Abstract
The design of a drug that successfully overcomes the constraints imposed by the blood–brain barrier (BBB, which acts as a gatekeeper to the entry of substances into the brain) requires an understanding of the biological firewall. It is also of utmost importance to understand the physicochemical properties of the said drug and how it engages the BBB to avoid undesired side effects. Since fewer than 5% of the tested molecules can pass through the BBB, drug development pertaining to brain-related disorders takes inordinately long to develop. Furthermore, in most cases it is also unsuccessful for allied reasons. Several drug delivery systems (DDSs) have shown excellent potential in drug delivery across the BBB while demonstrating minimal side effects. This mini-review summarizes key features of the BBB, recapitulates recent advances in our understanding of the BBB, and highlights existing strategies for the delivery of drug to the brain parenchyma.
1. Introduction
1.1. Blood–Brain Barrier (BBB)
The brain is the most complex and vital organ of the human body. For this reason, it is important to protect it against any insults that can possibly lead to infection, inflammation, improper activation, and even the death of cerebral cells.1 The CNS is composed of the brain and the spinal cord. These areas are protected by two main barriers: BBB and the blood–cerebrospinal fluid barrier (BCB).1 Using the term “barrier” to describe these interfaces seems misleading as these barriers of CNS act as a selectively permeable membrane and do not completely block all of the incoming compounds.2 The BBB is defined by a distinctive structure and an interaction between the acellular and cellular components in the brain. The primary function of the BBB is to make sure that there exists a suitable environment for the interaction and functioning of the neurons, which is important for maintaining homeostasis, regulating efflux and influx and protecting the brain from pathogenic agents.1 The BBB is composed of a continuous layer of endothelial cells connected through tight junctions (TJs), adherent junctions (AJs), and gap junctions (GJs). Among these junctions, TJs are the main compositional element of the BBB and provide enhanced trans-endothelial resistance in the BBB.3 These junctions allow for the controlled passage of the drugs across the BBB by tethering the adjacent cells so tightly that they occlude the intercellular space between them.2,3 The specialized cells found in the BBB are pericytes, astrocytes, microglia and adjacent neurons.1 From an anatomical viewpoint, pericytes are multifunctional mural cells that wrap around endothelial cells and regulate TJs, AJs and transcytosis across the BBB.4 These cells can be designated as member of the vascular smooth muscle cell lineage, and their main function is to regulate flow of blood in brain capillaries via contraction and relaxation of the walls of blood capillaries.3 On the contrary, astrocytes are glial cells that help neurons in maintaining homeostasis by regulating the neurotransmitter concentration and maintaining the redox potential and the removal of debris from the cerebrospinal fluid (CSF). Their interaction with the endothelial cells through projections in the basolateral cerebral capillaries leads to the formation of connections between the brain capillary and the adjacent neurons. Furthermore, microglia are the primary immune cells of the brain and can be activated in response to either systemic inflammation or trauma. These cells helps to clear neuronal or other cellular debris via phagocytosis.5 The basement membrane, on the other hand, consists of pericytes and endothelial cells which embody the surface of >99% of the capillaries found in the CNS. It is also rich in proteoglycans, namely, heparan sulfate and proteins such as collagen type IV and laminin3 (Figures 1 and 2).
Figure 1.
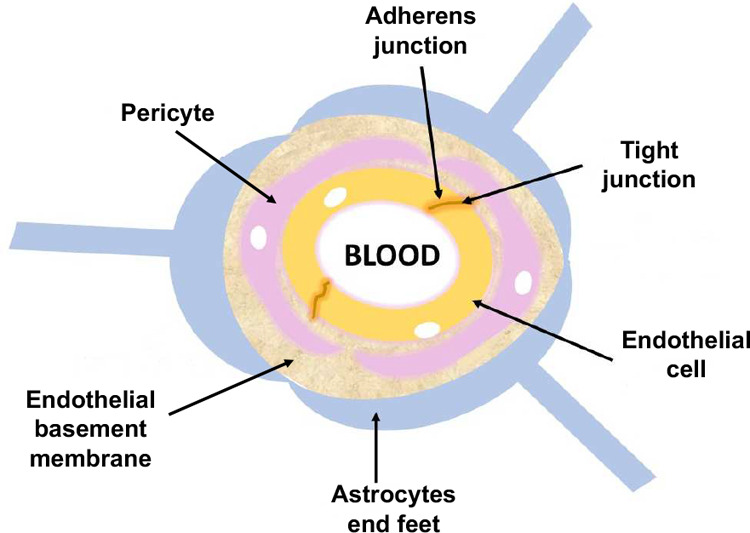
Schematic representation of the BBB.
Figure 2.
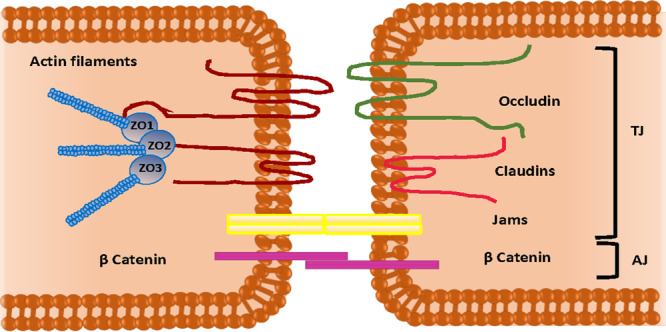
Schematic representation of tight junctions and adherence junctions between endothelial cells.
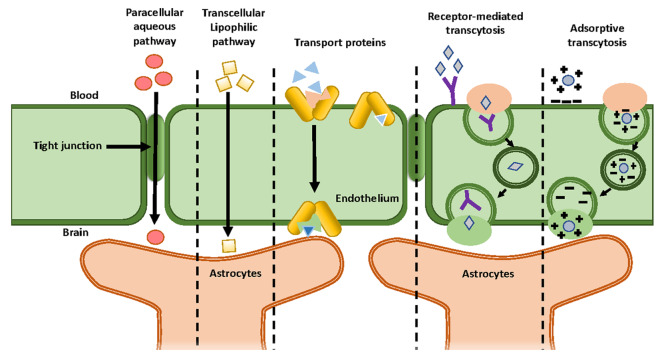
In addition to the physical barriers, there exist numerous carrier mechanisms which regulate the entry of essential compounds (such as glucose and amino acids) or the efflux of certain compounds which need to be excluded from the CNS.2 Such transportation of molecules across the BBB can be classified into three broad categories: a paracellular pathway, a transcellular pathway, and a carrier-mediated pathway. In the paracellular pathway, solutes passively diffuse across the BBB. As a consequence, net migration can take place only via concentration gradients.4 By contrast, the transcellular transportation system involves an energy expenditure (i.e., the hydrolysis of adenosine tripolyphosphate (ATP)) and includes mechanisms such as transcellular diffusion, active receptor-mediated transport, and transcytosis to transport the molecules. Most small molecules cross the BBB through a substrate-specific process known as an endogenous transporter or a carrier-mediated pathway (third route).4 This process occurs in the presence of a concentration gradient with the assistance of passable transporters. For example, carbon dots (CDs; nanosized particles (1–10 nm) with versatile surface chemistry) can be covalently conjugated with large drug molecules, resulting in the delivery of drugs via carrier-mediated transport.3 In the case of large molecules such as peptides and proteins, the passage through the BBB occurs via the endocytic mechanism.1,4 There are three subcategories in this type of transport: (a) transporter-mediated, (b) receptor-mediated, and (c) absorptive-mediated.1,4 Transporter-mediated transport is a nonspecific type of transport. GLUT-1 is an example of a glucose transporter which can also transport other hexoses as well. Also, LAT-1 is an amino acid transporter, and due to its nonspecific nature, it has the ability to carry and transport several types of amino acids.
