Abstract
Objective:
To explore the expression of miR-204 in patients with Osteoarthritis (OA) and its effect on chondrocytes.
Methods:
Cartilage tissues of patients with OA, and normal cartilage tissues of patients receiving emergent traumatic amputation were collected. Polymerase chain reaction (PCR) was used to quantify the expressions of miRNAs. Chondrocytes of patients with OA were isolated, cultured, and transfected with miR-204 mimics or miR-204 inhibitor, and cell models of over-expression and knockdown of miR-204 were constructed. MTT assay, clone formation test, trypan blue staining, and TdT-mediated dUTP Nick-End Labeling (TUNEL) staining were used to detect the effect of overexpression or knockdown of miR-204 on viability, proliferation, survival rate and apoptosis of chondrocytes.
Results:
miR-204 expression increased significantly in cartilage tissue of patients with OA. Results of MTT assay, clone formation test, and trypan blue staining showed that the over-expression of miR-204 inhibited the viability, proliferation, and survival rate, as well as promoted the apoptosis of chondrocytes. Whereas the knockdown of miR-204 improved the viability, proliferation, and survival rate of chondrocytes.
Conclusion:
The expression of miR-204 increased significantly in patients with OA and played a damaging role in chondrocytes. The knockdown of miR-204 may provide new approaches for clinical treatment of OA.
Keywords: Chondrocytes, Expression, miR-204, miRNA, Osteoarthritis
Introduction
Osteoarthritis (OA) is a degenerative joint disease characterized by articular cartilage degeneration and joint inflammation, with a high incidence rate in the middle-aged and elderly[1-3]. According to reports, the incidence is about 5% in people under 40 years old, 50% in people over 60 years old, and 80% in people over 75 years old[4,5]. Patients with OA suffer from joint pain, deformation, and dysfunction, which directly affect the activity of daily life and labor ability and severely reduce the quality of life[6,7]. At present, the etiology and pathogenesis of OA remain unknown. Many researchers believe that factors such as age, hormone, trauma, infection, obesity, inflammation, alcohol intake, vascular lesions, and genetic factors may break the imbalance between degradation and synthesis of chondrocytes, extracellular matrix, and subchondral bone, and subsequently cause the cartilage damage and ultimately leads to OA[8-10]. Chondrocytes are the only cells in cartilage with primary functions of maintaining integrity of cartilage and weight-bearing the articular cartilage, as well as keeping the balance of the internal environment during cartilage injury and remodeling[11,12]. Therefore, changes in the physiological function of chondrocytes play an essential role in the occurrence and development of OA. It is of great clinical significance to find effective methods for the treatment of OA in view of chondrocyte damage[13-15].
MicroRNA (miRNA) is a highly conserved, endogenous non-coding RNA with a length of approximately 25 bases[16]. It can interfere with the expression of the target gene by binding to the 3’ untranslated region (3’UTR) of the target mRNA, thereby affecting the proliferation, differentiation, apoptosis, aging, and individual development of cells, and participating in the occurrence and development of various diseases[17-19]. miR-363, miR-185, miR-217, miR-26b, miR-204 have been reported to regulate bone-related diseases, but no experiment has been conducted to explore the correlation between these five miRNAs and OA. Therefore, this study collected cartilage tissue from patients with OA, screened an important miRNA involved in OA, and discussed its effect on the biological function of chondrocytes in order to provide theoretical basis for the prevention and treatment of OA.
