Abstract
Background:
Mitral valve anomalies in children are rare but frequently severe, recalcitrant and not often amenable to primary repair, necessitating mechanical mitral valve replacement (M-MVR). This study examines outcomes of a cohort undergoing 1st M-MVR at <21 years of age.
Methods:
We queried the Pediatric Cardiac Care Consortium (PCCC), a multi-institutional US-based cardiac intervention registry, for patients undergoing 1st M-MVR for two-ventricle congenital heart disease. Survival and transplant status through 2014 were obtained from PCCC and linkage with the National Death Index and the Organ Procurement and Transplantation Network.
Results:
We identified 441 patients [median age 4.3 years (IQR: 1.3-10.1)] meeting study criteria. The commonest disease necessitating M-MVR was atrioventricular canal (44.3%). Early mortality (death <90 days post-M-MVR) was 11.1%; there was increased risk of early death if age at M-MVR was <2 years (OR 7.8; 95% CI:1.1-56.6) and with concurrent other mechanical valve placement (OR 8.5; 95% CI:2.0-35.6). In those surviving >90 days post-M-MVR, transplant-free survival was 76% at 20 years follow-up (median follow-up: 16.6, IQR: 11.9-21.3). Adjusted analysis in those who survived >90 days showed elevated risk of death/transplant for males (HR 1.5; 95% CI:1.0-2.3), age at M-MVR <2 years [10 year survival, HR 4.3 (95%CI: 1.2 - 15.1)], and non-bi-leaflet prosthesis placement (HR 2.4; 95% CI: 1.3-4.3).
Conclusions:
M-MVR is a viable strategy in children with unrepairable mitral valve disease. Age <2 years at 1st M-MVR is associated with significant early risk of death and poorer longterm survival.
Severe mitral valve (MV) abnormalities needing surgical intervention are rare in children, but in some, mitral valve replacement (MVR) becomes inevitable as the native valve is deemed unrepairable or repair fails [1–6]. In these cases, mechanical MVR (M-MVR) is most commonly performed as these are offered in smaller sizes and more durable than bio-prosthetic counterparts [7, 8]. While M-MVR can palliate serious MV abnormalities in children, those with M-MVR remain at risk for significant adverse events.
Outcomes, especially long-term, following M-MVR performed at <21 years of age are limited to single-institution or small samples due to the procedure’s rarity and lack of a reliable method to assess multi-institutional outcomes [4, 8, 9–10]. Thus, multi-institutional, registry-based datasets that can leverage other major event reporting resources may provide a better understanding of outcomes for this rare procedure. Understanding outcomes following M-MVR will assist with patient/family counseling and cardiac surgical decision-making.
The objective of this study is to better define transplant-free survival following 1st M-MVR performed for biventricular physiology congenital heart disease (CHD) at <21 years of age utilizing a multi-institutional registry (Pediatric Cardiac Care Consortium - PCCC) linked with the US National Death Index (NDI) and Organ Procurement and Transplantion Network (OPTN) to track survival and cardiac transplant status respectively. Ability to perform these linkages provided a unique opportunity to examine outcomes.
PATIENTS AND METHODS
Study Population:
The study included patients enrolled in the PCCC, a multi-institutional registry of children undergoing cardiac intervention [11–13]. The PCCC had acquired data from small and medium sized programs; representing up to 30% of all annual cardiac interventions performed in the US while the database was live between 1982 and 2011 [12]. Information collected by the registry included demographics, cardiac diagnoses, associated non-cardiac diagnoses, cardiac procedures and interventions and in-hospital outcomes.
The registry was queried for 1st M-MVR in children <21 years of age with underlying biventricular physiology CHD, who entered the database before April 15, 2003 (date of stricter implementation of Health Insurance Portability and Accountability Act, precluding linkage for patients entering the registry past this date). For those who entered PCCC prior to this date, M- MVR could occur through 2011. Patients were excluded if they had M-MVR in the setting of single ventricle CHD, MV disease due to connective tissue disorders, primary trauma to MV, rheumatic heart disease or endocarditis in a previously normal MV because of small numbers and unique clinical features in these groups. Patients were also excluded if they received initial M-MVR outside the PCCC to avoid immortal person-time bias because these patients would have had to survive to a second procedure to be included in PCCC, resulting in a bias if they were maintained in analyses [14]. Patients were not eligible if they were non-US residents, operated in non-US centers, lacked adequate identifiers for linkage or their operative record was unavailable or incomplete (Figure 1).
Figure 1:
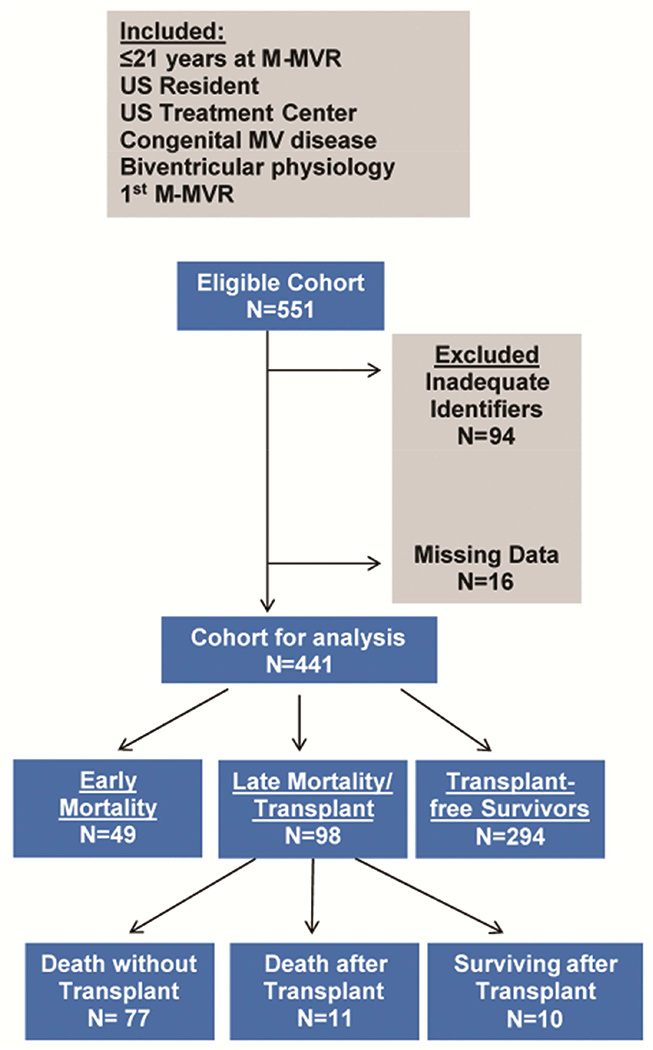
Inclusion/Exclusion Criteria
The PCCC was linked with NDI and OPTN to determine survival and cardiac transplant status through December 31, 2014. Identifiers used for linkage included first and middle name (when available), last name, date of birth, sex, state of last known residence, and state of birth. Sensitivity of this methodology for all PCCC patients sent for linkage reached 89% and 89.7% for NDI and OPTN respectively; specificity exceeded 99% in both cases [15].
