Abstract
Peptides are a promising new therapeutic approach for glioblastoma with potential for more effective targeting and fewer devastating side effects compared to conventional cancer therapies. With the specificity to target receptors which are uniquely or overexpressed on cancer cells as well as accurately targeting dysregulated signaling pathways, peptides demonstrate a high potential for the treatment of even the most aggressive cancers. By binding to these targets, peptides can be used to deliver drugs, serve as antagonists to various ligands, or, given some inherent anticancer activity, provide additional treatment options alone or in combination therapy. The highly specific targeting capacity of peptides is critical to achieve effective cancer treatment with limited side effects, and in preclinical studies peptides have shown to have both cell and blood brain barrier penetrating capacity. As tumor targeting peptides move beyond the preclinical setting, identification of additional glioblastoma-specific peptide ligands becomes imperative to expand the potential of this encouraging treatment strategy.
Introduction
One of the greatest challenges in cancer therapy is to selectively deliver chemotherapeutics to the cancer cells, thus achieving therapeutic efficacy while limiting damage to healthy tissues. The effective strategy of targeted drug delivery to tumor tissues is based on cellular heterogeneity which is characteristic of all organs and tissues including pathological tissues such as tumors. One of the biological properties of tumor cells is the expression or overexpression of a unique set of proteins or receptors on the cell surface. Activation of these receptors often results in deregulated signaling, increased cell proliferation and decreased cell apoptosis. In addition, these oncogenic mutations frequently stimulate invasion of tumor cells to neighboring tissues, tumor angiogenesis, migration and metastasis. Selective targeting of these tumor-specific receptors provides the opportunity for targeted drug delivery. Several types of tumor targeting ligands have been developed including antibodies, antibody fragments, polypeptides, small molecules, and others. In clinics, one of the most widely used tumor targeting ligands is an antibody. Antibodies can efficiently target the antigens that are overexpressed by cancer cells and deliver anticancer drugs specifically to the tumor tissue. However, some deficiencies of antibodies – such as limited stability in vivo, slow diffusion into the tumor tissue and high cost of production – limit their clinical use. Tumor-targeting peptides are an efficient alternative for selective targeting of tumor-specific receptors. Compared to antibodies, tumor-targeting peptides have better tumor/tissue penetration and are easy to synthesize and modify chemically to improve stability and pharmacokinetics. These peptides can be used for imaging and cancer diagnostics and also for targeted drug delivery. Furthermore, some peptides may be designed to have the ability to kill cancer cells and inhibit tumor progression.
GBM morphology and current treatment
Glioblastoma is an aggressive and lethal brain tumor characterized by the presence of poorly differentiated anaplastic cells surrounded by necrotic areas of brain tissue. Standard glioblastoma treatment includes surgical resection followed by radiotherapy and chemotherapy including temozolomide. However, these highly heterogenous tumors grow rapidly, invading and infiltrating neighboring healthy brain tissues, making complete tumor resection difficult. Tumor cells that escape surgical removal initiate formation of secondary glioblastoma lesions and tumor recurrence, which are more resistant to chemotherapy and radiotherapy than rapidly proliferating primary tumors. In addition to tumor recurrence and radioresistance and chemoresistance, treatment of glioblastoma is particularly challenging due to inability of drugs to cross the blood brain barrier (BBB) and pass through the blood brain tumor barrier (BBTB) which is formed in later stages of tumor growth. Although significant improvements in the development of drug nanocarriers – such as dendrimers, micelles and polymer-based nanoparticles – have been made, challenges in targeting glioblastoma cells remain.
Peptides
Peptides that are used for the targeted delivery of therapeu-ticagents canbe divided intothree distinct categories:tumor homing peptides, peptides targeting aberrant cellular signaling pathways and cell penetrating peptides (Figure 1).
Figure 1.