Materials and methods
Clinical data
According to the diagnostic criteria for OA from Chinese Orthopaedic Association[20], OA patients (n=24) who were treated in our hospital and underwent total knee arthroplasty from January 2016 to January 2018 were recruited, including 13 males and 11 females, aged (40-70) years, with the average age of (57.42±9.23) years. Inflammatory cartilage tissues from the patients were collected. In addition, 24 patients with emergent traumatic amputation treated in our hospital during the same period were selected, including 15 males and 9 females, aged (40-70) years, with the average age of (56.96±10.67) years. Normal cartilage tissues from the patients were enrolled as controls. Cartilage tissues of the overweight area of medial and lateral malleolus were collected from the patients in the two groups and stored in a refrigerator at -80°C. The study was approved by the Medical Ethics Committee of the hospital, and patients signed the written informed consent form. Exclusion criteria were as follows: According to data, medical history, X-ray, and laboratory examination, patients with tumors, tuberculosis, infection, rheumatoid arthritis, suppurative arthritis, osteoporosis, immune system diseases, connective tissue proliferative diseases, and diabetes were excluded.
Isolation, culture, and intervention of human primary chondrocytes
Isolated inflammatory cartilage tissues were immediately placed in a sterile serum bottle of DMEM culture solution (Corning, USA) containing serum, capped, then stored in an incubator, and send to the cell culture room for primary culture. Under aseptic conditions, the cartilage tissue was cut into approximately 1 mm3 tissue blocks by an ophthalmic scissor and washed three times with phosphate buffer (Solar Bio, China). The tissue blocks were covered with trypsin (Beyotime, China) and digested at 37°C for 30 min. Afterward, Type II collagenase (Sigma, USA) was added, and the tissues were digested at 37°C for 8 hours. Cells were collected every 4 hours for 2 times. The digested cells were centrifuged at 1500 r/min for 10 min to collect cell precipitates. Next, the cells were transferred to DMEM culture solution containing 10% fetal bovine serum (Thermo, USA), streptomycin 100 U/mL, and penicillin 100 U/mL, and cultured in an incubator (Thermo, USA) at 37°C and 5% CO2 and under saturated humidity. The cells were digested with trypsin when they reached 80-90% confluence, then passed to the next generation, and the third passage cells were used for subsequent experiments. The cells were treated with 10 ng/mL lipopolysaccharide (LPS) for 8 hours to construct inflammatory chondrocyte models for subsequent trials.
RNA extraction and reverse transcription
Cartilage tissues were placed in a mortar and ground with liquid nitrogen. miRNAs were separated according to instructions of a total miRNA extraction kit (Invitrogen, USA), and the concentration and purity were measured by a Nanodrop instrument (Thermo, USA). Then the RNAs were reverse-transcribed into cDNAs using a reverse transcription kit (ABI, USA).
Polymerase chain reaction (PCR) experiment
All reagents were added to the reaction system prepared according to the instruction manual of fluorescence quantitative kit (Novozyme, China). All primers were purchased from Shanghai GenePharma Co., Ltd. PCR amplification was carried out in a 20-µL reaction volume containing 10 µL of SYBR, 1µL of upstream primer (concentration 0.2 µM), 1 µL of downstream primer (concentration 0.2 µM), 1 µL of cDNA, 7 µL of ddH2O. PCR amplification reaction was carried out with U6 as the internal reference, and 4 replicates were made for each sample. The PCR conditions were as follows: 95°C for 3 min; 95°C for 5 s, 56°C for 30 s, 72°C for 25 s, for a total of 35 cycles; 65°C for 5 min; 95°C for 50 s. ABI7500 real-time quantitative PCR system was used to the experiment. Fluorescence signals were collected, and melting curves were plotted. 2-Δ CT was used to detect the relative expression of target genes. All primer sequences in this experiment are shown in [Table 1].
Table I.
