Abstract
Cirrhotic cardiomyopathy (CCM) is cardiac dysfunction in patients with end-stage liver disease in the absence of prior heart disease. First defined in 2005 during the World Congress of Gastroenterology, CCM criteria consisted of echo- cardiographic parameters to identify subclinical cardiac dysfunction in the absence of overt structural abnormalities. Significant advancements in cardiovascular imaging over the past 14 years, including the integration of myocardial deformation imaging into routine clinical practice to identify subclinical cardiovascular dysfunction, have rendered the 2005 CCM criteria obsolete. Therefore, new criteria based on contemporary cardiovascular imaging parameters are needed. In this guidance document, assembled by a group of multidisciplinary experts in the field, new core criteria based on contemporary cardiovascular imaging parameters are proposed for the assessment of CCM. This document provides a critical assessment of the diagnosis of CCM and ongoing assessment aimed at improving clinical outcomes, particularly surrounding liver transplantation. Key points and practice-based recommendations for the diagnosis of CCM are provided to offer guidance for clinicians and identify gaps in knowledge for future investigations.
The long recognized characteristic cardiovascular finding in end-stage liver disease (ESLD) is hyperdynamic function characterized by low systemic vascular resistance and high cardiac output state.(1) Despite the hyperdynamic circulation, the cardiac response to physiologic and pathophysiologic stimuli such as exercise, hemorrhage, infection, and surgery is abnormal, a condition termed “cirrhotic cardiomyopathy” (CCM).(2,3) Following the initial description of the syndrome three decades ago,(2) numerous studies have demonstrated the clinical relevance of CCM as a causative or contributory factor in the pathogenesis of conditions such as hepatorenal syndrome, decompensation of end-stage cirrhosis, and morbidity and mortality following surgery, transplantation, and infection.(4,5)
Although cellular and molecular pathogenic mechanisms underlying CCM have been elucidated in animal models, progress in the diagnosis and management of CCM has been hampered by lack of universally accepted diagnostic criteria.(6) At the Montreal 2005 World Congress of Gastroenterology, an expert consensus panel proposed preliminary criteria for diagnosis of CCM (Table 1a); but progress in modern concepts of ventricular dysfunction, especially over the past decade, has rendered these criteria obsolete. Accordingly, a group of multidisciplinary providers from hepatology, anesthesia, and cardiology have convened to form the Cirrhotic Cardiomyopathy Consortium (with the aim of proposing updated criteria for CCM based on modern concepts and knowledge of heart failure [HF]). The aim of this paper is to provide practice-based recommendations for the diagnosis of CCM in persons with cirrhosis, particularly surrounding liver transplantation. Key points are provided to offer guidance for clinicians and identify gaps in knowledge for future investigations.
TABLE 1.
Redefining Criteria for CCM
| a. World Congress of Gastroenterology Criteria (2005) | ||
| Systolic Dysfunction | Diastolic Dysfunction | Supportive Criteria |
| Any of the following | Any of the following | • Electrophysiological abnormalities |
| • Blunted contractile response on stress testing | • Deceleration time >200 milliseconds | • Abnormal chronotropic response |
| • LV ejection fraction <55% | • Isovolumetric relaxation time >80 milliseconds | • Electromechanical uncoupling |
| • Prolonged QTc interval | ||
| • E/A <1 | • Enlarged left atrium | |
| • Increased myocardial mass | ||
| • Increased BNP | ||
| • Increased proBNP | ||
| • Increased troponin I | ||
| b. Proposed criteria by the Cirrhotic Cardiomyopathy Consortium (2019) | ||
| Systolic Dysfunction | Advanced Diastolic Dysfunction† | Areas for Future Research Which Require Further Validation |
| Any of the following | ≥3 of the following | • Abnormal chronotropic or inotropic response§ |
| • LV ejection fraction ≤50% | • Septal e′ velocity <7 cm/second | • Electrocardiographic changes |
| • Absolute* GLS <18% | • E/e′ ratio ≥15 | • Electromechanical uncoupling |
| • LAVI >34 mL/m2 | • Myocardial mass change | |
| • TR velocity > 2.8 m/second‡ | • Serum biomarkers | |
| • Chamber enlargement | ||
| • CMRI∥ | ||
GLS is reported as a negative value in echocardiography reports. Changes in GLS should be described as changes in the absolute value.
Refer to Fig. 3 for echocardiographic changes in early diastolic dysfunction. They were not included in this table given their decreased specificity as they can occur due to aging.
In the absence of evidence of primary pulmonary hypertension or portopulmonary hypertension.
Examples include absence of or blunted contractile or diastolic reserve on exercise stress testing, dobutamine stress testing, or at rest on CMRI.
Myocardial extracellular volume as a surrogate for myocardial fibrosis can be assessed using this modality.
Abbreviation: e′, early diastolic mitral annular velocity.
CCM in the Spectrum of HF
The American Heart Association and the American College of Cardiology guidelines recommend a four- stage system to describe the syndrome of HF, which is applicable to both HF with preserved ejection fraction (> 50%) and HF with reduced ejection fraction (< 40%)(7):
Stage A: Patients at high risk for HF but without structural heart disease or symptoms of HF (e.g., patients with metabolic syndrome or some of its components such as diabetes mellitus or hypertension).
Stage B: Patients with structural heart disease in the absence of signs or symptoms of HF (e.g., patients with left ventricular [LV] remodeling).
Stage C: Structural heart disease with prior or current symptoms of HF.
Stage D: Patients with refractory HF not responsive to conventional medical or device therapy and requiring cardiac replacement, mechanical circulatory support, or palliation.