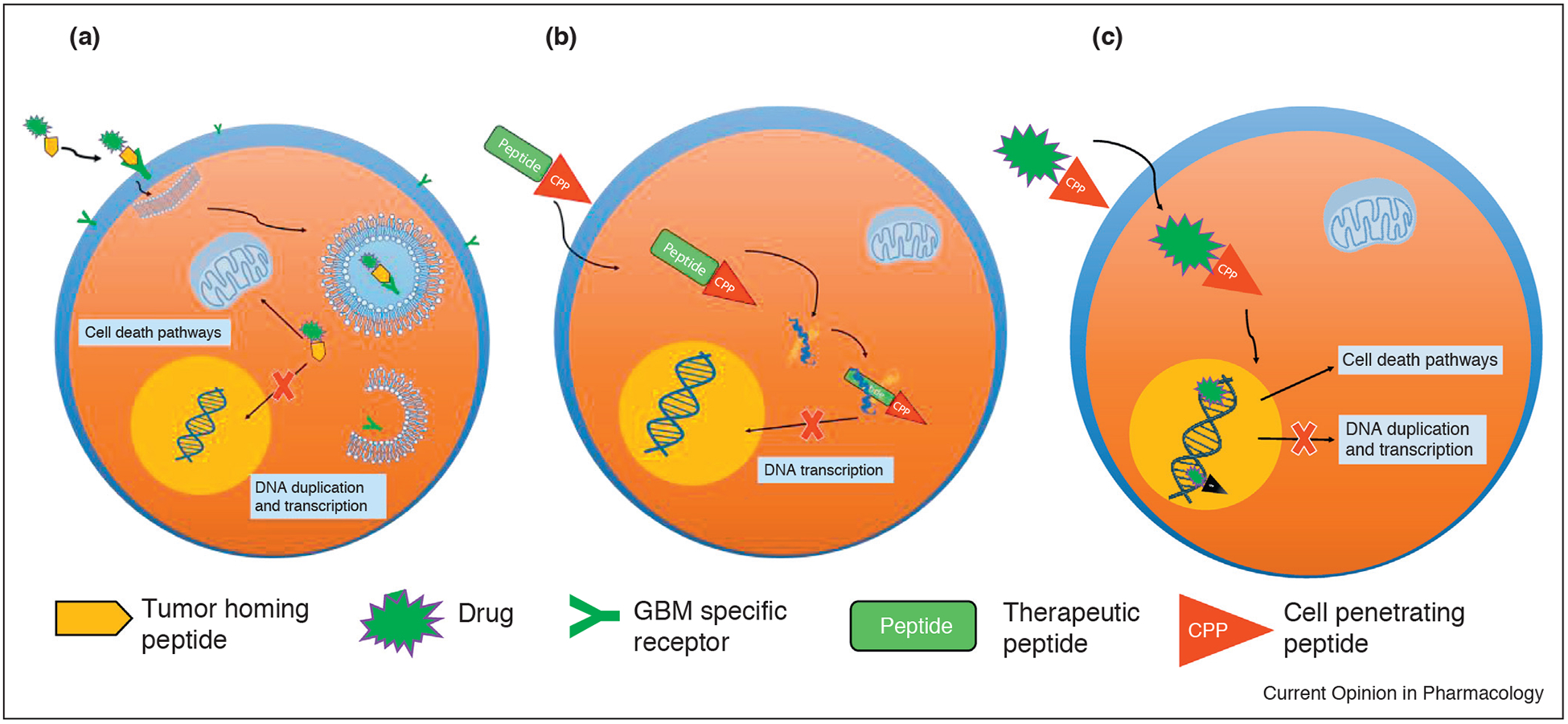
Peptides used for the targeted delivery of therapeutic agents. (a) Tumor homing peptides carrying a therapeutic load bind to the specific receptors on the cell surface and are endocytosed; drugs are released from endosomes inducing cell death or inhibiting DNA duplication; (b) Peptides targeting aberrant cellular signaling pathways are often conjugated with CPP and, after internalization, inhibit protein–protein interaction and DNA transcription, or cause cell death with some other mechanism; (c) Cell penetrating peptides are covalently coupled to various drug carriers and used for the targeted delivery of drugs.
Tumor homing peptides selectively bind to the molecules specifically expressed or overexpressed on the cell surface of the cancer cells. Binding of some of these peptides can also enhance or antagonize signal transduction pathways in cancer cells or tumor tissues.