PCR primer sequences.
| Primer | Sequence (5’ to 3’) |
|---|---|
| microRNA-363 | F: CGAATGTCCGTCACATCT |
| R: GTGCACGGGCAG AGGT | |
| microRNA-185 | F: GGATTGGAGAGAAAGGCAG |
| R: GTGCAGGGTCCGAGGT | |
| microRNA-217 | F: GGCGAGGTGAGGGTGAGGCAGAGTC |
| R: AATGCGGTTTATTTATGGCGAGATTG | |
| microRNA-26b | F: CAAAGGTCCATAGCAAGGGT |
| R: GCGACCTTGTCATGGTTTATAG | |
| microRNA-204 | F: AACCUGAUCCCGUCUGAGAUUG |
| R: CCGGAUCAAGAUUAGUUCGGUU | |
| U6 | F: TCGCTTCGGCAGCACATATAC |
| R: TATGGAACGCTTCACGAATTT |
Cell transfection
Cells were inoculated into a six-well plate, a 96-well plate, or a culture dish, respectively. When they reached 50-60% confluence, transfection was carried out according to instructions of Lipofectamine2000 reagent (Invitrogen, USA). Chondrocytes were transfected with miR-204 mimics, miR-204 inhibitor, mimics-NC, and inhibitor-NC, and subsequent experiments were performed after the transfection efficiency was verified.
Toluidine blue staining
Cartilage cells were identified by toluidine blue staining. The third passage chondrocytes were inoculated into a six-well plate (Nest, USA). When the cells reached 50% confluence, the culture solution was discarded, and the cells were washed with PBS, and fixed with 4% paraformaldehyde (Solar Bio, China) for 30 min at room temperature. After washing for 3 times, the cells were incubated with 1% toluidine blue staining solution. The staining solution was discarded after 4 hours, absolute ethyl alcohol was used for decolorization, and distilled water was used to terminate the reaction. Afterward, the cells on the six-well plate were observed under an inverted fluorescence microscope (Nikon, Japan) and photographed.
MTT assay
The third passage chondrocytes were inoculated into a 96-well plate (Nest, USA) at a density of 3000/well. The chondrocytes were transfected with miR-204 mimics or inhibitor on the next day, and MTT assay was performed 0, 12, 24, 36, and 48 h after transfection, respectively. A total of 20 µL of 5 mg/mL MTT solution (Biosharp, China) was added to each well. The cells were inoculated for 4 hours, the liquid was discarded, and 150 µL of dimethyl sulfoxide was added. After shaking for 10 min, the culture plate was placed on a microplate reader, and the optical density (OD) value was detected at 490 nm.
Clone formation test
Chondrocytes were inoculated into a six-well plate, transfected with miR-204 mimics or inhibitor, and cultured for 14 days. Then the cells were washed three times with PBS, fixed with 4% paraformaldehyde (Solar Bio, China) for 30 min, and incubated with 1 mL of crystal violet staining solution (Biosharp, China) for 30 min. After washed three times, the cells were observed under an optical microscope (Nikon, Japan) and photographed.
Trypan blue staining assay
Chondrocytes were inoculated into a six-well plate, transfected with miR-204 mimics or inhibitor, and digested for 24 h, and 0.4% trypan blue solution (Biosharp, China) was added to the cell suspension. After mixing, the cells were observed under an optical microscope. Dead cells were stained blue, and living cells were colorless. The living cells and dead cells were counted within 3 min.
TUNEL staining
Cartilage cells were inoculated into a culture dish, transfected with miR-204 mimics or inhibitor, and fixed with 4% paraformaldehyde for 30 min after 24 h. Washed three times with PBS, the cells were added with 30 µL of TdT-mediated dUTP Nick-End Labeling (TUNEL) reaction solution (Roche, Switzerland). The dish was placed in a wet box, incubated at 37°C for 1 h in the dark, and the cells were counterstained with DAPI solution (Solar Bio, China) for 20 min. After washed three times with PBS, the cells were observed under an inverted fluorescence microscope (Nikon, Japan) and photographed.
Statistical methods
All data were tested at least three times, and statistical analysis was carried out using Graphpad software (Graphpad, USA). The paired t-test was used for comparison between the two groups. A value of P<0.05 was considered statistically significant.