This strategy of staging HF highlights the importance of early identification and intervention in patients with stage A or B to prevent progression into stage C and D. Based on this classification, patients with ESLD or metabolic syndrome and its components without structural heart disease might be classified as stage A, whereas those considered to have CCM on the basis of LV remodeling and/or systolic or diastolic dysfunction in the absence of clinical HF symptoms might be classified as stage B HF. However, identification of stage C HF due to CCM in ESLD may be complicated by the fact that symptoms of HF may be masked or confounded by those of advanced cirrhosis, which can also limit functional capacity. This will affect prognosis as outcomes of those with stage C HF are significantly worse than those of stage A or B.(8) Therefore, accurate staging of HF due to CCM may require sophisticated investigation, perhaps to demonstrate hemodynamic effects of CCM in the setting of unclear symptomatic HF.
Redefining CCM Criteria: Alignment with Contemporary Metrics for Assessing Cardiac Dysfunction
LV SYSTOLIC FUNCTION
quantification. Hence, the 2005 CCM criteria define LV systolic dysfunction by the presence of a low LVEF (e.g., <55%) at rest and/or a blunted contractile response on myocardial stress testing (e.g., failure of LVEF to increase on stress testing by >5%).(9) However, assessment of impaired contractile response to stress testing is often limited in patients with ESLD. First, pharmacologic beta-blockade is common in this patient population. Second, and more importantly, the definition of impaired cardiac functional reserve has expanded beyond assessment of ejection fraction response and includes hemodynamic changes, other measures of contractile function, as well as impaired diastolic reserve.(9,10) Moreover, the vasodilatory state of ESLD results in decreased afterload and consequently normal or even increased LVEF. Therefore, while LVEF remains an important measure of global systolic function, additional surrogates are especially needed in patients with cirrhosis to assess cardiac contractility.
Echocardiographic strain imaging, also known as myocardial deformation imaging, has emerged as a means to objectively quantify regional myocardial contractile function. Strain can be categorized into circumferential, longitudinal, radial, and transverse strain, permitting more comprehensive assessment of contractile function beyond LVEF alone, which primarily reflects radial function. Because longitudinal contractile function is often impaired prior to loss of radial function, global longitudinal strain (GLS) can identify myocardial contractile dysfunction in those with preserved LVEF across a variety of pop- ulations.(11–13) GLS expresses myocardial longitudinal shortening as a percentage (i.e., change in length during systole as a proportion to baseline length at diastole), and it is typically described as a negative number because the normal myocardium shortens in the longitudinal plane during systole (Fig. 1).
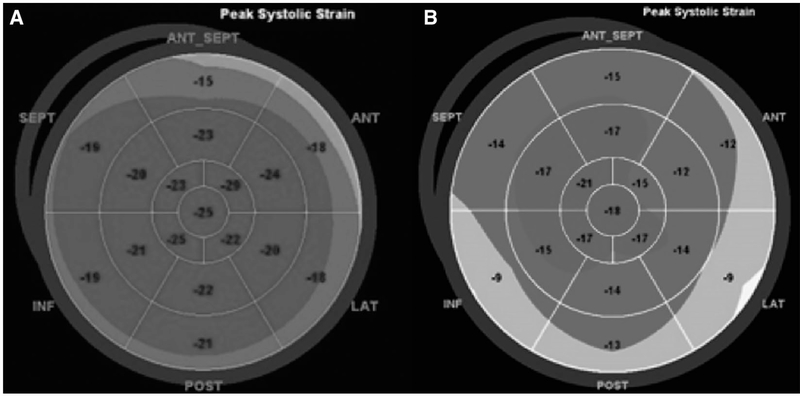
FIG. 1.
Illustration of strain imaging as a surrogate for cardiac systolic function. This “Bull’s eye” diagram of the myocardium shows (A) normal strain imaging (global longitudinal strain of −21%) and (B) abnormal strain imaging (global longitudinal strain of −14%). Diminished strain in patients with end stage liver disease in the absence of known heart disease is diagnostic of cirrhotic cardiomyopathy.
The American Society of Echocardiography (ASE) and the European Association of Cardiovascular Imaging (EACVI) guidelines define GLS less negative than −16% as abnormal, GLS −18% or greater (more negative) as normal, and GLS −16% to −18% as borderline in adults; changes in strain are described as absolute values to avoid confusion among providers/7,14,15 Although a limitation of GLS is that it varies with age and sex and is affected by LV loading conditions, the 2015 ASE guidelines for chamber quantification suggest GLS as an adjunct to assess LV systolic function in patients with normal LVEF. Data on strain imaging to detect CCM in patients with normal LVEF are limited and conflicting, with three studies showing normal longitudinal strain(16–18) and one multicenter study showing diminished longitudinal strain in one of two cohorts of patients with cirrhosis.(19) The latter study also showed that as liver disease advances, the GLS increases and correlates with impaired transplant-free survival. A plausible explanation for this finding is that as patients with ESLD become sicker, their systemic vascular resistance decreases, resulting in decreased afterload and increased cardiac contractility. However, strain imaging may still be considered to detect subclinical LV systolic dysfunction in the ESLD population.
CCM consortium recommendation
Absolute GLS (normal value 18% or greater) assessment should be considered to detect LV systolic function in patients with cirrhosis who have preserved LVEF (normal value >50%). Diminished LVEF or diminished GLS in the absence of known cardiac disease (e.g., other cardiomyopathies such as ischemic, rheumatic, etc.) should be considered diagnostic of CCM (Table 1b).