By contrast, receptor-mediated transport requires the binding of ligands to the receptor so that the transport of various macromolecules and proteins can occur across the cerebral endothelium. Examples include angiopep-2 and seq12 to transport different peptides as targeting groups. Whereas in the case of absorptive-mediated transport, since the surface of the cerebral endothelium is negatively charged, macromolecules and proteins with positive surface charge can attach or absorb to the surface of these cells, resulting in their transcytosis. Example includes TAT and K16ApoE as targeting groups1 (Figure 3).
Figure 3.
Schematic diagram of the different mechanisms for crossing the BBB.
1.2. Barriers to CNS Drug Delivery
In brain capillaries, intercellular clefts are almost nonexistent. Therefore, the transport must occur trans-cellularly. As a result, lipid-soluble solutes can freely diffuse through the endothelial membrane, resulting in their easy transport across the BBB.6 This results in the compromised therapeutic value of promising drugs which in turn have made cerebral disorders most refractory to therapeutic interventions. Lipid soluble drugs are preferred for drug delivery across the BBB.6 Due to the architecture of the BBB which acts as an interface between the aqueous blood environment and the aqueous brain interstitial fluid, the solubility of drugs in lipids is important for transporting molecules into the brain via passive diffusion.2
However, exceptions include doxorubicin and epipodophyllotoxin. These lipophilic drugs show poor permeability, thus posing difficulty in brain tumor treatment. This could be attributed to the active efflux mechanisms present in the membranes of the BBB. Moreover, increased lipid solubility may not guarantee the deposition of the drug in the brain because high lipid solubility can be positively correlated with enhanced accumulation of the drug at nontarget sites.2,6
The BBB has additional barriers as well. Solutes crossing the cerebral endothelial membrane, which is rich in mitochondria, are subsequently exposed to degrading enzymes such as neprilysin, enkephalin, and insulin-degrading enzymes.6 In addition, the BBB can also recognize and readily degrade peptides such as neuropeptides.7 Furthermore, the BBB is additionally reinforced by a high concentration of P-glycoproteins (Pgp) in the luminal membrane of the endothelium. This efflux transporter, a member of the B subfamily of the ATP-binding cassette proteins (ABC-B1), is a glycosylated protein that uses ATP hydrolysis to actively remove a wide range of drugs from the endothelial cytoplasm before they can cross the brain parenchyma8 (Figure 4). Drug molecules that are able to escape Pgp clearance are later exported out of the brain parenchyma via a second line of defense termed phase I and II metabolism. In this mechanism, enzymes either unmask or add reactive polar groups to the surface of the drug molecule (phase I modification), followed by the conjugation of anionic groups onto the drug molecules (phase II modification) to mark them for phase III expulsion. Removal occurs by either the multidrug resistance (MRP) protein or the breast cancer resistance protein (BCRP). The action of phase I and II metabolism reduces the lipid solubility of the drug molecule. This in return prevents the passage of the compound back into the brain parenchyma.2
Figure 4.
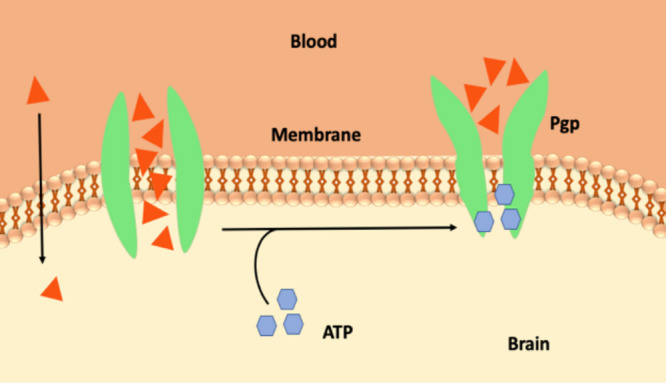
Schematic representation of the Pgp.
In addition to the previous large and complex set of barriers, there is a second barrier set that contributes to CNS complexity known as BCB.5 Physiologically, choroid plexus serves as a barrier in the brain by separating blood from CSF. A group of ependymal cells forms the choroid plexus. These cells line the ventricles of the brain and are responsible for the secretion of CSF.5 On the external surface of the brain, the ependymal cells fold over onto themselves to form a double layer which is called the arachnoid membrane.5 The arachnoid membrane is selectively permeable to hydrophilic substances. In a crucial evolutionary strategy, the choroid plexus and the arachnoid membrane work together at the barrier between the blood and the CSF. The BCB is also fortified by an active organic acid transport system in the choroid plexus which is capable of driving CSF-borne organic molecules into the blood5 (Figure 5). As a result of this system activity, penicillin, a narrow-spectrum antibiotic, and methotrexate, an antiviral agent, can be actively removed from the CSF and therefore are inhibited from diffusing into the brain parenchyma.5 These drugs exhibit poor lipid solubility and are thus restricted by the TJs between the cells that forms this barrier and require additional assistance for their transport across the BBB.2 Although these barriers (BBB and BCB) are very important for the normal functioning of the brain, they are also observed to provide multidrug resistance to the brain. This, in turn, acts as an obstacle in the delivery of therapeutics in the CNS. In addition, BBB permeability not only is dependent on the nature of the drug but also is affected by other factors. One of the factors is the blood/plasma concentration of the drug after a few hours of administration. This factor not only is dependent on the permeability across the BBB but also is related to (i) the rate of efflux from the brain, (ii) the absorption of drug onto the surface of the plasma protein, thus resulting in its retention in the blood, and (iii) the dissociation of the drug into its constituents and therefore reduced lipid solubility and transportation across the BBB via passive diffusion.2
Figure 5.
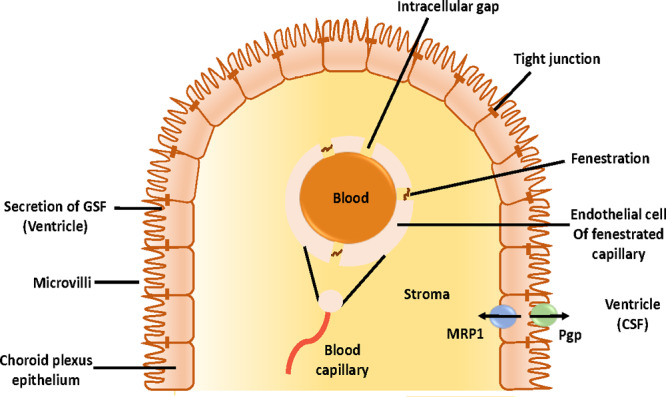
Schematic representation of the BCB.
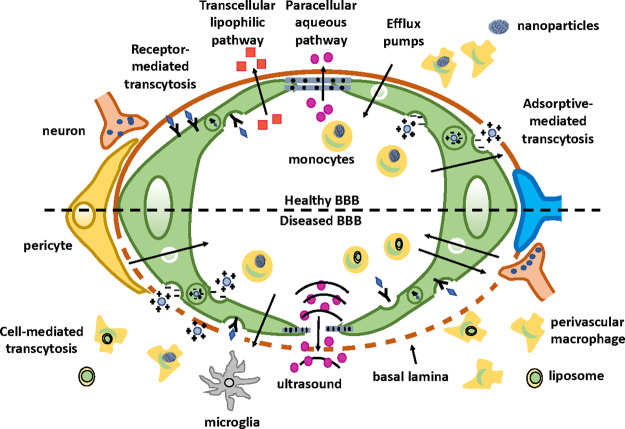
Alzheimer’s disease (AD), cerebral malignancies, multiple sclerosis, Parkinson’s disease (PD), and stroke are CNS disorders.1 These disorders arise from a combination of familial and sporadic factors, systemic pathologies, and abnormalities related to the development and functioning of the brain (Figure 6). When compared with other parts of the human body, the existing treatments for neurodegenerative and neurological diseases are comparatively unsuccessful due to the complexity of the brain and regulated entry into the cerebral mass. In the near future, the number of neuronal cases is expected to rise. Therefore, innovation and development of improved interventional strategies are essential.1 Despite some successful examples of brain drug delivery, only a few have passed the phase where they can be considered safe for human administration. Around 95% of the studied drugs cannot cross the BBB, and even if they overcome this interface, they are ineffective. This could be attributed to the fact that these drugs enter the brain so slowly that they are unable to reach therapeutic concentrations required to show their maximum effect.2 Thus, drug development for brain disease takes a longer time to develop and in most of the cases is unsuccessful.1 As previously described, BBB is a dynamic interface controlling the entry of molecules into the brain from the blood. The passage across the BBB can be improved by the chemical modification of drugs and prodrugs, regulating efflux transport, enhancing trans-cellular diffusion, or transiently disrupting the TJs complexes. This can be achieved via technologies such as the suppression of protein expression (TJs) by RNA interference (RNAi).1 Such discoveries open a new path for strategies and concepts about brain drug delivery.