Results
Expressions of miRNAs in cartilage tissues
Cartilage tissues of patients with OA and controls were collected, and the expressions of miR-363, miR-185, miR-204, miR-217, miR-26b in the two groups were detected. The results showed that there was no difference in the expression of miR-363 in the cartilage tissue of OA patients and the controls (Figure 1A). The expressions of miR-185 and miR-204 in cartilage tissue of OA patients were significantly higher than those of the controls (Figures 1B and 1C). Also, the expressions of miR-217 and miR-26b in cartilage tissue of OA patients were significantly lower than those of the controls (Figures 1D and 1E).
Figure 1.
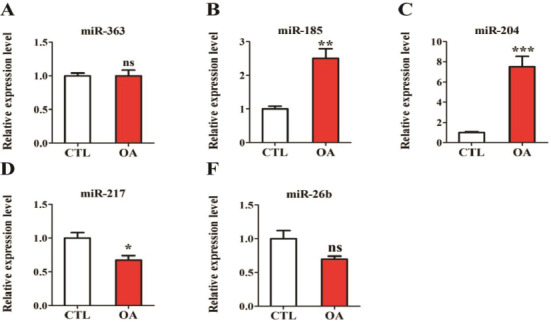
Expressions of five miRNAs in patients with OA. (A) miR-363 expression in patients with OA; (B) miR-185 expression in patients with OA; (C) miR-204 expression in patients with OA; (D) Expression of miR-217 in patients with OA; (E) Expression of miR-26b in patients with OA.
Transfection efficiency of miR-204 in chondrocytes of OA patients
As shown in [Figure 2A], toluidine blue staining showed that chondrocytes had positive staining. Chondrocytes of OA patients were transfected with miR-204 mimics, miR-204 inhibitor, mimics-NC, and inhibitor-NC, and the transfection efficiency of miR-204 was detected after 24 hours. As shown in [Figure 2B], miR-204 showed significantly higher expression in cells transfected with miR-204 mimics than those transfected with mimics-NC. As shown in [Figure 2C], miR-204 showed significantly lower expression in cells transfected with miR-204 inhibitor than those transfected with inhibitor -NC.
Figure 2.
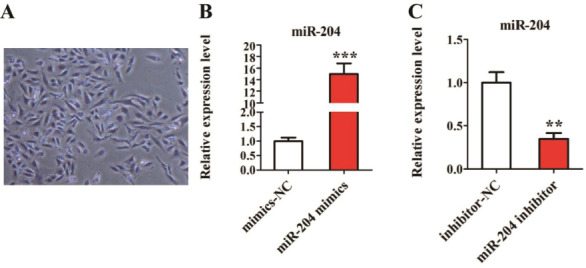
Transfection efficiency of miR-204 in chondrocytes of OA patients. (A) chondrocyte morphology under toluidine blue staining; (B) Expression of miR-204 in chondrocytes with over-expression of miR-204; (C) Expression of miR-204 in chondrocytes with knockdown of miR-204.
Effect of over-expression of miR-204 on chondrocytes
Chondrocytes of OA patients were transfected with miR-204 mimics and mimics-NC. MTT assay, clone formation test, trypan blue staining, and TUNEL staining were carried out. MTT assay showed that after transfected with miR-204 mimics, chondrocyte viability in OA patients was significantly reduced (Figure 3A). Clone formation assay demonstrated that the number of clone formation of chondrocytes transfected with miR-204 mimics was significantly lower than that transfected with mimics-NC, i.e. miR-204 inhibited the proliferation of chondrocytes (Figure 3B). As shown in [Figure 3C], trypan blue staining revealed that over-expression of miR-204 significantly reduced the survival rate of chondrocytes in patients with OA. Additionally, TUNEL staining results suggested that over-expression of miR-204 promoted chondrocyte apoptosis in patients with OA (Figure 3D).
Figure 3.