The following variables were collected from PCCC: age, weight, race/ethnicity, date of M-MVR & hospital discharge or death, M-MVR size & type (bi-leaflet versus non-bi-leaflet), cardiac diagnoses, underlying MV functional abnormality (stenosis, regurgitation, or both), and presence of chromosomal anomalies. Patients were assigned one of the following primary diagnoses based on findings in operative and clinical records: complete atrioventricular canal defect (CAVC), partial atrioventricular canal defect (PAVC), levo-transposition of the great arteries (l-TGA), Shone’s syndrome (SS) or primary mitral valve anomaly (MVA). SS was defined as MV disease (resulting in stenosis, regurgitation or both) associated with other left heart obstructive lesions such as supramitral ring, aortic/subaortic stenosis or coarctation. MVA was classified as isolated (iMVA) when there was no coexisting cardiac lesion or complex (cMVA) if hemodynamically significant cardiac lesions, except those previously listed as primary diagnoses, were coexistent. Pacemaker implantation within 90 days post M-MVR was noted.
As there were no clearly defined time periods related to changes in management of MVR, year of 1st M-MVR was classified into tertiles to balance the number of patients between groups to assess era effect. Age group classifications were based on examination of raw data. Early mortality was reported as death or heart transplant ≤90 days post M-MVR based on published guidelines for uniform reporting of valve surgery outcomes [16]. Long-term transplant-free survival was assessed in those who survived >90 days post M-MVR (long-term cohort). Causes of death were assigned using underlying cause of death from NDI.
As a secondary analysis, to assess the potential impact of M-MVR procedure on survival within diagnoses, we performed a comparative analysis between two groups: patients with CAVC, PAVC and l-TGA needing M-MVR and patients with similar underlying diagnoses who did not proceed to M-MVR. SS and MVA were excluded within this analysis as those diagnoses have increased heterogeneity. To enhance inter-group comparability, we restricted groups to patients with continuous participation for at least five years within PCCC after initial surgery date to ensure M-MVR, if it occurred in that time, would have been captured. Additionally children with M-MVR were required to have at least one other procedure in PCCC to guarantee that they would have been included in PCCC even without M-MVR. Transplant-free survival was compared from time of child’s first cardiac operation, making the assumption that underlying physiology dictated need for M-MVR and is the factor of interest related to survival. This assumption is likely stronger for children requiring M-MVR at younger ages, therefore we restricted this comparison to those who received both their first cardiac surgery and M-MVR at age <6 years.
Statistical Methods
Among patients with M-MVR, characteristics were compared between children who died ≤90 days post-M-MVR and long-term cohort using chi-squared tests (categorical variables), t-tests and Wilcoxon rank-sum tests (continuous variables). Adjusted odds ratios (OR) for associations between characteristics and early mortality were estimated using logistic regression. Covariates assessed were determined a priori and include sex, prosthetic valve size (mm) to body weight (kg) ratio (Sz/Wt), era, age at M-MVR, primary diagnosis, underlying regurgitation or stenosis, genetic conditions, other concurrent mechanical valve replacement, bileaflet prosthetic valve, and pacemaker placement within 90 days of M-MVR. Kaplan-Meier survival curves were generated for transplant-free survival. Factors related to long-term survival were assessed using Cox models. The proportional hazards assumption was assessed graphically and by including an interaction term with time in model. Age at M-MVR, Sz/Wt and pacemaker placement <90 days post-M-MVR violated the proportional hazards assumption so were included in the model using Heaviside functions and interaction terms with time. In a multivariable survival model, backward elimination was used to exclude covariates with p >0.1 from the adjusted model; covariates were kept despite a higher p-value if their exclusion meaningfully changed the estimates of other factors in the model. A random effect was included to account for correlation within M-MVR treatment center. Collinearity issues were assessed using a macro [17]. Comparison of survival after initial cardiac surgery between those with and without M-MVR within the CAVC, PAVC and l-TGA diagnoses groups was performed using Kaplan-Meier survival estimates and Cox models as described above.
A sensitivity analysis was conducted to assess potential differences between those included in the analysis and patients excluded because of inadequate identifiers or missing M-MVR clinical information. In-hospital death at M-MVR procedure was available for all children for comparison. Other available characteristics were also compared between the two groups and then used to estimate the inverse probability of having full information for inclusion; available variables included era, sex, age, MV functional abnormality, concurrent mechanical valve replacements, genetic conditions, and pacemaker placement within 90 days. This probability was applied as a weight to the children analyzed who survived the initial M-MVR so that they would represent themselves and children with missing information that were similar on the characteristics assessed in order to estimate long-term survival for the full underlying sample.
Analyses were conducted using SAS v9.4 (Cary, NC). This study was approved by Institutional Review Boards of Children’s Mercy Hospital and Emory University School of Medicine.