Figure 6.
Schematic diagram of diseased and healthy BBB.
Although there are many challenges, one of the biggest hurdles is to find an efficient vector that can easily cross the BBB and deliver the drug.1 Chemical drug delivery is attempting to increase the influx and decrease the efflux of drug by utilizing a metabolic approach which includes increasing the influx via passive diffusion and enhanced drug solubility in lipids.1 The use of nanotechnology could potentially improve noninvasive drug-delivery strategies that can further lead to the design of improved formulations. Such formulations will have a better chance of crossing the BBB than conventional drugs.1 Nanotechnology is an emerging field which utilizes principles from various disciplines toward the development of materials with one of its dimensions in the size range of 1–100 nm. Therefore, this review focuses on describing the importance of various nanotechnology-based carriers (i.e., nanocarriers and their application in delivering therapeutic molecules across the BBB).
2. Strategies for Enhanced CNS Drug Delivery
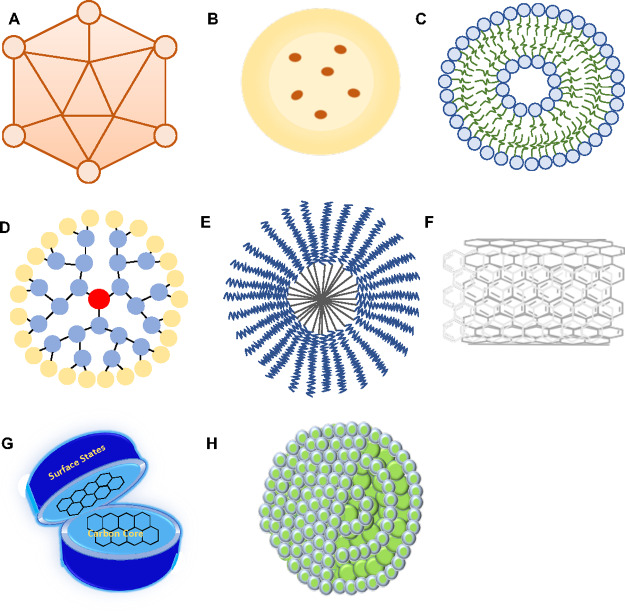
Pharmaceutical manipulation, BBB disruption, and other methods involving the use of nanocarriers are being utilized to facilitate the transport of therapeutic drug to the target site in the brain. Some of the strategies for drug delivery across the BBB are listed in detail below (Figure 7A–H).
Figure 7.
Schematic representation of (A) viral vectors, (B) polymeric nanoparticles, (C) liposomes, (D), dendrimers, (E) micelles, (F) carbon nanotubes, (G) carbon dots, and (H) carbon nano-onions.
2.1. Viral Vectors
Viral vectors have the ability to infect host cells by using their genetic material (RNA or DNA). These vectors have recently attracted a lot of attention owing to their ability to deliver the desired genes to patients suffering from neurological disorders. These DDSs are extensively used to transport nucleic acids to the brain parenchyma. The main advantage of using viral vectors compared to other drug delivery candidates in gene transduction (in the brain) is their high transfection efficiency (∼80%) and long-term expression of transgenes within the nondividing cells. Some of the viral vectors such as Lentivirus, herpes simplex virus (HSV), adenovirus (AdV) and adeno-associated virus (AAV) have achieved drug delivery into the brain. However, some of the limitations associated with the use of these vectors for drug delivery applications in addition to immunogenicity includes high cost of production and difficulties associated with manufacturing. Since viruses cannot passively cross the BBB, several administration routes such as stereotaxic injection and injection into the CSF have been developed to bypass the BBB. Hence, more research on their use, safety, and associated immunogenicity needs to be explored for the translation of viral vectors from preclinical to clinical studies.
In a study carried out by Shukla et al., the biodistribution and reticuloendothelial clearance of viruses were assessed in normal and cancer-bearing mice. It was reported that a new platform of potato virus X (PVX), a filamentous plant virus, was introduced. This virus showed up to a 30% signal in brain tumor sites, the colon, and mammary glands of the mice, while the remaining viral particles (70%) underwent reticuloendothelial clearance by the spleen and liver. Also, kidney and bile showed slower processing and clearance of these viral particles.9 In another study, the delivery of the glutamic acid decarboxylase gene in the AVV2 vector was studied using 42 PD patients. The study was conducted for 12 months in an open-label fashion. A reduction in levodopa-induced dyskinesias was observed at 12 months in a group injected with the AAV2- glutamic acid decarboxylase gene compared with the control. Hence, the clinical benefits were observed in patients injected with the AAV2-glutamic acid decarboxylase gene at 12 months.10
2.2. Polymeric Nanoparticles
Nanoparticles are materials that have at least one of their dimensions in the nanometer range.11 These have evolved to be one of the most successful candidates for drug delivery applications owing to their ability to undergo surface modification (e.g., PEGylation), nanosizing, bioactivity, controlled and sustained drug release, nontoxicity, bioavailability, biocompatibility, reticuloendothelial clearance bypass, and the encompassing of various active molecules including drugs, oligonucleotides, and peptides11 (Figure 7B). These nanoparticles have been extensively used as carriers for therapeutic drugs due to their efficient delivery across the BBB. Such entrapment of the drug via “nanoencasement” prevents phagocytosis, hence enhancing the blood circulation time.11 Some of the limitations associated with the use of these nanoparticles include low transfection efficiency, batch-to-batch variation, poor drug loading capacity and entrapment efficiency, and particle–particle aggregation increasing the complexity of handling these nanocarriers in both liquid and dry forms. Also, polycation nanocarriers have been observed to induce osmotic swelling and the subsequent rupture of the lysosome. This can eventually result in oxidative stress, protein aggregation, and mitochondrial dysfunction, subsequently resulting in cell death.12 Also, these nanocarriers can result in complement activation, thus inducing an inflammatory response.
