diabetes mellitus, cardiovascular disease and several types of cancer4–7. Circadian disruption of the endocrine system is one of the main mechanisms that mediates these circadian-related adverse consequences6–8. Many endocrine factors are known to show time-dependent variations; for example, TSH secretion exhibits a clear daily rhythmicity, and the HPT axis is under circadian control via the central circadian pacemaker in the suprachiasmatic nuclei (SCN) of the anterior hypothalamus9.
In this article, we review the interconnection between circadian clocks and thyroid function. We first describe the regulatory mechanisms controlling the HPT axis and provide an overview of the circadian system. We then summarize the current state of knowledge regarding the circadian regulation of the HPT axis. Furthermore, we discuss the connection between disruptions of central and peripheral circadian pacemakers and thyroid function. In addition, we highlight the possible link between thyroid cancer and disrupted circadian machinery.
HPT axis and thyroid hormone activation
In this section, we provide a brief overview of the regulatory mechanism of the HPT axis and highlight the importance of local activation of thyroid hormone. TSH, which is a pituitary-derived hormone that stimulates the thyroid gland to produce T4 and T3, is a non-covalently linked heterodimer glycoprotein that consists of α and β subunits. The release of TSH is controlled by the tripeptide hormone TRH (FIG. 1). Derived from a subset of neurons in the paraventricular nucleus of the hypothalamus, TRH is secreted from the hypothalamic median eminence to the anterior pituitary via the hypothalamic–hypophyseal portal system.1,2,10.
TRH binds to its membrane receptor on thyrotrophs in the pars distalis of the anterior pituitary and stimulates the synthesis of TSH by inducing mRNA expression of TSHA and TSHB, which encode the TSH α and β subunits, respectively11. The most sensitive marker of thyroid hormone action are blood levels of TSH, which are elevated in patients with hypothyroidism owing to reduced negative feedback suppression of TRH and TSH. The thyroid gland machinery is stimulated to produce thyroid hormones by TSH binding to the TSH receptor (a G protein-coupled receptor on the thyroid follicle cell membrane), which in turn stimulates the secondary messengers c AMP and inositol phosphate12.
In vertebrates, thyroid hormone is the only hormone that contains iodine. Iodine is taken up from the bloodstream into thyroid follicles. Thyroglobulin, which is a protein that is produced by thyroid epithelial cells, is secreted and stored in the follicular lumen. Thyroglobulin acts as a backbone for tyrosine residues that are subsequently iodinated. After internalization from follicular lumen into follicular cells, iodinated thyroglobulin is degraded through the lysosomal pathway, to release mainly T4 and some T3 into the bloodstream. These two iodothyronines circulate, bound reversibly to thyroid hormone-binding proteins: thyroxine-binding globulin, transthyretin, and albumin2. Unbound, also known as ‘free’, thyroid hormone is transported into target tissues by membrane transporters. The most important membrane transporter for the brain is the monocarboxylate transporter 8 (MCT8)13 and the most important transporters in other tissue are MCT8 and the organic anion transporter polypeptide OATP1C114.
As the mammalian thyroid gland predominantly produces the hormone precursor, T4, local activation of thyroid hormone at target tissues is an important mechanism of thyroid hormone action2,15. Following entry into the cell, thyroid hormone is metabolized by deiodinase enzymes. Type 1 iodothyronine deiodinase (DIO1) and type 2 iodothyronine deiodinase (DIO2) function principally as thyroid hormone activators, generating T3 from T4 by removing one iodine from the 5’ position of T415. They have variable levels of expression in tissues and cell types and in different species; DIO1 is commonly found in liver and kidney, and type 3 iodothyronine deiodinase (DIO3) is normally expressed in the central nervous system and placenta, while DIO2 is more widely expressed in humans. However, unlike humans, rodents express little or no DIO2 in their myocardium16.
DIO3 inactivates thyroid hormone by removing iodine from the 5 position of T4 and T3, which generates the inactive iodothyronines reverse T3 and T2, respectively16. Locally generated T3 is partially released from tissues to the circulation16. As thyroid hormones act on nuclear hormone receptors to regulate target gene expression, released T 3 and T4 act as part of a feedback loop that inhibits the production of hypothalamic-derived TRH and pars distalis-derived TSH (FIG. 1).
The thyroid hormone receptor has isoforms, TRα and TRβ. When T3-bound, these two receptors repress TRH and TSHB gene expression by binding to some thyroid hormone response elements (sequences containing [A/G]GGT[G/C/A]A) on DNA as either a thyroid hormone receptor monomer, thyroid hormone receptor homodimer, and/or thyroid hormone receptor –retinoid X receptor heterodimer in the TRH promoter17 or the TSHB promoter18,19. In the presence or absence of thyroid hormone, the thyroid hormone receptor complexes regulate positively or negatively target gene expression by interacting with co-activators or co-repressors, respectively2.
Circadian clocks and the master pacemaker
Circadian clocks are highly conserved, endogenous time-keeping mechanisms present in virtually all living organisms. These clocks generate self-sustained oscillations with an approximately 24-h period, referred to as ‘circadian rhythms’3. Circadian rhythms modulate multiple physiological and behavioral processes, including sleep–wake cycles, hormone release and metabolism3.
In mammals, these daily rhythms are primarily controlled by a population of neurons and astrocytes in the SCN, a paired structure located in the anterior hypothalamus above the optic chiasm20,21. The SCN, which is considered the master pacemaker, receives direct light information from the retina through the retinohypothalamic tract (FIG. 2). Within the retina, intrinsically photosensitive ganglion cells are primarily responsible for ‘circadian photoreception’ and the entrainment of the circadian pacemaker to environmental light–dark cycles22,23, while classical rod and cone photoreceptors have only a supportive role (of note, more than 50% of people who are totally blind have normal entrainment of the circadian pacemaker because their intrinsically photosensitive retinal ganglion cells are intact). Most peripheral tissues and cells also contain self-sustained circadian oscillators that, under normal conditions, are synchronized with the SCN clock via neural and humoral pathways (that is, the autonomous nervous system and the 24-h rhythms of glucocorticoid and melatonin levels)3,24,25.
Figure 2. The hypothalamic–pituitary–thyroid axis is under circadian regulation.
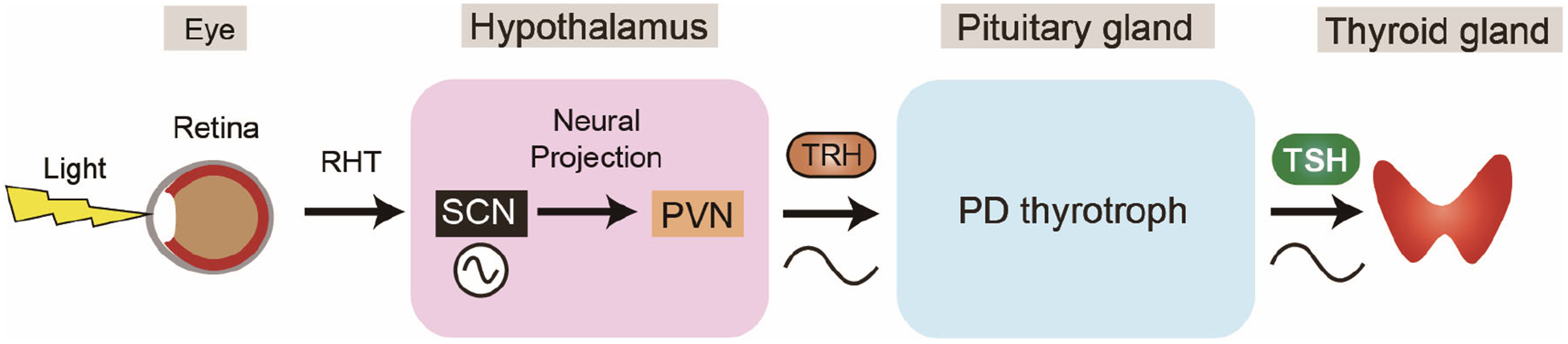
The suprachiasmatic nuclei (SCN) receives light information from the retina through the retino-hypothalamic tract (RHT) and outputs the circadian signal to hypothalamic paraventricular nucleus (PVN) via neural connections20,21. In humans, thyrotropin-releasing hormone (TRH) and TSH secretions exhibit circadian rhythms34,35.
Molecular mechanisms of the clockwork
The 2017 Nobel Prize for Physiology or Medicine was awarded for the discovery of the molecular mechanism driving circadian rhythms26. Briefly, circadian rhythms are generated by transcription–translation feedback loops consisting of circadian clock genes and proteins27 (FIG. 3). The basic helix-loop-helix proteins circadian locomotor output cycles kaput (CLOCK) and brain and muscle Arnt-like protein 1 (BMAL1) heterodimerize to form a transcriptional activator complex and activate the period (PER) and cryptochrome (CRY) repressor genes through E-box (CACGTG) enhancers, whose protein products in turn repress their own transcription28,29. The CLOCK–BMAL1 heterodimer also induces reverse strand of ERBα (REV-ERBα) and REV-ERBβ, and retinoic acid receptor-related orphan receptor-α (RORα), RORβ and RORγ, which regulate CLOCK and BMAL1 genes through ROR elements but exert opposite effects on gene transcription, thus constituting a second important interlocking feedback loop30–32 (FIG. 3).
Figure 3. The circadian transcriptional and translational feedback loop machinery in mammals.