RESULTS
A total of 441 children from 36 participating centers <21 years of age at 1st M-MVR were included (Figure 1). Median age at M-MVR was 4.3 years (IQR: 1.3-10.1). Over half the cohort (59.4%) were <6 years of age at M-MVR (Figure 2). The most prevalent cardiac diagnoses leading to M-MVR were CAVC or PAVC (44.3%) and most had underlying regurgitation (68.0%). Concurrent other mechanical valve replacement was performed in 7.7%. Pacemaker implantation was necessary within 90 days following M-MVR in 14.3% (Table 1).
Figure 2:
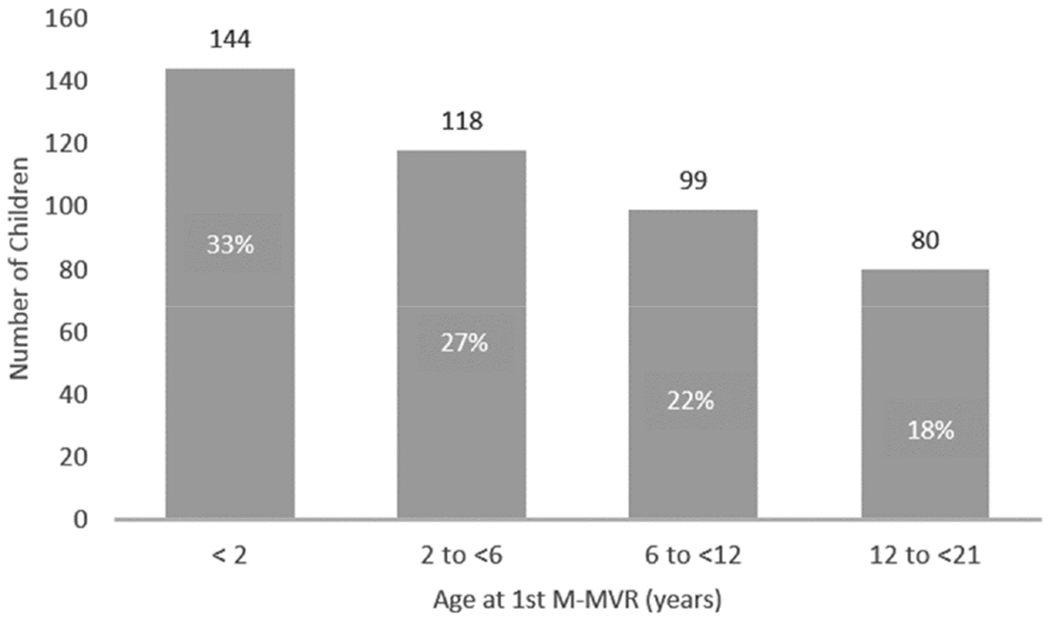
Age at 1st-M-MVR among eligible children treated <21 years of age for congenital mitral valve abnormalities in the PCCC
Table 1:
Cohort Characteristics: long-term cohort (survived >90 days) versus early mortality (died <90 days) post-1st M-MVR (unadjusted analysis)
| Total n=441 | Early Mortality n=49 | Long-term Cohort n=392 | Significant p-value | |
|---|---|---|---|---|
| M-MVR Era | ||||
| 1st Tertile: 1982-1992 | 133 (30.2) | 18 (36.7) | 115 (29.3) | |
| 2nd Tertile: 1993-1997 | 158 (35.8) | 19 (38.8) | 139 (35.5) | |
| 3rd Tertile: 1998-2011 | 150 (34.0) | 12 (24.5) | 138 (35.2) | |
| Male | 216 (49.0) | 25 (51.0) | 191 (48.7) | |
| Age at M-MVR (years) | ||||
| Median (IQR) | 4.3 (1.3-10.1) | 0.8 (0.4-2.0) | 5.1 (1.8-10.3) | <0.01 |
| Age (years) group | ||||
| <2 | 144 (32.6) | 36 (73.5) | 108 (27.6) | <0.01 |
| 2 - <6 | 118 (26.8) | 7 (5.9) | 111 (28.3) | |
| 6 - <12 | 99 (22.5) | 3 (6.1) | 96 (24.5) | |
| 12 - <21 | 80 (18.1) | 3 (6.1) | 77 (19.6) | |
| Sz/Wt Ratio (mm/kg) | ||||
| Median (IQR) | 1.6 (0.9-2.6) | 3.2 (2.1-3.7) | 1.5 (0.8-2.3) | <0.01 |
| >2 | 159 (36.6) | 37 (77.1) | 122 (31.5) | <0.01 |
| Missing | 6 | 1 | 5 | |
| Diagnostic Categories | ||||
| CAVC | 122 (27.7) | 23 (46.9) | 99 (25.3) | <0.01 |
| PAVC | 73 (16.6) | 2 (4.1) | 71 (18.1) | 0.01 |
| Shone Syndrome | 93 (21.9) | 14 (28.6) | 79 (20.2) | |
| l-TGA | 40 (9.1) | 3 (6.1) | 37 (9.4) | |
| MVA | 113 (25.6) | 7 (14.3) | 106 (27.0) | |
| Isolated (iMVA) | 52 (11.8) | 3 (6.1) | 49 (12.5) | |
| Complex (cMVA) | 61 (13.8) | 4 (8.2) | 57 (14.5) | |
| MV Functional Abnormality | ||||
| Mitral Stenosis | 86 (19.5) | 11 (22.5) | 75 (19.1) | |
| Mitral Regurgitation | 300 (68.0) | 32 (65.3) | 268 (68.4) | |
| Both | 55 (12.5) | 6 (12.2) | 49 (12.5) | |
| Mechanical Valve Type | ||||
| Bi-leaflet | 413 (94.7) | 46 (95.8) | 367 (94.6) | |
| Non-bi-leaflet | 23 (5.3) | 2 (4.2) | 21 (5.4) | |
| Missing | 5 | 1 | 4 | |
| Concurrent other mechanical valve replacement | 34 (7.7) | 6 (12.2) | 28 (7.1) | |
| Any | 26 (5.9) | 3 (6.1) | 23 (5.9) | |
| Aortic | 2 (0.5) | 1 (2.0) | 1 (0.3) | |
| Pulmonary | 6 (1.4) | 2 (4.1) | 4 (1.0) | |
| Tricuspid | ||||
| Genetic Conditions | ||||
| Down Syndrome | 79 (17.9) | 15 (30.6) | 64 (16.3) | 0.01 |
| Other | 36 (8.2) | 3 (6.1) | 33 (8.4) | |
| Pacemaker ≤ 90 days post-M-MVR | 63 (14.3) | 3 (6.1) | 60 (15.3) | |
Data presented as n (%) unless stated otherwise
Early mortality occurred in 11.1% (N=49), for whom median time to death was 5 days (IQR 1-16), resulting in 392 children in the long-term cohort. Figures 3A show the distribution of early mortality, late mortality and survivors by age groups. Differences in distribution of early versus late mortality within age groups are depicted in Figure 3B. Kaplan-Meier transplant-free survival is shown in Figure 4. No child in the early mortality cohort received heart transplant, however 5.4% of patients within the long-term cohort proceeded to cardiac transplantation.
