Introduction
Sturge-Weber syndrome (SWS) is a neurocutaneous disorder caused by a somatic mosaic mutation in GNAQ.1 SWS brain involvement causes seizures, stroke-like episodes and/or neurological deficits such as hemiparesis and intellectual disability. Seventy-five percent of infants with SWS develop seizures within the first year of life.2 Those with more severe epilepsy experience greater cognitive deficits and intellectual disability.3 There is ictal single-photon emission computed tomography (SPECT) evidence that, in SWS, the seizures themselves may contribute to the brain injury due to an abnormal hemodynamic response.4 The combination of impaired cortical blood flow and increased metabolic demand during a seizure may contribute to increased cortical damage.4 Early onset of seizures, before one year of age, has been associated with worse neurological outcome.2 Therefore delaying seizure onset may be beneficial for later clinical outcome.
Two approaches have been proposed to treat infants with SWS brain involvement; aggressive seizure management once seizures begin and presymptomatic treatment before the onset of seizures.5–7 Aggressive seizure management after onset usually involves an anti-epileptic medication to control seizures and abortive seizure management. Low dose aspirin may decrease the frequency and severity of stroke-like episodes and seizures.8 Although it makes sense that delaying the onset of seizures would be protective, actual data supporting the use of presymptomatic treatment with aspirin or antiepileptic drugs are limited.5,7
We aimed to further develop the hypothesis that presymptomatic treatment in infants with SWS brain involvement may delay seizure onset. To do so, we compared infants with SWS treated presymptomatically with infants treated after the onset of seizures.
Methods
Participants were obtained from a SWS research database consisting of patients with a port-wine birthmark who consented to research at the Kennedy Krieger Institute SWS Center (n = 336). The demographics of the database were similar to that of the US population. All selected subjects were born between 2006 and 2016 and had brain involvement confirmed by contrast-enhanced magnetic resonance imaging (MRI), Fig 1. All subjects had SWS neurological scores assigned prospectively at clinic visits to assess seizure frequency (seizure score), hemiparesis, visual field deficit, and cognitive function (supplementary Table 1).9 Presymptomatic individuals who had not yet developed seizures were given a score of 0 for the seizure subscore. Details on participants are noted in (supplementary Table 2). Age of seizure onset and medication records were collected retrospectively from the patients’ clinical records.
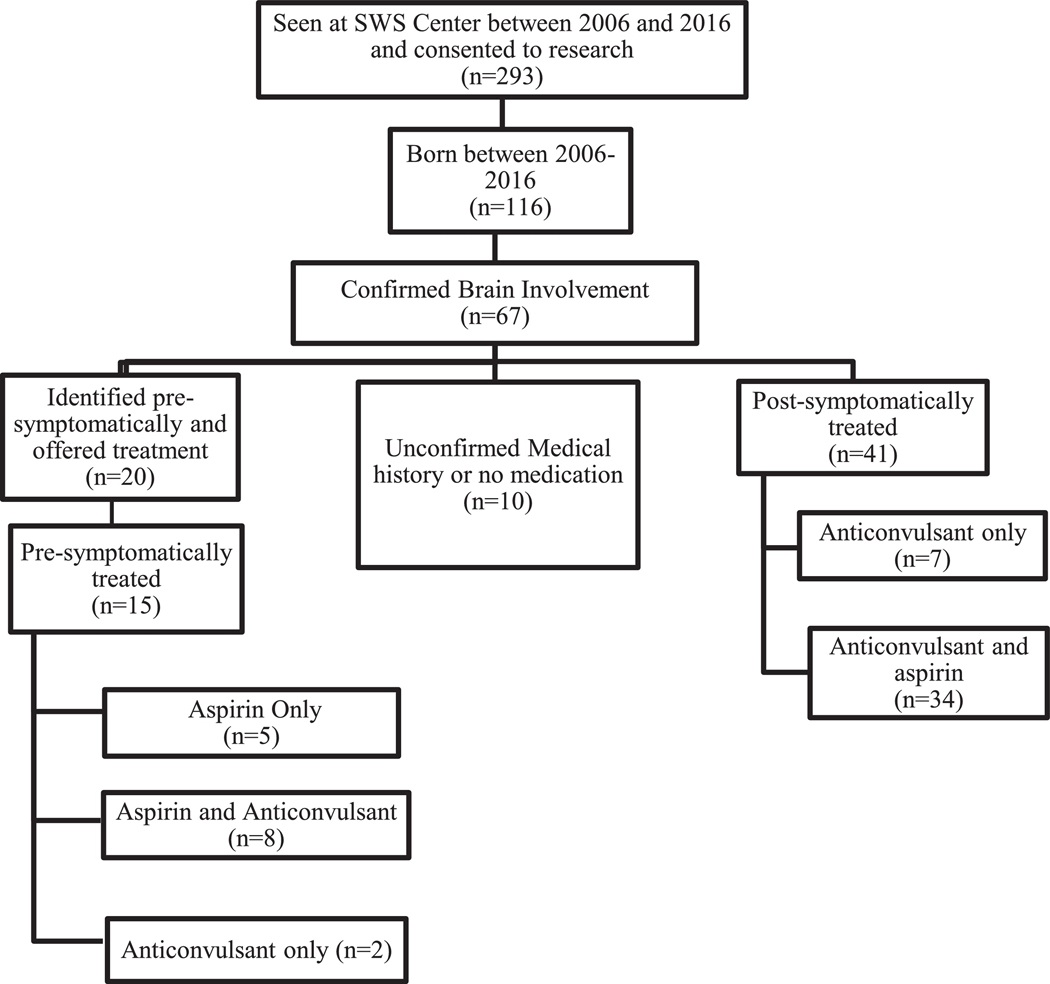
FIGURE 1.