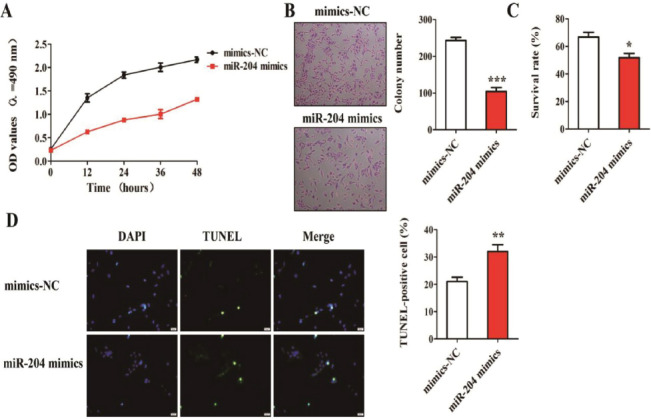
Effect of over-expression of miR-204 on biological function of chondrocytes in patients with OA. (A) Detection of cell viability using MTT assay; (B) Detection of cell proliferation using clone formation assay; (C) Detection of cell survival using trypan blue staining; (D) Detection of cell apoptosis using TUNEL staining.
Effect of knockdown of miR-204 on chondrocytes
Chondrocytes of OA patients were transfected with miR-204 inhibitor and inhibitor –NC. MTT assay, clone formation test, trypan blue staining, and TUNEL staining were carried out. MTT assay, clone formation test, and trypan blue staining showed that knockdown of miR-204 significantly increased viability, proliferation, and survival rate of chondrocytes in patients with OA (Figures 4A to 4C). Besides, TUNEL staining showed that knockdown of miR-204 significantly inhibited chondrocyte apoptosis in patients with OA (Figure 4D).
Figure 4.

Effect of knockdown of miR-204 on biological function of chondrocytes in patients with OA. (A) Detection of cell viability using MTT assay; (B) Detection of cell proliferation using clone formation test; (C) Detection of cell survival using trypan blue staining; (D) Detection of cell apoptosis using TUNEL staining.
Discussion
Modern medicine believes that the damage of articular cartilage is the main pathological change of OA, so its repair plays an essential role in the treatment of OA. Therefore, this study aimed to explore the expression of miR-204 in cartilage tissue of patients with OA, and to investigate the effects of miR-204 on chondrocyte viability, proliferation, survival rate, and apoptosis, to provide references for subsequent treatment of OA.
It has been reported that miR-363, miR-185, miR-217, miR-26b, miR-204 are involved in OA, fracture, and other bone-related diseases, but their correlation with the occurrence and development of OA has not been studied[21-25]. Therefore, we explored the expressions of miR-363, miR-185, miR-217, miR-26b, miR-204 in cartilage tissue of OA patients, and their effects on chondrocytes. PCR results showed that there was no difference in the expression of miR-363 in cartilage tissue of the OA patients and the controls. The expressions of miR-185 and miR-204 in cartilage tissue of OA patients were significantly higher than those of controls, whereas the expressions of miR-217 and miR-26b in cartilage tissue of OA patients were significantly lower than those of the controls. Among them, the differential expression of miR-204 in cartilage tissue of controls and OA patients was the most significant, suggesting that miR-204 might play an essential role in the occurrence and development of OA. Therefore, miR-204 was selected for subsequent research. Moreover, we speculated that miR-204 might become an important gene for predicting, diagnosing, and treating OA.