Figure 3.
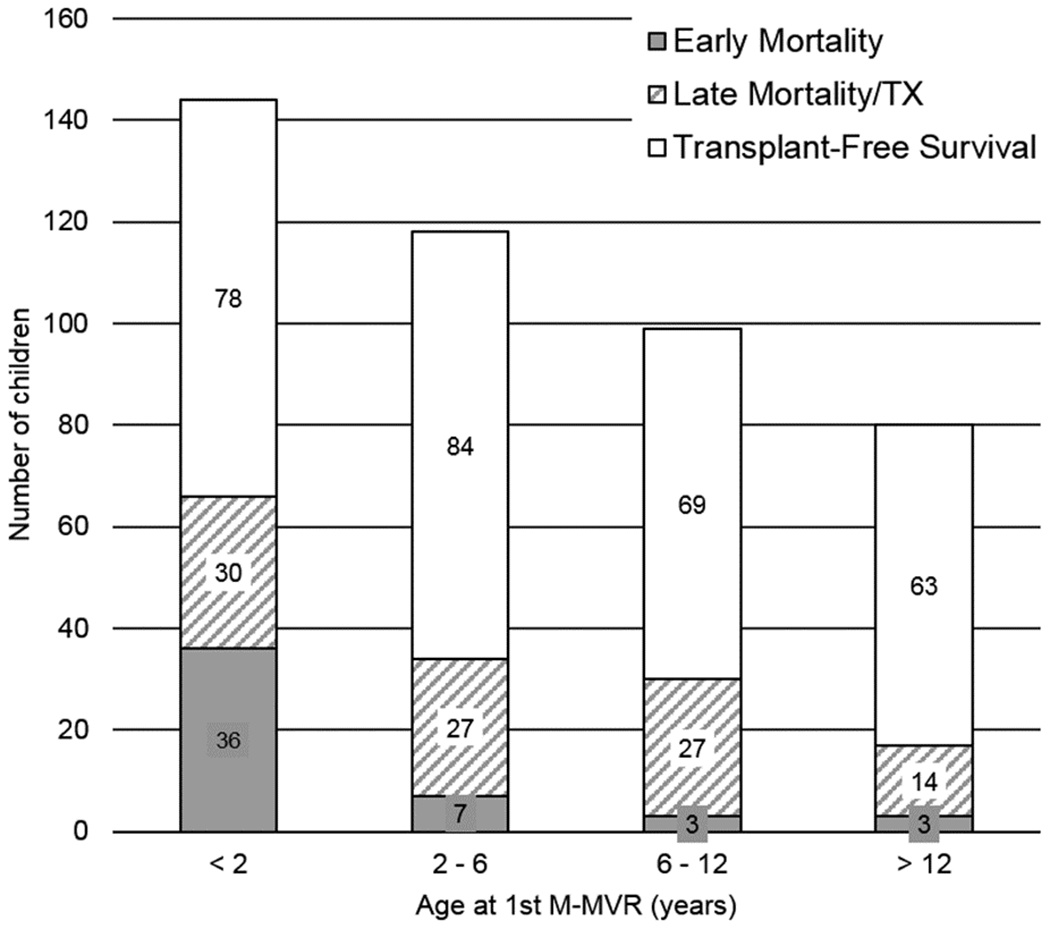
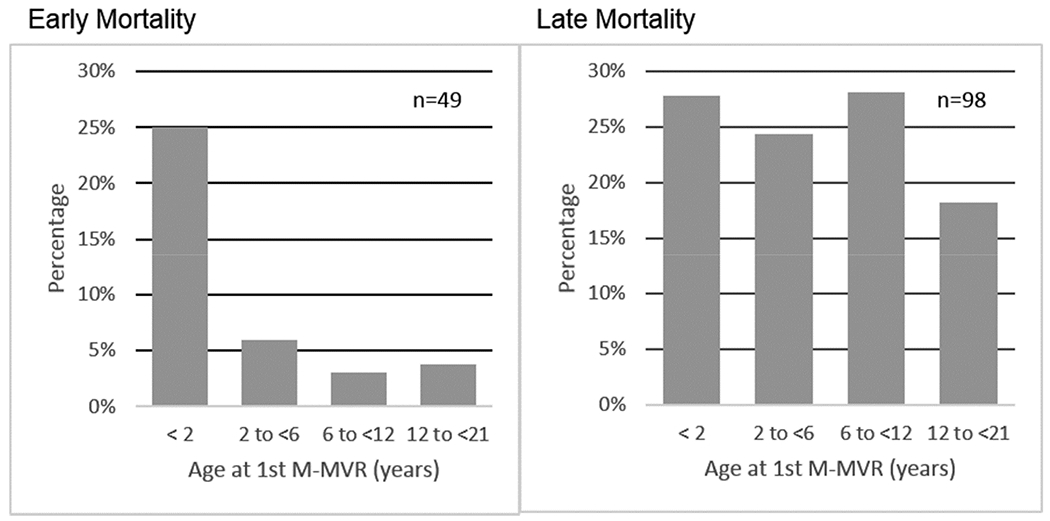
(a) Early mortality (≤90 days post-1st M-MVR), late mortality/cardiac transplant (>90 days post-1st M-MVR) and transplant free survivors by age at 1st-M-MVR. (b) Proportional illustration representing the age of mortality / cardiac transplant in patients undergoing M-MVR. Within cohort percentages in each age group are depicted on Y axis. Early mortality / cardiac transplant is predominant in those < 2 years of age at 1st M-MVR, late mortality / cardiac transplant is more evenly distributed across the age groups.
