Abstract
Background
Placebo and nocebo effects embody psychoneurobiological phenomena where behavioural, neurophysiological, perceptive and cognitive changes occur during the therapeutic encounter in the healthcare context. Placebo effects are produced by a positive healthcare context; while nocebo effects are consequences of negative healthcare context. Historically, placebo, nocebo and context-related effects were considered as confounding elements for clinicians and researchers. In the last two decades this attitude started to change, and the understanding of the value of these effects has increased. Despite the growing interest, the knowledge and the awareness of using the healthcare context to trigger placebo and nocebo effects is currently limited and heterogeneous among physiotherapists, reducing their translational value in the physiotherapy field.
Objectives
To introduce the placebo, nocebo and context-related effects by: (1) presenting their psychological models; (2) describing their neurophysiological mechanisms; (3) underlining their impact for the physiotherapy profession; and (4) tracing lines for future researches.
Conclusion
Several psychological mechanisms are involved in placebo, nocebo and context-related effects; including expectation, learning processes (classical conditioning and observational learning), reinforced expectations, mindset and personality traits. The neurophysiological mechanisms mainly include the endogenous opioid, the endocannabinoid and the dopaminergic systems. Neuroimaging studies have identified different brain regions involved such as the dorsolateral prefrontal cortex, the rostral anterior cingulate cortex, the periaqueductal gray and the dorsal horn of spine. From a clinical perspective, the manipulation of the healthcare context with the best evidence-based therapy represents an opportunity to trigger placebo effects and to avoid nocebo effects respecting the ethical code of conduct. From a managerial perspective, stakeholders, organizations and governments should encourage the assessment of the healthcare context aimed to improve the quality of physiotherapy services. From an educational perspective, placebo and nocebo effects are professional topics that should be integrated in the university program of health and medical professions. From a research perspective, the control of placebo, nocebo and context-related effects offers to the scientific community the chance to better measure the impact of physiotherapy on different outcomes and in different conditions through primary studies.
Keywords: Contextual factors, Placebo effect, Nocebo effect, Physical therapy modalities, Pain, Expectation, Conditioning, Rehabilitation, Therapeutic outcome, Learning
Background
Placebo and nocebo effects embody complex and distinct psychoneurobiological phenomena where behavioural, neurophysiological, perceptive and cognitive changes occur during therapeutic encounter, between the (physio) therapist and the patient, in the healthcare context [1]. Specifically, placebos and nocebos can be defined as inert treatments or active treatments that are not therapeutically effective for the disease or condition under cure (active placebo or nocebo). These treatments, if administered in a therapeutic and healthcare context, can produce remarkable effects, known as “placebo effects” or “nocebo effects” [2]. “Placebo effects” are produced by a positive healthcare context that can ameliorate the patient’s symptoms [1, 3]. Conversely, “nocebo effects” are produced by a negative healthcare context that can elevate the patient’s symptoms [4–6]. These effects can also occur when an active and therapeutically effective treatment is administered: indeed, any healthcare treatment (active or inert) that is administered in any healthcare contexts (e.g., medical, rehabilitative) can trigger contextual-related effects. Placebo, nocebo and contextual-related effects have been used as models to examine the body-mind interaction by investigating the impact of these phenomena on different bodily processes, diseases and individual behaviour [7, 8].
Indeed, the healthcare context is not a vacuum, but it is an enriched relational space created by several elements [9–15] – defined as contextual factors – such as: (1) the physiotherapist’s professionalism, mindset and appearance; (2) the patient’s beliefs, experiences and expectation about the disease and the therapy; (3) the words, gestures and behaviour presented in the physiotherapist-patient relationship during the therapeutic encounter; (4) the rituality, the invasiveness and the overt application of the intervention; (5) the furniture, the architectural design and the overall impression of the clinic [16]. These contextual factors have been suggested as responsible for a large non-specific component of treatment efficacy, directly affecting the quality of patient’s health-related outcomes (e.g., pain, disability, satisfaction, and experience) [16–20]. For instance, the same treatment (e.g., exercise) if associated with physiotherapist’s positive verbal suggestion (e.g., “This exercise will ameliorate your symptoms”) can reduce the pain and increase the strength of a patient. Whereas, if associated with verbal suggestion of uncertainty (e.g., “This exercise sometimes might reduce symptoms”), could even worsen pain and strength [17, 21, 22].
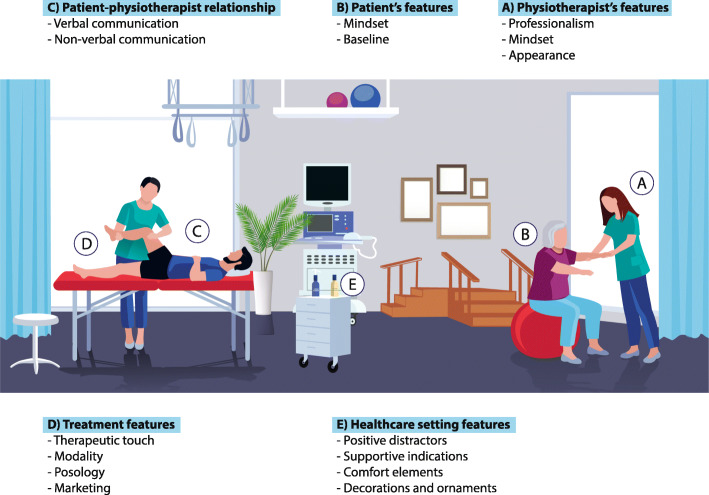
If compared with other healthcare professionals, the physiotherapist’s clinical action is deeply pervaded by placebo and nocebo effects for several reasons strongly related to the characteristics of physiotherapy administration [23]. Physiotherapists spend a significant amount of time with their patients in numerous sessions, intervening on different disorders (e.g., motor, cardio-respiratory, gastrointestinal, urogynecological or neurological), and therefore easily establishing an empathetic therapeutic relationship [24, 25]. Moreover, physiotherapists, more than medical doctors, interact with their patients using verbal (e.g., encouraging words) and non-verbal (e.g., communicative, non-therapeutic touch) elements [26, 27]. Every physiotherapy intervention, being it manual treatment, exercises or modalities, is naturally enriched by different contextual factors that can influence the trajectory of outcomes towards a positive or a negative result, depending on how they are managed by the physiotherapist [17] (Fig. 1).
Fig. 1.
Contextual factors in clinical practice. The following contextual factors were accepted as effective modifiers of physiotherapy outcomes. For a review of this topic see reviews [16, 17, 19]. a Physiotherapist’s features: professionalism (expertise, qualification, reputation, education, trining); mindset (behaviour, beliefs, expectation, previous experience); appearance (attire, uniform, white coat, trustworthiness). b Patient’s features: mindset (expectation, previous experience, history of treatment, preference, desire, and emotion); baseline (level of symptoms, comorbidity, health condition, gender, age). c Patient-physiotherapist relationship: verbal communication (positive message, tone of voice, active listening, suggestions of support and encouragement, language reciprocity, warmth, attention, care, empathetic interaction); non-verbal communication (eye contact, facial caring expression, smiling, posture, gestures, head nodding, forward leaning, open body orientation). d Treatment features: therapeutic touch (emotional, empathetic, affective); modality (level of invasiveness, open/overt application, observational/social learning); posology (personalized treatment, treatment delivered by the same physiotherapist, cleanliness, adequate length of the consultation, punctuality, flexibility with patient’s appointments, timely and efficient treatment, adequate frequency, duration and follow-up of therapy); marketing (brand, prize, novelty, rituality). e Healthcare setting features: positive distractors (natural lighting, low noise levels, relaxing and soft music, pleasing aromas, adequate temperature); supportive indications (highly visible and easy to read signs, parking information, accessible entrances, clear and consistent verbal or written directions, information desks and accessible electronic information); comfort element (windows and skylights, private therapeutic settings, good access to services, convenient clinic hours, location, parking, and available and approachable support staff); decorations and ornaments (nature artworks, green vegetation, flowers, water, plants, garden, colour)
Throughout the history of physiotherapy, placebo, nocebo and contextual-related effects have always been considered a challenging phenomena for two main reasons [28]. From a research perspective, these represented possible confounders capable of decreasing both internal and external validity of the studies conducted [29, 30]. From a clinical perspective, contextual factors symbolise troublesome and non-specific variables that can attenuate the therapeutic role of the specific interventions such as manual treatments, therapeutic exercise and modalities administration [31, 32].
It was at the end of the first decade of the twenty-first century that this attitude started to change. Indeed, the scientific community began to investigate the mechanisms of action of different therapeutic interventions like exercise and manual treatment of joint, soft and neural tissue, enlightening the weight of placebo and nocebo effects in such mechanisms and therefore their relevant role in physiotherapy [33, 34]. Emergent studies have suggested a mechanical and neurophysiological mechanism (peripheral, spinal and supraspinal) as the base of the effects induced by the therapeutic interventions adopted by the physiotherapists [35–37]. Considering the supraspinal mechanisms, placebo and nocebo effects were indicated as important top-down modulators of patient’s symptoms, mainly pain and motor performance [38–44], thus becoming elements that physiotherapists should attentively consider in their clinical practice [17].