Previous studies have shown that pathophysiological processes of OA, such as cartilage formation and differentiation, cartilage matrix degradation, and chondrocyte proliferation, apoptosis, and autophagy, play important roles in the occurrence and development of OA. Huang Z et al. revealed that miR-337-3p promoted proliferation of chondrocytes by regulating PTEN/AKT pathway, thus inhibiting OA[26]. Consistent with this, our study showed that knockdown of miR-204 significantly improved the viability, proliferation, and survival rate of chondrocytes in patients with OA. Therefore, we believe that knockdown of miR-204 may play a protective role in chondrocytes of OA by improving their viability, proliferation, and survival rate. Apoptosis is a cell death pattern mediated by programmed expression of specific genes, which is involved in various diseases. Chondrocyte apoptosis has been reported to be one of the important factors causing cartilage degradation in OA. Yang Y et al. stated that loganin inhibited IL-1β-induced chondrocyte lysis and apoptosis by regulating PI3K/Akt pathway, thereby reducing chondrocyte damage[3]. Liu Q et al. found that chondroitin sulfate in sturgeon bone protected chondrocytes via inhibiting apoptosis in OA[27]. Our study showed that over-expression of miR-204 could promote chondrocyte apoptosis in patients with OA, while knockdown of miR-204 could reduce the proportion of apoptotic cells. Therefore, we believed that knockdown of miR-204 might protect chondrocytes from apoptosis in patients with OA, but the specific mechanism needs to be further explored.
To sum up, miR-204 expression increased significantly in patients with OA. Moreover, it can reduce the viability, proliferation, and survival rate, as well as promote the apoptosis of chondrocytes, thus exerting the damaging effect on chondrocytes of OA. Therefore, the knockdown of miR-204 may be a new method for the treatment of OA. The effects of miR-204 on OA with animal models as well as the exploration of its mechanism should be further investigated in order to provide a theoretical basis for the development of novel and effective treatments for OA.
Authors’ contributions
XL, FG, and JY conceived and designed the study. XL, FG, WW, and JY were responsible for the collection, analysis, and interpretation of the data. XL drafted the manuscript. XL and JY revised the manuscript critically for important intellectual content. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of The 2nd Affiliated Hospital of Harbin Medical University. Signed written informed consents were obtained from the patients and/or guardians.
Footnotes
The authors have no conflict of interest.
Edited by: G. Lyritis
References
- 1.Huang BK, Tan W, Scherer KF, Rennie W, Chung CB, Bancroft LW. Standard and Advanced Imaging of Hip Osteoarthritis. What the Radiologist Should Know. Semin Musculoskelet Radiol. 2019;23:289–303. doi: 10.1055/s-0039-1681050. [DOI] [PubMed] [Google Scholar]
- 2.Eyles JP, Hunter DJ, Bennell KL, Dziedzic KS, Hinman RS, van der Esch M, Holden MA, Bowden JL. Joint Effort Initiative Members. Priorities for the effective implementation of osteoarthritis management programs:an OARSI international consensus exercise. Osteoarthritis Cartilage. 2019;27(9):1270–1279. doi: 10.1016/j.joca.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 3.Yang Y, Gu Y, Zhao H, Zhang S. Loganin Attenuates Osteoarthritis in Rats by Inhibiting IL-1beta-Induced Catabolism and Apoptosis in Chondrocytes Via Regulation of Phosphatidylinositol 3-Kinases (PI3K)/Akt. Med Sci Monit. 2019;25:4159–4168. doi: 10.12659/MSM.915064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lizaur-Utilla A, Miralles-Muñoz FA, Gonzalez-Parreño S, Lopez-Prats FA. Validation of the Spanish Version of the Knee Injury and Osteoarthritis Outcome Score (Koos) for Elderly Patients with Total Knee Replacement. J Orthop Res. 2019;37(10):2157–2162. doi: 10.1002/jor.24386. [DOI] [PubMed] [Google Scholar]