A study was focused on the use of curcumin-loaded polylactide-co-glycolic-acid (PLGA) nanoparticles to dissolve amyloid-β aggregates.13 This disintegration effect could be related to the ability of curcumin not only to inhibit the formation of new amyloid beta aggregates but also to dissolve or disintegrate the pre-existing ones. The encapsulation of this hydrophobic drug, curcumin, inside PLGA nanoencasement allowed us to overcome the low ability of curcumin to cross the BBB. The as-synthesized nanohybrid was reported to show no significant toxicity to the hippocampal cell culture when in vitro studies were performed. Also, a targeted delivery was achieved by decorating the PLGA surface with g7 ligands. In a different study, andrographolide-loaded human serum albumin (HSA) nanoparticles and polyethylcyanocrylate nanoparticles were used as anticancer agents. Andrographolide is a well-known anti-inflammatory compound derived from medicinal plant Andrographis paniculata, but limited bioavailability limits the further use of this drug. To overcome this challenge, andrographolide was encapsulated inside two different nanocarriers as mentioned previously. The experimental results showed enhanced permeability for human albumin-based nanoparticles compared to polyethylcyanocrylate nanoparticles.14 Thus, improved permeation of andrographolide upon encapsulation inside human serum albumin was observed, but in the case of polyethylcyanocrylate nanoparticles, disruption of the BBB membrane was reported. However, free andrographolide could not pass through the BBB model as predicted by in-silico studies as well. Therefore, HSA could serve as a promising candidate for the transport of andrographolide across the BBB.14
2.3. Liposomes
Liposomes are spherical delivery vehicles composed of amphiphilic lipid bilayers that can hold various therapeutic molecules including drugs, vaccines, nucleic acids, and proteins (Figure 7 C). In addition, the ease of surface modification, biocompatibility, amphiphilic nature, and enhanced circulation time make them an efficient DDS for the delivery of therapeutic medication.11 The application of liposomes mostly includes targeted drug delivery due to their ability to easily bypass the brain parenchyma and deliver drug to the targeted site.1 However, there are limitations associated with these nanocarriers, including the high cost of production, short half-life, low solubility, batch-to-batch variation, and leakage of the encapsulated drug during the storage process. Some of the safety issues arising due to liposome introduction includes hepatotoxicity as evident by an increased level of liver enzymes in the blood circulation upon liposome introduction and liposome-induced complement system activation (immunogenic response).12 These are also known to cause inflammation by inducing the upregulation of proinflammatory cytokines.12
Docetaxel passage across the BBB is restricted due to its pharmacological properties. Therefore, Shaw et al. developed docetaxel-loaded nanoliposomes via a lipid hydration method to treat solid tumors of brain. When tested in vitro, the formulation was readily taken up by C6 glioma cells. Compared to free docetaxel, this nanoformulation showed better pharmacokinetic results and enhanced uptake by the brain in rats, thus pointing toward the efficiency of liposomes to serve as a carrier for docetaxel to treat gliomas.15 In another study conducted by Rehman et al., a thermoresponsive lipid nanoparticulate delivery system was synthesized to hold anticancer drug paclitaxel. This formulation not only enhanced the BBB permeability of the drug but was also effective at targeting glioblastoma cells (the most aggressive type of brain tumor). Interestingly, the nanoensemble displayed a slower drug release profile at physiological temperature (37 °C). However, abrupt release was observed at 39 °C. This could be attributed to the increased diffusion of drug from the liposome at increased temperature.16 Therefore, liposome systems owing to their high biosafety can be employed for the treatment of CNS-related disorders.
2.4. Dendrimers
Dendrimers are tree-shaped synthetic molecules with multiple branched monomers radiating out from the central core.11 Excellent properties such as being nanosized and having a predictable molecular weight, low polydispersity, rigidity, and ease of surface modification make them a desirable candidate for applications in the treatment of neurodegenerative disorders.11,17,18 Dendrimers are generally used to deliver hydrophobic compounds to the desired areas in the brain11 (Figure 7D). However, the associated limitations include the high cost of synthesis, batch-to-batch variations, and difficulty in achieving targeted drug delivery. Safety issues related to cationic dendrimers includes high toxicity which can be ascribed to the interaction between the positively charged nanocarrier and the negatively charged cell surface. Such an interaction results in the creation of nanopores in the cell membrane and the subsequent leakage of cellular content and hence cell death.19
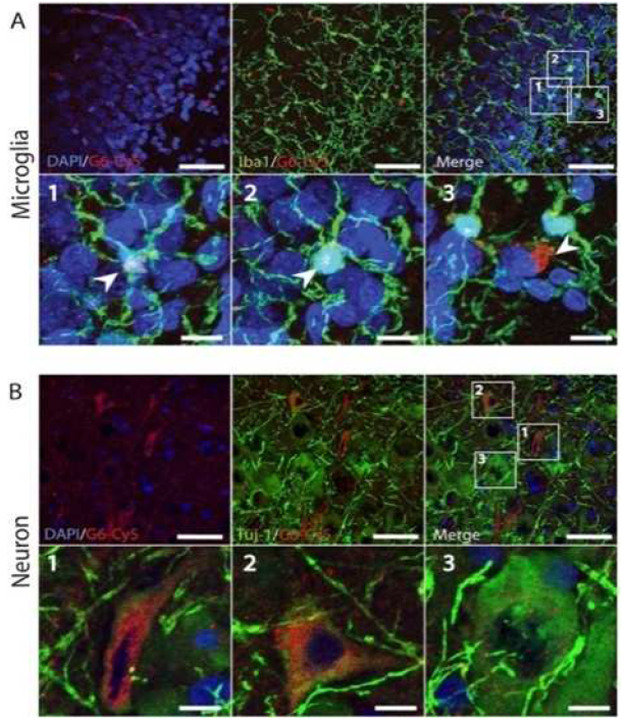
Fifth-generation amine-terminated (i.e., polyamidation) dendrimers are nanounifiers utilized to administer drug at the tumor site in the brain to treat gliomas.11,17 In this study, a theranostic composed of poly(amidoamine) (PAMAM) dendrimers multifunctionalized with poly(ethylene glycol) (PEG), anticancer drug chlorotoxin, and 3-(4′-hydroxyphenyl) propionic acid-OSu was prepared. Later, the nanoplatform was radiolabeled with 131I at the remaining terminals such that the multifunctional dendrimer can act as a good theranostic agent. The as-synthesized nanohybrid displayed good cytotoxicity against cancer cells and was specific to metallopeptidase 2 (MMP 2) overexpressing cancer cells. Thus, it can be concluded that this dendrimeric multifunctional nanocarrier has great potential to be used as a theranostic against human gliomas. In another study, generation 6 (G6) PAMAM dendrimers were used as a nanoplatform to see its effect in the canine model of hypothermic circulatory arrest (HCA)-induced brain injury.18 The experimental data revealed an increased blood circulation time and brain accumulation of the nanocarrier compared to generation 4 dendrimers (G4). This could be due to the smaller size of G4 dendrimers (∼4 nm) compared to G6 (∼7 nm). Moreover, increased levels of G6 were reported in cerebrospinal fluid indicating (CSF/blood: ∼20%) an enhanced uptake of G6 dendrimers compared to other nanoformulations. Also, reduced clearance by the kidneys and increased liver uptake were reported (Figure 9). Hence, the study pointed toward the importance of employing this nanosystem in large animal models to record its response and finally the potential of the clinical translation of this nanoformulation.18
Figure 9.
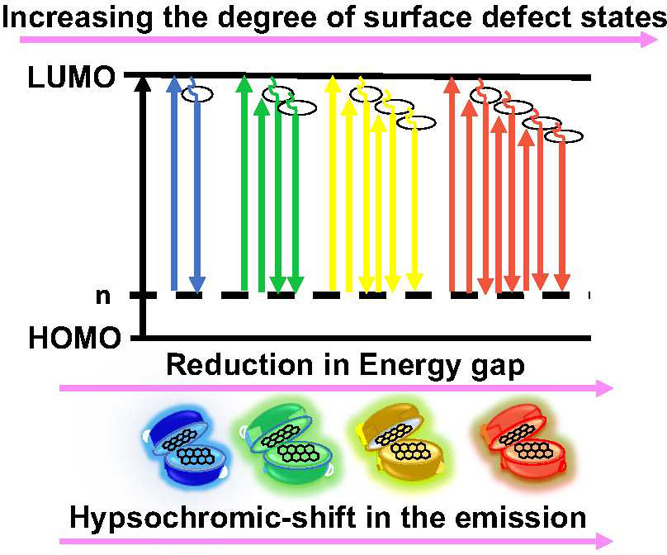
Schematic diagram of the size-dependent hypsochromic shift in the emission of CQDs.
Figure 8.
(A and B) Localization of the nanoformulation (G6-Cy5) in macrophages (Iba-1-positive) and neurons (TUJ1-positive) after 72 h of HCA. The images are from ref (18) and are reproduced after copyright permission from Elsiever.