Process of participant selection using our SWS Center research database. SWS: Sturge-Weber syndrome
During this period presymptomatic treatment was offered at our center to almost all infants diagnosed presymptomatically. Twenty participants with brain involvement were offered presymptomatic treatment and 15 of those 20 accepted. Four of the five who declined presymptomatic treatment were unilaterally affected; one participant, with a small unilateral, right-sided parietal-occipital area of SWS brain involvement, was presymptomatic at age two years and nine months when she presented. She was not put on any medication and remained seizure free at time of analysis (four years). The majority of postsymptomatically treated participants developed seizures either before diagnosis of brain involvement (n = 15) or before visiting our center (n = 22) making presymptomatic treatment impossible by that time. Postsymptomatic matches for the presymptomatically treated subjects were selected by study staff were blinded to their neurological scores based on the criteria outlined below.
For the first analysis, infants treated presymptomatically with low-dose aspirin (3 to 5 mg/kg/day) before seizure onset (n = 5) were compared with postsymptomatically treated infants (Supplementary Table 2). In the second analysis, presymptomatically treated infants (n = 8) received low-dose aspirin and low-dose anti-epileptic drugs and were compared with postsymptomatically treated infants (Supplementary Table 2). Subjects were taking either levetiracetam (n = 5), or oxcarbazepine (n = 3). One participant in the oxcarbazepine group began levetiracetam but switched due to behavioral side effects. The mean dosage of levetiracetam was 17 mg/kg/day (range = 7.58 mg/kg/day to 24.35 mg/kg/day) while the mean dose of oxcarbazepine was 23.14 mg/kg/day (range = 21.62 mg/kg/day to 25.59 mg/kg/day). The mean aspirin dose in the second analysis was 3.69 mg/kg/day (range = 2.14 mg/kg/day to 4.87 mg/kg/day). Vitamin B6 was prescribed to two of the five subjects on levetiracetam.
Each presymptomatic participant was matched with a postsymptomatic participant based on gender and brain involvement extent (number and location of lobes with abnormal leptomeningeal enhancement on MRI). Participants were also matched by age when neurological score was collected. For the presymptomatic participants, the neurological score from their most recent visit was used for analysis, and matched with the neurological score of the postsymptomatic participant taken closest to that age (Supplementary Table 3). SWS neurological scores and ages at seizure onset were compared between groups using the Wilcoxon-signed rank test and paired t tests, respectively. All analyses were completed using IBM SPSS Statistics 24.