LV DIASTOLIC FUNCTION
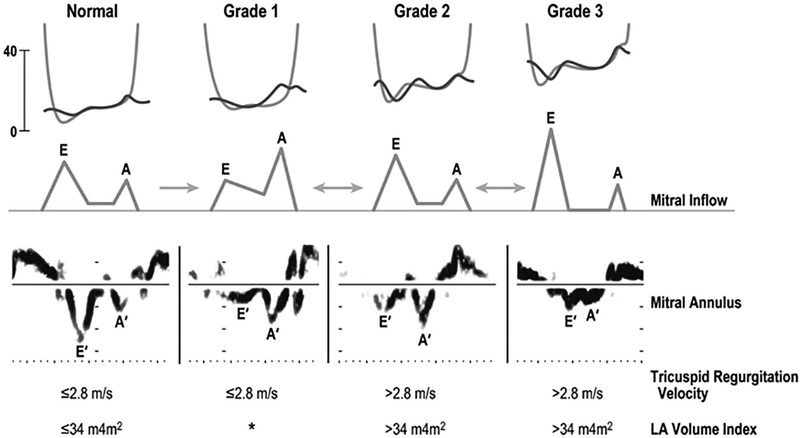
In 2016 the ASE issued new guidelines for assessment of LV diastolic function, which is part of the routine echocardiographic evaluation of LV function. These guidelines focus on screening for elevated LV filling pressures using a combination of four criteria: septal mitral annular early diastolic velocity (e’) by tissue Doppler <7 cm/second or lateral e’ velocity <10 cm/ second, mitral inflow early diastolic velocity (E) to e’ ratio >14 (using average e’) or 15 (using medial e’), left atrial (LA) volume index (LAVI) >34 mL/m2 and tricuspid regurgitation (TR) velocity >2.8 m/second (suggesting pulmonary hypertension).(20) Application of these modern criteria should supersede the 2005 Montreal CCM criteria, which rely on parameters that are impacted both by loading conditions and by heart rate, which can vary significantly in patients with ESLD (Table 1a). For example, the transmitral Doppler filling pattern (E/A ratio), which compares early (E) to late (A) diastolic filling, is dynamic and affected by preload; it improves with diuresis, dialysis, or paracentesis and worsens with volume overload. The E/A ratio is still a good diastolic parameter especially in patients with known diastolic dysfunction(17) and was recommended to be the first parameter to use in such patients. Moreover, all three parameters suggested by the 2005 CCM criteria (E/A ratio, deceleration, time and isovolumetric relaxation time) exhibit a U-shaped relationship with diastolic function (Fig. 2), such that normal patients and those with advanced diastolic dysfunction may have similar values. It can be difficult to distinguish normal from disease using each parameter alone, with a more distinctly different pattern for each variable only for milder forms of diastolic dysfunction. Therefore, using any of these three parameters in isolation to diagnose CCM may be problematic.
FIG. 2.
Changes in pressure, velocity, and volume during different grades of diastolic dysfunction diagram of left ventricle (LV)-left atrial (LA) pressure tracing (top), mitral inflow velocity (middle) and mitral annulus velocity (bottom) of normal and grade 1 to grade 3 diastolic dysfunction. Tricuspid regurgitation and LA volume index values of each category in general are shown at the bottom (with permission from The Echo Manual, 4th edition, Oh et al.)
Conversely, e’ (early diastolic mitral annular velocity) is a relatively preload-independent marker of diastolic function, reflecting the status of myocardial relaxation(21); thus, e’ is of specific importance in patients with ESLD and volume overload. Tissue Doppler imaging (TDI) is used to assess e’ from the mitral annulus, which is a marker of cardiac relaxation during diastole (Fig. 2). Myocardial relaxation, hence e’ velocity, is reduced in all forms of myocardial disease; and mitral inflow early diastolic velocity (E) is increased with higher filling pressure but reduced during early stage of diastolic dysfunction without increased filling pressure. Because reduced myocardial relaxation is one of the initial manifestations of myocardial dysfunction, it is reasonable to use reduced e’ velocity (< 7 cm/second of medial or septal e’ velocity) as a marker of initial diastolic dysfunction, especially accompanied by an E/A ratio < 0.8 (Fig. 3). The decline in E/A ratio is believed to follow the decline in e’ by several years. When the E/A ratio declines without a concurrent decline in e’, it is indicative of hypovolemia rather than diastolic dysfunction. E/e’ is another important component of the assessment of diastolic function and a marker of LV filling pressures. An E/e’ > 15 (using the medial e’) indicates an advanced diastolic dysfunction, although it may be falsely elevated in subjects with severe mitral annulus calcification.(22) Finally, LA volume is an indicator of both the severity and the duration of LV diastolic dysfunction/23 and LA size has proven to be a powerful predictor of outcome in several disease entities, including among patients with cirrhosis; but it can also be elevated with high output state without diastolic dysfunction.(24–27) Abnormalities in LAVI and E/e’ ratio have been shown to correlate with HF in liver transplant recipients(28) However, LAVI is a better marker of chronic elevation in LV filling pressures and does not reflect acute pressure changes; in contrast, LA strain may provide incremental information on LA function in addition to dynamic changes in LV filling pressures and may further contribute to the assessment of diastolic function (Fig. 4).(29,30) Importantly, the use of TR velocity to detect diastolic dysfunction may be limited in patients with ESLD. Because TR velocity is marker of pulmonary hypertension but cannot distinguish pulmonary venous (postcapillary) from pulmonary arterial hypertension (precapillary), it may be elevated in conditions that cause precapillary hypertension, including portopulmonary hypertension, in the absence of elevated LV filling pressures due to diastolic dysfunction. In this situation, the E/A ratio is <0.8 and E/e’ is <15.