2.5. Micelles
These are unilayered amphiphilic nanocarriers that allow the controlled release of encapsulated drugs20,21 (Figure 7E). These nanoparticles permit enhanced passage of hydrophobic drugs through the BBB for brain-related therapy.20,21 Micelles have also shown promising results as a nanocarrier for various active molecules such as small molecules and peptides. These nanoparticles have been observed to potentially treat AD as well as provide magnetic resonance for imaging brain inflammation and strokes. However, the associated limitations include the high cost of production, being prone to deformation and disassembly, high instablity, and an inability to be stored for future use. The safety issue associated with micelles includes the onset of a complement-induced immunogenic response (e.g., a Taxol-loaded micellar formulation).12
In a study carried out by Shiraishi et al., gadolinium-micelles (Gd-micelles) were prepared as an MRI contrast agent. After about half an hour of intravenous injection into a rat, clear contrast images of the ischemic hemisphere revealed increased permeability of the BBB and the distribution area of the nanoparticle in the ischemic hemisphere.20 Therefore, this nanoparticle can be considered to be a promising clinical diagnostic for finding recombinant human tissue plasminogen activator-dependent intracranial hemorrhage risks. In another study carried out by Yin et al., a nanodrug delivery system composed of doxorubicin-loaded poly(lactic-glycolic) acid-lysoGM1 micelles was developed, and a good encapsulation percentage (∼61%) of this low-solubility drug was achieved. This nanosystem could easily cross the BBB and accumulate inside the brain parenchyma through micropinocytosis and lysosomal pathways as evident by in vivo studies on mice and zebra fish21 (Figure 10). The nanoformulation showed an excellent antiglioma effect in rats, indicating its potential as an antiglioma drug.
Figure 10.
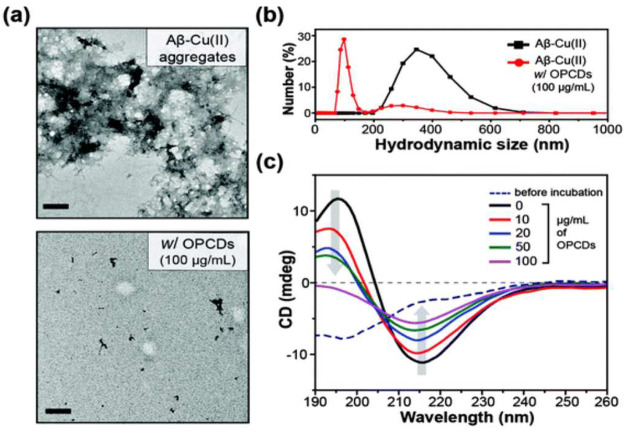
(a) High-resolution TEM image, (b) analysis of hydrodynamic size, and (c) secondary structure analysis of Cu(II)-induced amyloid-β aggregation in the absence and presence of OPCDs. The image is from ref (24) and is reproduced after copyright permission from the Royal Society of Chemistry.
2.6. Carbon Nanotubes (CNTs)
These nanostructures are composed of nanoscaled cylinders of graphene sheets. CNTs have emerged as a promising nanocarrier system and therapeutic agent in many brain-specific therapies. These promising activities of CNTs can be attributed to their ability to undergo surface functionalization easily with specific chemical compounds. Such surface modifications contribute to the variation in their physical and biological properties22 (Figure 7F). For instance, as observed in many in vitro and in vivo model experiments, polymer-coated carbon nanodots and chemically functionalized multiwalled carbon nanotubes (MWCNTs) are able to easily bypass BBB and also form an interface with neurons. This results in enhanced uptake of CNTs at the site of a tumor.22 However, the associated limitations include toxicity, no control over CNT length and chirality, batch-to-batch variation, polydispersity in CNT type, and a high cost of production. These nanocarriers have been reported to induce mesothelioma, lung inflammation upon accumulation, and complement-system activation.12 Also, studies on mice have shown that at a dose of 3 mg/mouse these nanocarriers displayed a carcinogenic effect.12
In a study carried out by Lohan et al., Berberine, an isoquinoline alkaloid and an anti-Alzheimer’s drug, was introduced on the surface of MWCNTs. MWCNTs were also surface modified with phospholipids and polysorbate to enhance their bioavailability. During in vitro experiments, no significant toxicity against SHSY-5Y cells was observed. Later, in vivo experiments pointed to the recovery of memory in rats after the 18th to 20th day of nanoformulation administration. Brain and plasma tissues of rats displayed the absorption of drug, indicating the crossing of BBB by the nanoformulation. Thus, drug-loaded phospholipid/polysorbate-coated MWCNTs can help in potentially reducing amyloid-β aggregation at the target site in AD patients.22
2.7. Carbon Dots (CDs)
As a new class of zero-dimensional carbonaceous nanomaterials, CDs display improved properties compared to traditional inorganic quantum dots or noble metal nanoclusters, making them promising luminescent nanocarriers and probes. One of the most intriguing characteristics of CDs is their tunable fluorescence (fully color-tunable fluorescent from the blue to the near-infrared regions) (Figure 9) and ease of surface modification for targeted delivery. These powerful tools may be used for chemo- or biosensing based on their tunable luminescence properties. CDs are often used in the biomedical field because of their array of physical and optical properties that include feasible fluorescence, adjustable stability, and water solubility. CDs have good biocompatibility and low toxicity, and their synthesis methods are cost-effective (Figure 7G). However, some of the limitations include photobleaching, instability, particle–particle aggregation, creation of a nonhomogeneous mixture, and their inability to be stored for very long times. CDs with sizes below 5 nm were observed to show more toxic effects than the ones with a size above 10 nm. This points toward the importance of size-induced toxicity. Also, trophic-level specific toxicity and oxidative stress were also reported.23
In a study, o-phenylenediamine (OPD)-based nitrogen-containing polyaromatic-surface-rich CDs were prepared. These CDs displayed Cu(II) chelation (responsible for AB aggregation and AD pathogenesis), acted as a β-sheet-rich structure breaker, and caused light-induced amyloid-β oxidation (Figure 10). Thus, multifunctional CDs can be employed to alleviate amyloid-β-related toxicity.24
2.8. Carbon Nano-onions (CNOs)
This carbonaceous zero-dimensional nanostructure exhibits unique electronic and structural features that highlight them as an excellent candidate for biomedical applications. These features includes a broad absorption band, a large surface area to volume ratio, the ability to reversibly accept multiple electrons, biocompatibility, and thermal stability.25 Because of the poor solubility of CNOs, they cannot be used in their pristine form. Hence, surface functionalization is carried out to avoid aggregation in organic and inorganic solvents due to intermolecular interactions (e.g., van der Waals forces) between the pristine nanostructures. These onion-like carbons are synthesized via thermal annealing of nanodiamonds at very high temperatures in an inert atmosphere and under high-pressure conditions and are on average composed of six to eight graphitic shells. Their average size is in the range 5 to 6 nm. The distance between two graphitic layers is 0.335 nm, and hence the structure is termed as graphitic (Figure 7H) . However, some of the limitations of using CNOs includes the inability to prepare well-dispersed CNOs in aqueous solution due to their hydrophobic nature which might result in bioaccumulation and hence the toxicity of these nanocarriers, batch-to-batch variation (size and number of graphitic shells depending on the synthesis method), and inability to easily bypass the BBB due to their high molecular weight, which might result in a disruption of the integrity of the BBB.25
In a study carried out by Sarkar et al., CNOs were developed to transport an anti-Alzheimer’s drug across the BBB.26 In their study, water-soluble CNOs (wsCNOs) in the size range of 25–50 nm were prepared and loaded with an acetylcholinesterase inhibitor. Thus, Trojan horse-like nanostructures were formed in where the inhibitor could be released in a sustained fashion at physiological pH. Then, these wsCNOs were tested for their cargo ability to cross the BBB and inhibit the acetylcholinesterase enzyme at the target site in glioblastoma multiform induced mice (GBM) and the cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) murine model.