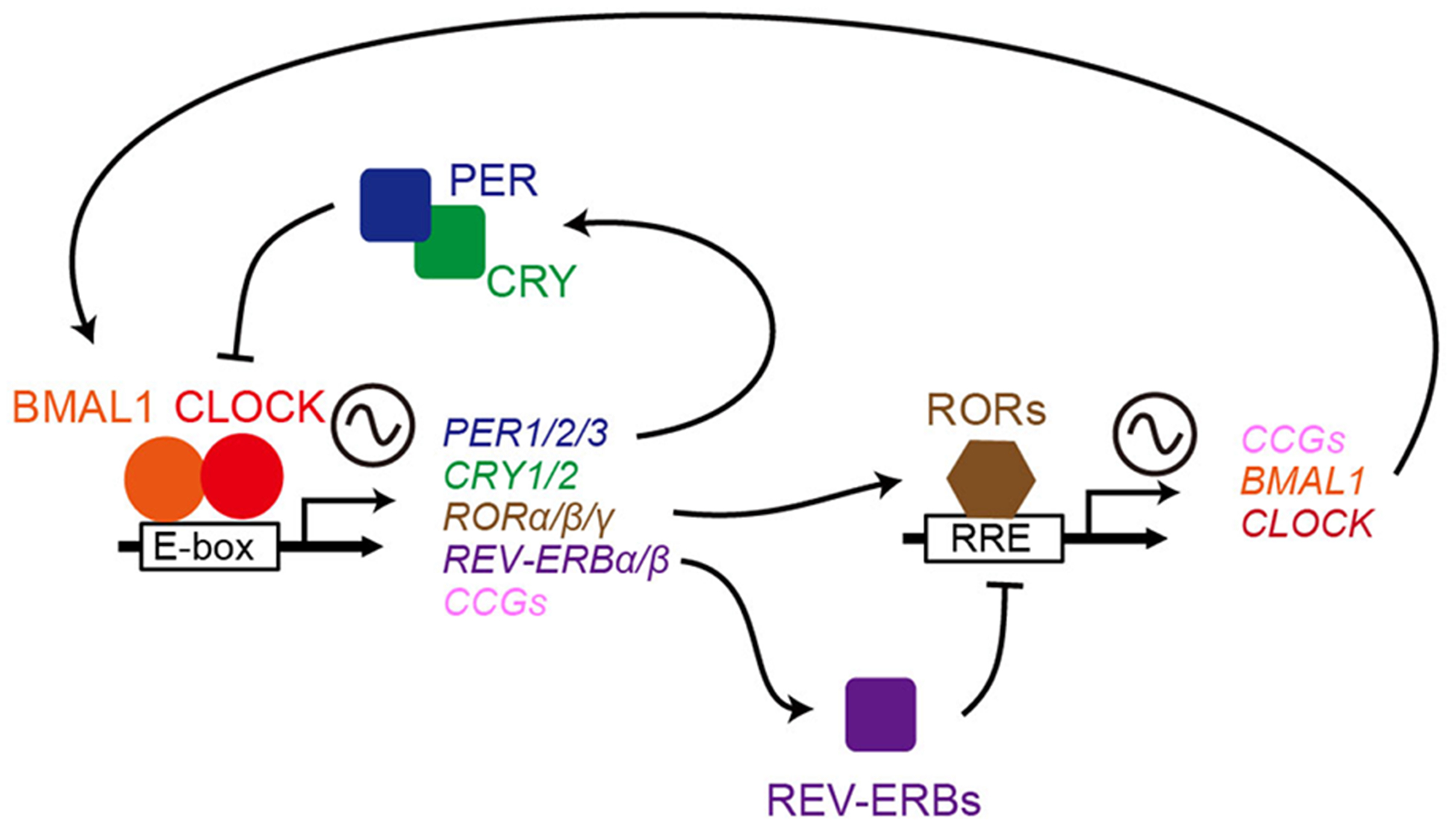
In the core loop, the CLOCK–BMAL1 complex binds E-boxes in promoters of target genes (PER1, PER2 and PER3, and CRY1 and CRY2, and clock-controlled genes [CCGs]) and activates their transcription. PERs and CRYs form complexes to inhibit CLOCK–BMAL1-mediated transcription. A second loop involves additional pairs of transcription factors such as Reverse strand of Erbα (REV-ERBα) and REV-ERBβ and retinoic acid receptor-related orphan receptor-α (RORα), RORβ and RORγ. CLOCK–BMAL1 also induces REV-ERB proteins and RORs, which regulate CLOCK and BMAL1 through ROR-elements (RREs) but exert opposite effects on transcription27.
The transcription–translation feedback loops of circadian clock genes drive rhythmic expression of many clock-controlled genes. The latter represent between 3% and 16% of the transcriptome in a given tissue33. As clock-controlled genes include genes that modulate transcription, signal transduction, protein turnover and metabolism, the circadian clocks influence various cellular, organ and physiological functions in a manner that depends on the time of day.
Circadian regulation of the HPT axis
Evidence of TSH rhythms.
In this section we describe how the internal circadian clock regulates the mammalian HPT axis and thyroid function. As is the case for other pituitary hormones, TSH secretion is partly controlled by the central circadian pacemaker in the SCN34,35 (FIG. 2). In humans, circulating TSH levels exhibit a clear daily rhythm. Plasma concentrations begin to rise in the late afternoon or early evening before sleep onset, and reach maximal levels during the early part of the night. Following the nighttime peak in TSH, plasma TSH concentration then declines during the rest of the sleep period until reaching low daytime levels.
Multiple studies that examined sleep deprivation and acute shifts of the sleep period have reported that TSH release into the blood is inhibited during sleep36,37. The interaction between the circadian-controlled nocturnal rise in TSH and the inhibitory effect of sleep is illustrated in the lower panel in FIG. 4, which shows the mean profile of plasma TSH levels in healthy young men who were sampled at 20-min intervals over a 53-h period, including an 8-h period of ‘normal’ average night sleep, a night of total sleep deprivation and an 8-h period of daytime recovery sleep36. TSH release, which is normally inhibited during sleep, continues to occur during nocturnal sleep deprivation. Therefore, morning plasma TSH levels are roughly twice as high in humans who have had a sleepless night than in humans who have had a night of normal sleep36.
Figure 4. Temporal changes of plasma TSH in human.
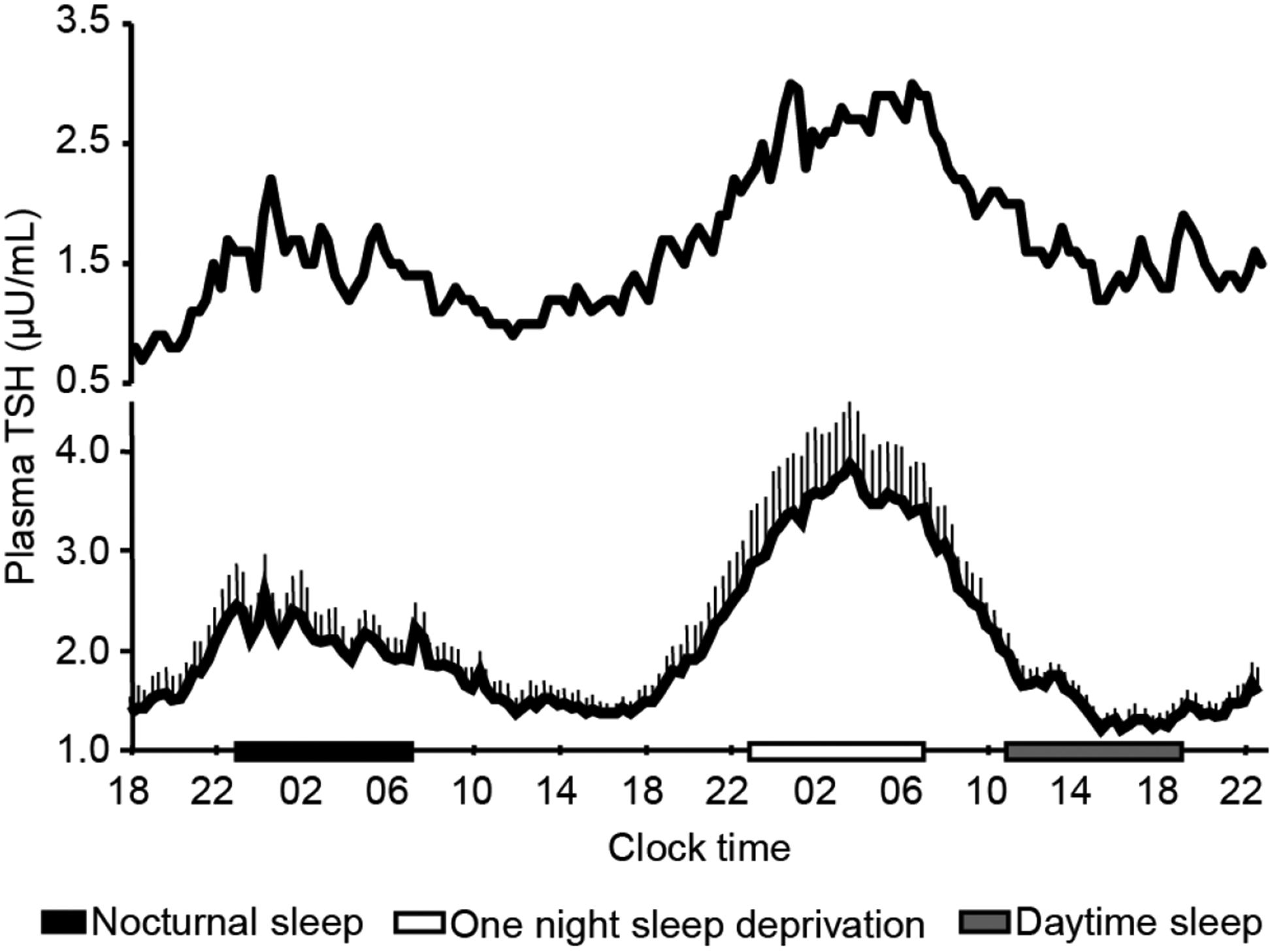
Upper panel shows an individual profile of plasma TSH sampled at 20-min intervals for 53 h in a healthy young male volunteer. The sampling period included one night of nocturnal sleep (black bar), one night of total sleep deprivation (open bar) and one 8-h period of daytime recovery sleep (grey bar). Note that sleep deprivation was associated with a near twofold rise in nocturnal TSH levels, uncovering an inhibitory effect of sleep under normal condition. Pulsatile TSH variations are apparent throughout the sampling period. Lower panel shows mean (plus standard error of the mean) levels of plasma TSH from 11 healthy young men. The symbols are the same as in upper panel. Note that daytime recovery sleep did not suppress TSH below the daytime levels. Modified from previous data36.
The upper panel in FIG. 4 illustrates a representative individual plasma TSH profile. It shows that a low amplitude ultradian rhythm of TSH is also present in humans, which reflects the pulsatile release of the hormone34,38 (Table 1). This ultradian rhythm seems to be influenced by the sleep stage such as the slow-wave sleep38 and food intake39. Thyroid hormone and glucocorticoid have also been to modulate the pulsatile secretion of TSH in healthy men40.
Table 1.
Effect of circadian clock on thyroid function.
| Circadian status | Intervention | species | Effect on thyroid function | references |
|---|---|---|---|---|
| Intact | None | Human | Rhythmic TSH secretion | 34,35,38 |
| Pacemaker ablation | SCN lesion | Rat | Rhythmic TSH secretion abolished and rhythmic Trα expression abolished | 53,54 |
| Circadian disruption | Recurrent sleep restriction | Human | Decreased TSH rise at night | 37 |
| Clock molecules | Increased expression of DEC1 | Human | Promoting thyroid cancer aggressiveness | 107 |
| Clock molecules | Rev-erbα | Mouse | Circadian regulation of Tshb expression | 56 |
Mechanisms.