Results
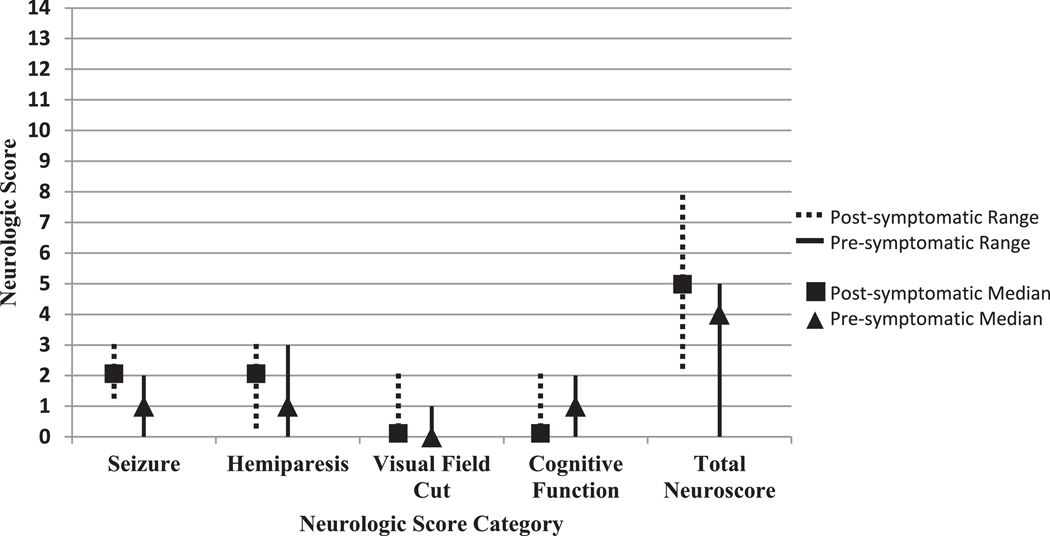
For analysis 1, differences between the neurological subscores between the presymptomatic participants and their postsymptomatic counterparts were not significant, Fig 2. Two of the five presymptomatic analysis 1 participants had side effects that may have been due to aspirin. These included small amounts of bruising which resolved with a decrease in medication dose, and one participant who developed a rash the doctor suspected was petechial around age 1.5 months of age, at which time aspirin was discontinued.
FIGURE 2.
Medians of the neuroscores of presymptomatic treatment (aspirin only) versus postsymptomatic treatment. Graph of the medians and ranges of the neurological scores of those presymptomatically treated with aspirin only and postsymptomatically treated. *There were significant differences between the two groups (Wilcoxon-signed rank test).
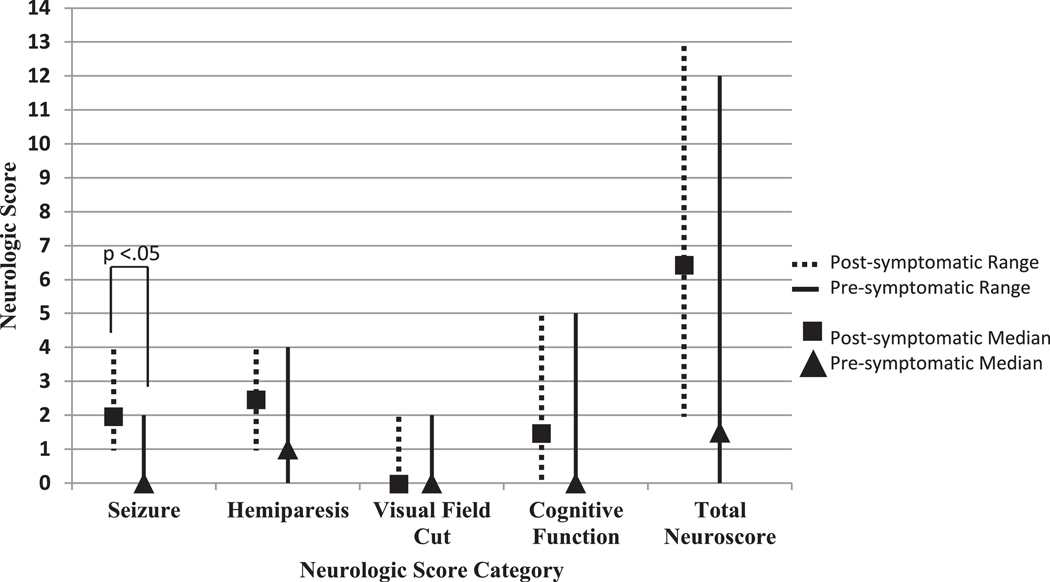
However, in analysis 2 (aspirin and anti-epileptic), seizure scores were significantly less in presymptomatically treated subjects (median seizure score = 0, 95% confidence interval [0, 2]) than in postsymptomatically treated infants (median = 2, P = 0.02, 95% confidence interval [1, 2.]), Fig 3. Although extent of brain involvement was similar between groups, four presymptomatically treated subjects had yet to develop seizures at the time of analysis, Supplementary Table 4. Participants who had not yet experienced seizure onset were older at the time of analysis (Mean = 27.5 SEM 6.7 months) than their postsymptomatic counterparts were at their seizure onset (Mean = 6.0 SEM 1.4 months, P =.03). Additionally, two other presymptomatic participants with seizure onset showed an older age of onset than their postsymptomatic matches. Side effects seen consisted of irritability, sleep disruptions, or increased behavior difficulties while on antiepileptic drugs; these were noted in three participants and all were resolved after medication adjustments. There were no adverse side effects reported in this group pertaining to aspirin use. Fifty percent of the presymptomatic participants in analysis 2 showed abnormal electroencephalography (EEG) findings (asymmetries but no ictal activity) before treatment or onset of seizures, Supplementary Table 4.
FIGURE 3.
Medians of the neuroscores of presymptomatic treatment (aspirin and anti-epileptic) versus postsymptomatic treatment. Graph of the medians and ranges of the neurological scores of those presymptomatically treated with both aspirin and antiepileptic drugs versus those postsymptomatically treated. *The seizure subscore is significantly lower for those given presymptomatic treatment (Wilcoxon-signed rank test, P < 0.05).
Discussion