Despite the growing interest in the physiotherapy field [16, 17], the knowledge and the awareness of using contextual factors to enhance placebo and avoid nocebo effects is still limited among physiotherapists [45]. The same unawareness has also been shown among other healthcare providers such as nurses and physicians [46, 47]. Moreover, the lack of a clear education about this professional topic in physiotherapy university courses reduces their perceived translational value and their relevance for physiotherapy practice [16, 48].
The aim of this narrative review is to offer a general overview of placebo, nocebo and contextual factors effects by: (1) presenting the psychological models behind their effects; (2) describing the neurophysiological mechanisms involved; (3) underlining the impact for the physiotherapy profession (clinical, managerial and educational); and (4) tracing lines for future researches.
To this end, the narrative review style has been selected, citing both primary studies (e.g., clinical trial) and secondary studies (e.g., systematic review, narrative review) on placebo and nocebo effects from different healthcare fields (e.g., medicine, nursing, physiotherapy) as previously reported [16, 17, 19]. Some studies specifically focus on the role of inert substances in producing positive (placebo) or negative (nocebo) effects, whereas others refer to the effect of the therapeutic context when an active treatment is administered (context-related effects).
Main text
The psychobiological determinants of placebo, nocebo and context-related effects
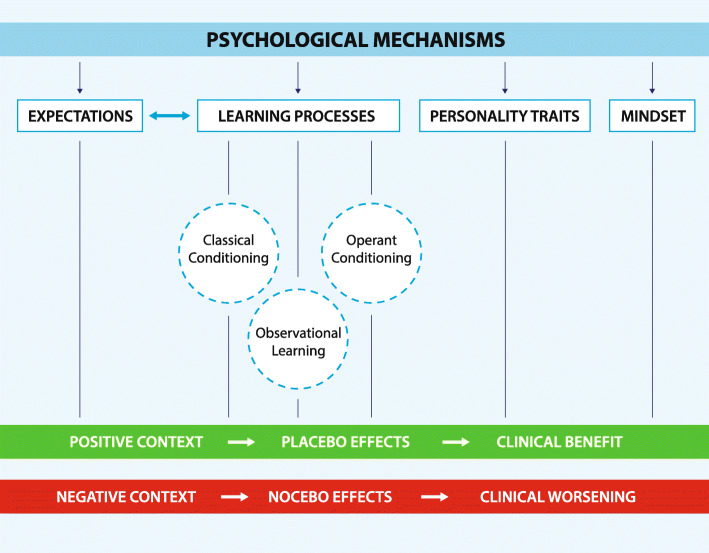
There are several psychological mechanisms involved in placebo, nocebo and context-related effects including: expectation, learning processes such as classical conditioning and observational learning, reinforced expectations, mindset and personality traits (Fig. 2) [1].
Fig. 2.
Psychobiological determinants of placebo, nocebo and context-related effects. Principal psychobiological determinants of placebo, nocebo and context related effects
Expectation refers to one’s anticipation of a future event. The expectations of individuals are a powerful modulator of their cognitive, emotional and physical experiences [49]. These are continuously shaped and updated according to the inputs coming from the surrounding environment [50]. Contextual factors summarized in Fig. 1 represent the key elements that are likely to influence patients’ expectations within the healthcare setting [17]. Verbal suggestions are the simplest and most direct way to shape expectations, therefore, a large body of evidence has used either positive or negative verbal information to modulate expectations and elicit placebo and nocebo responses. For example, Kam-Hansen et al. (2014) demonstrated that placebo labelled as active treatment (positive context expectation) and active treatment labelled as placebo (drug effect) had comparable healing effects on migraine attacks [51]. Similar findings were reported on post-surgical pain of thoracotomized patients [52] and on patients suffering from irritable bowel syndrome [53]. A recent study conducted on chronic neck pain patients has shown neck pain modulation according to contextual changes. Specifically, when positive verbal suggestions were associated with the neck manipulation, patients reported a significant reduction in pain. This was the case for both the real or the sham neck handling. A similar, but weaker effect was shown in relation to neutral information, whereas pain worsened when negative information was given [54].
Pavlovian classical conditioning [55] represents another mechanism that is strongly involved in placebo and nocebo effects, and associated positive and negative contexts [56–58]. Benedetti et al. (2003) showed that placebo administration (saline injection) after real drug preconditioning (sumatriptan injection) mimicked the effects of the drug on growth hormone (GH) secretion. In contrast, positive expectations alone without prior conditioning led to no effect on GH [59]. These findings, together with other classical conditioning studies [56, 60, 61], have demonstrated that verbally induced expectations alone have no effect on autonomous physiological processes such as hormonal plasma production and the immune system. However, a positive context created by a placebo administration after real drug preconditioning can elicit autonomic responses.
Conditioning can also function as an expectation booster, leading to reinforced expectations. To this end, a conditioning procedure can be used to create an association between an inert treatment and pain amelioration by reducing the intensity of the stimulation during the learning phase. During the recall phase, stimuli intensity is set back to baseline but is generally perceived as less painful [62]. Montgomery and Kirsch (1997) further investigated conditioning response when participants were overtly told the nature of the placebo and of the preconditioning. Interestingly, analgesic and hyperalgesic responses were no longer present. This suggests that conditioning alone was insufficient to elicit placebo and nocebo effects and that conscious expectations were necessary; indicating that conditioning served as a reinforcer to enhance expectation [63].
Observational learning represents another mechanism associated with placebo responsiveness. Colloca and Benedetti (2009) explored observational learning mechanisms in electric shock induced pain [64]. At first, participants observed a demonstrator taking part in the experiment and whom was instructed to shown analgesic and hyperalgesic effects when the painful stimuli were preceded by a green and by a red light, respectively. Then, participants underwent the same procedure they had observed and, as expected, they reported analgesia (pain decrease) and hyperalgesia (pain increase) in response to green and red cues accordingly. The same experiment was conducted using classical conditioning procedure as well as verbal suggestions alone. The magnitudes of placebo and nocebo effects were comparable between classical conditioning and observational learning procedures. On the contrary, the effect was much smaller for verbal suggestions alone. The role of observational learning in both positive social context (placebo) [65, 66] and negative social context (nocebo) [65–68] has been reiterated in later studies.
Up to date evidence has introduced a new mechanism involved in placebo and nocebo effects, namely operant conditioning [69]. This new paradigm consisted in rewarding and punishing participants when they responded ‘correctly’ to painful stimuli. Coloured cues preceded each stimulus, and dependently on the cue colour, the experimenter wanted the subjects to respond either with analgesia or hyperalgesia. Participants were rewarded or punished, via positive and negative writings displayed on the screen, accordingly on whether they responded as desired by the experimenter. When stimuli were presented in the absence of rewards and ‘punishments’, analgesia and hyperalgesia phenomena persisted, suggesting that operant conditioning functions as a learning process eliciting placebo and nocebo effects [69].
Other factors may interact with placebo and nocebo effects, including mindset and personality traits. Here, mindset refers to a broad set of viewpoints that compose one’s outlook on life. Multiple studies revealed that increasing the level of optimism in one’s mindset can positively change both subjective and objective measures of one’s health and wellbeing [70–72].
Concerning personality traits, suggestibility appears to make individuals more or less susceptible to the positive and/or negative context, leading to stronger placebo and nocebo responses [73]. Similarly, optimistic and pessimistic personality seems to facilitate placebo and nocebo effects, respectively [74]. Whereas, high trait and state anxiety tend to promote nocebo responses [75, 76]. With this knowledge in mind, no personality trait has yet been identified that can reliably predict if someone will respond to placebo (placebo responder) or not (placebo non responder) [1].
The neurophysiological mechanisms of placebo, nocebo and context-related effects
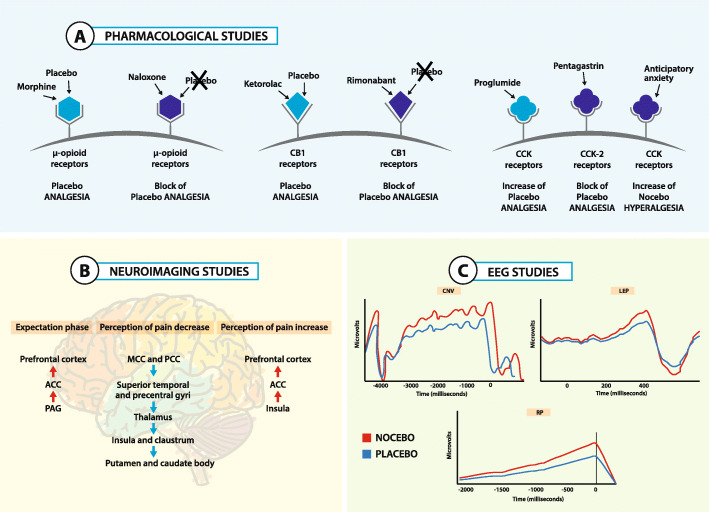
Over the past decade, the neurophysiological mechanisms underlying placebo, nocebo and context-related effects have begun to be extensively identified, using different approaches ranging from pharmacology to neuroimaging [17, 48, 77–79]. Pain and Parkinson Disease (PD) have been used as main models to understand the neurobiology of positive (placebo), negative (nocebo) and context-related effects [80, 81]. So far, three major questions have been addressed: (1) which are the neurobiological pathways activated by inert substances; (2) which are the brain regions that trigger these effects; and (3) which are the dynamical and temporal changes that occur in the brain before and after the administration of a placebo treatment (Fig. 3).