The mechanisms generating ultradian TSH rhythms are still unclear41,42. Several studies have reported no intergender differences in circadian and ultradian TSH release patterns43,44; however, a paper from 2015 based on 324,750 outpatients with no known thyroid disease found that females have higher plasma TSH levels than males45.
In addition to the well-characterized rhythm in plasma levels of TSH, daily variations in plasma thyroid hormone levels have also been reported in the humans and in wild-type rats46,47. Some studies, however, have not detected these variations48,49. This discrepancy is thought to result from differences in experimental conditions and analytical tools between research groups50. It should also be noted that nocturnal rodents might not be an ideal model to explore the mechanisms underlying the human temporal control of TSH and thyroid hormone release. The reason that laboratory rodents are not an ideal model is because they undergo multiphasic sleep during the light phase whereas humans have a consolidated nocturnal sleep period.
Neural projections from the SCN to TRH neurons in the paraventricular nucleus form the anatomical basis for the daily rhythms in TRH synthesis and secretion, and thus are responsible for rhythmic TSH secretion from the pars distalis9,51–53 (FIG. 2). Indeed, in rats, an SCN lesion abolishes the rhythmicity of circulating TSH and thyroid hormones, which suggests that the SCN is involved in the regulation of the HPT axis53,54 (Table 1); however, peripheral clocks can also regulate rhythmic transcription of genes that regulate hormone secretion, for example, human growth hormone (GH)55. Indeed, in the mouse TαT1.1 thyrotroph cell line, the circadian rhythmicity of Tshb expression is regulated by the local circadian clock (REV-ERBα) along with the Tr complex co-activator nuclear receptor corepressor 1 (NCOR1)56 (Table 1). Of note, however, hypophysectomy extinguishes the rhythmicity of circulating thyroid hormone levels, but not the rhythmicity of circadian clock genes (Per1 and Bmal1) in rat thyroid57. These data suggest that the daily rhythm of thyroid hormone release from the thyroid gland is regulated by the central circadian pacemaker in the SCN via rhythmic TSH secretion, rather than by the local circadian clock in the thyroid gland.
Some studies have demonstrated that genes encoding thyroid hormone receptors exhibit rhythmic expression in mouse white adipose tissue (Thra and Thrb), brown adipose tissue (Thra), kidney (Thra), and liver (Thra)58–60. Furthermore, real-time qPCR has shown rhythmic expression of thyroid hormone receptors in rat liver (Thra and Thrb)54. A lesion study in rats showed that the rhythmic expression of liver TRα is regulated by the SCN, whereas that of TRβ is affected by food intake54. Interestingly, in humans, mice and rats, the Thra overlaps with Rev-erba, which is on the opposite DNA strand61. This finding lead to the speculation that circadian Thra expression is mediated by an antisense mechanism61; however, strand-specific RNA-sequencing revealed that Thra does not show a clear oscillation in mouse liver62. Arrhythmic expression of both Thr genes has also been reported in mouse skeletal muscle58. These discrepancies could be due to tissue-specific expression patterns or the analytical methods used.
Circadian rhythms in thyroid diseases
In the previous section, we described the link between circadian clocks and thyroid function. Here we consider the relationship between circadian rhythms and thyroid diseases, specifically hypothyroidism and hyperthyroidism.
Hypothyroidism.
A reduced production of thyroid hormones is the central feature of hypothyroidism. Loss of the thyroid gland function through a destructive processes including autoimmunity (Hashimoto disease), irradiation, or surgical ablation, and congenital defects in hormone synthesis or gland development are termed ‘primary hypothyroidism’. Central hypothyroidism is caused by dysfunction of the pituitary (secondary hypothyroidism) or the hypothalamus (tertiary hypothyroidism). Defects or damage to brain regions results in insufficient stimulation of the normal thyroid gland. Approximately 99.9% of cases of hypothyroidism are caused by primary hypothyroidism, but it depends on age63,64.
The term ‘subclinical hypothyroidism’ is applied to primary hypothyroidism when TSH is high but free T4 is still in the normal range. In this form of mild hypothyroidism, total TSH secretion is increased above the upper limit of normal while in severe hypothyroidism the increase could be as high as ~200-fold65. The daily TSH secretion pattern is sustained in mild hypothyroidism, but markedly high serum TSH levels in severe cases obliterate the detection of rhythmicity66,67. A study from 2016 revealed higher serum TSH levels and an elevated risk of subclinical hypothyroidism in night shift workers than non-night shift workers68 (FIG. 5a). However, because the authors of this study measured serum TSH levels at only one time point during the day, careful evaluation is necessary as this alteration could be caused by a phase shift of the TSH rhythm. Autoimmune thyroid disorders are also associated with shift work69.
Figure 5. Circadian disruption can drive thyroid diseases.
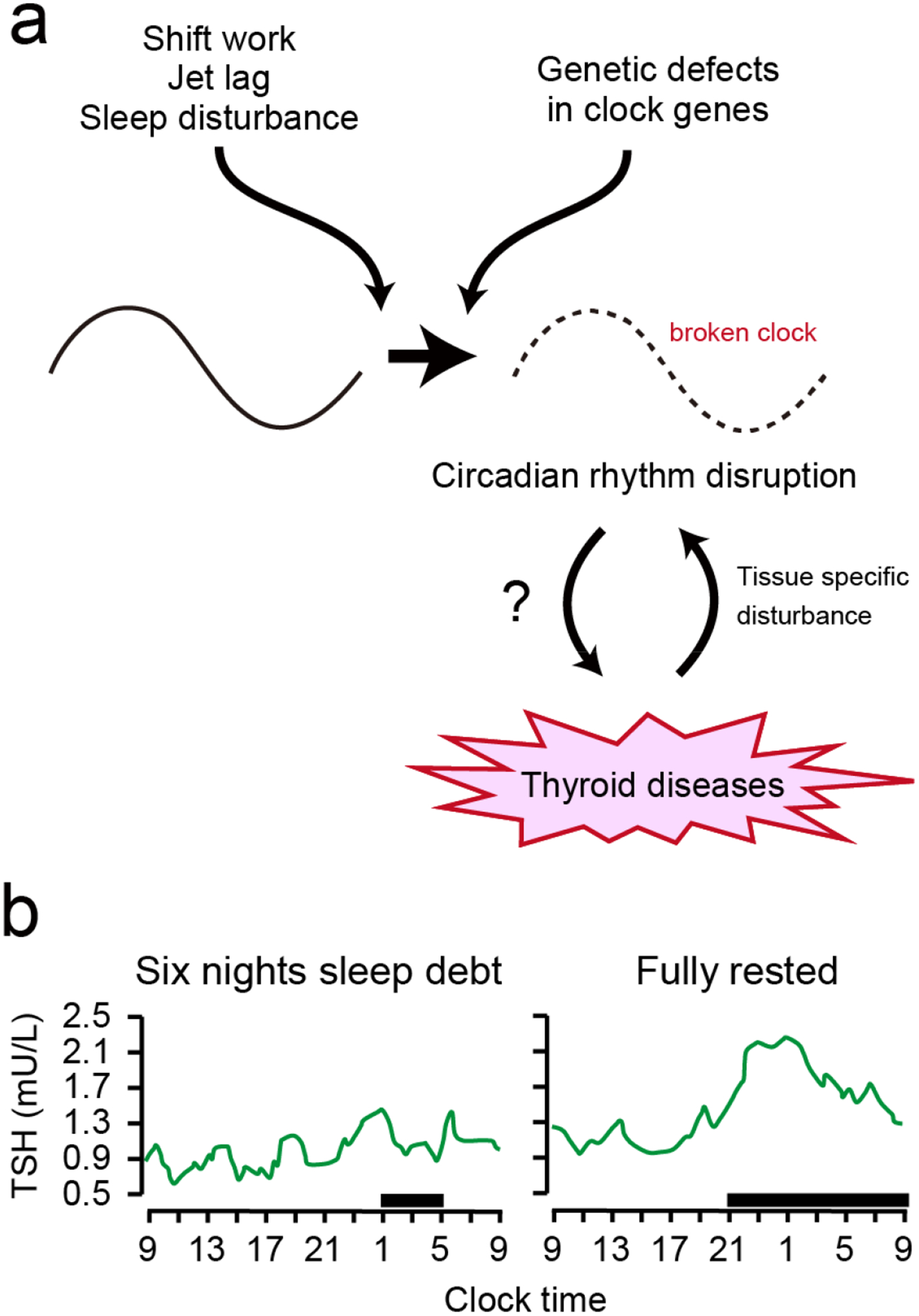
(a) Endogenous factors, such as genetic defects, and exogenous factors such as chronic shift work, jet lag and sleep disturbance, disrupt the endogenous circadian timing system. A faulty circadian clock is thought to increase the risk of thyroid diseases7,8. Thyroid diseases also perturb the circadian system in a tissue-specific manner68. (b) Effects of a sleep debt achieved by six nights of bedtime restriction to 4 h per night and a fully rested condition on TSH concentration (modified from Ref37). Shaded areas show bedtime periods.
In central hypothyroidism caused by inactivating mutations in TRH and TRH receptor, serum TSH concentration is within subnormal or mildly increased levels70. In such cases, however, circulating T4 levels are diminished. Cranial irradiation therapy for brain tumors and leukemia also cause abnormal TRH secretion patterns, leading to disrupted or absent TSH rhythm71. Glycosylation influences the bioactivity and half-life of glycoprotein hormones72, and the pars distalis TSH glycans are mainly biantennary and sulfated complexes73. Diminished T4 levels accompanied by elevated or normal TSH levels in central hypothyroidism are caused by a reduction in the bioactivity of TSH due to increased sialylation of glycan74. As TRH regulates sulfation and sialylation of TSH glycan75, TRH deficiency also induces abnormal glycosylation of TSH, resulting in loss of the normal circadian pattern of TSH secretion71,76.
Defects in thyroid hormone receptors cause reduced sensitivity to the hormone77. In patients with a mutation in THRB, elevated serum T4 and T3 and slightly elevated or normal TSH are observed due to a reduced negative feedback of the HPT axis77. Enlargement of the thyroid gland and increased synthesis of thyroid hormone result from an increase in TSH bioactivity78. In most patients, basal serum TSH concentration is normal and the circadian rhythm is preserved79.