Peptides targeting aberrant cellular signaling pathways have been identified that have the ability to regulate oncogenic signaling pathways that govern cancer cell function, evasion of apoptosis, and increased proliferation. Therefore, well-designed peptides and their derivatives can be incorporated into drug delivery systems and specifically target the diverse molecular pathways in tumor microenvironments with increased selectivity resulting in improved tumor therapy.
Cell penetrating peptides (CPPs) are short peptides derived from insect, viral or mammalian proteins that have the ability to pass through the cell membrane. These peptides can be covalently coupled to various drug carriers and used for the targeted delivery of drugs, imaging agents, oligonucleotides, nanoparticles and liposomes to the specific molecular targets inside cancer cells.
Tumor homing peptides
Proteomic and genomic studies have shown that glioblas-tomas have increased expression of specific proteins on the cell surface compared to normal brain tissues [1••]. These proteins regulate protein synthesis, cellular movement and various cellular functions important in glioblas-toma progression. Furthermore, these membrane proteins are also involved in receptor-mediated endocytosis, signaling, and transcytosis, which is the essential route for transport of proteins and large molecules across the blood brain barrier (BBB). Therefore, peptides can be designed to target specific receptors and facilitate the transport of drugs or nanocarriers across the BBB.
One of the receptors that is overexpressed in glioblastoma cells is low density lipoprotein receptor related protein (LRP). LRP is a multifunctional receptor for natural ligands that binds receptor-associated protein (RAP), human melanotransferrin (p97), lactoferrin [2], and synthetic peptides, such as peptide analogue of ApoE3 [3] and angiopep-2 (ANG) [4]. Angiopep-2 was used to modify polyethylene glycol-based nanoparticles (PEG-NP) loaded with paclitaxel (ANG-PEG-NP-PTX) [5]. The in vivo antitumor efficacy studies have shown that ANG-PEG-NP-PTX reduces tumor size two-fold as compared to Taxol and PEG-NP [6]. The clinical potential of ANG-PEG-NP-PTX for glioma treatment was confirmed in survival studies which showed that survival time of ANG-PEG-NP-PTX-treated animals was 37 days, significantly longer than that of mice treated with PEG-NP-PTX (30 days), Paclitaxel (25 days) or saline (22 days). ANG conjugated with PTX, also known as ANG1005, was already evaluated in clinical trials for recurrent malignant gliomas and for brain metastases from advanced breast cancer [7]. To transport small molecule drugs and genetic material to the brain, ANG has been conjugated to various carriers (summarized in Table 1).
Table 1.
Selected peptides used for glioblastoma targeting and therapy
| Peptide name | Sequence | Origin and target | Carrier/Conjugate | Cargo/drug | Ref. |
|---|---|---|---|---|---|
| Tumor homing peptides | |||||
| Angiopep-2 (ANG) | Polyethylene glycol co-poly (e-caprolactone) (PEG-PCL) copolymer nanoparticles | Paclitaxel | [6] | ||
| Cell penetrating peptides: PepFect 14 (PF14) and the amphipathic peptide PepFect 28 (PF28) Liposomes | siRNA | [26*] | |||
| TFFYGGSRGKRNNFKTEEY | Derived from the Kunitz domains of aprotinin, a protease inhibitor, that targets low density lipoprotein receptor related protein (LRP) | Human tumor necrosis factor-related apoptosis-inducing ligand (pEGFP- hTRAIL) and paclitaxel (PTX) | |||
| [27] | |||||
| PEGylated oxidized multi-walled carbon nanotubes (O-MWNTs) | Doxorubicin | [28] | |||
| Poly(amidoamine) dendrimers | Doxorubicin | [29] | |||
| Gold nanoparticles | Doxorubicin | [30] | |||
| Chlorotoxin | MCMPCFTTDHQMARKCDDCC GGKGRGKCYGPQCLCR |