Figure 4:
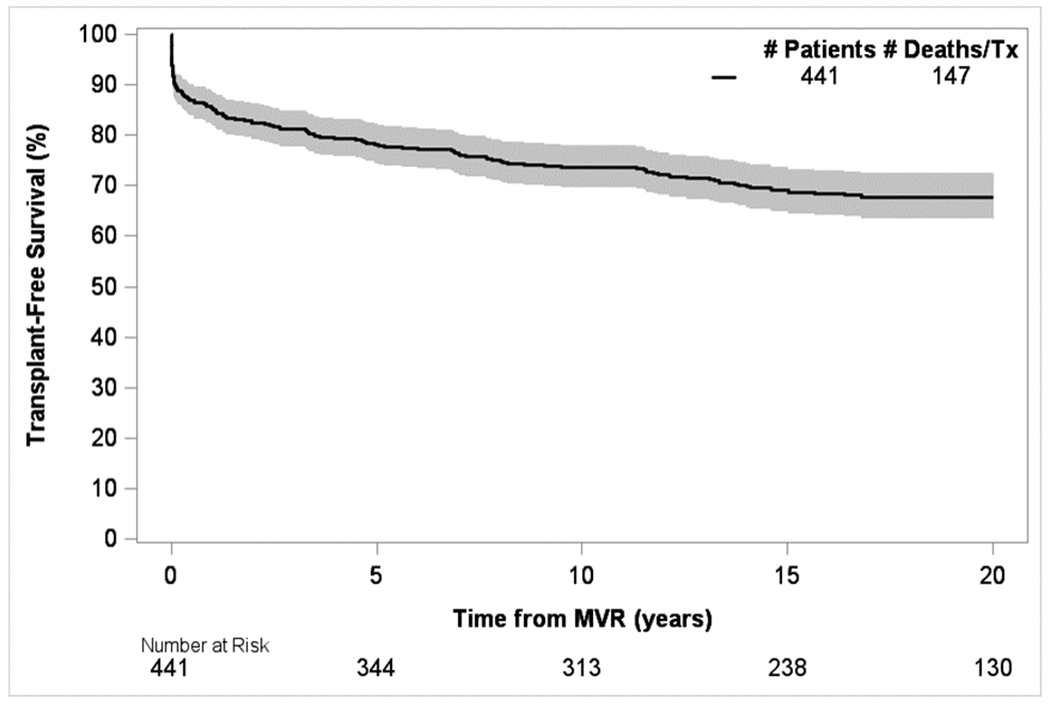
Overall transplant-free survival (including early and late mortality / cardiac transplant) after 1st M-MVR
Early Mortality Characteristics
Early mortality occurred predominantly in those <2 years (p<0.01), Sz/Wt ratio >2 (p<0.01), with Down syndrome (p=0.01), and with CAVC (p<0.01) in comparison to other diagnoses. The risk of early mortality was lower in patients with PAVC (unadjusted analyses - Table 1). In the adjusted model including all assessed covariates, age <2 years at M-MVR [(OR: 7.8 (1.1–56.6)] and concurrent other mechanical valve placement [(OR 8.5 (2.0–35.6)] were related to increased early mortality.
Long-Term Cohort
In those surviving >90 days post-M-MVR, long-term transplant-free survival at 20 years was 76% with a median length of follow-up of 16.6 years (IQR: 11.9-21.3). Adjusted analyses in this group showed that males [HR 1.5; 95% CI: 1.0-2.3], age at M-MVR <2 years [10 year survival, HR 4.3 (95% CI: 1.2-15.1)] and use of a non-bi-leaflet mechanical prosthesis [HR 2.4; 95% CI: 1.3-4.3] were associated with a higher rate of death or transplant (Table 2).
Table 2:
Predictors of death/cardiac transplant >90 days post-1st M-MVR
| Adjusted Hazards Ratio | 95% CI | Significant p-value | |
|---|---|---|---|
| Male vs female | 1.5 | 1.0 – 2.3 | 0.05 |
| Sz/wt >2 vs ≤ 2 | |||
| 1 year post-M-MVR | 1.5 | 0.5–4.3 | |
| 10 years post-M-MVR | 0.3 | 0.1–0.8 | 0.02 |
| Age <2 years vs. 12 - <21 years | |||
| 1 year post-M-MVR | 1.8 | 0.4–7.0 | |
| 10 years post-M-MVR | 4.3 | 1.2–15.1 | 0.02 |
| Age 2 - <6 years vs. 12 - <21 years | |||
| 1 year post-M-MVR | 1.7 | 0.6 –4.8 | |
| 10 years post-M-MVR | 1.7 | 0.6–4.3 | |
| Age 6 - <12 years vs. 12 - <21 years | |||
| 1 year post-M-MVR | 2.0 | 0.7–5.3 | |
| 10 years post-M-MVR | 1.9 | 0.8–4.8 | |
| Concurrent other valve replacement vs. none | 1.8 | 0.9–3.5 | |
| Non-bi-leaflet vs. bi-leaflet valve | 2.4 | 1.3 –4.3 | <0.01 |
| Pacemaker ≤90 days following M-MVR vs. none | |||
| 1 year post-M-MVR | 1.7 | 0.9–3.0 | |
| 10 years post-M-MVR | 1.1 | 0.6–2.1 | |
Time of hazard ratio assessment for variables violating the proportional hazards assumption. Age and Sz/Wt were modeled using Heaviside functions; other syndromes and pacemaker implantation were modeled using a continuous term for interaction with the natural log of time.