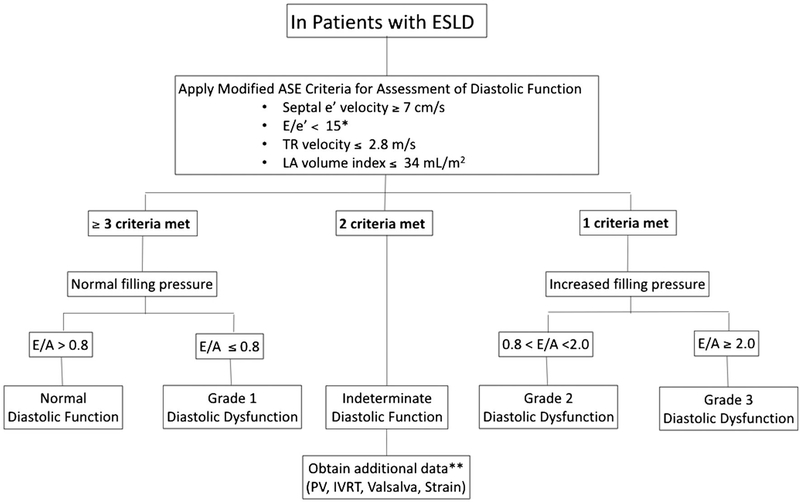
FIG. 3.
Evaluation of diastolic function in patients with end-stage liver disease (A simplified algorithm, revised from the 2016 ASE guideline. [Adapted from Oh JK et al.(33) Submitted to JACC Imaging]). *In this algorithm, only medial annulus velocity is recommended. After applying the modified criteria, filling pressure is first assessed, then diastolic function is graded based on E/A ratio. **For values of PV, IVRT, and strain assessment in patients with indeterminate diastolic function, refer to Fig. 4. Advanced diastolic dysfunction (grade 2 or 3) in patients with ESLD in the absence of known heart disease is diagnostic of cirrhotic cardiomyopathy. Abbreviations: LA, left atrium; PV, pulmonary vein; IVRT, isovolumetric relaxation time.
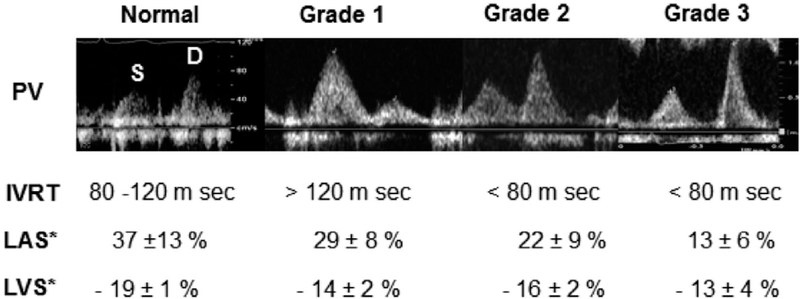
FIG. 4.
Additional assessment to reclassify patients with indeterminate diastolic function based on Fig. 3 into normal versus different grades of diastolic dysfunction. Pictured are still frames of pulmonary vein pressures, values of isovolumetric relaxation (IVRT), Left atrial systolic strain (LAS), and LV global longitudinal strain (LVS) for normal and different stages of diastolic function.
Diastolic dysfunction is relatively common in the elderly and individuals with hypertension, coronary artery disease, or diabetes mellitus. Therefore, it is critical to exclude coexisting comorbidities responsible for diastolic dysfunction when CCM is determined. Diastolic parameters in normal controls based on age have been published.(31) The 2016 ASE guideline was created to make diastolic function simpler but is very specific for advanced diastolic dysfunction and increased the proportion of the patients with normal diastolic function in the general population.(32) The Mayo Clinic echocardiography laboratory has proposed a revised, simpler diagnostic algorithm (Fig. 3) to improve sensitivity and practicality.(33)
CCM consortium recommendation
We endorse clinical implementation of contemporary ASE/EACVI guidelines with a minor revision for assessment of diastolic dysfunction in patients with ESLD. For simplicity, we recommend using the medial e’ velocity preferably, rather than averaging both lateral and medial e’ velocities. Advanced diastolic dysfunction defined by the aforementioned criteria in the absence of known cardiac disease should be considered diagnostic of CCM (Table 1b). Decreased e’ velocity alone or with decreased E/A ratio may be an early marker(s) of diastolic dysfunction in CCM; however, these decrements can also occur due to aging. Notably, lower thresholds (with higher value) of e’ velocity than those outlined in the ASE criteria may detect diastolic dysfunction in younger CCM patients with greater sensitivity, though further research is needed. TR velocity alone should not be used to diagnose diastolic dysfunction in CCM, especially with portopulmonary hypertension.
Potential Additional Markers of CCM
CONTRACTILE RESERVE
Abnormal or blunted contractile reserve is often defined as failure to augment ejection fraction by >5% in response to stress.(10) Although frequently considered a marker of diffuse coronary disease or hypertensive response, abnormal contractile reserve may indicate subclinical LV systolic dysfunction in patients with myocardial disease in the absence of epicardial coronary artery disease.(34) This blunted response to stress can occur in CCM; however, there is no universal definition for “blunted response to stress,” which limits its diagnostic utility in clinical practice. In addition, abnormal diastolic reserve on exercise testing can be identified by an increase in E/e’ suggestive of increased filling pressures; this is observed in patients with HF with preserved ejection fraction as well as those with other myocardial disease.(35,36) Early stages of CCM may not manifest as overt LV dysfunction with decreased LVEF and severe diastolic dysfunction. Therefore, absence of contractile or diastolic reserve may be a useful marker of subclinical LV dysfunction in early-stage CCM or more profoundly limited in those with more advanced stages of chronic high-output HF or myocardial fibrosis that might be expected in CCM.