Venom of the scorpion Leiurusquinquestriatus. that targets chloride channels and matrix metalloproteinase-2 (MMP-2) | Iron oxide nanoparticle core, coated with a | Green fluorescent protein (GFP) | [31] |
| copolymer of chitosan, polyethylene glycol | encoding DNA | ||||
| (PEG), and polyethylenimine (PEI) | |||||
| Liposomes | Doxorubicin | [32] | |||
| Pep1-L | ACGEMGWGWVRCGGSLCW | Synthetic peptide that targets interleukin 13 receptor a2 (IL13Ra2) | Direct conjugation | Actinium-225, an alpha particle emitter | [11*] |
| PEGylated nanoparticles | Paclitaxel | [12] | |||
| Peptides targeting aberrant cellular signaling pathways | |||||
| LP4 | SWTWEKKLETAVNLAW TAGNSNKWTWK | Synthetic peptide that targets voltage-dependent anion channel 1 (VDAC1) | Conjugated with cell penetrating peptide Antp (Antp-LP4) | Direct inhibition | [15*] |
| NBD | TALDWSWLQTE | Synthetic peptide that targets NEMO binding domain of IKK complex | Conjugated with cell penetrating peptide Antp (Antp-LP4) | Direct inhibition of NF-kB activity | [17] |
| H1 | WPGSGNELKRAFAALRDQI | Synthetic peptide targeting c-Myc | Conjugated with cell penetrating peptide Bac and ELP | Direct inhibition of c-Myc transcriptional activity | [21] |
| Cell penetrating peptides | |||||
| TAT peptide | Polyamidoamine (PAMAM) dendrimer and bacterial magnetic particles (BMPs) | ||||
| siRNA expression plasmid, | [22] | ||||
| GRKKRRQRRRPQ | Transactivator of transcription (TAT) of human immunodeficiency virus | downregulation of EGFR through the electrostatic interactions | |||
| Liposomes | Plasmid encoding green fluorescent protein (pEGFP-N1) | [23] | |||
| Syn B | RGGRLSYSRRRFSTSTGR | Antimicrobial peptide protegrin 1 (PG-1) | Direct conjugation | Doxorubicin Benzylpenicillin (B-Pc) | [24][25] |
| Direct conjugation | |||||
| Penetratin peptide (Antp) | RQIKIWFQNRRMKWKK | Transcription factor antennapedia | Direct conjugation | Doxorubicin | [24] |
Another class of glioma specific peptides were identified while studying glioma-specific chloride channels. It was determined that these channels are preferentially expressed in glioblastoma and that high-grade tumors display more of this channel type than low tumor grades. The first peptide that was identified to bind specifically to these channels was chlorotoxin, a 36-amino acid peptide identified from the venom of the scorpion Leiurus quin-questriatus [8]. Using a recombinant chlorotoxin, it was soon discovered that chlorotoxin has a high affinity for surface-bound matrix metalloproteinase-2 (MMP-2) iso-forms, which are highly expressed in glioblastomas, but normally not expressed in brain tissue. Targeting properties of chlorotoxin to glioblastomas were demonstrated for the delivery of several anticancer agents (see Table 1).
Interleukin-13 receptor α2 (IL-13Rα2) is a glioblastoma-associated plasma membrane receptor, which is expressed in 75% of glioblastomas, but absent in normal brain tissue, making it a potential therapeutic target for glioblastoma treatment. IL-13Rα2-targeting peptide, peptide-1 linear (Pep-1L) was designed to function as a molecular scaffold specifically targeting IL-13Rα2 expressing glioblastoma cells. In vivo studies confirmed that intravenously injected cy5.5 conjugated Pep-1L or [64Cu] Pep-1L in mice accumulates in glioblastoma, specifically binding IL-13Rα2 [9,10]. Pep-1L conjugated to cytotoxic a-particle emitting radioisotopes and Pep-1L-decorated paclitaxel-particle drug delivery systems exhibited remarkable anti-glioblastoma efficacy with an extended median survival time in mice [11•,12].
There are many other peptides that bind to various glioblastoma specific targets, such as vascular endothelial growth factor receptor 2 (VEGFR-2), epidermal growth factor receptor (EGFR), and others reviewed elsewhere in this issue.