Fig. 3.
Neurophysiological mechanisms of placebo, nocebo and context-related effects. Principal neurobiological mechanisms of placebo, nocebo and context related effects. a Pharmacological studies. The opioid and cannabinoid systems are involved in placebo and nocebo effects. In some circumstances, placebo analgesia occurs through the activation of the opioid system and can be reversed by naloxone. In other circumstances, placebo analgesia occurs through the activation of the cannabinoid system and can be reversed by rimonabant. Anticipatory anxiety can activate the pro-nociceptive cholecystokinin (CCK) system, leading to nocebo hyperalgesia. The pro-nociceptive CCK effect can be reversed by proglumide and agonized by pentagastrin. b Neuroimaging studies. The activation and deactivation of different brain regions during placebo analgesia and nocebo hyperalgesia have been described trough different brain imaging studies. c Electrophysiological studies. Electroencephalographic (EEG) studies showed the dynamical studies showed the dynamical and temporal changes that occur in the brain before and after the administration of inert treatments
Pharmacological studies have demonstrated that the administration of an inert substance activates the endogenous opioid system and the endocannabinoid system. The observation that μ-opioid antagonists (e.g., naloxone) [56, 82, 83] and CB1-antagonist (e.g., rimonabant) [84] block some types of placebo analgesia has been documented by conditioning protocols using opioid drugs like morphine or cannabinoid drugs like ketorolac, respectively. Indirect confirmation of the involvement of the opioid system has been confirmed by the study of the anti-opioid action of cholecystokinin (CCK). Indeed, it has been demonstrated that CCK antagonist (e.g., proglumide) and CCK-2 agonist (e.g., pentagastrin) produce opposite effects on pain: the former enhances placebo analgesic effects while the latter disrupts them [85–89]. Also nocebo hyperalgesia seems to be modulated by the activation of the opioid system as it can be reversed by CCK antagonist. Moreover, placebos and nocebos also modulate the synthesis of prostaglandins, an important target of analgesic drugs [90]. On the whole, these pharmacological studies support the notion that inert substances and drugs may share common biochemical pathways.
Neuroimaging studies have identified different brain regions that contribute to placebo, nocebo and context related effects [91–99]. Different brain regions are activated during the “expectation phase”, when a positive or negative effect is foreseen, and during the “perception phase”, when analgesic or hyperalgesic effects are experienced. In particular, when a positive beneficial effect is expected, activation of anterior cingulate cortex (ACC), precentral and lateral prefrontal cortex and periaqueductal gray (PAG) has been documented. On the other hand, when placebo analgesia is experienced deactivation has been found in different brain regions such as the mid- and posterior cingulate cortex, superior temporal and precentral gyri, the anterior and posterior insula, the claustrum and putamen, and the thalamus and caudate body [100]. As for nocebo effects, when hyperalgesia is expected and experienced, an increased activity in different brain regions involved in pain processing and emotion regulation, such as prefrontal cortex (PFC), ACC and insula, has been documented [101–106]. Context-related effects have been extensively documented using the so-called “open-hidden” design, in which patients receive a real analgesic drug but they are either aware (open condition) or unaware (hidden condition) of receiving it. In these studies, it has been demonstrated that the open condition, that is a condition that maximizes the context-related effects, produced analgesic effect along with deactivation of the pain matrix and activation of dorsolateral prefrontal cortex (DLPFC) and rostral Anterior Cingulate Cortex (rACC). On the contrary, patients in the hidden condition, that is a condition that dramatically reduces the context-related effects, exhibited no changes in pain perception and no pain matrix deactivation [107].
Furthermore, the involvement of the dopaminergic system has been documented in pain [108, 109] and PD patients [110]. In placebo analgesia, an increase in dopamine binding to D2/D3 receptors and in opioid binding to μ-opioid receptors occurs in the nucleus accumbens, whereas a decreased binding to the same receptors is present in nocebo hyperalgesia [108, 109]. In PD, when patients experienced a motor improvement after a placebo administration, a large amount of dopamine was released in the dorsal motor striatum, suggesting a relationship between the amount of dorsal striatal dopamine release and clinical benefit. On the whole, these studies demonstrate that a complex network of brain regions is activated when placebos or nocebos are expected and positive or negative effects are experienced [111–113].
Recently, high temporal resolution techniques, such as electroencephalographic (EEG), has been used to determine changes in brain activity when placebo and nocebo phenomena arise. Studies on pain have revealed insights into the time-by-time and fast changes that occur before and after the administration of a sham treatment. Interestingly, as already documented by neuroimaging studies, placebos and nocebos change EEG brain activity during both the expectation and the perception phases. For example, the expectation of receiving no painful or painful stimuli respectively decrease or increase the amplitude of the contingent negative variation (CNV), an EEG slow negative wave that represents an objective measure of expectation of a specific incoming event (e.g., expectation of analgesia or hyperalgesia) [114]. Considering the “perception phase”, placebo treatments produce a decrease in laser-evoked potential (LEP), that represents an early measure of nociceptive processes, since it occurs after 200–250 ms after a painful stimulation [115]. Also motor electrophysiological potentials related to motor preparation and fatigue, such as the readiness potential (RP), can be affected by a placebo administration in healthy volunteers [116] and PD patients [117], demonstrating that the brain changes that occur when a placebo is administered can be triggered in different ways and in different times.
Even if modern brain imaging techniques have been fundamental in the understanding of the placebo and nocebo effects, further researches are needed to fully understand the underpinnings of these phenomena and the clinical implications and application of these findings.
Clinical, managerial and educational implications for physiotherapy profession
In accordance with the evidence reported in this review, the analysis of placebo, nocebo and context-related effects may lead to new therapeutic strategies that are capable of improving the professional action of the physiotherapist and of influencing management and education.
From a clinical perspective, the integration of the contextual factors with the best evidence-based therapy represents an opportunity to stimulate placebo effects and to prevent nocebo effects; boosting therapy effectiveness. This would be done in accordance with the ethical and deontological code of conduct [118, 119]. Indeed, contextual factors pervade every clinical action (e.g., history taking, physical examination, therapy administration, prognosis communication and revaluation) and directly affect the quality of health-related outcomes [16, 17]. A positive context (e.g., an encouraging verbal suggestion during a joint manipulation [54], or an empathetic therapeutic relationship during a modalities administration [120]), can ameliorate patients’ clinical outcomes by triggering placebo effects. Instead a negative context (e.g., a detrimental verbal indication delivered during a joint manipulation [54] or during a muscle strength test [121, 122]), can worsen patients’ symptoms by stimulating nocebo effects. Examples of clinical application of contextual factors are presented in Table 1.
Table 1.
Clinical application of contextual factors. The table presents examples of use of contextual factors aimed to enhance placebo effects and avoid nocebo effects. Adapted from [16, 17, 19]
| Contextual factors | Actions to enhance (placebo effects) | Actions to avoid (nocebo effects) |
|---|---|---|
| (A) Physiotherapist’s features |
• improve physiotherapist’s professionalism; • be aware of physiotherapist’s mindset; • promote physiotherapist’s appearance; |
• overlook physiotherapist’s professionalism; • be unaware of physiotherapist’s mindset; • disregard physiotherapist’s appearance; |
| (B) Patient’s features |
• examine patients’ mindset; • analyse patients’ baseline; |
• neglect patients’ mindset; • ignore patients’ baseline; |
| (C) Patient-physiotherapist relationship |
• manage verbal communication; • optimise non-verbal communication; |
• neglect verbal communication; • ignore non-verbal communication; |
| (D) Treatment features |
• amplify the rituality of treatments; • be aware of therapeutic touch; • consider the modality/posology of treatment; • use marketing of treatment; |
• limit the rituality of treatments; • be unaware of therapeutic touch; • omit the modality/posology of treatment; • neglect marketing of treatment; |
| (E) Healthcare setting features |
• adopt positive distractors and supportive indications; • use comfort elements, decorations and ornaments; |
• avoid positive distractors and supportive indications; • omit comfort elements, decorations and ornaments; |
From a clinical point of view, it is imperative to distinguish between the changes in patients’ symptoms resulting from placebo and nocebo effects, and those rising from other variables [123]. Possible confounders include the natural history (the spontaneous relief of the dysfunction and symptom modifications) and the regression to the mean (a statistical event caused by selection biases). Moreover, the patient’s and clinician’s confirmation biases during the description of clinical symptoms as well as unrevealed effects of simultaneous treatments have been reported as other confounders [1, 124].