Decreased sensitivity to thyroid hormone caused by THRA mutations manifests in affected tissues expressing mainly the THRA gene80. The clinical features of such patients are dysmorphic features, skeletal dysplasia, growth retardation and intellectual deficit80. The associated biochemical abnormalities include low or low to normal T4 concentrations and high or high to normal T3 concentrations80.
Hyperthyroidism.
In marked contrast to hypothyroidism, hyperthyroidism is characterized by excessive quantities of thyroid hormones in the presence of normal thyroid hormone receptor expression50. Common conditions causing hyperthyroidism include overstimulation of the thyroid gland by immunoglobulins that bind to the TSH receptor, activating mutations of the TSH receptor and (although rarely) TSH producing pituitary tumors50. Circadian rhythmicity of serum TSH levels in hyperthyroidism is not expected as TSH is suppressed and diurnal rhythm of thyroid hormone does not occur because hormone synthesis and secretion is under the control of immunoglobulins81. In rare instances of hyperthyroidism that result from TSH producing pituitary adenomas, the TSH secretion pattern is more irregular, but the diurnal rhythm is preserved82.
Thyroid hormone and gene expression
Next, we describe how thyroid hormone deficiency and excess affect the circadian clock. When wheel running activity rhythms were studied under constant condition, surgical removal of the thyroid and parathyroid glands shortens the period of circadian activity rhythms in rats83,84, and thiourea and thyroxine administration lengthens the period of circadian activity rhythms in hamsters and rats84,85(Table 2). However, as knocking out Thrb does not directly alter the circadian behaviour of mice86, effects of thyroid hormones on circadian behaviour might be mediated by Trα. It is interesting to note that Trα is predominantly expressed in the heart87.
Table 2.
Effect of thyroid status on the circadian system.
| Thyroid status | Symptoms or treatment | species | Effect on the circadian system | references |
|---|---|---|---|---|
| Hypothyroidism | Thyroidectomy | Rat | Shortened free-running period of locomotor activity rhythm, decreased amplitude of clock genes (Bmal1, Per2, Nr1d1 and Rora) in heart, and altered Per2 expression (in the bed nucleus and amygdala, not in the SCN) | 83,84,88,89 |
| Hyperthyroidism | T4 administration | Rat | Lengthened free-running period of locomotor activity rhythm | 84 |
| Hyperthyroidism | Thiourea administration | Hamster | Lengthened free-running period of locomotor activity rhythm | 85 |
| Hyperthyroidism | T3 administration | Mouse | Altered clock gene (Bmal1, Nr1d1 and Rora) expressions in heart | 88 |
| Thyroid cancer | Cancer | Human | Abnormal clock gene expressions | 113 |
In rats, the expression patterns of circadian clock genes (Per2, Bmal1, Rev-erba, and Rora) and clock-controlled genes (Pdk4 and Ucp3) in the heart are affected by hyperthyroidism and hypothyroidism, which suggests that chronic alterations in thyroid status affect the circadian clock and metabolic function in this organ88(Table 2). In the rat brain, thyroidectomy affects the expression of PER2 in the bed nucleus of the stria terminalis, as well as the amygdala, while it does not affect the SCN clock89(Table 2). Moreover, co-culture with thyroid gland does not modulate the circadian rhythms of Rat-1 fibroblasts90. These data indicate that abnormal serum levels of thyroid hormone affects the circadian clocks in peripheral tissues, which might be dependent on tissue-specific thyroid hormone receptor regulation.
The circadian clock and tumorigenesis
In this section, we describe the molecular link between circadian clocks and cancer. Disruption of the circadian clocks due to shift work or travel across time zones leads to circadian desynchrony, or jet lag, which reflects a mismatch between the internal biological clock and external time cues91 (BOX 1; FIG. 5a).
Box 1. Central clock versus peripheral clocks.
The mammalian circadian system is comprised of the central master pacemaker located in the hypothalamic suprachiasmatic nucleus (SCN) and a multitude of peripheral circadian clocks that use the same molecular machinery as the SCN clock and receive synchronizing inputs from the central clock. When disconnected from the central clock, the peripheral clocks are capable of generating self-sustained circadian oscillations under constant environmental conditions for at least a few cycles25. Circadian misalignment occurs when the central clock is not aligned with the peripheral clocks and/or when distinct peripheral clocks are not in synchrony with each other91. Circadian misalignment can happen when a stimulus that affects a peripheral tissue (such as the feeding schedule for the liver) does not affect another peripheral tissue (for example, those that are not sensitive or less sensitive to dietary cues)3.
Chronic circadian disruption has long-term consequences on our health and increases the risk of cancers in the immune, skeletal, digestive and reproductive organs that require cell proliferation, metabolism, and DNA damage repair to maintain daily function7. In fact, variants of various clock genes (PER1, PER2 and PER3, and CRY1 and CRY2, in addition to REV-ERB genes and ROR genes) are associated with thyroid cancer in humans7. Circadian disruption also affects endocrine functions, including glucocorticoid, melatonin, and pituitary hormone)8. Specifically, in the HPT axis, disruption of plasma levels of TSH and T3 have been observed in shift work, jet lag and chronic sleep debt in humans37,68,92,93 (Table 1; FIG. 5b). The normal evening rise of plasma levels of TSH is decreased or absent in human in a sleep debt condition achieved by bedtime restriction for 4 h for six nights compared with a fully rested condition (allowed 12 h in bed per night for six nights)37 (Table 1; FIG. 5b).
Because chronic circadian disruption has long-term consequences on our health7, it is necessary to evaluate the effects of long-term circadian and sleep disruption on the HPT axis. Some studies suggest that elevated concentrations of serum TSH is linked to the incidence of human thyroid cancer94–96; however, it is not clear if this is causative or the consequence of the cancer. Furthermore, two 2018 cohort studies that investigated circadian disruption in flight attendants and flight crews did not provide convincing evidence of an association with elevated risk of thyroid cancers97,98. Therefore, further studies are indeed required to clarify the relationship between circadian disruption and thyroid cancer further studies are indeed required to clarify the relationship between circadian disruption and thyroid cancer.
Loss of cell cycle regulation also leads to the development of cancer99–101. It remains unclear whether, and if so to what extent, circadian clocks are involved in this process; however, synchronization of circadian clock function in tumor cells impinges on the cell cycle and suppresses cellular growth99. Emerging evidence suggests molecular connections between the circadian clock and cell cycle regulators, such as Wee1 (which inhibits the G2/M transition by phosphorylation of CDK1), and Cyclin B1 (Ccnb1) in melanoma cells. For example, Clock/Bmal1 activates Wee1 in mouse liver, and PER influences the transcription of WEE1 and CCNB1 in human cancer cell100,101.
PER1 and PER2 are necessary for transcriptional activation of cell cycle checkpoint genes in several mouse tissues102. Bmal1 positively regulates the p53 tumor suppressor pathway and has anti-tumor activity in human pancreatic cancer103. Genetic manipulations in mice have demonstrated the direct relationship between tumor and clock genes through activation of cell cycle in spontaneous and radiation-induced tumor development in liver, ovary, kidney104, lymphoid tissue105 and lung106. In the case of thyroid cancer, increased expression of the circadian clock component DEC1 (differentially expressed in chondrocytes 1), which targets the E-box, is reported to drive the expression of many cell cycle-related genes and promotes thyroid cancer in humans107(Table 1).
Circadian clocks in thyroid nodules and thyroid cancer.
In this section, we explore the potential link between circadian clocks and the development of thyroid cancer. Although the aforementioned studies suggest that there could be a causal link from disruption of the circadian system to cancer, other studies have established a link in the opposite direction108,109. Multiple aspects of tumours, including the RAS and MYC oncogenes, can induce dysregulation of circadian clocks in human cancer cell lines108,109. While it remains unclear whether the circadian clock is actually disrupted within human tumors in vivo, it is important to characterize the profiles of clock disruption in human cancers in order to develop novel anti-tumor strategies.
Thyroid nodules, defined as any discrete mass in the thyroid gland, can often represent an abnormal growth of thyroid cells in discrete regions within the thyroid gland, are often detected in humans, but most are non-cancerous (benign)110. Well-differentiated thyroid cancer constitutes the vast majority (more than 90%) of all thyroid cancers111,112. Poorly differentiated thyroid carcinoma is less common than well-differentiated thyroid cancer but is more aggressive111,112. Robust circadian oscillation of clock genes has been observed in primary cultured thyrocytes established from healthy human thyroid tissue and benign nodules113; however, the up-regulation of BMAL1 and down-regulation of CRY2 have been detected in tissue samples from well-differentiated thyroid cancer, and dramatic changes in circadian clock genes expression has been reported in poorly differentiated thyroid carcinoma113. Therefore, it is hypothesized that the circadian clock machinery in the thyroid could be altered during malignant transformation113 (Table 2). Thyroid nodules are detected in up to 65% of the general populations and are usually benign110. Mutational analysis and gene expression analyses are two common molecular tests for malignancy. Mutational analysis involves sequencing specific proto-oncogenes, such as BRAF and RAS, for possible mutations. In contrast, gene expression analysis examines specific gene sets, called ‘gene expression classifiers’. However, because of the low positive predictive value, refinement in molecular testing strategies with improved diagnostic performance is required110. As alterations in the clock machinery are observed in malignant transformation of thyroid nodules, it is hypothesized that characterization of circadian clock genes expression might help improve pre-operative diagnosis of thyroid nodules. This hypothesis should be addressed in future studies.
Seasonal rhythms and thyroid function
In addition to circadian rhythms, thyroid function is intimately linked to seasonal rhythms. In this section we discuss the link between seasonal rhythms and thyroid function. Most animals exhibit seasonal changes in physiology and behaviour, such as reproduction, migration and hibernation, in order to adapt to seasonal changes in environment114. In rats and hamsters, the circadian rhythms of levels of TSH and thyroid hormones in the blood exhibit seasonal changes115,116.
Seasonality has also been reported in humans, including alterations in mood, immune function, duration and amplitude of melatonin secretion, endocrine function and gene expression117,118. Some studies in humans reported that levels of thyroid hormone in the circulation decrease during the summer119,120. Although several studies have demonstrated that humans exhibit seasonality in circulating levels of TSH119,121, other cohort studies have argued that TSH has little seasonality45,122. In thyrotoxicosis, the peak incidence of diagnosis of hyperthyroidism probably occurs during the summer because the symptoms of heat intolerance in patients with hyperthyroidism are more noticeable during the summer period123. Thyroid cancers exhibit an annual rhythm, with markedly more patients presenting with the disease during the late autumn and winter124. The underlying mechanisms of seasonal regulation of these thyroid diseases remains to be elucidated.