Peptides targeting aberrant cellular signaling pathways
Glioblastomas are very heterogeneous and have a complex pathogenesis that involves alterations and/or mutations of some key cellular signaling pathways involved in cell proliferation, apoptosis, migration, invasion, and angiogenesis. Therefore, using peptides targeted to over-expressed oncogenes or signaling molecules involved in regulation of these aberrant pathways provides a promising therapeutic strategy for glioblastoma treatment. In this review, we focus on therapeutic peptides that inhibit specific molecular pathways in glioblastoma and have anticancer activity themselves.
One of the hallmarks of cancer is cellular signaling reprogramming, resulting in evasion of apoptosis, promoting proliferation and survival, and facilitating tumor development and metastasis. The voltage-dependent anion channel 1 (VDAC1), which is overexpressed in glioblastoma cells, plays a key role in cell energy metabolism, regulates mitochondria-mediated apoptosis by interacting with anti-apoptotic proteins, and protects glioblastoma cells from cell death [13,14]. Shteinfer-Kuzmine et al. [15•] designed VDAC1-based peptides targeting these interactions, thereby inhibiting the anti-apoptotic effects of these proteins, activating mitochondria-mediated pathways, and inducing apoptosis. They demonstrated that VDAC-1 based peptides that were conjugated to transferrin receptor or CPP strongly inhibit tumor growth in the orthotopic glioblastoma mouse model.
Another example of aberrant signaling in glioblastoma includes constitutive activation of the NF-kB pathway, which has been shown to promote cell proliferation, angiogenesis, tumor growth, and survival [16]. To target the NF-κB pathway, Friedmann-Morvinski et al. [17] designed a peptide targeting NEMO [called NEMO-binding domain (NBD)] that blocks the interaction of NEMO with the IKK (IκB)-kinase complex, and inhibits NF-κB activity. NBD treatment slowed tumor growth in mouse and human glioblastoma models and extended survival time from 30 to more than 50 days in mice, confirming that the NF-κB pathway is a promising target for glioblastoma treatment.
Other preclinical evaluations include peptides which target c-Myc, a transcriptional regulator that controls cell growth, proliferation, apoptosis, and tumorigenesis. It has been found that deregulated c-Myc expression correlates with the grade of malignancy and that glioma cancer stem cells express c-Myc at much higher levels than non-stem cells [18,19]. To inhibit the c-Myc pathway, a peptide derived from helix 1 (H1) of the helix-loop-helix region of c-Myc (H1) [20], which inhibits the c-Myc signaling pathway, was fused with a cell penetrating peptide and thermo-responsive elastin-like polypeptide (ELP) [21]. When this cell penetrating peptide ELP-H1 (CPP-ELP-H1) was combined with focused hyperthermia of the intracerebral gliomas in vivo, an 80% reduction in tumor volume and at least double the median survival time with full regression in 80% of animals were attained.
Cell penetrating peptides
The BBB and cell plasma membrane are impermeable to therapeutic macromolecules due to the large size and inherently poor penetration capabilities of these molecules. These limitations can be addressed by incorporating CPPs, which promote crossing the BBB and plasma membrane, into peptide-based drug delivery systems. Numerous preclinical studies with CPP-based drug delivery systems demonstrated the efficacy in various cancer models, including glioblastoma.
CPPs, derived from human immunodeficiency virus type1 (HIV-1) transcriptional activator TAT protein (TAT), were conjugated to polyamidoamine (PAMAM) dendrimers loaded with siRNA and demonstrated enhanced therapeutic effect of siRNA in vivo [22]. That TAT can deliver genetic material was further confirmed in studies by Gupta et al. [23] which demonstrated that TAT decorated liposomes can efficiently deliver a plasmid encoding green fluorescent protein (pEGFP-N1) to intracranial human brain tumor xenografts in nude mice.
Other in vivo studies demonstrated that SynB1 CPPs, derived from natural peptides called protegrins, and Antennapedia homeodomain-derived penetratin peptide (Antp), can enhance the delivery of the anticancer drug doxorubicin across the BBB, indicating their therapeutic potential for glioblastoma treatment [24,25]. While CPPs are capable of transporting into cells a wide variety of cargoes, there is still concern about the non-specificity of CPP. Specificity of CPP-derived therapeutics can be improved by combining them with tumor homing ligands or other targeted delivery systems, therefore, ensuring efficient and selective drug delivery.