Adjusted model includes all variables listed in the table and a random effect for M-MVR treatment center. Era of M-MVR, MV regurgitation and/or stenosis, and primary diagnoses evaluated with these covariates had p>0.2 and did not meaningfully contribute to the model.
Causes of death were predominantly cardiac related directly to underlying CHD or other circulatory system disease such as stroke, endocarditis (Table 3).
Table 3:
Causes of early and late deaths post-1st M-MVR
| Total N (%) | Death ≤90 days post-M-MVR | Death >90 days post-M-MVR | ||
|---|---|---|---|---|
| After transplant | No transplant | |||
| Total deaths | 137 | 49 | 11 | 77 |
| Congenital Heart Disease | 70 (51.1) | 30 | 4 | 36 |
| Diseases of the Circulatory System | 36 (26.3) | 9 | 6 | 21 |
| Other Congenital Malformation | 14 | 8 | 0 | 6 |
| Deformation/Chromosomal Abnormality | (10.2) | |||
| External Causes of Injury | 3 (2.2) | 0 | 0 | 3 |
| Infections/Parasitic Diseases | 6 (4.4) | 0 | 1 | 5 |
| Other | 8 (5.8) | 2 | 0 | 6 |
Sensitivity Analysis
Compared with children included in the analysis, those excluded for missing information were more likely to have undergone M-MVR in the earliest era and be male. They were also more likely to die at the 1st M-MVR procedure (in-hospital mortality) (Supplemental Table 1). However, among survivors to hospital discharge after M-MVR, after applying inverse probability weighting, the transplant-free survival estimates did not meaningfully change from the original, non-weighted estimates across 20 years of follow up (Supplemental Table 2).
Additional Impact of MVR on Survival within Diagnoses
For this analysis 2,023 patients with CAVC, 754 with PAVC, and 178 with l-TGA < 6 years of age at 1st operation were identified. Of these 70 (3.5%), 19 (2.5%), and 14 (7.9%) respectively proceeded to require M-MVR (systemic atrio-ventricular valve replacement in l-TGA) over 5 years of follow up. Unadjusted transplant-free survival in CAVC at 20 years was 78.8% in those without M-MVR and 60.1% in those with M-MVR (p=0.0004) (Figure 5a). Hazard for death in CAVC with M-MVR remained higher for ~17 years of follow up; however, hazards for both groups were declining and converging over time (Figure 5b). Among those with CAVC requiring M-MVR, increased hazard for death persisted after adjustment for sex, era of first surgery, age at first surgery, and presence of Down syndrome.
Figure 5.
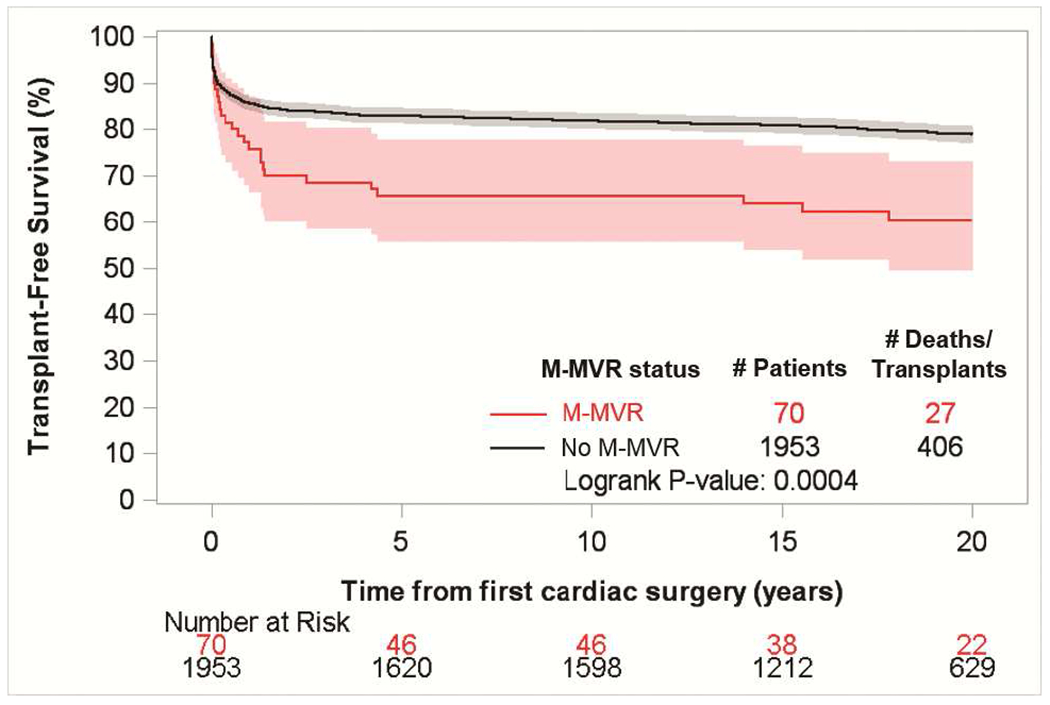
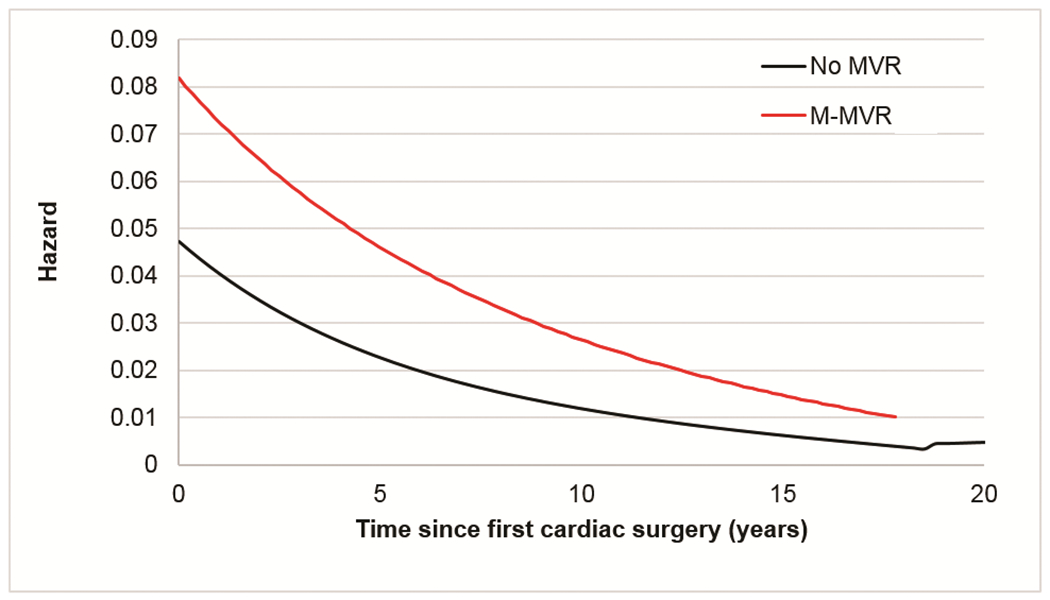
(a) Transplant-free survival from 1st cardiac surgery in patients with underlying CAVC compared between those receiving M-MVR before the age of 6 years and patients who did not require M-MVR (b) Hazard of death / cardiac transplant over time from 1st cardiac surgery in patients < 6 years of age with underlying CAVC who had M-MVR compared to patients who did not require M-MVR.