From a managerial perspective, placebo, nocebo and context-related effects could help policy decision-makers during the design of the healthcare setting [16, 17]. Taking into account contextual factors offers the opportunity to significantly improve patients’ perception of physiotherapy services in terms of quality and overall satisfaction [16, 17]. Thus, at multiple levels (e.g., private or public services, inpatients or outpatients units), stakeholders, organizations and governments should encourage assessment and management of contextual factors [125, 126]. The positive context around the treatment (e.g., respect of timetable of physiotherapy treatment, a quiet setting) can impact the overall patients’ satisfaction and perception of their health care experiences by enhancing the attractiveness of a specific physiotherapy service [20, 127]. A positive healthcare context invites patients to choose, return to and recommend the physiotherapy service. Moreover, it increases the adherence to prescribed treatments and follow-ups [20, 127]. Instead, a negative healthcare context (e.g., a noisy environment, an overcrowded setting) improves the likelihood of patients’ dissatisfaction, the abandonment of the service and the withdrawing from the treatment plan [20, 127].
From an educational perspective, contextual factors (e.g., patient-clinician relationship) are underestimated during the majority of physiotherapy degrees. Awareness and practice of these professional topics should be strengthened and steadily integrated in teaching programs (e.g., core curriculum and core competence) and activities (e.g., skill-labs, role-playing), aiming to prepare the students for a better management of the psychosocial component in the clinical practice [16, 128]. In physiotherapy education, contextual factors help students to consider the therapeutic outcome as a complex, not predictable and nonlinear result of multiple interactions between different variables (e.g., clinicians, patients and healthcare setting) that evolve during the therapeutic encounter [129] in a positive or negative way through placebo and nocebo effects. Emerging evidence has suggested that awareness of contextual factors corresponds to good diagnostic skills and therapeutic reasoning [130–134], thus suggesting to academics and lecturers an additional teaching instrument for the development of students’ clinical ability.
Emerging lines for future research
With an increased understanding of contextual factors, and placebo and nocebo effects, the physiotherapy scientific community can measure the impact of physiotherapeutic interventions with greater precision in primary studies.
In contrast to the simplicity of creating a pharmaceutical placebo (where the active component of the treatment is removed), devising physiotherapy placebos is a significant challenge. At first, the aim should be to identify the best physiotherapy placebos for existing treatments. Various studies tried to develop [135, 136] and validate [137–139] a sham placebo technique. The principle obstacles are the needs to assess the patients’ blinding, their expectations and their priori (real) inertness [140–142]. However, a novel sham procedure has recently been validated [143] and applied in clinical settings; both to patients with migraines [144] and those with cervicogenic headache [145], thus paving the way for a series of further studies.
Secondly, the impact of contextual factors on patient outcomes should be examined. The task of designing a proper trial continues to be inconclusive among scholars [78, 146]. Primary studies should follow a research agenda aimed to estimate the effect of contextual factors on various patient’s clinical outcomes and in various health conditions. Using randomized clinical trials, there is a need to compare the same physiotherapy treatment performed in a neutral and enriched context [16]; measuring the change of subjective (e.g., pain, disability, expectation and satisfaction) and objective (e.g., heart rate variability, salivary cortisol, electromyographic activity) outcomes.
Thirdly, researchers should examine the patient’s perception of contextual factors. Although the patient’s viewpoint on placebo interventions has been investigated through surveys and qualitative interviews [47, 147], only study in this field has accounted for the influence of the patient’s viewpoint so far; it investigated contextual factors in patients with musculoskeletal pain [148]. Moreover, an item bank of contextual factors has been presented to evaluate the patient’s viewpoint regarding the overall healthcare experience [149]. This preliminary finding represents an initial phase for the creation of a questionnaire that: classifies patients based on their preferred contextual factors; and helps clinicians to enrich the physiotherapy treatment with specific contextual elements.
Conclusions
In summary (Table 2), this narrative review provides a stimulus for reflection on the role and strength of placebo, nocebo and context-related effects surrounding the administration of a physiotherapy treatment. On one hand, a positive healthcare context can significantly improve therapeutic effectiveness. On the other hand, a negative context can manifest adverse effects. However, the research on contextual factors is still at an early stage, and it constitutes an emerging field for investigation. Findings in this area of research could generate new psychologically appreciative treatments and thus create opportunity for growth for the physiotherapy profession.
Table 2.
Key points on placebo, nocebo and context-related effects
| Take-home message | |
|
• Placebo and nocebo effects are psychoneurobiological phenomena respectively produced by a positive and a negative healthcare context around the treatment; • The healthcare context is composed by contextual factors such as the feature of: the physiotherapist; the patient; the patient-physiotherapist relationship; the treatment; and the healthcare setting. • The psychological determinants of placebo and nocebo effects include: expectation; learning (classical conditioning and observational learning); reinforced expectations; mindset; and personality traits. • The neurophysiological mechanisms of placebo and nocebo effects involve different systems (the endogenous opioid, the endocannabinoid, and the dopaminergic) and brain regions (dorsolateral prefrontal cortex, the rostral anterior cingulate cortex, the periaqueductal gray, and the dorsal horn of spine). • From a clinical perspective, the manipulation of the healthcare context with the best evidence-based therapy represents an opportunity to trigger placebo effects and to avoid nocebo effects respecting the ethical and deontological code of conduct. • From a managerial perspective, stakeholders, organizations and governments should encourage the assessment of the healthcare context aimed to improve the quality of physiotherapy services. • From an educational perspective, placebo and nocebo effects are professional topics that should be integrated in the university program of health and medical professions. • From a research perspective, the control of placebo, nocebo and context-related effects offers to the scientific community the chance to better measure the impact of physiotherapy on different outcomes and in different conditions through primary studies. |
Acknowledgements
The authors want to thank Samuele Graffiedi and Mattia Mirandola for their valuable advices during the advancement of this manuscript.
Abbreviations
- GH
growth hormone
- PD
Parkinson disease
- CCK
Cholecystokinin
- ACC
Anterior cingulate cortex
- PAG
Periaqueductal gray
- PFC
Prefrontal cortex
- DLPFC
Dorsolateral prefrontal cortex
- rACC
Rostral anterior cingulate cortex
- EEG
Electroencephalographic
- LEP
Laser-evoked potential
- CNV
Contingent negative variation
- RP
Readiness potential
Authors’ contributions
All authors conceived, designed, drafted and approved the final manuscript.
Funding
The authors declare that they have no funding for this review.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Giacomo Rossettini, Email: giacomo.rossettini@gmail.com.
Eleonora Maria Camerone, Email: camerone.eleonora@gmail.com.
Elisa Carlino, Email: elisa.carlino@gmail.com.
Fabrizio Benedetti, Email: fabrizio.benedetti@unito.it.
Marco Testa, Email: marco.testa@unige.it.
References
- 1.Benedetti F. Placebo and the new physiology of the doctor-patient relationship. Physiol Rev. 2013;93:1207–1246. doi: 10.1152/physrev.00043.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colloca L, Barsky AJ. Placebo and Nocebo effects. N Engl J Med. 2020;382:554–561. doi: 10.1056/NEJMra1907805. [DOI] [PubMed] [Google Scholar]