Conclusion and perspective
This review has focused on the application of peptides that not only bind to receptors specifically expressed on cancer cells and peptides targeting aberrant cellular signaling pathways, but also on peptides that demonstrate a high capacity to deliver cargo into tumor cells and penetrate the BBB, with a specific focus on glioblastoma. Cancer cell targeting peptides provide excellent means for the delivery of a variety of anticancer therapeutics, such as small molecule drugs, oligonucleotides, radio-nucleotides, cytotoxic peptides, and others. While peptide-mediated drug delivery increases the efficacy of cancer treatment and decreases side effects in healthy tissues, there are still some limitations, such as stability in circulation, and, in the case of CPPs, non-specificity, since the CPP will be likely taken up by circulating blood cells before it reaches the tumor site. Stability of the peptides can be improved by chemical modification or conjugation of the peptides with macromolecules and nanocarriers that will make them resistant to proteolytic degradation. One of the possibilities to improve the specificity of CPP-based therapeutics is to conjugate them with tumor homing peptides, which are meant to target cells or tissue-specific receptors. This is especially important – and challenging – in the treatment of glioblastomas, which are difficult to treat because of their heterogeneity. Despite great advances and successful clinical applications of some peptides for cancer treatment, there is a need for identification of additional glioblastoma-specific peptide-ligands. In the future, peptide targets could be identified based on the molecular and genetic profile of an individual patient, allowing for a personalized, targeted approach with fewer of the devastating side effects of standard cancer therapies.
Acknowledgements
We would like to thank Dr. Bettye Sue Hennington and Lindsey Turner for manuscript editing, and Sonja Dragojevic for technical editing and help with creating the figure.
Funding
National Science Foundation PFI: AIR-TT; Award Number: 1640519.
Footnotes
Conflict of interest statement
D. Raucher is the CEO of Thermally Targeted Technology Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.••. Shergalis A, Bankhead A 3rd, Luesakul U, Muangsin N, Neamati N: Current challenges and opportunities in treating glioblastoma. Pharmacol Rev 2018, 70:412–414. [DOI] [PMC free article] [PubMed] [Google Scholar]; Comprehensive and useful review discussing novel drug delivery methods, including prodrugs and nanoparticles for the treatment of glioblastoma.
- 2.Papademetriou IT, Porter T: Promising approaches to circumvent the blood-brain barrier: progress, pitfalls and clinical prospects in brain cancer. Ther Deliv 2015, 6:989–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Markoutsa E, Papadia K, Giannou AD, Spella M, Cagnotto A, Salmona M, Stathopoulos GT, Antimisiaris SG: Mono and dually decorated nanoliposomes for brain targeting, in vitro and in vivo studies. Pharm Res 2014, 31:1275–1289. [DOI] [PubMed] [Google Scholar]
- 4.Demeule M, Regina A, Che C, Poirier J, Nguyen T, Gabathuler R, Castaigne JP, Beliveau R: Identification and design of peptides as a new drug delivery system for the brain. J Pharmacol Exp Ther 2008, 324:1064–1072. [DOI] [PubMed] [Google Scholar]