3. Other Strategies for Enhanced Drug Delivery across the BBB
3.1. Lipoproteins
Low-density lipoprotein-receptor-related proteins (LRP-1 and LRP-2) are used as carriers. LRP, a multifunctional endocytic receptor, allows the internalization and degradation of ligands that are involved in metabolic pathways. It also allows the endocytosis of various secreted proteins by directly interacting with them or by activating various signal pathways, resulting in the degradation of these proteins.27 Although nanoparticles have been employed for drug delivery across the BBB, the exact mechanism of the transcytosis is not clearly known yet. One possible mechanism could be the ability of surfactant-coated nanoparticles such as polysorbate 80-coated polybutyl-cyanoacrylate nanoparticles to absorb apolipoproteins (such as B and E) from the bloodstream and employ LRP to cross the brain parenchyma via transcytosis.28 These proteins have been observed to prevent polymeric nanostructure-induced complement activation presumably based on their interaction with these nanostructures.12 Interestingly, Angiochem Inc. has been developing angiopep peptides to enhance the uptake of therapeutic agents by the brain parenchyma. ANG1005, the most advanced peptide, is formed by the chemical conjugation of Angiopep-2 with three molecules of paclitaxel and is used to treat brain cancers. Adenosine receptor (AR) substrate modulates BBB permeability and can be used to enhance the uptake of drug by either activating the A2A receptors or by restricting the entry of neurotoxic substances into the CNS.28
3.2. Ultrasound and Microbubbles
Sonoporation with focused ultrasound and added intravenously injected microbubbles (MBs) allow more location-specific drug delivery.29 Microbubbles and ultrasound, which result in sonopermeation, the formation of pores, and other structural changes in the permeability of the BBB, can be used to target the tumor area or other parts of the brain. Pore formation does not require high-energy ultrasound and hence does not cause any damage to the tertiary tissues, thereby enhancing nanoparticle or noncompound entry into the brain parenchyma.29 However, such temporary disruption of the BBB is risky and may damage the integrity of the BBB. Compromised integrity of the BBB may lead to the uncontrolled influx of drugs and toxins into the BBB as a result of the loss of homeostatic function of this barrier to forming cells.29
4. Nanocarriers in Clinical Trials
The unique properties (mentioned in detail in section 2) of these nanomaterials make them a suitable candidate for targeted drug delivery through the most complicated organs of the human body, such as the brain. These manipulated nanocarriers have the ability to efficiently overcome the BBB.30 The associated advantages include the encapsulation and delivery of hydrophobic drugs, reduced systemic side effects, increased therapeutic efficacy, decreased therapeutic dose, and sustained and controlled release of the therapeutic drug at the destined location while minimizing the toxic effects of the encapsulated or adsorbed therapeutic agents. Table 1 summarizes some of the promising nanocarriers in clinical trials relevant to the treatment of neurodegenerative diseases, whereas Figure 11 summarizes the existing obstacles or requirements for the clinical translation of these nanocarriers.
Table 1. Advantages of Nanocarriers in the Treatment of Neurodegenerative Disease30−34.
| type of nanocarrier used for clinical trial | size range (nm) | neurodegenerative disease | drug | applications and advantages |
|---|---|---|---|---|
| polymeric nanoparticle | 1–100 | Alzheimer’s disease | MEM-PEG-PLGA-NPs | reduction of β-amyloid plaques, disruption of β-amyloid (Aβ) aggregates |
| Parkinson’s disease | PLGA-NPs | reach lysosomes and restore their impaired function | ||
| liposome | 50–450 | Huntington’s disease | apolipoprotein E (ApoE) amino acid | increase cellular drug uptake as a versatile “nanovehicle” to deliver drug across the BBB |
| dendrimers | 1.5–10 | Alzheimer’s disease | KLVFF peptides | prevent Aβ-induced spatial memory impairments |
| carbon nanotubes (CNTs) | 1–100 | stroke | aggregated single-walled CNTs | ability to repair damaged neural tissue |
| micelles | 10–100 | Alzheimer’s disease | resveratrol-loaded polymeric micelles | protect cells against Aβ-induced oxidative stress and apoptosis |
Figure 11.
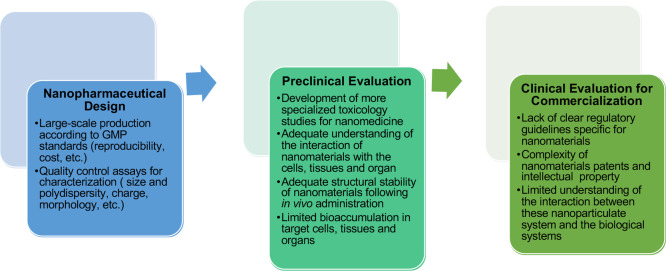
Existing obstacles or requirements for the clinical translation of a drug.35
5. Conclusions and Outlook
Brain diseases and correlated illnesses lack proper treatment because of their inability to cross the BBB. For an administered drug to show its effect at the target site, it first needs to pass through this highly selective barrier. The development of a drug small enough to circulate past this “gate” is still being researched and tested. Diseases such as AD have no cure due to the inability of these drug molecule to cross the BBB. Thus, the application of nanotechnology is significant in CNS-related disorders when speaking of delivering drugs to the brain. DDS includes polymeric nanoparticles, liposomes, dendrimers, micelles, and carbon nanotubes. These nanocarriers are known to deliver drug at a certain location, providing protection against enzymatic degradation and resulting in minimized immunogenicity, enhanced plasma stability and solubility, and drug release in a controlled and sustained manner and are small enough to cross the BBB and avoid reticuloendothelial clearance. Understanding how nanoparticles function in greater detail will help to develop new forms of treatment for brain diseases that currently limit research related to CNS disorders. Moreover, the selective targeting of the drug only to cerebral circulation will allow enhanced uptake of the compound by the brain, allowing minimized toxicity to other organs of the body. This may include designing vectors that are able to release drug only in response to an external stimulus (e.g., pH or photoactivation by a laser beam). For drug delivery, the nanocarrier should remain stable in the blood, should be able to bypass renal clearance and plasma protein binding, should be able to penetrate the BBB, and should be able to overcome active efflux by Pgp or other mechanisms. While currently we have some solutions to these problems individually, the perfect candidate for the treatment of CNS disorders would be the one that is able to solve all of these issues at once. While there are several techniques described in the literature to deliver drug molecules across the BBB, only a handful of them have resulted in clinical translation as mentioned in section 4. These limited clinical translations can be ascribed to an expensive and time-consuming process, scalability complexities, and biocompatibility and safety issues. Therefore, a better understanding of the interactions among different factors related to nanoparticle structure will allow the development of more specific and successful drug delivery system for more efficient delivery of therapeutic drug and diagnostic molecules across the brain parenchyma. Overall, these nanocarriers have the potential to show major impact in CNS-related disorders. However, there are still many issues that need to be addressed. These, in addition to previously mentioned ones, include cost, hurdles related to manufacturing and commercialization, and a reduction in the complexity of the nanosystem and regulatory standards. To summarize, the future of these nanocarriers in the field of treatment of CNS-related disorders and bypassing the BBB to deliver therapeutic doses of the drug is very promising.
Acknowledgments
M.N. and J.A. acknowledge NIH grant number 1SC3 GM111200 01A1. Research reported in this article was supported by the National Institute of General Medical Sciences of the National Institutes of Health under linked award numbers RL5GM118969 and UL1GM118970. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. M. N. and J.A. thank Drs Lourdes Echegoyen and Steve Aley for the opportunity to conduct the lab. J.A. thanks K. C. Rodriguez (B.S., UTEP). This mini-review is an outcome of a 1-year research-driven course under the Freshman Year Research Intensive Sequence (FYRIS) series sponsored by the National Institutes of Health. Therefore, the students were divided into 2 large groups consisting of 12 and 11 students, respectively. Later, the research articles were provided, which are studied and summarized here in this mini-review after the experimental part of the FYRIS laboratory every week. J.A. is the teaching assistant for this course and teaches the laboratory under the supervision of M.N. J.A. came up with the idea, researched, collected research articles, and supervised and guided the students. G.G.B. and S.M.A. volunteered to teach the laboratory. N.A. provided suggestions while writing the manuscript. All work was performed under the great supervision of M.N.