- 3.Colloca L. The placebo effect in pain therapies. Annu Rev Pharmacol Toxicol. 2019;59:191–211. doi: 10.1146/annurev-pharmtox-010818-021542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blasini M, Corsi N, Klinger R, Colloca L. Nocebo and pain: an overview of the psychoneurobiological mechanisms. Pain Rep. 2017;2:e585. doi: 10.1097/PR9.0000000000000585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klinger R, Blasini M, Schmitz J, Colloca L. Nocebo effects in clinical studies: hints for pain therapy. Pain Rep. 2017;2:e586. doi: 10.1097/PR9.0000000000000586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kravvariti E, Kitas GD, Mitsikostas DD, Sfikakis PP. Nocebos in rheumatology: emerging concepts and their implications for clinical practice. Nat Rev Rheumatol. 2018;14:727–740. doi: 10.1038/s41584-018-0110-9. [DOI] [PubMed] [Google Scholar]
- 7.Carlino E, Frisaldi E, Benedetti F. Pain and the context. Nat Rev Rheumatol. 2014;10:348–355. doi: 10.1038/nrrheum.2014.17. [DOI] [PubMed] [Google Scholar]
- 8.Carlino E, Benedetti F. Different contexts, different pains, different experiences. Neuroscience. 2016;338:19–26. doi: 10.1016/j.neuroscience.2016.01.053. [DOI] [PubMed] [Google Scholar]
- 9.Ongaro G, Kaptchuk TJ. Symptom perception, placebo effects, and the Bayesian brain. Pain. 2019;160:1–4. doi: 10.1097/j.pain.0000000000001367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geuter S, Koban L, Wager TD. The cognitive neuroscience of placebo effects: concepts, predictions, and physiology. Annu Rev Neurosci. 2017;40:167–188. doi: 10.1146/annurev-neuro-072116-031132. [DOI] [PubMed] [Google Scholar]
- 11.Ashar YK, Chang LJ, Wager TD. Brain mechanisms of the placebo effect: an affective appraisal account. Annu Rev Clin Psychol. 2017;13:73–98. doi: 10.1146/annurev-clinpsy-021815-093015. [DOI] [PubMed] [Google Scholar]
- 12.Kaptchuk TJ. Placebo studies and ritual theory: a comparative analysis of Navajo, acupuncture and biomedical healing. Philos Trans R Soc Lond Ser B Biol Sci. 2011;366:1849–1858. doi: 10.1098/rstb.2010.0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller FG, Kaptchuk TJ. The power of context: reconceptualizing the placebo effect. J R Soc Med. 2008;101:222–225. doi: 10.1258/jrsm.2008.070466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balint M. The doctor, his patient, and the illness. Lancet. 1955;268:683–688. doi: 10.1016/s0140-6736(55)91061-8. [DOI] [PubMed] [Google Scholar]
- 15.Di Blasi Z, Harkness E, Ernst E, Georgiou A, Kleijnen J. Influence of context effects on health outcomes: a systematic review. Lancet. 2001;357:757–762. doi: 10.1016/s0140-6736(00)04169-6. [DOI] [PubMed] [Google Scholar]
- 16.Testa M, Rossettini G. Enhance placebo, avoid nocebo: how contextual factors affect physiotherapy outcomes. Man Ther. 2016;24:65–74. doi: 10.1016/j.math.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 17.Rossettini G, Carlino E, Testa M. Clinical relevance of contextual factors as triggers of placebo and nocebo effects in musculoskeletal pain. BMC Musculoskelet Disord. 2018;19:27. doi: 10.1186/s12891-018-1943-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geri T, Viceconti A, Minacci M, Testa M, Rossettini G. Manual therapy: exploiting the role of human touch. Musculoskelet Sci Pract. 2019;44:102044. doi: 10.1016/j.msksp.2019.07.008. [DOI] [PubMed] [Google Scholar]
- 19.Palese A, Rossettini G, Colloca L, Testa M. The impact of contextual factors on nursing outcomes and the role of placebo/nocebo effects: a discussion paper. Pain Rep. 2019;4(3):e716. doi: 10.1097/PR9.0000000000000716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rossettini G, Latini TM, Palese A, Jack SM, Ristori D, Gonzatto S, Testa M. Determinants of patient satisfaction in outpatient musculoskeletal physiotherapy: a systematic, qualitative meta-summary, and meta-synthesis. Disabil Rehabil. 2020;42:460–472. doi: 10.1080/09638288.2018.1501102. [DOI] [PubMed] [Google Scholar]
- 21.Colloca L, Corsi N, Fiorio M. The interplay of exercise, placebo and nocebo effects on experimental pain. Sci Rep. 2018;8:14758. doi: 10.1038/s41598-018-32974-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rossettini G, Emadi Andani M, Dalla Negra F, Testa M, Tinazzi M, Fiorio M. The placebo effect in the motor domain is differently modulated by the external and internal focus of attention. Sci Rep. 2018;8:12296. doi: 10.1038/s41598-018-30228-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miciak M, Gross DP, Joyce A. A review of the psychotherapeutic 'common factors' model and its application in physical therapy: the need to consider general effects in physical therapy practice. Scand J Caring Sci. 2012;26:394–403. doi: 10.1111/j.1471-6712.2011.00923.x. [DOI] [PubMed] [Google Scholar]
- 24.Miciak M, Mayan M, Brown C, Joyce AS, Gross DP. The necessary conditions of engagement for the therapeutic relationship in physiotherapy: an interpretive description study. Arch Physiother. 2018;8:3. doi: 10.1186/s40945-018-0044-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miciak M, Mayan M, Brown C, Joyce AS, Gross DP. A framework for establishing connections in physiotherapy practice. Physiother Theory Pract. 2019;35:40–56. doi: 10.1080/09593985.2018.1434707. [DOI] [PubMed] [Google Scholar]
- 26.Bolioli A, Alloatti F, Guadalupi M, Lanzi RI, Pregnolato G, Turolla A. How do physiotherapists and patient talk? Developing the RiMotivAzione dialogue corpus. CEUR Workshop Proceedings. 2019;2481..
- 27.Roberts LC, Whittle CT, Cleland J, Wald M. Measuring verbal communication in initial physical therapy encounters. Phys Ther. 2013;93:479–491. doi: 10.2522/ptj.20120089. [DOI] [PubMed] [Google Scholar]
- 28.Rossettini G, Testa M. Manual therapy RCTs: should we control placebo in placebo control? Eur J Phys Rehabil Med. 2018;54:500–501. doi: 10.23736/S1973-9087.17.05024-9. [DOI] [PubMed] [Google Scholar]
- 29.Menke JM. Manual therapy researchers are misled by natural history and placebo effects. Spine (Phila Pa 1976) 2014;39:1997. doi: 10.1097/BRS.0000000000000585. [DOI] [PubMed] [Google Scholar]
- 30.Kamper SJ. Control groups: linking evidence to practice. J Orthop Sports Phys Ther. 2018;48:905–906. doi: 10.2519/jospt.2018.0706. [DOI] [PubMed] [Google Scholar]
- 31.Kamper SJ, Williams CM. The placebo effect: powerful, powerless or redundant? Br J Sports Med. 2013;47:6–9. doi: 10.1136/bjsports-2012-091472. [DOI] [PubMed] [Google Scholar]
- 32.Benz LN, Flynn TW. Placebo, nocebo, and expectations: leveraging positive outcomes. J Orthop Sports Phys Ther. 2013;43:439–441. doi: 10.2519/jospt.2013.0105. [DOI] [PubMed] [Google Scholar]
- 33.Bialosky JE, George SZ, Bishop MD. How spinal manipulative therapy works: why ask why? J Orthop Sports Phys Ther. 2008;38:293–295. doi: 10.2519/jospt.2008.0118. [DOI] [PubMed] [Google Scholar]
- 34.Bialosky JE, Bishop MD, Price DD, Robinson ME, George SZ. The mechanisms of manual therapy in the treatment of musculoskeletal pain: a comprehensive model. Man Ther. 2009;14:531–538. doi: 10.1016/j.math.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bialosky JE, Beneciuk JM, Bishop MD, Coronado RA, Penza CW, Simon CB, et al. Unraveling the mechanisms of manual therapy: modeling an approach. J Orthop Sports Phys Ther. 2018;48:8–18. doi: 10.2519/jospt.2018.7476. [DOI] [PubMed] [Google Scholar]
- 36.Bialosky JE, Bishop MD, Penza CW. Placebo mechanisms of manual therapy: a sheep in Wolf’s clothing? J Orthop Sports Phys Ther. 2017;47:301–304. doi: 10.2519/jospt.2017.0604. [DOI] [PubMed] [Google Scholar]
- 37.Newell D, Lothe LR, Raven TJL. Contextually aided recovery (CARe): a scientific theory for innate healing. Chiropr Man Therap. 2017;25:6. doi: 10.1186/s12998-017-0137-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Villa-Sánchez B, Emadi Andani M, Menegaldo G, Tinazzi M, Fiorio M. Positive verbal suggestion optimizes postural control. Sci Rep. 2019;9:6408. doi: 10.1038/s41598-019-42888-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Darnall BD, Colloca L. Optimizing placebo and minimizing Nocebo to reduce pain, Catastrophizing, and opioid use: a review of the science and an evidence-informed clinical toolkit. Int Rev Neurobiol. 2018;139:129–157. doi: 10.1016/bs.irn.2018.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Damien J, Colloca L, Bellei-Rodriguez C-É, Marchand S. Pain modulation: from conditioned pain modulation to placebo and Nocebo effects in experimental and clinical pain. Int Rev Neurobiol. 2018;139:255–296. doi: 10.1016/bs.irn.2018.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klinger R, Stuhlreyer J, Schwartz M, Schmitz J, Colloca L. Clinical use of placebo effects in patients with pain disorders. Int Rev Neurobiol. 2018;139:107–128. doi: 10.1016/bs.irn.2018.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fiorio M. Modulation of the motor system by placebo and Nocebo effects. Int Rev Neurobiol. 2018;139:297–319. doi: 10.1016/bs.irn.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 43.Villa-Sánchez B, Emadi Andani M, Fiorio M. The role of the dorsolateral prefrontal cortex in the motor placebo effect. Eur J Neurosci. 2018;48:3410–3425. doi: 10.1111/ejn.14217. [DOI] [PubMed] [Google Scholar]