SWS is a devastating disease with a progressive nature. Efforts should be made to identify neuroprotective interventions that could positively impact outcome in early stages of the disease. We hypothesized that low-dose antiepileptic drugs and aspirin may be effective in delaying seizure onset in SWS infants. This hypothesis is supported by lower seizure scores and older age of seizure onset in the presymptomatically treated infants. Most notably, four of the infants treated presymptomatically with antiepileptic drugs and aspirin had yet to develop seizures at ages 14 to 39 months.
This study is limited by its small sample size, lack of a placebo control group, and use of seizure score rather than long-term neuropsychologic testing as the outcome measure. Extent of brain involvement in SWS is associated with severity of seizures and neurological outcome. Bilaterally affected subjects made up a larger percent of the aspirin and anti-epileptic study subjects than what would normally be seen in a SWS population, with 50% of subjects showing bilateral brain involvement compared with the average 15%.10 This may be due to ascertainment bias. However, the percentage of bilaterally affected subjects was equally spread between those presymptomatically and postsymptomatically treated, Supplementary Table 3, so we do not believe this would have biased estimates of association. On the other hand, the one subject in our database not treated presymptomatically who has remained seizure free has a smaller area of brain involvement than typical for most SWS patients. Presymptomatic treatment may be most beneficial for patients with extensive unilateral or bilateral brain involvement who are at greatest risk for severe seizures and neurological deficits. Further-more, leptomeningeal enhancement is not the only variable of brain involvement that may influence a participant’s severity of brain involvement and therefore this could introduce other possible selection bias. EEG data were also limited, but did demonstrate abnormalities before the onset of seizure in some subjects.
Our preliminary study’s small sample size and lack of placebo control group prevents robust conclusions but does allow us to hone our hypothesis and use these data as the basis of a multicentered, prospective, and rigorous clinical research trial, the planning of which is currently underway. Future studies should investigate EEGs as a potential biomarker and use additional MRI findings for better stratification of subjects based on brain involvement.
Conclusions
Without intervention, 75% of children with SWS brain involvement will develop seizures in the first year of life, and those with early seizure onset have a worse neurological outcome.2 Therefore continued efforts, building upon these data, are urgently needed to develop presymptomatic treatments.