IMPAIRED EXERCISE TESTING
Cardiopulmonary exercise testing (CPET) to assess exercise tolerance and functional aerobic capacity provides valuable diagnostic and prognostic information in patients with HF. The peak oxygen consumption (pVO2) determined by CPET can be used to stratify risk and assess treatment response as well as to identify candidates for device, pharmacologic, and advanced HF therapies.(37,38) For example, a pVO2 of <14 mL/kg/minute is associated with a 1-year survival of 48% and has been adopted as a selection criterion for heart transplantation and LV assist devices.(39,40)
In addition, the measurement of physiologic variables in CPET beyond heart rate and blood pressure response permits differentiation of multiple mechanisms that can affect pVO2 and exercise tolerance, including cardiovascular performance (i.e., stroke volume limitation), pulmonary gas exchange, peripheral vascular function, skeletal muscle metabolism, hematologic conditions that affect arterial or mixed venous oxygen content, or deconditioning. Therefore, CPET may be a useful test to identify patients who may have cardiovascular limitation in CCM and to differentiate those patients with advanced liver disease who may have severe exercise limitation and low pVO2 due to other mechanisms. One limitation is that patients with CCM may have high-output HF, in which oxygen consumption is increased; but this may be offset by other physiologic changes.
CHAMBER ENLARGEMENT
Chronic high-output HF, which may occur in the setting of liver disease, results in cardiac structural and physiologic changes. Increased systemic vasodilatation leading to higher preload may result in LV chamber dilatation. Compared to patients with normal output, those with high-output HF have increased LV end diastolic dimension and LV mass index suggestive of eccentric remodeling.(41) These changes can be readily detected by echocardiography and progressive changes can be serially monitored.
RIGHT VENTRICULAR DYSFUNCTION
The physiologic changes that result in high cardiac output and increased preload also affect pulmonary blood flow and both left-sided and right-sided cardiac filling pressures. Increased LV filling pressures will also increase mean pulmonary artery pressure and cause pulmonary venous hypertension and worsen pulmonary hypertension in those with predisposing conditions, such as preexisting portopulmonary hypertension. In high-output conditions, right atrial pressure and mean pulmonary artery pressure are more than twice as high as in patients with normal cardiac output.(41) Chronic exposure to these hemodynamic changes will lead to right-sided chamber enlargement and right HF; patients with CCM may also present with right HF in addition to changes in LV structure and function.
BIOMARKERS
The search for accurate diagnostic and prognostic noninvasive cardiac biomarkers is ongoing in the HF literature. Although many of these may translate into the CCM population, this will require further study. Well-described biomarkers for subclinical and clinical HF include brain natriuretic peptide (BNP), propeptide N-terminal prohormone (NT-proBNP), and cardiac troponins (either T or I).(42) BNP and NT-proBNP have been associated with the severity of ESLD and portal hypertension, and their secretion reflects systemic attempts at natriuresis. They also reflect the severity of diastolic and systolic cardiac abnormalities(43–45) as well as mortality in clinical HF(46) and in CCM.(43) An elevated troponin level in the patient with cirrhosis has been associated with cardiovascular events as well as patient and graft sur- vival(47–49) and is well known to predict cardiac mortality in acute coronary syndrome(50) and nonischemic heart disease.(51)
Circulatory modulations in ESLD likely drive cardiac dysfunction in patients with cirrhosis. Activation of the renin-angiotensin-aldosterone system as well as the sympathetic/adrenergic and vasopressin systems suggest that biomarkers such as nitric oxide, endothelin, adrenomedullin, copeptin (provasopressin), vasoactive peptide, endocannabinoids, and bile salts may also be of value in diagnostic assays for CCM risk.
Cardiac remodeling may be measured by levels of galectin-3 and soluble suppression of tumorgenic- ity-2 (ST-2, member of the interleukin [IL]-1 family, directly interacting with cardioprotective IL-33). These markers have been shown to reflect cardiac inflammatory and fibrotic remodeling.(52,53) However, galectin-3, and soluble ST-2 have also been shown to be markers for liver inflammation and fibrosis, which may limit their applicability to CCM.(54)
In addition to highly sensitive C-reactive protein associated with cardiac disease (and other inflammatory conditions), other inflammatory markers have been studied in HF and CCM including IL-6, IL-8, tumor necrosis factor-alpha, lipopolysaccharide binding protein, vascular endothelial growth factor, and soluble urokinase-type plasminogen activator receptor, some of which may provide additive insult to the circulatory dysfunction of portal hypertension.(45,55)
ELECTRO CARDIOGRAPHY
Echocardiography findings are of limited value in CCM. Prolongation of the QT interval (>440 milliseconds) is the most common ECG finding in cirrhosis and one of the previous supportive criteria for CCM in the 2005 Montreal criteria (Table 1a). Prolonged QTc, however, can be seen in up to 50% of patients with ESLD, and its utility in predicting poor outcomes is controversial.(56) In general, prolonged QTc interval seems to improve after liver transplantation, though limited data support this assertion.(56) A short interval from peak T wave to end T wave (<50 milliseconds) reflecting ventricular repolarization has been linked with poor outcomes in one small study(57) but certainly requires more study. Unlike the situation in patients without cirrhosis, prolonged QTc is not considered a risk factor for torsade de pointes ventricular tachycardia in cirrhosis. Atrial fibrillation is common in ESLD and increases with increasing Model for End-Stage Liver Disease score.(58) Atrial fibrillation and other dysrhythmias have been associated with increased cardiovascular events in the perioperative period and worse long-term outcomes.(59,60)
CARDIAC MAGNETIC RESONANCE IMAGING
Cardiac magnetic resonance imaging (CMRI) is a noninvasive method for assessing LV function and myocardial strain. T1 images provide diagnostic and prognostic value of fibrosis and are particularly relevant for their relation to diastolic dysfunction in HF, and thus, they may have a potential role in detecting CCM. Increasing signal of the myocardium on T2-weighted images is a marker of acute myocardial inflammation and injury. Contrast-enhanced CMRI has the potential to demonstrate subclinical myocardial changes prior to the onset of clinical LV dysfunction and is also able to detect edema. Extracellular volume fraction (ECVf) of the myocardium and fibrosis measurements can be quantified, possibly predicting who may recover after liver transplantation and who may not. For example, ECVf has been shown to increase with Child-Pugh class, and ECVf >31.2% was a predictor of poor outcome after liver transplantation in one study.(61) It is noteworthy that, in the same study, patients with less advanced cirrhosis (Child-Pugh score <8) had slightly higher ECVf than healthy controls but significantly lower ECVf than patients with more advanced disease.(61) This observation suggests that myocardial changes such as myocardial fibrosis in patients with cirrhosis can be evolving even before ESLD develops. Low T2* mapping, specifically with a T2* < 15 milliseconds, has been associated with poor liver transplant outcomes including posttransplant heart failure and death.(62) CMRI may also detect inotropic incompetence to pharmacological stress related to intrinsic myocardial dysfunction response at rest and during dobutamine stress at low to intermediate doses.(63) Thus, CMRI may be more sensitive than conventional methods (e.g., dobutamine stress echocardiography) at detecting subclinical myocardial dysfunction in CCM. Finally, CMRI can provide a comprehensive evaluation of myocardial function in a single exam independent of the acoustic window and without radiation. Thus, in ESLD, prospective trials are needed to determine the potential role of CMRI in the cardiac evaluation and management strategy for patients with cirrhosis, in particular surrounding selection criteria for liver transplantation.