- 5.Ahn H, Sorkpor S, Miao H, Zhong C, Jorge R, Park L, Abdi S, Cho RY. Home-based self-administered transcranial direct current stimulation in older adults with knee osteoarthritis pain:An open-label study. J Clin Neurosci. 2019;66:61–65. doi: 10.1016/j.jocn.2019.05.023. [DOI] [PubMed] [Google Scholar]
- 6.Iijima H, Suzuki Y, Aoyama T, Takahashi M. Quadriceps Weakness in Individuals with Coexisting Medial and Lateral Osteoarthritis. JB JS Open Access. 2019;4(1):0028. doi: 10.2106/JBJS.OA.18.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsehaie J, van der Oest MJW, Poelstra R, Selles RW, Feitz R, Slijper HP, Hovius SER, Porsius JT Hand-Wrist Study Group. Positive experience with treatment is associated with better surgical outcome in trapeziometacarpal osteoarthritis. J Hand Surg Eur. 2019;44(7):714–721. doi: 10.1177/1753193419851777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang D, Marom N, Coxe FR, Kalia V, Burge AJ, Jones KJ, Rodeo SA, Williams RJ., 3rd Preoperative Grades of Osteoarthritis and Meniscus Volume Correlate with Clinical Outcomes of Osteochondral Graft Treatment for Cartilage Defects in the Knee. Cartilage. 2019:1947603519852402. doi: 10.1177/1947603519852402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang S, Pillinger MH, Krasnokutsky S, Barbour KE. The Association Between Asymptomatic Hyperuricemia and Knee Osteoarthritis:Data from the Third National Health and Nutrition Examination Survey. Osteoarthritis Cartilage. 2019;27(9):1301–1308. doi: 10.1016/j.joca.2019.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hussain SM, Dawson C, Wang Y, Tonkin AM, Chou L, Wluka AE, Cicuttini FM. Vascular pathology and osteoarthritis:A systematic review. J Rheumatol. 2020;47(5):748–760. doi: 10.3899/jrheum.181236. [DOI] [PubMed] [Google Scholar]
- 11.Mokuda S, Nakamichi R, Matsuzaki T, Ito Y, Sato T, Miyata K, Inui M, Olmer M, Sugiyama E, Lotz M, et al. Wwp2 maintains cartilage homeostasis through regulation of Adamts5. Nat Commun. 2019;10:2429. doi: 10.1038/s41467-019-10177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen YY, Chen Y, Wang WC, Tang Q, Wu R, Zhu WH, Li D, Liao LL. Cyclin D1 regulates osteoarthritis chondrocyte apoptosis via WNT3/beta-catenin signalling. Artif Cells Nanomed Biotechnol. 2019;47:1971–1977. doi: 10.1080/21691401.2019.1593853. [DOI] [PubMed] [Google Scholar]
- 13.Ragni E, Perucca Orfei C, De Luca P, Viganò M, Colombini A, Lugano G, Bollati V, de Girolamo L. miR-22-5p and miR-29a-5p Are Reliable Reference Genes for Analyzing Extracellular Vesicle-Associated miRNAs in Adipose-Derived Mesenchymal Stem Cells and Are Stable under Inflammatory Priming Mimicking Osteoarthritis Condition. Stem Cell Rev. 2019;15(5):743–754. doi: 10.1007/s12015-019-09899-y. [DOI] [PubMed] [Google Scholar]
- 14.Hahm SC, Song E, Jeon H, Yoon YW, Kim J. Transcutaneous Electrical Nerve Stimulation Reduces Knee Osteoarthritic Pain by Inhibiting Spinal Glial Cells in Rats. Phys Ther. 2019;99(9):1211–1223. doi: 10.1093/ptj/pzz076. [DOI] [PubMed] [Google Scholar]