- 44.Corsi N, Emadi Andani M, Sometti D, Tinazzi M, Fiorio M. When words hurt: verbal suggestion prevails over conditioning in inducing the motor nocebo effect. Eur J Neurosci. 2019;50:3311–3326. doi: 10.1111/ejn.14489. [DOI] [PubMed] [Google Scholar]
- 45.Rossettini G, Palese A, Geri T, Fiorio M, Colloca L, Testa M. Physical therapists’ perspectives on using contextual factors in clinical practice: findings from an Italian national survey. PLoS One. 2018;13:e0208159. doi: 10.1371/journal.pone.0208159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palese A, Cadorin L, Testa M, Geri T, Colloca L, Rossettini G. Contextual factors triggering placebo and nocebo effects in nursing practice: findings from a national cross-sectional study. J Clin Nurs. 2019;28:1966–1978. doi: 10.1111/jocn.14809. [DOI] [PubMed] [Google Scholar]
- 47.Linde K, Atmann O, Meissner K, Schneider A, Meister R, Kriston L, et al. How often do general practitioners use placebos and non-specific interventions? Systematic review and meta-analysis of surveys. PLoS One. 2018;13:e0202211. doi: 10.1371/journal.pone.0202211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cadorin L, Rossettini G, Testa M, Geri T, Palese A. The awareness of contextual factors, placebo and nocebo effects among nursing students: findings from a cross-sectional study. Nurse Educ Pract. 2020;42:102670. [DOI] [PubMed]
- 49.Benedetti F, Carlino E, Pollo A. How placebos change the patient’s brain. Neuropsychopharmacology. 2011;36:339–354. doi: 10.1038/npp.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wiech K. Deconstructing the sensation of pain: the influence of cognitive processes on pain perception. Science. 2016;354:584–587. doi: 10.1126/science.aaf8934. [DOI] [PubMed] [Google Scholar]
- 51.Kam-Hansen S, Jakubowski M, Kelley JM, Kirsch I, Hoaglin DC, Kaptchuk TJ, Burstein R. Labeling of Medication and Placebo Alters the Outcome of Episodic Migraine Attacks. Sci Transl Med. 2014;6:218ra5. doi: 10.1126/scitranslmed.3006175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pollo A, Amanzio M, Arslanian A, Casadio C, Maggi G, Benedetti F. Response expectancies in placebo analgesia and their clinical relevance. Pain. 2001;93:77–84. doi: 10.1016/S0304-3959(01)00296-2. [DOI] [PubMed] [Google Scholar]
- 53.Vase L, Robinson ME, Verne GN, Price DD. The contributions of suggestion, desire, and expectation to placebo effects in irritable bowel syndrome patients. An empirical investigation. Pain. 2003;105:17–25. doi: 10.1016/s0304-3959(03)00073-3. [DOI] [PubMed] [Google Scholar]
- 54.Malfliet A, Lluch Girbés E, Pecos-Martin D, Gallego-Izquierdo T, Valera-Calero A. The influence of treatment expectations on clinical outcomes and cortisol levels in patients with chronic neck pain: an experimental study. Pain Pract. 2019;19:370–381. doi: 10.1111/papr.12749. [DOI] [PubMed] [Google Scholar]
- 55.Pavlov IP, Thompson WH. The work of the digestive glands. London: C. Griffin; 1902. [Google Scholar]
- 56.Amanzio M, Benedetti F. Neuropharmacological dissection of placebo analgesia: expectation-activated opioid systems versus conditioning-activated specific subsystems. J Neurosci. 1999;19:484–494. doi: 10.1523/JNEUROSCI.19-01-00484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Babel P, Adamczyk W, Swider K, Bajcar EA, Kicman P, Lisinska N. How classical conditioning shapes placebo analgesia: hidden versus open conditioning. Pain Med. 2018;19:1156–1169. doi: 10.1093/pm/pnx177. [DOI] [PubMed] [Google Scholar]
- 58.Colloca L, Sigaudo M, Benedetti F. The role of learning in nocebo and placebo effects. Pain. 2008;136:211–218. doi: 10.1016/j.pain.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 59.Benedetti F, Pollo A, Lopiano L, Lanotte M, Vighetti S, Rainero I. Conscious expectation and unconscious conditioning in analgesic, motor, and hormonal placebo/nocebo responses. J Neurosci. 2003;23:4315–4323. doi: 10.1523/JNEUROSCI.23-10-04315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goebel MU, Trebst AE, Steiner J, Xie YF, Exton MS, Frede S, Canbay AE, Michel MC, Heemann U, Schedlowski M. Behavioral conditioning of immunosuppression is possible in humans. FASEB J. 2002;16:1869–1873. doi: 10.1096/fj.02-0389com. [DOI] [PubMed] [Google Scholar]
- 61.Jensen KB, Kaptchuk TJ, Kirsch I, Raicek J, Lindstrom KM, Berna C, Gollub RL, Ingvar M, Kong J. Nonconscious activation of placebo and nocebo pain responses. Proc Natl Acad Sci U S A. 2012;109:15959–15964. doi: 10.1073/pnas.1202056109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Krummenacher P, Candia V, Folkers G, Schedlowski M, Schönbächler G. Prefrontal cortex modulates placebo analgesia. PAIN®. 2010;148(3):368–374. doi: 10.1016/j.pain.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 63.Montgomery GH, Kirsch I. Classical conditioning and the placebo effect. Pain. 1997;72:107–113. doi: 10.1016/s0304-3959(97)00016-x. [DOI] [PubMed] [Google Scholar]
- 64.Colloca L, Benedetti F. Placebo analgesia induced by social observational learning. Pain. 2009;144:28–34. doi: 10.1016/j.pain.2009.01.033. [DOI] [PubMed] [Google Scholar]
- 65.Hunter T, Siess F, Colloca L. Socially induced placebo analgesia: a comparison of a pre-recorded versus live face-to-face observation. Eur J Pain. 2014;18:914–922. doi: 10.1002/j.1532-2149.2013.00436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Świder K, Bąbel P. The effect of the type and colour of placebo stimuli on placebo effects induced by observational learning. PLoS One. 2016;11:e0158363. doi: 10.1371/journal.pone.0158363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bajcar EA, Bąbel P. How does observational learning produce placebo effects? A Model Integrating Research Findings. Front Psychol. 2018;9:2041. doi: 10.3389/fpsyg.2018.02041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Swider K, Bąbel P. The effect of the sex of a model on nocebo hyperalgesia induced by social observational learning. Pain. 2013;154:1312–1317. doi: 10.1016/j.pain.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 69.Adamczyk WM, Wiercioch-Kuzianik K, Bajcar EA, Bąbel P. Rewarded placebo analgesia: a new mechanism of placebo effects based on operant conditioning. Eur J Pain. 2019;23:923–935. doi: 10.1002/ejp.1360. [DOI] [PubMed] [Google Scholar]
- 70.Crum AJ, Salovey P, Achor S. Rethinking stress: the role of mindsets in determining the stress response. J Pers Soc Psychol. 2013;104:716–733. doi: 10.1037/a0031201. [DOI] [PubMed] [Google Scholar]
- 71.Crum AJ, Langer EJ. Mind-set matters: exercise and the placebo effect. Psychol Sci. 2007;18:165–171. doi: 10.1111/j.1467-9280.2007.01867.x. [DOI] [PubMed] [Google Scholar]
- 72.Crum AJ, Corbin WR, Brownell KD, Salovey P. Mind over milkshakes: mindsets, not just nutrients, determine ghrelin response. Health Psychol. 2011;30:424–429. doi: 10.1037/a0023467. [DOI] [PubMed] [Google Scholar]
- 73.De Pascalis V, Chiaradia C, Carotenuto E. The contribution of suggestibility and expectation to placebo analgesia phenomenon in an experimental setting. Pain. 2002;96:393–402. doi: 10.1016/S0304-3959(01)00485-7. [DOI] [PubMed] [Google Scholar]
- 74.Geers AL, Helfer SG, Kosbab K, Weiland PE, Landry SJ. Reconsidering the role of personality in placebo effects: dispositional optimism, situational expectations, and the placebo response. J Psychosom Res. 2005;58:121–127. doi: 10.1016/j.jpsychores.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 75.Colloca L, Pine DS, Ernst M, Miller FG, Grillon C. Vasopressin boosts placebo analgesic effects in women: a randomized trial. Biol Psychiatry. 2016;79:794–802. doi: 10.1016/j.biopsych.2015.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Woo KY. Unravelling nocebo effect: the mediating effect of anxiety between anticipation and pain at wound dressing change. J Clin Nurs. 2015;24:1975–1984. doi: 10.1111/jocn.12858. [DOI] [PubMed] [Google Scholar]
- 77.Carlino E, Vase L, Piedimonte A. Mechanisms of Placebo and Nocebo. In: Mitsikostas D, Benedetti F, editors. Placebos and Nocebos in Headaches. Headache. Springer, Cham. 2019. pp. 43–55. [Google Scholar]
- 78.Carlino E, Vase L. Can knowledge of placebo and Nocebo mechanisms help improve randomized clinical trials? Int Rev Neurobiol. 2018;138:329–357. doi: 10.1016/bs.irn.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 79.Dodd S, Dean OM, Vian J, Berk M. A review of the theoretical and biological understanding of the Nocebo and placebo phenomena. Clin Ther. 2017;39:469–476. doi: 10.1016/j.clinthera.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 80.Carlino E, Piedimonte A, Benedetti F. Nature of the placebo and nocebo effect in relation to functional neurologic disorders. Handb Clin Neurol. 2016;139:597–606. doi: 10.1016/B978-0-12-801772-2.00048-5. [DOI] [PubMed] [Google Scholar]