Need for M-MVR did not affect long-term survival for PAVC or l-TGA.
COMMENT
This study describes long-term outcomes for children who underwent 1st M-MVR for biventricular physiology CHD, utilizing a multi-institutional database linked with national death and heart transplant registries.
Early Mortality
Early mortality (≤ 90 days post 1st M-MVR) was 11.1%, although this is likely a slight underestimate given the higher in-hospital mortality in patients without adequate information for linkage to long-term follow up data. Adjusted analysis revealed age <2 years at M-MVR and concurrent other mechanical valve replacements as risk factors. Other single-institution and smaller cohort studies have reported 11-36% early mortality following M-MVR in children; young age, those with CAVC, higher Sz/Wt ratio or prosthetic valve to MV annulus diameters were reported as risk factors [2–5,7,9–10,18,19]. Increased early hazard in younger ages and those with concurrent other valve replacement in our study is likely reflecting more severe underlying pathology, technical surgical challenges and possibly the additional risk from post-operative myocardial dysfunction or impediment of leaflet mobility due to the relative increased prosthetic valve burden. The Sz/Wt mismatch was most likely due to lack of availability of smaller-sized prostheses for the youngest children. Recent FDA approval of smaller (15 mm) mechanical prostheses and its impact on early mortality in young children will need to be assessed [20].
Long-Term Cohort
There is ongoing risk for premature death in those surviving the early period post-M-MVR. Only ~76% of those who survived >90 days post-M-MVR were alive without needing heart transplant at 20 years of follow-up with males at a slight disadvantage. Conditioning surviving to 90 days post-M-MVR, young age (<2 years at M-MVR) was associated with lower, long-term, transplant-free survival as well. This implies that even if hemodynamics following M-MVR are tolerated in the early post-operative period by this age group, residual long-term hazard remains. This may reflect increased reoperative or additional morbidity burden faced by young children.
Irrespective of diagnoses, those with non-bi-leaflet prosthesis had poorer long-term survival, potentially due to intrinsic valve-related complications. Bi-leaflet prostheses were most commonly used and were associated with the most favorable long-term outcomes. While limited valve types in this cohort restrict drawing definite conclusions, this finding is similar to previous reports [6,8]. Non-bi-leaflet prostheses are seldom used in current era but children previously implanted with such may still face higher risk.
Survival by Diagnostic Category
Cardiac diagnoses did not influence early or late mortality following M-MVR in the adjusted model. However, within the CAVC cohort we noted higher mortality in those needing M-MVR. This may reflect more severe underlying pathology leading to the need for M-MVR plus additional complications related to the care of a child with M-MVR. It is reassuring that M-MVR did not increase mortality in those with PAVC and l-TGA.
Overall, M-MVR is a challenge in children with an early hazard of 11.1% mortality in the first 90 postoperative days and an additional 24% progressing to mortality/heart transplant over 20 years (~5% requiring cardiac transplantation). However, in unrepairable MV disease cases where the systemic atrioventricular valve is the main limiting factor in achieving biventricular physiology, M-MVR is a viable option with satisfactory 20-year survival.
Future
Though M-MVR can restore MV functionality, it comes with costs: notable early hazard and slow, long-term attrition. It remains to be seen, whether newer primary MV repair modalities, smaller mechanical prostheses, durable bio-engineered valves or catheter-based interventions will improve outcomes in young children.
Limitations
Data collection was limited to available variables within the registry and by the incomplete nature of long-term follow up via NDI/OPTN linkage methodology. Patients with incomplete identifiers or missing clinical records had to be excluded and did differ from those included in inhospital mortality, era of M-MVR and sex. However, application of inverse probability weighting suggested that the inclusion of these patients would not have impacted long-term survival estimates although we cannot dismiss the possibility that important covariates that would differ between groups and would impact survival were not included, such as underlying diagnosis. Additionally, re-interventions or additional procedures outside the PCCC could not be tracked precluding analysis of re-interventions. Finally, the study is non-population based; therefore, characteristics of participating centers could introduce bias. However, previous studies comparing subgroups within the PCCC to other US registries with greater coverage have revealed similar case distributions. While causes of death could be classified into broad categories, details and events leading to death could not be ascertained.
Conclusions
Despite these limitations, this study describes long-term, historical outcomes of the largest multi-institutional cohort of children and adolescents who underwent their 1st M-MVR in the US over a 20+year span. M-MVR demonstrates notable early mortality (~11%) within the first 90 days post-replacement, especially in those <2 years of age or needing concurrent other mechanical valve replacement. Those who survived >90 days faced continuous adversities (~24%) due to death or need for heart transplant over almost 20 years of follow-up. Male sex, age <2 years at M-MVR and use of non-bi-leaflet prostheses were risk factors for poorer long-term survival.