- 5.Xin H, Jiang X, Gu J, Sha X, Chen L, Law K, Chen Y, Wang X, Jiang Y, Fang X: Angiopep-conjugated poly(ethylene glycol)-co-poly(epsilon-caprolactone) nanoparticles as dual-targeting drug delivery system for brain glioma. Biomaterials 2011, 32:4293–4305. [DOI] [PubMed] [Google Scholar]
- 6.Xin H, Sha X, Jiang X, Zhang W, Chen L, Fang X: Anti-glioblastoma efficacy and safety of paclitaxel-loading Angiopep-conjugated dual targeting PEG-PCL nanoparticles. Biomaterials 2012, 33:8167–8176. [DOI] [PubMed] [Google Scholar]
- 7.Li F, Tang SC: Targeting metastatic breast cancer with ANG1005, a novel peptide-paclitaxel conjugate that crosses the blood-brain-barrier (BBB). Genes Dis 2017, 4:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soroceanu L, Gillespie Y, Khazaeli MB, Sontheimer H: Use of chlorotoxin for targeting of primary brain tumors. Cancer Res 1998, 58:4871–4879. [PubMed] [Google Scholar]
- 9.Pandya H, Gibo DM, Garg S, Kridel S, Debinski W: An interleukin 13 receptor alpha 2-specific peptide homes to human glioblastoma multiforme xenografts. Neuro Oncol 2012, 14:6–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sai KKS, Sattiraju A, Almaguel FG, Xuan A, Rideout S, Krishnaswamy RS, Zhang J, Herpai DM, Debinski W, Mintz A: Peptide-based PET imaging of the tumor restricted IL13RA2 biomarker. Oncotarget 2017, 8:50997–51007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.•. Sattiraju A, Solingapuram Sai KK, Xuan A, Pandya DN, Almaguel FG, Wadas TJ, Herpai DM, Debinski W, Mintz A: IL13RA2 targeted alpha particle therapy against glioblastomas. Oncotarget 2017, 8:42997–43007. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study highlights the importance of Interleukin-13 receptor alpha 2 (IL13RA2), which is a glioblastoma restricted receptor that is abundantly overexpressed in glioblastoma but absent in normal brain tissue. In this work, authors evaluated the potential of a Pep-1L peptide that targets IL13RA2 conjugated to Actinium-225, an alpha particle emitter, to treat glioblastoma in mice. It has been shown that treated mice have significantly greater overall survival than the control group.
- 12.Wang B, Lv L, Wang Z, Jiang Y, Lv W, Liu X, Wang Z, Zhao Y, Xin H, Xu Q: Improved anti-glioblastoma efficacy by IL-13Rα2 mediated copolymer nanoparticles loaded with paclitaxel. Sci Rep 2015, 5:16589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maldonado EN, Lemasters JJ: Warburg revisited: regulation of mitochondrial metabolism by voltage-dependent anion channels in cancer cells. J Pharmacol Exp Ther 2012, 342:637–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shoshan-Barmatz V, De Pinto V, Zweckstetter M, Raviv Z, Keinan N, Arbel N: VDAC, a multi-functional mitochondrial protein regulating cell life and death. Mol Aspects Med 2010, 31:227–285. [DOI] [PubMed] [Google Scholar]
- 15.•. Shteinfer-Kuzmine A, Arif T, Krelin Y, Tripathi SS, Paul A, Shoshan-Barmatz V: Mitochondrial VDAC1-based peptides: attacking oncogenic properties in glioblastoma. Oncotarget 2017, 8:31329–31346. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study evaluates efficiency of peptides inhibiting voltage-dependent anion channel 1 (VDAC1)– a key component of cell energy, metabolism and apoptosis regulation – in glioblastoma treatment. Peptide-treated tumors showed decreased expression of metabolism-related enzymes, and elevated levels of apoptotic proteins resulting in tumor growth inhibition.