Seasonal reproduction of animals ensures the survival of offspring. A 2008 functional genomic analysis of seasonal breeding animals revealed unexpected roles of TSH and thyroid hormone in the regulation of seasonality125. Long-day stimulus induces TSH production in the pars tuberalis of the pituitary gland in birds125. TSH derived from the pars tuberalis acts on the hypothalamus to induce DIO2 and downregulate DIO3125 (FIG. 6). The switch from DIO2 to DIO3 fine-tunes local bioactive thyroid hormone concentrations within the hypothalamus, which in turn regulates seasonal reproduction in birds and mammals126–129. Thyrotrophs in the pars tuberalis lack TRH receptors and thyroid hormone receptors, which makes pars tuberalis-derived TSH independent from the HPT axis in mammals73,130.
Figure 6. Pars tuberalis-derived TSH regulates seasonal thyroid hormone function.
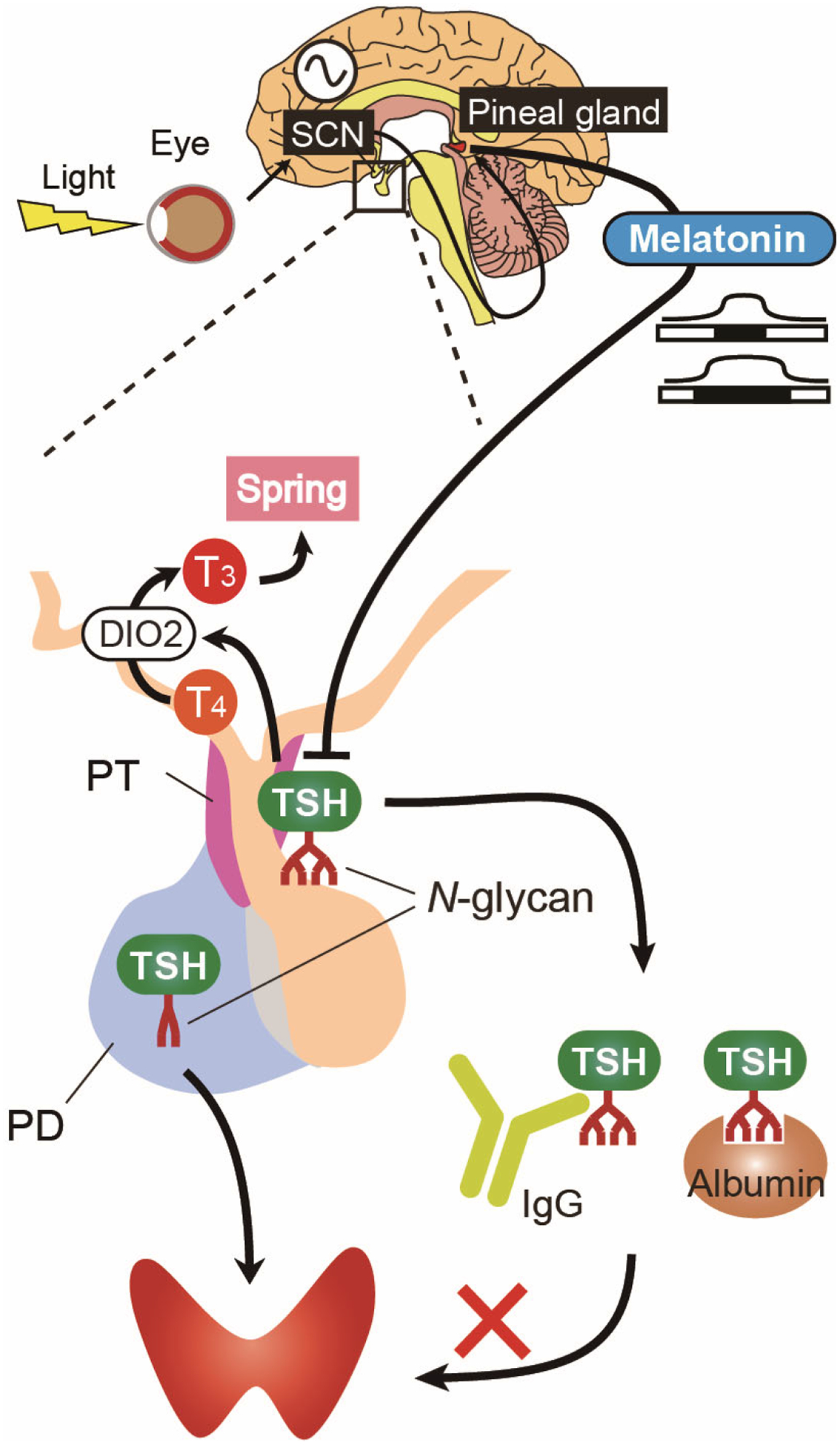
Light information received by the eyes is transmitted to the pineal gland via the suprachiasmatic nuclei (SCN). Pineal melatonin secretion pattern reflects the length of nights and suppresses pars tuberalis-derived TSH expression. Long days increase production of pars tuberalis-derived TSH, which acts on ependymal cells in the hypothalamus to induce type 2 iodothyronine deiodinase (DIO2) expression through the TSH receptor–Gsα–cAMP signaling pathway. DIO2-induced thyroid hormone activation, through the generation of T3 from T4, transmits the springtime signal128,132. Pars distalis-derived TSH stimulates the thyroid gland. Pars tuberalis-derived TSH has tissue-specific N-glycans and forms macro-TSH complexes with immunoglobulin (IgG) and albumin in the circulation. The macro-TSH complexes are unable to stimulate the thyroid gland, and this feature prevents functional crosstalk between the two TSHs, thus preventing the production of seasonal thyroid gland overactivity73.
The hormone melatonin, which is released by the pineal gland, provides an endocrine representation of seasonal information (such as changes in day length) via the SCN–pineal pathway in mammals131. Studies using knockout mice revealed that thyrotrophs in the pars tuberalis are the targets of nocturnal melatonin; specifically, pars tuberalis-derived TSH is negatively regulated by melatonin via the MT1 melatonin receptor128,132 (FIG. 6). A 2014 murine study revealed that pars tuberalis TSH is secreted into the circulation at a level similar to pars distalis-derived TSH, but has minimal effects on the thyroid gland73 (BOX 2; FIG. 6). Hibernation in mammals, including primates and rodents, is also an important adaptation strategy133,134. A 2018 in hamsters demonstrated that Dio2 expression is decreased in the brown adipose tissue during hibernation135, suggesting important roles for thyroid hormone in various seasonal adaptation mechanisms.
Box 2. Pars tuberalis TSH and macro-TSH.
TSH is a glycoprotein72. A 2014 study in mice revealed that TSH derived from pars tuberalis is secreted into the circulation but has little bioactivity in the thyroid gland73. TSH from the pars tuberalis has decreased bioactivity because it forms macro-TSH complexes with immunoglobulin (IgG) and albumin in the circulation73. These complexes form as the result of differences in N-glycosylation patterns between TSH derived from the pars tuberalis (with sialylated multibranched N-glycans) and TSH derived from the pars distalis (with sulfated bi-antennary N-glycans)73 (FIG. 6). Therefore, tissue-specific glycosylation of TSH in the pars tuberalis and pars distalis compartmentalizes TSH function, thereby preventing functional crosstalk within the body. These tissue-specific features of glycosylation seem to be due to tissue-specific expression of glycosyltransferases73.
In humans, primary or central hypothyroidism and hyperthyroidism are sometimes associated with high serum levels of macro-TSH complex (that is, high molecular weight TSH-IgG complex with reduced bioactivity)140–142. However, high serum levels of TSH that result from the presence of macro-TSH complex are found in the absence of thyroid dysfunction and in the presence of normal thyroid hormone levels, although the underlying mechanism for this remains unclear143,144. The dynamics of circulating TSH in individuals with high TSH but without thyroid dysfunction are similar to those of TSH that is derived from pars tuberalis. That is, the circulating TSH in these individuals has sialylated multibranched N-glycans with low bioactivity and a long half-life74,75,141,142,145. Although seasonality has been reported in many human functions, such as mood, immune function, metabolism and hormone secretion, their underlying mechanisms remain unknown117,118. Therefore, the functional significance of human TSH derived from the pars tuberalis is of interest146.
Future directions
The precise role of circadian clocks in thyroid function and diseases needs to be addressed in future studies. Thyroid-specific conditional knockout of circadian clock genes and/or circadian clock manipulation within the functional thyroid tissue derived from embryonic or pluripotent stem cells would provide an opportunity to test the link between circadian clock and thyroid function and disease136.
The disruption of circadian clocks has been implicated in various diseases, and accordingly the identification of circadian clock modulators might provide new opportunities for treatment of clock-related disorders137. Studies from 2018 and 2019 have demonstrated that agonists of the REV-ERB proteins are specifically lethal to cancer cells and that a selective CK2 inhibitor inhibits cancer cell growth138,139. Therefore, pharmacological modulation of the circadian machinery may be an innovative and selective strategy for treating a wide spectrum of cancers, including thyroid cancer139.
Conclusions
To better adapt to daily environmental changes, animals have evolved circadian clock for the regulation of various physiological and endocrine rhythms3. The HPT axis is controlled by the circadian system, with a predominant role for the central pacemaker in the SCN, as well as the sleep-wake cycle34,35,53,54. Thyroid status such as hypothyroidism, hyperthyroidism, and thyroid cancer affects peripheral circadian clocks83–90. Thus, thyroid function and circadian clocks are interconnected. Epidemiologic studies have suggested that chronic circadian disruption has long-term consequences on our health and is an important lifestyle factor that contributes to the risk of developing obesity, diabetes, cancer, and cardiovascular disease4–7. Indeed, chronic sleep dept disrupts rhythmic TSH secretion37. Generalized disruption of the endocrine system is one of the important mechanisms thought to mediate the adverse effects of circadian misalignment6–8. Although several studies suggested that elevated serum TSH level is linked to the incidence of human thyroid cancer94–96, relationship between circadian disruption and thyroid cancer require further investigation. By contrast, perturbation of oncogene can induce dysregulation of circadian clock108,109 and disrupted circadian clock gene expression is reported during thyroid nodule malignant transformation113. Since disruption of circadian clocks has been implicated in various diseases137, pharmacological modulation of the circadian clock machinery might provide a new strategy for the treatment of thyroid diseases139.