Biographies

Jyoti Ahlawat is a third-year Ph.D. student pursuing her doctorate degree in the field of chemistry. Her research work is centered around nanotechnology-enabled therapeutics. She is the lead researcher in the creation and development of nanohybrids which can be used to increase the bioavailability of selected phytochemicals. Presently, she is also working on the development of nanomaterials with amyloidogenic fibril disintegrating properties.
Gileydis Guillama Barroso graduated with a bachelor’s degree in chemistry from the University of Havana.
Shima Masoudi Asil is a first-year Ph.D. student pursuing her doctorate degree in the field of environmental science and engineering from UTEP under the guidance of Dr. Mahesh Narayan.
Melinda Alvarado is pursuing a bachelor’s degree in the College of Science, UTEP.
Isabela Armendariz is pursuing a bachelor’s degree in the College of Science, UTEP
Jose Bernal is pursuing a bachelor’s degree in the College of Science, UTEP.
Ximena Carabaza is pursuing a bachelor’s degree in the College of Science, UTEP.
Stephanie Chavez is pursuing a bachelor’s degree in the College of Science, UTEP.
Paulina Cruz is pursuing a bachelor’s degree in the College of Science, UTEP.
Vassti Escalante is pursuing a bachelor’s degree in the College of Science, UTEP.
Savana Estorga is pursuing a bachelor’s degree in the College of Science, UTEP.
Daniel Fernandez is pursuing a bachelor’s degree in the College of Science, UTEP.
Carolina Lozano is pursuing a bachelor’s degree in the College of Science, UTEP.
Martin Marrufo is pursuing a bachelor’s degree in the College of Science, UTEP.
Nabeel Ahmad is an associate professor of biotechnology, serving as the head of department in the School of Biotechnology, IFTM University, Moradabad, India.
Sergio Negrete is pursuing a bachelor’s degree in the College of Science, UTEP.
Karyme Olvera is pursuing a bachelor’s degree in the College of Science, UTEP.
Ximena Parada is pursuing a bachelor’s degree in the College of Science, UTEP.
Brianna Portillo is pursuing a bachelor’s degree in the College of Science, UTEP.
Andrea Ramirez is pursuing a bachelor’s degree in the College of Science, UTEP.
Raul Ramos is pursuing a bachelor’s degree in the College of Science, UTEP.
Veronica Rodriguez is pursuing a bachelor’s degree in the College of Science, UTEP.
Paola Rojas is pursuing a bachelor’s degree in the College of Science, UTEP.
Jessica Romero is pursuing a bachelor’s degree in the College of Science, UTEP.
David Suarez is pursuing a bachelor’s degree in the College of Science, UTEP.
Graciela Urueta is pursuing a bachelor’s degree in the College of Science, UTEP.
Stephanie Viel is pursuing a bachelor’s degree in the College of Science, UTEP.

Mahesh Narayan is currently serving as a professor in the Department of Chemistry and Biochemistry at the University of Texas at El Paso. In summary, he has authored and co-authored 75 research and review articles and book chapters in the areas of free radical biology, protein–structure function, oxidative folding and protein misfolding, halogen bonding, and in silico drug design for a collective citation count of 3103. Five other manuscripts have been submitted, are under revision, or are being prepared. He retains corresponding authorship of 84% of the papers he has published post-tenure. His work has been recognized through invitations to speaking engagements in over 15 international forums and by recognition in a variety of media outlets. Currently, he serves on the editorial board of PLOS One (Public Library of Science) and the editorial board of Cell Biochemistry and Biophysics (Springer) starting in 2016. He has been invited to deliver lectures at the Institute of NanoChemistry and NanoBiology of Shanghai University.
Author Contributions
# M.A., I.A., J.B., X.C., S.C., P.C., V.E., S.E., D.F., C.L., M.M., S.N., K.O., X.P., B.P., A.R., R.R., V.R., P.R., J.R., D.S., G.U., and S.V. contributed equally.
The authors declare no competing financial interest.
References
- Barchet T. M.; Amiji M. M. Challenges and opportunities in CNS delivery of therapeutics for neurodegenerative diseases. Expert Opin. Drug Delivery 2009, 6 (3), 211–225. 10.1517/17425240902758188. [DOI] [PubMed] [Google Scholar]
- Habgood M.; Ek J. Delivering drugs into the brain: barriers and possibilities. Ther. Delivery 2010, 1 (4), 483–488. 10.4155/tde.10.58. [DOI] [PubMed] [Google Scholar]
- Zhou Y.; Peng Z.; Seven E. S.; Leblanc R. M. Crossing the blood-brain barrier with nanoparticles. J. Controlled Release 2018, 270, 290–303. 10.1016/j.jconrel.2017.12.015. [DOI] [PubMed] [Google Scholar]
- He Q.; Liu J.; Liang J.; Liu X.; Li W.; Liu Z.; Ding Z.; Tuo D. Towards improvements for penetrating the blood–brain barrier—recent progress from a material and pharmaceutical perspective. Cells 2018, 7 (4), 24. 10.3390/cells7040024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redzic Z. Molecular biology of the blood-brain and the blood-cerebrospinal fluid barriers: similarities and differences. Fluids Barriers CNS 2011, 8 (1), 3. 10.1186/2045-8118-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davson H.; Segal M.. Special Aspects of the Blood–Brain Barrier. Physiology of the CSF and Blood–Brain Barriers; CRC Press: Boca Raton, FL, 1996; pp 303–485. [Google Scholar]
- Lee M.; Jayant R. Penetration of the blood-brain barrier by peripheral neuropeptides: new approaches to enhancing transport and endogenous expression. Cell Tissue Res. 2019, 375 (1), 287–293. 10.1007/s00441-018-2959-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savolainen J.; Edwards J. E.; Morgan M. E.; McNamara P. J.; Anderson B. D. Effects of a P-glycoprotein inhibitor on brain and plasma concentrations of anti-human immunodeficiency virus drugs administered in combination in rats. Drug Metab. Dispos. 2002, 30 (5), 479–482. 10.1124/dmd.30.5.479. [DOI] [PubMed] [Google Scholar]
- Shukla S.; Wen A. M.; Ayat N. R.; Commandeur U.; Gopalkrishnan R.; Broome A.-M.; Lozada K. W.; Keri R. A.; Steinmetz N. F. Biodistribution and clearance of a filamentous plant virus in healthy and tumor-bearing mice. Nanomedicine 2014, 9 (2), 221–235. 10.2217/nnm.13.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niethammer M.; Tang C. C.; LeWitt P. A.; Rezai A. R.; Leehey M. A.; Ojemann S. G.; Flaherty A. W.; Eskandar E. N.; Kostyk S. K.; Sarkar A., Long-term follow-up of a randomized AAV2-GAD gene therapy trial for Parkinson’s disease. JCI Insight 2017, 2( (7), ), 10.1172/jci.insight.90133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlawat J.; Henriquez G.; Narayan M. Enhancing the delivery of chemotherapeutics: role of biodegradable polymeric nanoparticles. Molecules 2018, 23 (9), 2157. 10.3390/molecules23092157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfram J.; Zhu M.; Yang Y.; Shen J.; Gentile E.; Paolino D.; Ferrari M. Safety of nanoparticles in medicine. Curr. Drug Targets 2015, 16 (14), 1671–1681. 10.2174/1389450115666140804124808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbara R.; Belletti D.; Pederzoli F.; Masoni M.; Keller J.; Ballestrazzi A.; Vandelli M. A.; Tosi G.; Grabrucker A. M. Novel Curcumin loaded nanoparticles engineered for Blood-Brain Barrier crossing and able to disrupt Abeta aggregates. Int. J. Pharm. 2017, 526 (1–2), 413–424. 10.1016/j.ijpharm.2017.05.015. [DOI] [PubMed] [Google Scholar]