- 16.Raychaudhuri B, Han Y, Lu T, Vogelbaum MA: Aberrant constitutive activation of nuclear factor kappaB in glioblastoma multiforme drives invasive phenotype. J Neuro Oncol 2007, 85:39–47. [DOI] [PubMed] [Google Scholar]
- 17.Friedmann-Morvinski D, Narasimamurthy R, Xia Y, Myskiw C, Soda Y, Verma IM: Targeting NF-kappaB in glioblastoma: a therapeutic approach. Sci Adv 2016, 2:e1501292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herms JW, von Loewenich FD, Behnke J, Markakis E, Kretzschmar HA: c-Myc oncogene family expression in glioblastoma and survival. Surg Neurol 1999, 51:536–542. [DOI] [PubMed] [Google Scholar]
- 19.Wang J, Wang H, Li Z, Wu Q, Lathia JD, McLendon RE, Hjelmeland AB, Rich JN: c-Myc is required for maintenance of glioma cancer stem cells. PLoS One 2008, 3:e3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Draeger LJ, Mullen GP: Interaction of the bHLH-zip domain of c-Myc with H1-type peptides. Characterization of helicity in the H1 peptides by NMR. J Biol Chem 1994, 269:1785–1793. [PubMed] [Google Scholar]
- 21.Bidwell GL 3rd, Perkins E, Hughes J, Khan M, James JR, Raucher D: Thermally targeted delivery of a c-Myc inhibitory polypeptide inhibits tumor progression and extends survival in a rat glioma model. PLoS One 2013, 8:e55104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han L, Zhang A, Wang H, Pu P, Jiang X, Kang C, Chang J: Tat-BMPs-PAMAM conjugates enhance therapeutic effect of small interference RNA on U251 glioma cells in vitro and in vivo. Hum Gene Ther 2010, 21:417–426. [DOI] [PubMed] [Google Scholar]
- 23.Gupta B, Levchenko TS, Torchilin VP: TAT peptide-modified liposomes provide enhanced gene delivery to intracranial human brain tumor xenografts in nude mice. Oncol Res 2007, 16:351–359. [DOI] [PubMed] [Google Scholar]
- 24.Rousselle C, Clair P, Lefauconnier JM, Kaczorek M, Scherrmann JM, Temsamani J: New advances in the transport of doxorubicin through the blood-brain barrier by a peptide vector-mediated strategy. Mol Pharmacol 2000, 57:679–686. [DOI] [PubMed] [Google Scholar]
- 25.Rousselle C, Clair P, Temsamani J, Scherrmann JM: Improved brain delivery of benzylpenicillin with a peptide-vector-mediated strategy. J Drug Target 2002, 10:309–315. [DOI] [PubMed] [Google Scholar]
- 26.•. Srimanee A, Arvanitidou M, Kim K, Hallbrink M, Langel U: Cell- penetrating peptides for siRNA delivery to glioblastomas. Peptides 2018, 104:62–69. [DOI] [PubMed] [Google Scholar]; In this study, several siRNA carriers have been constructed using cell-penetrating peptides. These constructs show a two-fold increase in gene silencing efficiency compared to control peptide and improved glioblastoma specificity.
- 27.Sun X, Pang Z, Ye H, Qiu B, Guo L, Li J, Ren J, Qian Y, Zhang Q, Chen J, Jiang X: Co-delivery of pEGFP-hTRAIL and paclitaxel to brain glioma mediated by an angiopep-conjugated liposome. Biomaterials 2012, 33:916–924. [DOI] [PubMed] [Google Scholar]
- 28.Ren J, Shen S, Wang D, Xi Z, Guo L, Pang Z, Qian Y, Sun X, Jiang X: The targeted delivery of anticancer drugs to brain glioma by PEGylated oxidized multi-walled carbon nanotubes modified with angiopep-2. Biomaterials 2012, 33:3324–3333. [DOI] [PubMed] [Google Scholar]
- 29.Han S, Zheng H, Lu Y, Sun Y, Huang A, Fei W, Shi X, Xu X, Li J, Li F: A novel synergetic targeting strategy for glioma therapy employing borneol combination with angiopep-2-modified, DOX-loaded PAMAM dendrimer. J Drug Target 2018, 26:86–94. [DOI] [PubMed] [Google Scholar]
- 30.Ruan S, Yuan M, Zhang L, Hu G, Chen J, Cun X, Zhang Q, Yang Y, He Q, Gao H: Tumor microenvironment sensitive doxorubicin delivery and release to glioma using angiopep-2 decorated gold nanoparticles. Biomaterials 2015, 37:425–435. [DOI] [PubMed] [Google Scholar]
- 31.Kievit FM, Veiseh O, Fang C, Bhattarai N, Lee D, Ellenbogen RG, Zhang M: Chlorotoxin labeled magnetic nanovectors for targeted gene delivery to glioma. ACS Nano 2010, 4:4587–4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiang Y, Liang L, Wang X, Wang J, Zhang X, Zhang Q: Chloride channel-mediated brain glioma targeting of chlorotoxin-modified doxorubicine-loaded liposomes. J Control Release 2011, 152:402–410. [DOI] [PubMed] [Google Scholar]