Supplementary Material
Key points.
The hypothalamic–pituitary–thyroid axis is controlled by the central circadian pacemaker located in the suprachiasmatic nucleus.
Daily TSH secretion profiles are often disrupted in patients with hypothyroidism or hyperthyroidism.
Circadian dysfunction caused by shift work, travel across time zones or irregular sleep-wake cycles might be a novel lifestyle risk factor for disturbances in thyroid homeostasis in modern societies.
Disruption of circadian clock genes in vivo and in vitro disturb cell cycle progression.
The circadian clock is thought to be disrupted in well-differentiated thyroid cancer.
Acknowledgements
This work was supported by the JSPS KAKENHI Grant-in-Aid for Specially Promoted Research (26000013), for Young Scientists (B) (17K15574), the Human Frontier Science Program (RGP0030/2015), and the National Institutes of Health (PO1 AG-11412 and R01 DK-15070). WPI-ITbM is supported by the World Premier International Research Center Initiative (WPI), MEXT, Japan.
Footnotes
Competing interests
The authors declare no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fekete C & Lechan RM Central regulation of hypothalamic-pituitary-thyroid axis under physiological and pathophysiological conditions. Endocr. Rev 35, 159–194 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ortiga-Carvalho TM, Chiamolera MI, Pazos-Moura CC & Wondisford FE Hypothalamus-pituitary-thyroid axis. Compr. Physiol 6, 1387–1428 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Mohawk JA, Green CB & Takahashi JS Central and peripheral circadian clocks in mammals. Annu. Rev. Neurosci 35, 445–462 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scheer FAJL, Hilton MF, Mantzoros CS & Shea SA Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sci. U. S. A 106, 4453–4458 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davidson AJ et al. Chronic jet- lag increases mortality in aged mice. Curr. Biol 16, 914–916 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buxton OM et al. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci. Transl. Med 4, 129ra43 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kettner NM, Katchy CA & Fu L Circadian gene variants in cancer. Ann. Med 46, 208–220 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bellastella A et al. Endocrine secretions under abnormal light-dark cycles and in the blind. Horm. Res 49, 153–157 (1998). [DOI] [PubMed] [Google Scholar]
- 9.Kalsbeek A & Fliers E Daily regulation of hormone profile in Handbook of experimental pharmacology (eds. Kramer A & Merrow M) 217, 185–226 (Springer Berlin Heidelberg, 2013). [DOI] [PubMed] [Google Scholar]
- 10.Pierce JG Eli lilly lecture: The subunits of pituitary thyrotropin—their relationship to other glycoprotein hormones. Endocrinology 89, 1331–1344 (1971). [DOI] [PubMed] [Google Scholar]
- 11.Shupnik MA, Greenspan SL & Ridgway EC Transcriptional regulation of thyrotropin subunit genes by thyrotropin-releasing hormone and dopamine in pituitary cell culture. J. Biol. Chem 261, 12675–12679 (1986). [PubMed] [Google Scholar]
- 12.Vassart G & Dumont JE The thyrotropin receptor and the regulation of thyrocyte function and growth. Endocrine Reviews 13, 596–611 (1992). [DOI] [PubMed] [Google Scholar]
- 13.Friesema ECH et al. Identification of monocarboxylate transporter 8 as a specific thyroid hormone transporter. J. Biol. Chem 278, 40128–40135 (2003). [DOI] [PubMed] [Google Scholar]
- 14.Pizzagalli F et al. Identification of a novel human organic anion transporting polypeptide as a high affinity thyroxine transporter. Mol. Endocrinol 16, 2283–2296 (2002). [DOI] [PubMed] [Google Scholar]
- 15.Schweizer U, Weitzel JM & Schomburg L Think globally: Act locally. New insights into the local regulation of thyroid hormone availability challenge long accepted dogmas. Mol. Cell. Endocrinol 289, 1–9 (2008). [DOI] [PubMed] [Google Scholar]
- 16.Gereben B et al. Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr. Rev 29, 898–938 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hollenberg AN et al. The human thyrotropin-releasing hormone gene is regulated by thyroid hormone through two distinct classes of negative thyroid hormone response elements. Mol. Endocrinol 9, 540–550 (1995). [DOI] [PubMed] [Google Scholar]
- 18.Franklyn JA, Wood DF, Balfour NJ & Sheppard MC Effect of triiodothyronine treatment on thyrotrophin β- and α-messenger RNAs in the pituitary of the euthyroid rat. Mol. Cell. Endocrinol 60, 1–5 (1988). [DOI] [PubMed] [Google Scholar]
- 19.Cohen O, Flynn TR & Wondisford FE Ligand-dependent antagonism by retinoid X receptors of inhibitory thyroid hormone response elements. J. Biol. Chem 270, 13899–13905 (1995). [DOI] [PubMed] [Google Scholar]
- 20.Ralph MR, Foster RG, Davis FC & Menaker M Transplanted suprachiasmatic nucleus determines circadian period. Science 247, 975–978 (1990). [DOI] [PubMed] [Google Scholar]
- 21.Brancaccio M et al. Cell-autonomous clock of astrocytes drives circadian behavior in mammals. Science 192, 187–192 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Panda S et al. Melanopsin is required for non-image-forming photic responses in blind mice. Science 301, 525–527 (2003). [DOI] [PubMed] [Google Scholar]
- 23.Hattar S et al. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature 424, 76–81 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balsalobre A, Damiola F & Schibler U A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell 93, 929–937 (1998). [DOI] [PubMed] [Google Scholar]
- 25.Yamazaki S et al. Resetting central and peripheral circadian oscillators in transgenic rats. Science 288, 682–685 (2000). [DOI] [PubMed] [Google Scholar]
- 26.Sehgal A Physiology flies with time. Cell 171, 1232–1235 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Takahashi JS Molecular components of the circadian clock in mammals. Diabetes, Obes. Metab 17, 6–11 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gekakis N et al. Role of the CLOCK protein in the mammalian circadian mechanism. Science 280, 1564–1569 (1998). [DOI] [PubMed] [Google Scholar]
- 29.Hogenesch JB, Gu Y-Z, Jain S & Bradfield CA The basic-helix-loop-helix-PAS orphan MOP3 forms transcriptionally active complexes with circadian and hypoxia factors. Proc. Natl. Acad. Sci. U. S. A 95, 5474–5479 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cho H et al. Regulation of circadian behaviour and metabolism by REV-ERB-α and REV-ERB-β. Nature 485, 123–127 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ueda HR et al. A transcription factor response element for gene expression during circadian night. Nature 418, 534–539 (2002). [DOI] [PubMed] [Google Scholar]
- 32.Preitner N et al. The orphan nuclear receptor REV-ERBα controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 110, 251–260 (2002). [DOI] [PubMed] [Google Scholar]
- 33.Zhang R, Lahens NF, Ballance HI, Hughes ME & Hogenesch JB A circadian gene expression atlas in mammals: Implications for biology and medicine. Proc. Natl. Acad. Sci. U. S. A 111, 16219–16224 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weeke J & Gundersen HJ Circadian and 30 minutes variations in serum TSH and thyroid hormones in normal subjects. Acta. Endocrinol. (Copenh) 89, 659–672 (1978). [DOI] [PubMed] [Google Scholar]
- 35.Copinschi G & Challet E Endocrine rhythms, the sleep-wake cycle, and biological clocks in Endocrinology: Adult and Pediatric (eds. Jameson JL & Groot L. De) 147–173.e9 (Elsevier, 2016). [Google Scholar]
- 36.Van Cauter E & Spiegel K Circadian and sleep control of hormonal secretions in Regulation of sleep and circadian rhythms (eds. Turek FW & Zee PC) 397–426 (Marcel Dekker, Inc., 1999). [Google Scholar]
- 37.Spiegel K, Leproult R & Van Cauter E Impact of sleep debt on metabolic and endocrine function. Lancet 354, 1435–1439 (1999). [DOI] [PubMed] [Google Scholar]
- 38.Gronfier C & Brandenberger G Ultradian rhythms in pituitary and adrenal hormones: Their relations to sleep. Sleep Med. Rev 2, 17–29 (1998). [DOI] [PubMed] [Google Scholar]
- 39.Romijn JA et al. Pulsatile secretion of thyrotropin during fasting: A decrease of thyrotropin pulse amplitude. J. Clin. Endocrinol. Metab 70, 1631–1636 (1990). [DOI] [PubMed] [Google Scholar]
- 40.Brabant G et al. Circadian and pulsatile thyrotropin secretion in euthyroid man under the influence of thyroid hormone and glucocorticoid administration. J. Clin. Endocrinol. Metab 65, 83–88 (1987). [DOI] [PubMed] [Google Scholar]
- 41.Samuels MH, Veldhuis JD, Henry P & Ridgway EC Pathophysiology of pulsatile and copulsatile release of thyroid-stimulating hormone, luteinizing hormone, follicle-stimulating hormone, and α -Subunit. J. Clin. Endocrinol. Metab 71, 425–432 (1990). [DOI] [PubMed] [Google Scholar]
- 42.Samuels MH, Henry P, Luther M & Ridgway EC Pulsatile TSH secretion during 48-hour continuous TRH infusions. Thyroid 3, 201–206 (1993). [DOI] [PubMed] [Google Scholar]
- 43.Brabant G et al. Physiological regulation of circadian and pulsatile thyrotropin secretion in normal man and woman. J. Clin. Endocrinol. Metab 70, 403–409 (1990). [DOI] [PubMed] [Google Scholar]
- 44.Roelfsema F et al. Thyrotropin secretion profiles are not different in men and women. J. Clin. Endocrinol. Metab 94, 3964–3967 (2009). [DOI] [PubMed] [Google Scholar]
- 45.Ehrenkranz J et al. Circadian and circannual rhythms in thyroid hormones: determining the TSH and free T4 reference intervals based upon time of day, age, and sex. Thyroid 25, 954–961 (2015). [DOI] [PubMed] [Google Scholar]
- 46.Lucke C, Hehrmann R, von Mayersbach K & von zur Muhlen A Studies on circadian variations of plasma TSH, thyroxine and triiodothyronine in man. Acta Endocrinol 86, 81–88 (1977). [DOI] [PubMed] [Google Scholar]