Supplementary Material
Acknowledgments
Funding
This work was supported by grants from the National Institute of Neurological Disorders and Stroke (NINDS) (National Institutes of Health [NIH] U54NS065705 to Dr. Lawton; Sturge-Weber Project PI Dr. Comi) and from Celebrate Hope Foundation (to Dr. Comi). The Brain Vascular Malformation Consortium (U54NS065705) is a part of the NIH Rare Diseases Clinical Research Network (RDCRN), supported through the collaboration between the NIH Office of Rare Diseases Research (ORDR) at the National Center for Advancing Translational Science (NCATS) and the NINDS.
Footnotes
Group Information: All collaborators are part of the Rare Diseases Clinical Research Network (RDCRN) Brain and Vascular Malformation Consortium (BVMC) SWS Investigator Group which is sponsored by the National Institutes of Health:
Karen L. Ball, Brian J. Fisher: The Sturge-Weber Foundation, Houston, TX; Jim I. Koenig: National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD; Michael T. Lawton: Department of Neurosurgery, Barrow Neurological Institute, Phoenix Children’s Hospital, Phoenix, AZ; Warren D. Lo: Department of Neurology, Nationwide Children’s Hospital, Columbus, OH Douglas A. Marchuk: Molecular Genetics and Microbiology Department, Duke University School of Medicine, Durham, NC; Daniel K. Miles: Department of Neurology, Pediatric Epilepsy, New York University Langone Health, New York, NY; Marsha A. Moses: Vascular Biology Program, Boston Children’s Hospital, Boston, MA and Department of Surgery, Harvard Medical School and Boston Children’s Hospital, Boston, MA; Angus A. Wilfong: Department of Neurology, Barrow Neurological Institute, Phoenix Children’s Hospital, Phoenix AZ.
Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.pediatrneurol.2018.04.009.
Conflicts of interest
The authors declare no conflict of interest.
References
- 1.Shirley MD, Tang H, Gallione CJ, et al. Sturge-Weber syndrome and port-wine stains caused by somatic mutation in GNAQ. N Engl J Med. 2013;368:1971–1979. 10.1056/NEJMoa1213507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sujansky E, Conradi S. Sturge-Weber syndrome: age of onset of seizures and glaucoma and the prognosis for affected children. J Child Neurol. 1995;10:49–58. 10.1177/088307389501000113. [DOI] [PubMed] [Google Scholar]
- 3.Arzimanoglou A, Aicardi J. The epilepsy of Sturge-Weber syndrome: clinical features and treatment in 23 patients. Acta Neurol Scand. 1992;86:18–22. [DOI] [PubMed] [Google Scholar]
- 4.Aylett SE, Neville BG, Cross JH, Boyd S, Chong WK, Kirkham FJ. Sturge-Weber syndrome: cerebral haemodynamics during seizure activity. Dev Med Child Neurol. 1999;41:480–485. 10.1111/j.1469-8749.1999.tb00641.x. [DOI] [PubMed] [Google Scholar]
- 5.Lance EI, Sreenivasan AK, Zabel TA, Kossoff EH, Comi AM. Aspirin use in Sturge-Weber syndrome: side effects and clinical out-comes. J Child Neurol. 2013;28:213–218. 10.1177/0883073812463607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sudarsanam A, Ardern-Holmes SL. Sturge-Weber syndrome: from the past to the present. Eur J Paediatr Neurol. 2014;18:257–266. 10.1016/j.ejpn.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Ville D, Enjolras O, Chiron C, Dulac O. Prophylactic antiepileptic treatment in Sturge-Weber disease. Seizure. 2002;11:145–150. 10.1053/seiz.2001.0629. [DOI] [PubMed] [Google Scholar]
- 8.Maria BL, Neufeld JA, Rosainz LC, et al. Central nervous system structure and function in Sturge-Weber syndrome: evidence of neurologic and radiologic progression. J Child Neurol. 1998;13:606–618. 10.1177/088307389801301204. [DOI] [PubMed] [Google Scholar]
- 9.Kelley TM, Hatfield LA, Lin DD, Comi AM. Quantitative analysis of cerebral cortical atrophy and correlation with clinical severity in unilateral Sturge-Weber syndrome. J Child Neurol. 2005;20:867–870. 10.1177/08830738050200110201. [DOI] [PubMed] [Google Scholar]
- 10.Bebin EM, Gomez MR. Prognosis in Sturge-Weber disease: comparison of unihemispheric and bihemispheric involvement. J Child Neurol. 1988;3:181–184. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.