Supplementary Material
Abbreviations
- MV
Mitral Valve
- MVR
Mitral Valve Replacement
- M-MVR
Mechanical-Mitral Valve Replacement
- CHD
Congenital Heart Disease
- PCCC
Pediatric Cardiac Care Consortium
- NDI
National Death Index
- OPTN
Organ Procurement and Transplant Network
- CAVC
Complete atrioventricular canal
- PAVC
Partial atrioventricular canal
- l-TGA
levo-Transpositon of the great arteries
- SS
Shone’s syndrome
- MVA
Mitral valve anomaly
- iMVA
isolated Mitral valve anomaly
- cMVA
complex Mitral valve anomaly
- Sz/Wt
prosthetic valve (mm) to body weight (kg) ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ackermann K, Balling G, Eicken A, Gunther T, Schreiber C, Hess J. Replacement of the systemic atrioventricular valve with a mechanical prosthesis in children aged less than 6 years: Late clinical results of survival and subsequent replacement. J Thorac Cardiovasc Surg 2007;134:750–756. [DOI] [PubMed] [Google Scholar]
- 2.Beierlein W, Becker V, Yates R, et al. Long-term follow-up after mitral valve replacement in childhood: poor event-free survival in the young child. Eur J Cardiothorac Surg 2007;31:860–865. [DOI] [PubMed] [Google Scholar]
- 3.Henaine R, Nloga J, Wautot F, et al. Long-Term Outcome After Annular Mechanical Mitral Valve Replacement in Children Aged Less Than Five Years. Ann Thorac Surg 2010;90:1570–1576. [DOI] [PubMed] [Google Scholar]
- 4.Caldarone CA, Raghuveer G, Hills CB, et al. Long-term survival after mitral valve replacement in children aged <5 years: a multi-institutional study. Circulation 2001; 104:I-143-I-147. [DOI] [PubMed] [Google Scholar]
- 5.Vohra HA, Laker S, Stumper O, et al. Predicting the performance of mitral prostheses implanted in children under 5 years of age. Eur J Cardiothorac Surg 2006;29:688–692. [DOI] [PubMed] [Google Scholar]
- 6.Kojori F, Chen R, Caldarone CA, et al. Outcomes of mitral valve replacement in children: A competing-risks analysis. J Thorac Cardiovasc Surg 2004;128:703–709. [DOI] [PubMed] [Google Scholar]
- 7.Brown JW, Fiore AC, Ruzmetov M, Eltayeb O, Rodefeld MD, Turrentine MW. Evolution of mitral valve replacement in children: a 40-year experience. Ann Thorac Surg 2012;93:626–633. [DOI] [PubMed] [Google Scholar]
- 8.Masuda M, Kado H, Matsumoto T, et al. Mitral valve replacement using bileaflet mechanical prosthetic valve in the first year of life. Jpn J Thorac Cardiovasc Surg 2000;48:643–647. [DOI] [PubMed] [Google Scholar]
- 9.Alsoufi B, Manlhiot C, McCrindle BW, et al. Results after mitral valve replacement with mechanical prostheses in young children. J Thorac Cardiovasc Surg 2010;139:1189–1196. [DOI] [PubMed] [Google Scholar]
- 10.Raghuveer G, Caldarone CA, Hills CB, Atkins DL, Belmont JM, Moller JH. Predictors of prosthesis survival, growth, and functional status following mechanical mitral valve replacement in children aged <5 years, a multi-institutional study. Circulation 2003;108: II174–179. [DOI] [PubMed] [Google Scholar]
- 11.Moller JH. Using Data to Improve Quality: the Pediatric Cardiac Care Consortium. Congenit Heart Dis 2016;11:19–25. [DOI] [PubMed] [Google Scholar]
- 12.Vinocur JM, Moller JH, Kochilas LK. Putting the Pediatric Cardiac Care Consortium in context: evaluation of scope and case mix compared with other reported surgical datasets. Circ Cardiovasc Qual Outcomes 2012;5:577–579. [DOI] [PubMed] [Google Scholar]
- 13.Spector LG, Menk JS, Knight JH, et al. Trends in Long-term Morality After Congenital Heart Surgery. J Am Coll Cardiol 2018;71:2434–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mi X, Hammill BG, Curtis LH, Lai EC, Setoguchi S. Use of the landmark method to address immortal person-time bias in comparative effectiveness research: a simulation study. Stat Med 2016;35:4824–4836. [DOI] [PubMed] [Google Scholar]
- 15.Spector LG, Menk JS, Vinocur JM, et al. In-Hospital Vital Status and Heart Transplants After Intervention for Congenital Heart Disease in the Pediatric Cardiac Care Consortium: Completeness of Ascertainment Using the National Death Index and United Network for Organ Sharing Datasets. J Am Heart Assoc 2016;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akins CW, Miller DC, Turina MI, et al. Guidelines for Reporting Mortality and Morbidity After Cardiac Valve Interventions. Ann Thorac Surg 2008;85:1490–1495. [DOI] [PubMed] [Google Scholar]
- 17.Zack M, Singleton J, Wall K, Delaney K. %Collin: Collinearity diagnostics using the information matrix. 2011
- 18.Eble BK, Fiser WP, Simpson P, Dugan J, Drummond-Webb JJ, Yetman AT. Mitral valve replacement in children: predictors of long-term outcome. Ann Thorac Surg 2003;76: 853–859. [DOI] [PubMed] [Google Scholar]
- 19.Selamet Tierney ES, Pigula FA, Berul CI, Lock JE, del Nido PJ, McElhinney DB. Mitral valve replacement in infants and children 5 years of age or younger: Evolution in practice and outcome over three decades with a focus on supra-annular prosthesis implantation. J Thorac Cardiovasc Surg 2008;136:954–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.FDA Premarket Approval Database: SJM Masters Series Mechanical Heart Valve, 15mm HP. Available at https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P810002s101. Accessed April 16, 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