- 47.Jordan D, Rousset B, Perrin F, Fournier M & Orgiazzi J Evidence for circadian variations in serum thyrotropin, 3,5,3’-triiodothyronine, and thyroxine in the rat. Endocrinology 107, 1245–1248 (1980). [DOI] [PubMed] [Google Scholar]
- 48.Fukuda H et al. Nyctohemeral and sex-related variations in plasma thyrotropin, thyroxine, and triiodothyronine. Endocrinology 97, 1424–1431 (1975). [DOI] [PubMed] [Google Scholar]
- 49.Azukizawa M, Eugene Pekary A, Hershman JM & Parker DC Plasma thyrotropin, thyroxine, and triiodothyronine relationships in man. J. Clin. Endocrinol. Metab 43, 533–542 (1976). [DOI] [PubMed] [Google Scholar]
- 50.Roelfsema F & Veldhuis JD Thyrotropin secretion patterns in health and disease. Endocr. Rev 34, 619–657 (2013). [DOI] [PubMed] [Google Scholar]
- 51.Mazzoccoli G et al. The hypothalamic-pituitary-thyroid axis and melatonin: Possible interactions in the control of body temperature. Neuroendocrinol. Lett 25, 368–372 (2004). [PubMed] [Google Scholar]
- 52.Covarrubias L et al. In vitro TRH release from hypothalamus slices varies during the diurnal cycle. Neurochem. Res 19, 845–850 (1994). [DOI] [PubMed] [Google Scholar]
- 53.Kalsbeek A, Fliers E, Franke AN, Wortel J & Buijs RM Functional connections between the suprachiasmatic nucleus and the thyroid gland as revealed by lesioning and viral tracing techniques in the rat. Endocrinology 141, 3832–3841 (2000). [DOI] [PubMed] [Google Scholar]
- 54.Zandieh Doulabi B et al. Diurnal variation in rat liver thyroid hormone receptor (TR)-α messenger ribonucleic acid (mRNA) is dependent on the biological clock in the suprachiasmatic nucleus, whereas diurnal variation of TRβ1 mRNA is modified by food intake. Endocrinology 145, 1284–1289 (2004). [DOI] [PubMed] [Google Scholar]
- 55.Vakili H, Jin Y & Cattini PA Evidence for a circadian effect on the reduction of human growth hormone gene expression in response to excess caloric intake. J. Biol. Chem 291, 13823–13833 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aninye IO, Matsumoto S, Sidhaye AR & Wondisford FE Circadian regulation of Tshb gene expression by Rev-Erbα (NR1D1) and nuclear corepressor 1 (NCOR1). J. Biol. Chem 289, 17070–17077 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fahrenkrug J, Georg B, Hannibal J & Jørgensen HL Hypophysectomy abolishes rhythms in rat thyroid hormones but not in the thyroid clock. J. Endocrinol 233, 209–216 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang X et al. Nuclear receptor expression links the circadian clock to metabolism. Cell 126, 801–810 (2006). [DOI] [PubMed] [Google Scholar]
- 59.Hatanaka F et al. Genome-wide profiling of the core clock protein BMAL1 targets reveals a strict relationship with metabolism. Mol. Cell. Biol 30, 5636–5648 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nikolaeva S et al. The circadian clock modulates renal sodium handling. J. Am. Soc. Nephrol 23, 1019–1026 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Munroe SH & Lazar MA Inhibition of c-erbA mRNA splicing by a naturally occurring antisense RNA. J. Biol. Chem 266, 22083–22086 (1991). [PubMed] [Google Scholar]
- 62.Vollmers C et al. Circadian oscillations of protein-coding and regulatory RNAs in a highly dynamic mammalian liver epigenome. Cell Metab 16, 833–845 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Benvenga S, Klose M, Vita R & Feldt-Rasmussen U Less known aspects of central hypothyroidism: Part 2 – Congenital etiologies. J. Clin. Transl. Endocrinol 14, 5–11 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Benvenga S, Klose M, Vita R & Feldt-Rasmussen U Less known aspects of central hypothyroidism: Part 1 – Acquired etiologies. J. Clin. Transl. Endocrinol 14, 25–33 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Biondi B & Cooper DS The clinical significance of subclinical thyroid dysfunction. Endocr. Rev 29, 76–131 (2008). [DOI] [PubMed] [Google Scholar]
- 66.Adriaanse R, Brabant G, Prank K, Endert E & Wiersinga WM Circadian changes in pulsatile TSH release in primary hypothyroidism. Clin. Endocrinol. (Oxf) 37, 504–510 (1992). [DOI] [PubMed] [Google Scholar]
- 67.Roelfsema F et al. Thyrotropin secretion in mild and severe primary hypothyroidism is distinguished by amplified burst mass and basal secretion with increased spikiness and approximate entropy. J. Clin. Endocrinol. Metab 95, 928–934 (2010). [DOI] [PubMed] [Google Scholar]
- 68.Moon SH, Lee BJ, Kim SJ & Kim HC Relationship between thyroid stimulating hormone and night shift work. Ann. Occup. Environ. Med 28, 53 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Magrini A et al. Shift work and autoimmune thyroid disorders. Int. J. Immunopathol. Pharmacol 19, 31–36 (2006). [PubMed] [Google Scholar]
- 70.Adriaanse R, Brabant G, Endert E & Wiersinga MM Pulsatile untreated thyrotropin pituitary release in patients disease. J. Clin. Endocrinol. Metab 77, 205–209 (1993). [DOI] [PubMed] [Google Scholar]
- 71.Rose SR Cranial irradiation and central hypothyroidism. Trends Endocrinol. Metab 12, 97–104 (2001). [DOI] [PubMed] [Google Scholar]
- 72.Baenziger JU & Green ED Pituitary glycoprotein hormone oligosaccharides: Structure, synthesis and function of the asparagine-linked oligosaccharides on lutropin, follitropin and thyrotropin. Biochim. Biophys. Acta - Rev. Biomembr 947, 287–306 (1988). [DOI] [PubMed] [Google Scholar]
- 73.Ikegami K et al. Tissue-specific posttranslational modification allows functional targeting of thyrotropin. Cell Rep 9, 801–809 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Persani L, Ferretti E, Borgato S, Faglia G & Beck-Peccoz P Circulating thyrotropin bioactivity in sporadic central hypothyroidism. J. Clin. Endocrinol. Metab 85, 3631–3635 (2000). [DOI] [PubMed] [Google Scholar]
- 75.Gesundheit N, Magner JA, Chen T & Weintraub BD Differential sulfation and sialylation of secreted mouse thyrotropin (TSH) subunits: Regulation by TSH releasing hormone. Endocrinology 119, 455–463 (1986). [DOI] [PubMed] [Google Scholar]
- 76.Darzy KH & Shalet SM Circadian and stimulated thyrotropin secretion in cranially irradiated adult cancer survivors. J. Clin. Endocrinol. Metab 90, 6490–6497 (2005). [DOI] [PubMed] [Google Scholar]
- 77.Refetoff S, Weiss RE & Usala SJ The syndromes of resistance to thyroid hormone. Endocr. Rev 14, 348–399 (1993). [DOI] [PubMed] [Google Scholar]
- 78.Persani L et al. Evidence for the secretion of thyrotropin with enhanced bioactivity in syndromes of thyroid hormone resistance. J. Clin. Endocrinol. Metab 78, 1034–1039 (1994). [DOI] [PubMed] [Google Scholar]
- 79.Custro N, Scafidi V & Notarbartolo A Pituitary resistance to thyroid hormone action with preserved circadian rhythm of thyrotropin in a postmenopausal woman. J. Endocrinol. Invest 15, 121–126 (1992). [DOI] [PubMed] [Google Scholar]
- 80.Moran C & Chatterjee K Resistance to thyroid hormone due to defective thyroid receptor alpha. Best Pract. Res. Clin. Endocrinol. Metab 29, 647–657 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.DeGroot LJ Graves’ disease and the manifestations of thyrotoxicosis in Endotext (eds. Feingold KR et al. ) 1–77 (MDText.com, Inc., 2000). [Google Scholar]
- 82.Roelfsema F, Pereira AM, Keenan DM, Veldhuis JD & Romijn JA Thyrotropin secretion by thyrotropinomas is characterized by increased pulse frequency, delayed and disorderliness. J. Clin. Endocrinol. Metab 93, 4052–4057 (2008). [DOI] [PubMed] [Google Scholar]
- 83.Schull J et al. Effects of thyroidectomy, parathyroidectomy and lithium on circadian wheelrunning in rats. Physiol. Behav 42, 33–39 (1988). [DOI] [PubMed] [Google Scholar]
- 84.McEachron DL, Lauchlan CL & Midgley DE Effects of thyroxine and thyroparathyroidectomy on circadian wheel running in rats. Pharmacol. Biochem. Behav 46, 243–249 (1993). [DOI] [PubMed] [Google Scholar]
- 85.Beasley LJ & Nelson RJ Thyroid gland influences the period of hamster circadian oscillations. Experientia 38, 870–871 (1982). [DOI] [PubMed] [Google Scholar]
- 86.Dkhissi-Benyahya O, Gronfier C, De Vanssay W, Flamant F & Cooper HM Modeling the role of mid-wavelength cones in circadian responses to light. Neuron 53, 677–687 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gloss B et al. Cardiac ion channel expression and contractile function in mice with deletion of thyroid hormone receptor α or β. Endocrinology 142, 544–550 (2001). [DOI] [PubMed] [Google Scholar]
- 88.Peliciari-Garcia RA, Bargi-Souza P, Young ME & Nunes MT Repercussions of hypo and hyperthyroidism on the heart circadian clock. Chronobiol. Int 35, 147–159 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Amir S & Robinson B Thyroidectomy alters the daily pattern of expression of the clock protein, PER2, in the oval nucleus of the bed nucleus of the stria terminalis and central nucleus of the amygdala in rats. Neurosci. Lett 407, 254–257 (2006). [DOI] [PubMed] [Google Scholar]
- 90.Noguchi T, Ikeda M, Ohmiya Y & Nakajima Y A dual-color luciferase assay system reveals circadian resetting of cultured fibroblasts by co-cultured adrenal glands. PLoS ONE 7, e37093 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Arendt J Managing jet lag: Some of the problems and possible new solutions. Sleep Med. Rev 13, 249–256 (2009). [DOI] [PubMed] [Google Scholar]
- 92.Gary KA et al. Total sleep deprivation and the thyroid axis: Effects of sleep and walking activity. Aviat. Space Environ. Med 67, 513–519 (1996). [PubMed] [Google Scholar]
- 93.Hirschfeld U et al. Progressive elevation of plasma thyrotropin during adaptation to simulated jet lag: effects of treatment with bright light or zolpidem. J. Clin. Endocrinol. Metab 81, 3270–3276 (1996). [DOI] [PubMed] [Google Scholar]