CCM consortium recommendation
Measurement of serum biomarkers may be of clinical relevance in conjunction with imaging-based markers for the diagnosis of CCM, but further studies on the role and threshold for diagnosis are needed. ECG findings have little value in the diagnosis of CCM. Studies looking at combined multimodality imaging techniques for CCM are needed.
Proposed Future Directions
The true burden of CCM among patients with ESLD is currently unknown. Prior studies evaluated CCM prevalence and estimated it to be approximately 50%(5); however, this estimate was based on the old criteria. In order to advance understanding of the natural history and impact of CCM on clinical outcomes, updated standardized criteria for diagnosis are imminently needed. We believe that evidence exists to support adaptation of routine strain and TDI for the assessment of LV systolic and diastolic function in cirrhosis. Whether or not additional markers of myocardial injury including serum biomarkers, advanced cardiac imaging, submaximal exercise testing, contractile reserve on myocardial stress imaging, markers of right ventricular dysfunction, and ECG abnormalities improve evaluation of CCM has yet to be determined. Overall, better evaluation of CCM has the potential to improve long-term outcomes in patients with cirrhosis, in particular surrounding liver transplantation, with the ultimate goal of improving recipient selection and maximizing the benefit of scarce donor organs.
CCM consortium recommendation
We recommend that echocardiographic exams in patients with cirrhosis and in liver transplant recipients include TDI and strain imaging. We propose that echocardiographic imaging be performed in wait-list candidates at 6-month intervals and in intervals of 6, 12, and 24 months posttransplant in all patients with any degree of pretransplant systolic or diastolic dysfunction based on the aforementioned criteria. This longitudinal follow-up is essential in order to identify patients whose cardiac dysfunction worsens pretransplant or persists posttransplant (i.e., persistent stage B HF). Early interventions in these patients can include adaptation of stricter posttransplant systolic blood pressure targets (e.g., <120 versus <130 mm Hg) and using antihypertensive medications that have the potential to prevent further cardiac remodeling (e.g., angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, or beta blockers) instead of using amlodipine as a first-line antihypertensive medication, which is commonly practiced in post-liver transplant settings. Therefore, early interventions may be beneficial for improving diastolic function,(64) preventing the development of more advanced stages of heart failure,(7) and subsequently decreasing cardiac events and improving long-term posttransplant survival.(60,65)
Conclusion
CCM Consortium recommendations based upon expert multidisciplinary review of the available body of evidence suggest a new definition of CCM in patients with ESLD (Table 1). A significant knowledge gap in the study of cardiovascular outcomes in patients with CCM persists and requires further studies. Modification of these new CCM criteria based on CCM-specific investigations should be undertaken in the future. The unique physiology of ESLD can profoundly influence accurate diagnosis, management, and outcomes of underlying cardiac pathology and requires a careful evidence-based and multidisciplinary approach to the prevention, diagnosis, and treatment of CCM.
Acknowledgments
Supported by the National Institutes of Health’s National Heart, Lung, and Blood Institute (K23HL136891, to L.B.V).
Abbreviations
- ASE
American Society of Echocardiography
- BNP
brain natriuretic peptide
- CCM
cirrhotic cardiomyopathy
- CMRI
cardiac magnetic resonance imaging
- CPET
cardiopulmonary exercise testing
- E/A ratio
early to late diastolic filling ratio
- ECG
electrocardiography
- ECVf
extracellular volume fraction
- E/e’
ratio of mitral peak velocity of earlyfilling to early diastolic mitral annular velocity
- ESLD
end-stage liver disease
- GLS
global longitudinal strain
- HF
heart failure
- IL
interleukin
- LA
left atrium
- LAVI
LA volume index
- LV
left ventricle
- LVEF
left ventricle ejection fraction
- NT-proBNP
propeptide N-terminal prohormone
- pVO2
peak oxygen consumption
- TDI
tissue Doppler imaging
- TR
tricuspid regurgitation
Footnotes
Potential conflict of interest: Nothing to report.