- 81.Frisaldi E, Piedimonte A, Benedetti F. Placebo and nocebo effects: a complex interplay between psychological factors and neurochemical networks. Am J Clin Hypnosis. 2015;57:267–284. doi: 10.1080/00029157.2014.976785. [DOI] [PubMed] [Google Scholar]
- 82.Eippert F, Bingel U, Schoell ED, Yacubian J, Klinger R, Lorenz J, Büchel C. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron. 2009;63:533–543. doi: 10.1016/j.neuron.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 83.Ellerbrock I, Wiehler A, Arndt M, May A. Nocebo context modulates long-term habituation to heat pain and influences functional connectivity of the operculum. Pain. 2015;156:2222–2233. doi: 10.1097/j.pain.0000000000000297. [DOI] [PubMed] [Google Scholar]
- 84.Benedetti F, Amanzio M, Rosato R, Blanchard C. Nonopioid placebo analgesia is mediated by CB1 cannabinoid receptors. Nat Med. 2011;17:1228–1230. doi: 10.1038/nm.2435. [DOI] [PubMed] [Google Scholar]
- 85.Benedetti F, Amanzio M, Casadio C, Oliaro A, Maggi G. Blockade of nocebo hyperalgesia by the cholecystokinin antagonist proglumide. Pain. 1997;71:135–140. doi: 10.1016/s0304-3959(97)03346-0. [DOI] [PubMed] [Google Scholar]
- 86.Benedetti F. The opposite effects of the opiate antagonist naloxone and the cholecystokinin antagonist proglumide on placebo analgesia. Pain. 1996;64:535–543. doi: 10.1016/0304-3959(95)00179-4. [DOI] [PubMed] [Google Scholar]
- 87.Benedetti F, Amanzio M, Maggi G. Potentiation of placebo analgesia by proglumide. Lancet. 1995;346:1231. doi: 10.1016/s0140-6736(95)92938-x. [DOI] [PubMed] [Google Scholar]
- 88.Benedetti F, Amanzio M, Thoen W. Disruption of opioid-induced placebo responses by activation of cholecystokinin type-2 receptors. Psychopharmacology (Berl) 2011;213:791–797. doi: 10.1007/s00213-010-2037-y. [DOI] [PubMed] [Google Scholar]
- 89.Benedetti F, Amanzio M, Vighetti S, Asteggiano G. The biochemical and neuroendocrine bases of the hyperalgesic nocebo effect. J Neurosci. 2006;26:12014–12022. doi: 10.1523/JNEUROSCI.2947-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Benedetti F, Durando J, Vighetti S. Nocebo and placebo modulation of hypobaric hypoxia headache involves the cyclooxygenase-prostaglandins pathway. Pain. 2014;155:921–928. doi: 10.1016/j.pain.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 91.Kong J, Gollub RL, Rosman IS, Webb JM, Vangel MG, Kirsch I, Kaptchuk TJ. Brain activity associated with expectancy-enhanced placebo analgesia as measured by functional magnetic resonance imaging. J Neurosci. 2006;26:381–388. doi: 10.1523/JNEUROSCI.3556-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lieberman MD, Jarcho JM, Berman S, Naliboff BD, Suyenobu BY, Mandelkern M, Mayer EA. The neural correlates of placebo effects: a disruption account. Neuroimage. 2004;22:447–455. doi: 10.1016/j.neuroimage.2004.01.037. [DOI] [PubMed] [Google Scholar]
- 93.Petrovic P, Kalso E, Petersson KM, Andersson J, Fransson P, Ingvar M. A prefrontal non-opioid mechanism in placebo analgesia. Pain. 2010;150:59–65. doi: 10.1016/j.pain.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 94.Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and opioid analgesia-- imaging a shared neuronal network. Science. 2002;295:1737–1740. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- 95.Price DD, Craggs J, Verne GN, Perlstein WM, Robinson ME. Placebo analgesia is accompanied by large reductions in pain-related brain activity in irritable bowel syndrome patients. Pain. 2007;127:63–72. doi: 10.1016/j.pain.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 96.Tracey I. Getting the pain you expect: mechanisms of placebo, nocebo and reappraisal effects in humans. Nat Med. 2010;16:1277–1283. doi: 10.1038/nm.2229. [DOI] [PubMed] [Google Scholar]
- 97.Wager TD, Atlas LY, Leotti LA, Rilling JK. Predicting individual differences in placebo analgesia: contributions of brain activity during anticipation and pain experience. J Neurosci. 2011;31:439–452. doi: 10.1523/JNEUROSCI.3420-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303:1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- 99.Wager TD, Scott DJ, Zubieta JK. Placebo effects on human mu-opioid activity during pain. Proc Natl Acad Sci U S A. 2007;104:11056–11061. doi: 10.1073/pnas.0702413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Palermo S, Benedetti F, Costa T, Amanzio M. Pain anticipation: an activation likelihood estimation meta-analysis of brain imaging studies. Hum Brain Mapp. 2015;36:1648–1661. doi: 10.1002/hbm.22727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chua P, Krams M, Toni I, Passingham R, Dolan R. A functional anatomy of anticipatory anxiety. Neuroimage. 1999;9:563–571. doi: 10.1006/nimg.1999.0407. [DOI] [PubMed] [Google Scholar]
- 102.Keltner JR, Furst A, Fan C, Redfern R, Inglis B, Fields HL. Isolating the modulatory effect of expectation on pain transmission: a functional magnetic resonance imaging study. J Neurosci. 2006;26:4437–4443. doi: 10.1523/JNEUROSCI.4463-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kong J, Gollub RL, Polich G, Kirsch I, Laviolette P, Vangel M, Rosen B, Kaptchuk TJ. A functional magnetic resonance imaging study on the neural mechanisms of hyperalgesic nocebo effect. J Neurosci. 2008;28:13354–13362. doi: 10.1523/JNEUROSCI.2944-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Koyama T, Tanaka YZ, Mikami A. Nociceptive neurons in the macaque anterior cingulate activate during anticipation of pain. Neuroreport. 1998;9:2663–2667. doi: 10.1097/00001756-199808030-00044. [DOI] [PubMed] [Google Scholar]
- 105.Porro CA, Baraldi P, Pagnoni G, Serafini M, Facchin P, Maieron M, Nichelli P. Does anticipation of pain affect cortical nociceptive systems? J Neurosci. 2002;22:3206–3214. doi: 10.1523/JNEUROSCI.22-08-03206.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Porro CA, Cettolo V, Francescato MP, Baraldi P. Functional activity mapping of the mesial hemispheric wall during anticipation of pain. Neuroimage. 2003;19:1738–1747. doi: 10.1016/s1053-8119(03)00184-8. [DOI] [PubMed] [Google Scholar]
- 107.Bingel U, Wanigasekera V, Wiech K, Ni Mhuircheartaigh R, Lee MC, Ploner M, Tracey I. The effect of treatment expectation on drug efficacy: imaging the analgesic benefit of the opioid remifentanil. Sci Transl Med. 2011;3:70ra14. doi: 10.1126/scitranslmed.3001244. [DOI] [PubMed] [Google Scholar]
- 108.Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK. Individual differences in reward responding explain placebo-induced expectations and effects. Neuron. 2007;55:325–336. doi: 10.1016/j.neuron.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 109.Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK. Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Arch Gen Psychiatry. 2008;65:220–231. doi: 10.1001/archgenpsychiatry.2007.34. [DOI] [PubMed] [Google Scholar]
- 110.Frisaldi E, Carlino E, Lanotte M, Lopiano L, Benedetti F. Characterization of the thalamic-subthalamic circuit involved in the placebo response through single-neuron recording in Parkinson patients. Cortex. 2014;60:3–9. doi: 10.1016/j.cortex.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 111.de la Fuente-Fernández R. The placebo-reward hypothesis: dopamine and the placebo effect. Parkinsonism Relat Disord. 2009;3:S72–S74. doi: 10.1016/S1353-8020(09)70785-0. [DOI] [PubMed] [Google Scholar]
- 112.de la Fuente-Fernández R, Stoessl AJ. The placebo effect in Parkinson’s disease. Trends Neurosci. 2002;25:302–306. doi: 10.1016/s0166-2236(02)02181-1. [DOI] [PubMed] [Google Scholar]
- 113.de la Fuente-Fernández R, Ruth TJ, Sossi V, Schulzer M, Calne DB, Stoessl AJ. Expectation and dopamine release: mechanism of the placebo effect in Parkinson’s disease. Science. 2001;293:1164–1166. doi: 10.1126/science.1060937. [DOI] [PubMed] [Google Scholar]
- 114.Piedimonte A, Guerra G, Vighetti S, Carlino E. Measuring expectation of pain: contingent negative variation in placebo and nocebo effects. Eur J Pain. 2017;21:874–885. doi: 10.1002/ejp.990. [DOI] [PubMed] [Google Scholar]
- 115.Carlino E, Torta DME, Piedimonte A, Frisaldi E, Vighetti S, Benedetti F. Role of explicit verbal information in conditioned analgesia. Eur J Pain. 2015;19:546–553. doi: 10.1002/ejp.579. [DOI] [PubMed] [Google Scholar]
- 116.Piedimonte A, Benedetti F, Carlino E. Placebo-induced decrease in fatigue: evidence for a central action on the preparatory phase of movement. Eur J Neurosci. 2015;41:492–497. doi: 10.1111/ejn.12806. [DOI] [PubMed] [Google Scholar]
- 117.Carlino E, Piedimonte A, Romagnolo A, Guerra G, Frisaldi E, Vighetti S, Lopiano L, Benedetti F. Verbal communication about drug dosage balances drug reduction in Parkinson's disease: behavioral and electrophysiological evidences. Parkinsonism Relat Disord. 2019;65:184–189. doi: 10.1016/j.parkreldis.2019.06.015. [DOI] [PubMed] [Google Scholar]