- 15.Tian J, Cheng C, Zhang F. Can biomarkers differentiate psoriatic arthritis from osteoarthritis? Ann Rheum Dis. 2019 doi: 10.1136/annrheumdis-2019-215745. annrheumdis-2019-215745. [DOI] [PubMed] [Google Scholar]
- 16.Xia P, Gu R, Zhang W, Shao L, Li F, Wu C, Sun Y. MicroRNA-200c promotes osteogenic differentiation of human bone mesenchymal stem cells through activating the AKT/beta-Catenin signaling pathway via downregulating Myd88. J Cell Physiol. 2019;234(12):22675–22686. doi: 10.1002/jcp.28834. [DOI] [PubMed] [Google Scholar]
- 17.Han Q, Li C, Cao Y, Bao J, Li K, Song R, Chen X, Li J, Wu X. CBX2 is a functional target of miRNA let-7a and acts as a tumor promoter in osteosarcoma. Cancer Med. 2019;8:3981–3991. doi: 10.1002/cam4.2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bellavia D, Salamanna F, Raimondi L, De Luca A, Carina V, Costa V, Alessandro R, Fini M, Giavaresi G. Deregulated miRNAs in osteoporosis:effects in bone metastasis. Cell Mol Life Sci. 2019;76(19):3723–3744. doi: 10.1007/s00018-019-03162-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y, Chen G, Liu H, Li Z, Yang Q, Gu X, Du Z, Zhang G, Wang J. Integrated bioinformatics analysis of miRNA expression in Ewing sarcoma and potential regulatory effects of miR-21 via targeting ALCAM/CD166. Artif Cells Nanomed Biotechnol. 2019;47:2114–2122. doi: 10.1080/21691401.2019.1620760. [DOI] [PubMed] [Google Scholar]
- 20.Lo PC, Lin FC, Tsai YC, Lin SK. Traditional Chinese medicine therapy reduces the risk of total knee replacement in patients with knee osteoarthritis. Medicine (Baltimore) 2019;98:15964. doi: 10.1097/MD.0000000000015964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li M, Luo R, Yang W, Zhou Z, Li C. miR-363-3p is activated by MYB and regulates osteoporosis pathogenesis via PTEN/PI3K/AKT signaling pathway. In Vitro Cell Dev Biol Anim. 2019;55:376–386. doi: 10.1007/s11626-019-00344-5. [DOI] [PubMed] [Google Scholar]
- 22.Cui Q, Xing J, Yu M, Wang Y, Xu J, Gu Y, Nan X, Ma W, Liu H, Zhao H. Mmu-miR-185 depletion promotes osteogenic differentiation and suppresses bone loss in osteoporosis through the Bgn-mediated BMP/Smad pathway. Cell Death Dis. 2019;10:172. doi: 10.1038/s41419-019-1428-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang L, Zeng Z, Kang N, Yang JC, Wei X, Hai Y. Circ-VANGL1 promotes the progression of osteoporosis by absorbing miRNA-217 to regulate RUNX2 expression. Eur Rev Med Pharmacol Sci. 2019;23:949–957. doi: 10.26355/eurrev_201902_16981. [DOI] [PubMed] [Google Scholar]
- 24.Hu H, Zhao C, Zhang P, Liu Y, Jiang Y, Wu E, Xue H, Liu C, Li Z. miR-26b modulates OA induced BMSC osteogenesis through regulating GSK3beta/beta-catenin pathway. Exp Mol Pathol. 2019;107:158–164. doi: 10.1016/j.yexmp.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 25.Wang CG, Liao Z, Xiao H, Liu H, Hu YH, Liao QD, Zhong D. LncRNA KCNQ1OT1 promoted BMP2 expression to regulate osteogenic differentiation by sponging miRNA-214. Exp Mol Pathol. 2019;107:77–84. doi: 10.1016/j.yexmp.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 26.Huang Z, Zhang N, Ma W, Dai X, Liu J. MiR-337-3p promotes chondrocytes proliferation and inhibits apoptosis by regulating PTEN/AKT axis in osteoarthritis. Biomed Pharmacother. 2017;95:1194–1200. doi: 10.1016/j.biopha.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 27.Liu Q, Wang J, Sun Y, Han S. Chondroitin sulfate from sturgeon bone protects chondrocytes via inhibiting apoptosis in osteoarthritis. Int J Biol Macromol. 2019;134:1113–1119. doi: 10.1016/j.ijbiomac.2019.05.110. [DOI] [PubMed] [Google Scholar]