The Cirrhotic Cardiomyopathy Consortium is a multidisciplinary international group whose focus is to improve the understanding of cirrhotic cardiomyopathy, its management and outcomes. The group met in October 2018 at the Mayo Clinic (Minnesota) and subsequently worked together to develop this document.
Assessment of LV systolic function has traditionally focused on the LV ejection fraction (LVEF)
REFERENCES
- 1).Kowalski HJ, Abelmann WH. The cardiac output at rest in Laennec’s cirrhosis. J Clin Invest 1953;32:1025–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Lee SS. Cardiac abnormalities in liver cirrhosis. West J Med 1989;151:530–535. [PMC free article] [PubMed] [Google Scholar]
- 3).Ma Z, Lee SS. Cirrhotic cardiomyopathy: getting to the heart of the matter. Hepatology 1996;24:451–459. [DOI] [PubMed] [Google Scholar]
- 4).Wong F, Liu P, Lilly L, Bomzon A, Blendis L. Role of cardiac structural and functional abnormalities in the pathogenesis of hyperdynamic circulation and renal sodium retention in cirrhosis. Clin Sci (Lond) 1999;97:259–267. [PubMed] [Google Scholar]
- 5).Wiese S, Hove JD, Bendtsen F, Moller S. Cirrhotic cardiomyopathy: pathogenesis and clinical relevance. Nat Rev Gastroenterol Hepatol 2014;11:177–186. [DOI] [PubMed] [Google Scholar]
- 6).Liu H, Jayakumar S, Traboulsi M, Lee SS. Cirrhotic cardiomyopathy: implications for liver transplantation. Liver Transpl 2017;23:826–835. [DOI] [PubMed] [Google Scholar]
- 7).Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Drazner MH, et al. ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;2013(62):e147–e239. [DOI] [PubMed] [Google Scholar]
- 8).Shah AM, Claggett B, Loehr LR, Chang PP, Matsushita K, Kitzman D, et al. Heart failure stages among older adults in the community: the Atherosclerosis Risk in Communities Study. Circulation 2017;135:224–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Lancellotti P, Pellikka PA, Budts W, Chaudhry FA, Donal E, Dulgheru R, et al. The clinical use of stress echocardiography in non-ischaemic heart disease: recommendations from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr 2017;30:101–138. [DOI] [PubMed] [Google Scholar]
- 10).Marwick TH, Gillebert TC, Aurigemma G, Chirinos J, Derumeaux G, Galderisi M, et al. Recommendations on the use of echocardiography in adult hypertension: a report from the European Association of Cardiovascular Imaging (EACVI) and the American Society of Echocardiography (ASE). J Am Soc Echocardiogr 2015;28:727–754. [DOI] [PubMed] [Google Scholar]
- 11).Ternacle J, Berry M, Alonso E, Kloeckner M, Couetil JP, Rande JL, et al. Incremental value of global longitudinal strain for predicting early outcome after cardiac surgery. Eur Heart J Cardiovasc Imaging 2013;14:77–84. [DOI] [PubMed] [Google Scholar]
- 12).Sjoli B, Grenne B, Smiseth OA, Edvardsen T, Brunvand H. The advantage of global strain compared to left ventricular ejection fraction to predict outcome after acute myocardial infarction. Echocardiography 2011;28:556–563. [DOI] [PubMed] [Google Scholar]
- 13).Lauridsen TK, Alhede C, Crowley AL, Kisslo J, Sorensen LL, Hansen TF, et al. Two-dimensional global longitudinal strain is superior to left ventricular ejection fraction in prediction of outcome in patients with left-sided infective endocarditis. Int J Cardiol 2018;260:118–123. [DOI] [PubMed] [Google Scholar]
- 14).Cheng S, Larson MG, McCabe EL, Osypiuk E, Lehman BT, Stanchev P, et al. Age- and sex-based reference limits and clinical correlates of myocardial strain and synchrony: the Framingham Heart Study. Circ Cardiovasc Imaging 2013;6:692–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:233–270. [DOI] [PubMed] [Google Scholar]
- 16).Chen Y, Chan AC, Chan SC, Chok SH, Sharr W, Fung J, et al. A detailed evaluation of cardiac function in cirrhotic patients and its alteration with or without liver transplantation. J Cardiol 2016;67:140–146. [DOI] [PubMed] [Google Scholar]
- 17).Sampaio F, Pimenta J, Bettencourt N, Fontes-Carvalho R, Silva AP, Valente J, et al. Systolic and diastolic dysfunction in cirrhosis: a tissue-Doppler and speckle tracking echocardiography study. Liver Int 2013;33:1158–1165. [DOI] [PubMed] [Google Scholar]
- 18).Rimbas RC, Baldea SM, Guerra R, Visolu SI, Rimbas M, Pop CS, et al. New definition criteria of myocardial dysfunction in patients with liver cirrhosis: a speckle tracking and tissue Doppler imaging study. Ultrasound Med Biol 2018;44:562–574. [DOI] [PubMed] [Google Scholar]
- 19).Jansen C, Cox A, Schueler R, Schneider M, Lehmann J, Praktiknjo M, et al. Increased myocardial contractility identifies patients with decompensated cirrhosis requiring liver transplantation. Liver Transpl 2018;24:15–25. [DOI] [PubMed] [Google Scholar]
- 20).Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2016;17:1321–1360. [DOI] [PubMed] [Google Scholar]
- 21).Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quinones MA. Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol 1997;30:1527–1533. [DOI] [PubMed] [Google Scholar]