- 118.Enck P, Bingel U, Schedlowski M, Rief W. The placebo response in medicine: minimize, maximize or personalize? Nat Rev Drug Discov. 2013;12:191–204. doi: 10.1038/nrd3923. [DOI] [PubMed] [Google Scholar]
- 119.Finniss DG, Kaptchuk TJ, Miller F, Benedetti F. Biological, clinical, and ethical advances of placebo effects. Lancet. 2010;375:686–695. doi: 10.1016/S0140-6736(09)61706-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fuentes J, Armijo-Olivo S, Funabashi M, Miciak M, Dick B, Warren S, Rashiq S, Magee DJ, Gross DP. Enhanced therapeutic alliance modulates pain intensity and muscle pain sensitivity in patients with chronic low back pain: an experimental controlled study. Phys Ther. 2014;94:477–489. doi: 10.2522/ptj.20130118. [DOI] [PubMed] [Google Scholar]
- 121.Pfingsten M, Leibing E, Harter W, Kröner-Herwig B, Hempel D, Kronshage U, Hildebrandt J. Fear-avoidance behavior and anticipation of pain in patients with chronic low back pain: a randomized controlled study. Pain Med. 2001;2:259–266. doi: 10.1046/j.1526-4637.2001.01044.x. [DOI] [PubMed] [Google Scholar]
- 122.Zech N, Seemann M, Grzesiek M, Breu A, Seyfried TF, Hansen E. Nocebo effects on muscular performance - an experimental study about clinical situations. Front Pharmacol. 2019;10:219. doi: 10.3389/fphar.2019.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hartman SE. Why do ineffective treatments seem helpful? A brief review. Chiropr Osteopat. 2009;17:10. doi: 10.1186/1746-1340-17-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Benedetti F, Carlino E, Piedimonte A. Increasing uncertainty in CNS clinical trials: the role of placebo, nocebo, and Hawthorne effects. Lancet Neurol. 2016;15:736–747. doi: 10.1016/S1474-4422(16)00066-1. [DOI] [PubMed] [Google Scholar]
- 125.Solvang PK, Hanisch H, Reinhardt JD. The rehabilitation research matrix: producing knowledge at micro, meso, and macro levels. Disabil Rehabil. 2017;39:1983–1989. doi: 10.1080/09638288.2016.1212115. [DOI] [PubMed] [Google Scholar]
- 126.Kringos DS, Sunol R, Wagner C, Mannion R, Michel P, Klazinga NS, Groene O. DUQuE Consortium The influence of context on the effectiveness of hospital quality improvement strategies: a review of systematic reviews. BMC Health Serv Res. 2015;15:277. doi: 10.1186/s12913-015-0906-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Batbaatar E, Dorjdagva J, Luvsannyam A, Amenta P. Conceptualisation of patient satisfaction: a systematic narrative literature review. Perspect Public Health. 2015;135:243–250. doi: 10.1177/1757913915594196. [DOI] [PubMed] [Google Scholar]
- 128.Schedlowski M, Enck P, Rief W, Bingel U. Neuro-bio-behavioral mechanisms of placebo and Nocebo responses: implications for clinical trials and clinical practice. Pharmacol Rev. 2015;67:697–730. doi: 10.1124/pr.114.009423. [DOI] [PubMed] [Google Scholar]
- 129.Durning SJ, Artino AR, Pangaro LN, van der Vleuten C, Schuwirth L. Perspective: redefining context in the clinical encounter: implications for research and training in medical education. Acad Med. 2010;85:894–901. doi: 10.1097/ACM.0b013e3181d7427c. [DOI] [PubMed] [Google Scholar]
- 130.McBee E, Ratcliffe T, Picho K, Artino AR, Schuwirth L, Kelly W, Masel J, van der Vleuten C, Durning SJ. Consequences of contextual factors on clinical reasoning in resident physicians. Adv Health Sci Educ Theory Pract. 2015;20:1225–1236. doi: 10.1007/s10459-015-9597-x. [DOI] [PubMed] [Google Scholar]
- 131.Durning SJ, Artino AR, Boulet JR, Dorrance K, van der Vleuten C, Schuwirth L. The impact of selected contextual factors on experts’ clinical reasoning performance (does context impact clinical reasoning performance in experts?) Adv Health Sci Educ Theory Pract. 2012;17:65–79. doi: 10.1007/s10459-011-9294-3. [DOI] [PubMed] [Google Scholar]
- 132.McBee E, Ratcliffe T, Picho K, Schuwirth L, Artino AR, Yepes-Rios AM, Masel J, van der Vleuten C, Durning SJ. Contextual factors and clinical reasoning: differences in diagnostic and therapeutic reasoning in board certified versus resident physicians. BMC Med Educ. 2017;17:211. doi: 10.1186/s12909-017-1041-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Durning S, Artino AR, Pangaro L, van der Vleuten CPM, Schuwirth L. Context and clinical reasoning: understanding the perspective of the expert’s voice. Med Educ. 2011;45:927–938. doi: 10.1111/j.1365-2923.2011.04053.x. [DOI] [PubMed] [Google Scholar]
- 134.McBee E, Ratcliffe T, Schuwirth L, O'Neill D, Meyer H, Madden SJ, Durning SJ. Context and clinical reasoning : understanding the medical student perspective. Perspect Med Educ. 2018;7:256–263. doi: 10.1007/s40037-018-0417-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Michener LA, Kardouni JR, Lopes Albers AD, Ely JM. Development of a sham comparator for thoracic spinal manipulative therapy for use with shoulder disorders. Man Ther. 2013;18:60–64. doi: 10.1016/j.math.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 136.Lougee H, Johnston RG, Thomson OP. The suitability of sham treatments for use as placebo controls in trials of spinal manipulative therapy: a pilot study. J Bodyw Mov Ther gennaio. 2013;17:59–68. doi: 10.1016/j.jbmt.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 137.Michener LA, Kardouni JR, Sousa CO, Ely JM. Validation of a sham comparator for thoracic spinal manipulation in patients with shoulder pain. Man Ther. 2015;20:171–175. doi: 10.1016/j.math.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Vernon H, MacAdam K, Marshall V, Pion M, Sadowska M. Validation of a sham manipulative procedure for the cervical spine for use in clinical trials. J Manip Physiol Ther. 2005;28:662–666. doi: 10.1016/j.jmpt.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 139.Vernon HT, Triano JJ, Ross JK, Tran SK, Soave DM, Dinulos MD. Validation of a novel sham cervical manipulation procedure. Spine J. 2012;12:1021–1028. doi: 10.1016/j.spinee.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bizzarri P, Buzzatti L, Cattrysse E, Scafoglieri A. Thoracic manual therapy is not more effective than placebo thoracic manual therapy in patients with shoulder dysfunctions: a systematic review with meta-analysis. Musculoskelet Sci Pract. 2018;33:1–10. doi: 10.1016/j.msksp.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 141.Puhl AA, Reinhart CJ, Doan JB, Vernon H. The quality of placebos used in randomized, controlled trials of lumbar and pelvic joint thrust manipulation-a systematic review. Spine J. 2017;17:445–456. doi: 10.1016/j.spinee.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 142.Cerritelli F, Verzella M, Cicchitti L, D’Alessandro G, Vanacore N. The paradox of sham therapy and placebo effect in osteopathy: A systematic review. Medicine (Baltimore) 2016;95:e4728. doi: 10.1097/MD.0000000000004728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chaibi A, Šaltytė Benth J, Bjørn RM. Validation of placebo in a manual therapy randomized controlled trial. Sci Rep. 2015;5:11774. doi: 10.1038/srep11774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chaibi A, Benth JŠ, Tuchin PJ, Russell MB. Chiropractic spinal manipulative therapy for migraine: a three-armed, single-blinded, placebo, randomized controlled trial. Eur J Neurol. 2017;24:143–153. doi: 10.1111/ene.13166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chaibi A, Knackstedt H, Tuchin PJ, Russell MB. Chiropractic spinal manipulative therapy for cervicogenic headache: a single-blinded, placebo, randomized controlled trial. BMC Res Notes. 2017;10:310. doi: 10.1186/s13104-017-2651-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Vase L, Amanzio M, Price DD. Nocebo vs. placebo: the challenges of trial design in analgesia research. Clin Pharmacol Ther. 2015;97:143–150. doi: 10.1002/cpt.31. [DOI] [PubMed] [Google Scholar]
- 147.Hardman DI, Geraghty AW, Lewith G, Lown M, Viecelli C, Bishop FL, et al. From Substance to Process: A Meta-Ethnographic Review of How Healthcare Professionals and Patients Understand Placebos and Their Effects in Primary Care. Health (London). 2020;24:315–40. [DOI] [PubMed]
- 148.Rossettini G, Palese A, Geri T, Mirandola M, Tortella F, Testa M. The knowledge of contextual factors as triggers of placebo and Nocebo effects in patients with musculoskeletal pain: findings from a National Survey. Front Psychiatry. 2019;10:478. doi: 10.3389/fpsyt.2019.00478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Greco CM, Yu L, Johnston KL, Dodds NE, Morone NE, Glick RM, Schneider MJ, Klem ML, McFarland CE, Lawrence S, Colditz J, Maihoefer CC, Jonas WB, Ryan ND, Pilkonis PA. Measuring nonspecific factors in treatment: item banks that assess the healthcare experience and attitudes from the patient’s perspective. Qual Life Res. 2016;25:1625–1634. doi: 10.1007/s11136-015-1178-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.