- 94.Polyzos SA et al. Serum thyrotropin concentration as a biochemical predictor of thyroid malignancy in patients presenting with thyroid nodules. J. Cancer Res. Clin. Oncol 134, 953–960 (2008). [DOI] [PubMed] [Google Scholar]
- 95.Haymart MR et al. Higher serum thyroid stimulating hormone level in thyroid nodule patients is associated with greater risks of differentiated thyroid cancer and advanced tumor stage. J. Clin. Endocrinol. Metab 93, 809–814 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Boelaert K et al. Serum thyrotropin concentration as a novel predictor of malignancy in thyroid nodules investigated by fine-needle aspiration. J. Clin. Endocrinol. Metab 91, 4295–4301 (2006). [DOI] [PubMed] [Google Scholar]
- 97.Pinkerton LE et al. Melanoma, thyroid cancer, and gynecologic cancers in a cohort of female flight attendants. Am. J. Ind. Med 61, 572–581 (2018). [DOI] [PubMed] [Google Scholar]
- 98.Liu GS et al. Thyroid cancer risk in airline cockpit and cabin crew: a meta-analysis. Cancers Head Neck 3, 7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kiessling S et al. Enhancing circadian clock function in cancer cells inhibits tumor growth. BMC Biol 15, 1–18 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Matsuo T et al. Control mechanism of the circadian clock for timing of cell division in vivo. Science 302, 255–260 (2003). [DOI] [PubMed] [Google Scholar]
- 101.Gery S et al. The circadian gene Per1 plays an important role in cell growth and DNA damage control in human cancer cells. Mol. Cell 22, 375–382 (2006). [DOI] [PubMed] [Google Scholar]
- 102.Kowalska E et al. NONO couples the circadian clock to the cell cycle. Proc. Natl. Acad. Sci. U. S. A 110, 1592–1599 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jiang W et al. The circadian clock gene Bmal1 acts as a potential anti-oncogene in pancreatic cancer by activating the p53 tumor suppressor pathway. Cancer Lett 371, 314–325 (2016). [DOI] [PubMed] [Google Scholar]
- 104.Lee S, Donehower LA, Herron AJ, Moore DD & Fu L Disrupting circadian homeostasis of sympathetic signaling promotes tumor development in mice. PLoS ONE 5, e10995 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fu L, Pelicano H, Liu J, Huang P & Lee CC The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell 111, 41–50 (2002). [DOI] [PubMed] [Google Scholar]
- 106.Papagiannakopoulos T et al. Circadian rhythm disruption promotes lung tumorigenesis. Cell Metab 24, 324–331 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gallo C et al. The bHLH transcription factor DEC1 promotes thyroid cancer aggressiveness by the interplay with NOTCH1. Cell Death Dis 9, 871 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Relógio A et al. Ras-mediated deregulation of the circadian clock in cancer. PLoS Genet 10, e1004338 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Altman BJ et al. MYC disrupts the circadian clock and metabolism in cancer cells. Cell Metab 22, 1009–1019 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Durante C et al. The diagnosis and management of thyroid nodules. JAMA 319, 914–924 (2018). [DOI] [PubMed] [Google Scholar]
- 111.Sherman SI Thyroid carcinoma. Lancet 361, 501–511 (2003). [DOI] [PubMed] [Google Scholar]
- 112.Kitahara CM & Sosa JA The changing incidence of thyroid cancer. Nat. Rev. Endocrinol 12, 646–653 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mannic T et al. Circadian clock characteristics are altered in human thyroid malignant nodules. J. Clin. Endocrinol. Metab 98, 4446–4456 (2013). [DOI] [PubMed] [Google Scholar]
- 114.Nakane Y & Yoshimura T Photoperiodic regulation of reproduction in vertebrates. Annu. Rev. Anim. Biosci 7, 173–194 (2018). [DOI] [PubMed] [Google Scholar]
- 115.Ottenweller JE, Tapp WN, Pitman DL & Natelson BH Adrenal, thyroid, and testicular hormone rhythms in male golden hamsters on long and short days. Am. J. Physiol. Regul. Integr. Comp. Physiol 253, R321–R328 (1987). [DOI] [PubMed] [Google Scholar]
- 116.Wong CC et al. Influence of age, strain and season on diurnal periodicity of thyroid stimulating hormone, thyroxine, triiodothyronine and parathyroid hormone in the serum of male laboratory rats. Eur. J. Endocrinol 102, 377–385 (1983). [DOI] [PubMed] [Google Scholar]
- 117.Wirz-Justice A Seasonality in affective disorders. Gen. Comp. Endocrinol 258, 244–249 (2018). [DOI] [PubMed] [Google Scholar]
- 118.Dopico XC et al. Widespread seasonal gene expression reveals annual differences in human immunity and physiology. Nat. Commun 6, 1–13 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Maes M et al. Components of biological variation, including seasonality, in blood concentrations of TSH, TT3, FT4, PRL, cortisol and testosterone in healthy volunteers. Clin. Endocrinol 46, 587–598 (1997). [DOI] [PubMed] [Google Scholar]
- 120.Smals AGH, Ross HA & Kloppenborg PWC Seasonal variation in serum T3 and T4 levels in man. J. Clin. Endocrinol. Metab 44, 998–1001 (1977). [DOI] [PubMed] [Google Scholar]
- 121.Bellastella A et al. Circannual rhythms of plasma growth hormone, thyrotropin and thyroid hormones in prepuberty. Clin. Endocrinol 20, 531–537 (1984). [DOI] [PubMed] [Google Scholar]
- 122.Gullo D et al. Seasonal variations in TSH serum levels in athyreotic patients under L-thyroxine replacement monotherapy. Clin. Endocrinol 87, 207–215 (2017). [DOI] [PubMed] [Google Scholar]
- 123.Buchinger W, Semlitsch G, Pongratz R & Rainer BHF Jahreszeitliche variationen im auftreten der hyperthyreose. Acta Med. Austriaca 27, 51–53 (2000). [PubMed] [Google Scholar]
- 124.Akslen LA & Sothern RB Seasonal variations in the presentation and growth of thyroid cancer. Br. J. Cancer 77, 1174–1179 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nakao N et al. Thyrotrophin in the pars tuberalis triggers photoperiodic response. Nature 452, 317–322 (2008). [DOI] [PubMed] [Google Scholar]
- 126.Yoshimura T et al. Light-induced hormone conversion of T4 to T3 regulates photoperiodic response of gonads in birds. Nature 426, 178–181 (2003). [DOI] [PubMed] [Google Scholar]
- 127.Yamamura T, Hirunagi K, Ebihara S & Yoshimura T Seasonal morphological changes in the neuro-glial interaction between gonadotropin-releasing hormone nerve terminals and glial endfeet in Japanese quail. Endocrinology 145, 4264–4267 (2004). [DOI] [PubMed] [Google Scholar]
- 128.Ono H et al. Involvement of thyrotropin in photoperiodic signal transduction in mice. Proc. Natl. Acad. Sci. U. S. A 105, 18238–18242 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hanon EA et al. Ancestral TSH mechanism signals summer in a photoperiodic mammal. Curr. Biol 18, 1147–1152 (2008). [DOI] [PubMed] [Google Scholar]
- 130.Bockmann J et al. Thyrotropin expression in hypophyseal pars tuberalis-specific cells is 3,5,3’-triiodothyronine, thyrotropin-releasing hormone, and Pit-1 independent. Endocrinology 138, 1019–1028 (1997). [DOI] [PubMed] [Google Scholar]
- 131.Arendt J Melatonin and the mammalian pineal gland (Chapman & Hall, 1995). [Google Scholar]
- 132.Yasuo S, Yoshimura T, Ebihara S & Korf HW Melatonin transmits photoperiodic signals through the MT1 melatonin receptor. J. Neurosci 29, 2885–2889 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Heldmaier G, Ortmann S & Elvert R Natural hypometabolism during hibernation and daily torpor in mammals. Respir. Physiol. Neurobiol 141, 317–329 (2004). [DOI] [PubMed] [Google Scholar]
- 134.Geiser F & Turbill C Hibernation and daily torpor minimize mammalian extinctions. Naturwissenschaften 96, 1235–1240 (2009). [DOI] [PubMed] [Google Scholar]
- 135.Gautier C et al. Gene expression profiling during hibernation in the European hamster. Sci. Rep 8, 1–17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Antonica F et al. Generation of functional thyroid from embryonic stem cells. Nature 491, 66–71 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tamai TK et al. Identification of circadian clock modulators from existing drugs. EMBO Mol. Med 10, e8724 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Oshima T et al. Cell-based screen identifies a new potent and highly selective CK2 inhibitor for modulation of circadian rhythms and cancer cell growth. Sci. Adv 5, 1–16 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sulli G et al. Pharmacological activation of REV-ERBs is lethal in cancer and oncogene-induced senescence. Nature 553, 351–355 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Biro J Specific binding of thyroid-stimulating hormone by human serum globulins. J. Endocrinol 88, 339–349 (1980). [DOI] [PubMed] [Google Scholar]
- 141.Spitz IM et al. Increased high-molecular-weight thyrotropin with impaired biologic activity in a euthyroid man. N. Engl. J. Med 304, 278–282 (1981). [DOI] [PubMed] [Google Scholar]
- 142.DeCherney GS, Gesundheit N, Gyves PW, Showalter CR & Weintraub BD Alterations in the sialylation and sulfation of secreted mouse thyrotropin in primary hypothyroidism. Biochem. Biophys. Res. Commun 159, 755–762 (1989). [DOI] [PubMed] [Google Scholar]
- 143.Loh TP et al. Macro-thyrotropin : a case report and review of literature. J. Clin. Endocrinol. Metab 97, 1823–1828 (2012). [DOI] [PubMed] [Google Scholar]
- 144.Tamaki H et al. Novel thyrotropin ( TSH ) -TSH antibody complex in a woman and her neonates. Thyroid 5, 299–304 (1995). [DOI] [PubMed] [Google Scholar]
- 145.Constant RB & Weintraub BD Differences in the metabolic clearance of pituitary and serum thyrotropin (TSH) derived from euthyroid and hypothyroid rats: Effects of chemical deglycosylation of pituitary TSH. Endocrinology 119, 2720–2727 (1986). [DOI] [PubMed] [Google Scholar]
- 146.Asa SL, Kovacs K & Bilbao JM The pars tuberalis of the human pituitary. Virchows Arch. A 399, 49–59 (1983). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