- Guccione C.; Oufir M.; Piazzini V.; Eigenmann D. E.; Jähne E. A.; Zabela V.; Faleschini M. T.; Bergonzi M. C.; Smiesko M.; Hamburger M. Andrographolide-loaded nanoparticles for brain delivery: Formulation, characterisation and in vitro permeability using hCMEC/D3 cell line. Eur. J. Pharm. Biopharm. 2017, 119, 253–263. 10.1016/j.ejpb.2017.06.018. [DOI] [PubMed] [Google Scholar]
- Shaw T. K.; Mandal D.; Dey G.; Pal M. M.; Paul P.; Chakraborty S.; Ali K. A.; Mukherjee B.; Bandyopadhyay A. K.; Mandal M. Successful delivery of docetaxel to rat brain using experimentally developed nanoliposome: a treatment strategy for brain tumor. Drug Delivery 2017, 24 (1), 346–357. 10.1080/10717544.2016.1253798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman M.; Madni A.; Shi D.; Ihsan A.; Tahir N.; Chang K.; Javed I.; Webster T. Enhanced blood brain barrier permeability and glioblastoma cell targeting via thermoresponsive lipid nanoparticles. Nanoscale 2017, 9 (40), 15434–15440. 10.1039/C7NR05216B. [DOI] [PubMed] [Google Scholar]
- Zhao L.; Zhu J.; Cheng Y.; Xiong Z.; Tang Y.; Guo L.; Shi X.; Zhao J. Chlorotoxin-conjugated multifunctional dendrimers labeled with radionuclide 131I for single photon emission computed tomography imaging and radiotherapy of gliomas. ACS Appl. Mater. Interfaces 2015, 7 (35), 19798–19808. 10.1021/acsami.5b05836. [DOI] [PubMed] [Google Scholar]
- Zhang F.; Magruder J. T.; Lin Y.-A.; Crawford T. C.; Grimm J. C.; Sciortino C. M.; Wilson M. A.; Blue M. E.; Kannan S.; Johnston M. V. Generation-6 hydroxyl PAMAM dendrimers improve CNS penetration from intravenous administration in a large animal brain injury model. J. Controlled Release 2017, 249, 173–182. 10.1016/j.jconrel.2017.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janaszewska A.; Lazniewska J.; Trzepiński P.; Marcinkowska M.; Klajnert-Maculewicz B. Cytotoxicity of Dendrimers. Biomolecules 2019, 9, 330. 10.3390/biom9080330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi K.; Wang Z.; Kokuryo D.; Aoki I.; Yokoyama M. A polymeric micelle magnetic resonance imaging (MRI) contrast agent reveals blood–brain barrier (BBB) permeability for macromolecules in cerebral ischemia-reperfusion injury. J. Controlled Release 2017, 253, 165–171. 10.1016/j.jconrel.2017.03.020. [DOI] [PubMed] [Google Scholar]
- Yin Y.; Wang J.; Yang M.; Du R.; Pontrelli G.; McGinty S.; Wang G.; Yin T.; Wang Y. Penetration of the blood–brain barrier and the anti-tumour effect of a novel PLGA-lysoGM1/DOX micelle drug delivery system. Nanoscale 2020, 12 (5), 2946–2960. 10.1039/C9NR08741A. [DOI] [PubMed] [Google Scholar]
- Ma X.; Zhong L.; Guo H.; Wang Y.; Gong N.; Wang Y.; Cai J.; Liang X. J. Multiwalled Carbon Nanotubes Induced Hypotension by Regulating the Central Nervous System. Adv. Funct. Mater. 2018, 28 (11), 1705479. 10.1002/adfm.201705479. [DOI] [Google Scholar]
- Yao K.; Lv X.; Zheng G.; Chen Z.; Jiang Y.; Zhu X.; Cai Z. Effects of Carbon Quantum Dots on Aquatic Environments: Comparison of Toxicity to Organisms at Different Trophic Levels. Environ. Sci. Technol. 2018, 52 (24), 14445–14451. 10.1021/acs.est.8b04235. [DOI] [PubMed] [Google Scholar]
- Chung Y. J.; Lee B. I.; Park C. B. Multifunctional carbon dots as a therapeutic nanoagent for modulating Cu (ii)-mediated β-amyloid aggregation. Nanoscale 2019, 11 (13), 6297–6306. 10.1039/C9NR00473D. [DOI] [PubMed] [Google Scholar]
- Plonska-Brzezinska M. E. Carbon Nano-Onions: A Review of Recent Progress in Synthesis and Applications. ChemNanoMat 2019, 5 (5), 568–580. 10.1002/cnma.201800583. [DOI] [Google Scholar]
- Pakhira B.; Ghosh M.; Allam A.; Sarkar S. Carbon nano onions cross the blood brain barrier. RSC Adv. 2016, 6 (35), 29779–29782. 10.1039/C5RA23534K. [DOI] [Google Scholar]
- May P.; Woldt E.; Matz R. L.; Boucher P. The LDL receptor-related protein (LRP) family: An old family of proteins with new physiological functions. Ann. Med. 2007, 39 (3), 219–228. 10.1080/07853890701214881. [DOI] [PubMed] [Google Scholar]
- Thomas F. C.; Taskar K.; Rudraraju V.; Goda S.; Thorsheim H. R.; Gaasch J. A.; Mittapalli R. K.; Palmieri D.; Steeg P. S.; Lockman P. R. Uptake of ANG1005, a novel paclitaxel derivative, through the blood-brain barrier into brain and experimental brain metastases of breast cancer. Pharm. Res. 2009, 26 (11), 2486–2494. 10.1007/s11095-009-9964-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher D. G.; Price R. J., Recent Advances in the Use of Focused Ultrasound for Magnetic Resonance Image-Guided Therapeutic Nanoparticle Delivery to the Central Nervous System. Front. Pharmacol. 2019, 10, 10.3389/fphar.2019.01348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poovaiah N.; Davoudi Z.; Peng H.; Schlichtmann B.; Mallapragada S.; Narasimhan B.; Wang Q. Treatment of neurodegenerative disorders through the blood–brain barrier using nanocarriers. Nanoscale 2018, 10 (36), 16962–16983. 10.1039/C8NR04073G. [DOI] [PubMed] [Google Scholar]
- Calzoni E.; Cesaretti A.; Polchi A.; Di Michele A.; Tancini B.; Emiliani C. Biocompatible polymer nanoparticles for drug delivery applications in cancer and neurodegenerative disorder therapies. J. Funct. Biomater. 2019, 10 (1), 4. 10.3390/jfb10010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Re F.; Cambianica I.; Zona C.; Sesana S.; Gregori M.; Rigolio R.; La Ferla B.; Nicotra F.; Forloni G.; Cagnotto A. Functionalization of liposomes with ApoE-derived peptides at different density affects cellular uptake and drug transport across a blood-brain barrier model. Nanomedicine 2011, 7 (5), 551–559. 10.1016/j.nano.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Xiang C.; Zhang Y.; Guo W.; Liang X.-J., Biomimetic carbon nanotubes for neurological disease therapeutics as inherent medication. Acta Pharm. Sin. B 2020,10, 239. 10.1016/j.apsb.2019.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X.; Ji C.; Xu H.; Li X.; Ding H.; Ye M.; Zhu Z.; Ding D.; Jiang X.; Ding X. Resveratrol-loaded polymeric micelles protect cells from Aβ-induced oxidative stress. Int. J. Pharm. 2009, 375 (1–2), 89–96. 10.1016/j.ijpharm.2009.03.021. [DOI] [PubMed] [Google Scholar]
- Hua S.; De Matos M. B.; Metselaar J. M.; Storm G. Current trends and challenges in the clinical translation of nanoparticulate nanomedicines: pathways for translational development and commercialization. Front. Pharmacol. 2018, 9, 790. 10.3389/fphar.2018.00790. [DOI] [PMC free article] [PubMed] [Google Scholar]