- 22).Abudiab MM, Chebrolu LH, Schutt RC, Nagueh SF, Zoghbi WA. Doppler echocardiography for the estimation of LV filling pressure in patients with mitral annular calcification. JACC Cardiovasc Imaging 2017;10:1411–1420. [DOI] [PubMed] [Google Scholar]
- 23).Tsang TS, Barnes ME, Gersh BJ, Bailey KR, Seward JB. Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden. Am J Cardiol 2002;90:1284–1289. [DOI] [PubMed] [Google Scholar]
- 24).Poulsen MK, Dahl JS, Henriksen JE, Hey TM, Hoilund-Carlsen PF, Beck-Nielsen H, et al. Left atrial volume index: relation to long-term clinical outcome in type 2 diabetes. J Am Coll Cardiol 2013;62:2416–2421. [DOI] [PubMed] [Google Scholar]
- 25).Meris A, Amigoni M, Uno H, Thune JJ, Verm a A, Kober L, et al. Left atrial remodelling in patients with myocardial infarction complicated by heart failure, left ventricular dysfunction, or both: the VALIANT Echo study. Eur Heart J 2009;30:56–65. [DOI] [PubMed] [Google Scholar]
- 26).Cesari M, Frigo AC, Tonon M, Angeli P. Cardiovascular predictors of death in patients with cirrhosis. Hepatology 2018;68:215–223. [DOI] [PubMed] [Google Scholar]
- 27).Merli M, Torromeo C, Giusto M, Iacovone G, Riggio O, Puddu PE. Survival at 2 years among liver cirrhotic patients is influenced by left atrial volume and left ventricular mass. Liver Int 2017;37:700–706. [DOI] [PubMed] [Google Scholar]
- 28).Dowsley TF, Bayne DB, Langnas AN, Dumitru I, Windle JR, Porter TR, et al. Diastolic dysfunction in patients with end-stage liver disease is associated with development of heart failure early after liver transplantation. Transplantation 2012;94:646–651. [DOI] [PubMed] [Google Scholar]
- 29).Huynh QL, Kalam K, Iannaccone A, Negishi K, Thomas L, Marwick TH. Functional and anatomic responses of the left atrium to change in estimated left ventricular filling pressure. J Am Soc Echocardiogr 2015;28:1428–1433. [DOI] [PubMed] [Google Scholar]
- 30).Singh A, Addetia K, Maffessanti F, Mor-Avi V, Lang RM. LA strain for categorization of LV diastolic dysfunction. JACC Cardiovasc Imaging 2017;10:735–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31).Caballero L, Kou S, Dulgheru R, Gonjilashvili N, Athanassopoulos GD, Barone D, et al. Echocardiographic reference ranges for normal cardiac Doppler data: results from the NORRE Study. Eur Heart J Cardiovasc Imaging 2015;16: 1031–1041. [DOI] [PubMed] [Google Scholar]
- 32).Almeida JG, Fontes-Carvalho R, Sampaio F, Ribeiro J, Bettencourt P, Flachskampf FA, et al. Impact of the 2016 ASE/ EACVI recommendations on the prevalence of diastolic dysfunction in the general population. Eur Heart J Cardiovasc Imaging 2018;19:380–386. [DOI] [PubMed] [Google Scholar]
- 33).Oh JK, Miranda WR, Bird JG, Kane GC. A proposal for revised echocardiographic algorithm to assess diastolic function and filling pressure. JACC Imaging. Submitted for publication. [Google Scholar]
- 34).Nasis A, Moir S, Meredith IT, Barton TL, Nerlekar NL, Wong DT, et al. Abnormal left ventricular contractile response to exercise in the absence of obstructive coronary artery disease is associated with resting left ventricular long-axis dysfunction. J Am Soc Echocardiogr 2015;28:95–105. [DOI] [PubMed] [Google Scholar]
- 35).Kim SA, Shim CY, Kim JM, Lee HJ, Choi DH, Choi EY, et al. Impact of left ventricular longitudinal diastolic functional reserve on clinical outcome in patients with type 2 diabetes mellitus. Heart 2011;97:1233–1238. [DOI] [PubMed] [Google Scholar]
- 36).Rustad LA, Amundsen BH, Slordahl SA, Stoylen A. Upright bicycle exercise echocardiography in patients with myocardial infarction shows lack of diastolic, but not systolic, reserve: a tissue Doppler study. Eur J Echocardiogr 2009;10:503–508. [DOI] [PubMed] [Google Scholar]
- 37).Balady GJ, Arena R, Sietsema K, Myers J, Coke L, Fletcher GF, et al. Clinician’s guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation 2010;122:191–225. [DOI] [PubMed] [Google Scholar]
- 38).Albouaini K, Egred M, Alahmar A, Wright DJ. Cardiopulmonary exercise testing and its application. Postgrad Med J 2007; 83:675–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).Mancini DM, Eisen H, Kussmaul W, Mull R, Edmunds LH Jr., Wilson JR. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation 1991;83:778–786. [DOI] [PubMed] [Google Scholar]
- 40).Mehra MR, Canter CE, Hannan MM, Semigran MJ, Uber PA, Baran DA, et al. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: a 10-year update. J Heart Lung Transplant 2016;35:1–23. [DOI] [PubMed] [Google Scholar]
- 41).Reddy YNV, Melenovsky V, Redfield MM, Nishimura RA, Borlaug BA. High-output heart failure: a 15-year experience. J Am Coll Cardiol 2016;68:473–482. [DOI] [PubMed] [Google Scholar]
- 42).Grewal J, McKelvie RS, Persson H, Tait P, Carlsson J, Swedberg K, et al. Usefulness of N-terminal pro-brain natriuretic peptide and brain natriuretic peptide to predict cardiovascular outcomes in patients with heart failure and preserved left ventricular ejection fraction. Am J Cardiol 2008;102:733–737. [DOI] [PubMed] [Google Scholar]
- 43).Farr M, Schulze PC. Recent advances in the diagnosis and management of cirrhosis-associated cardiomyopathy in liver transplant candidates: advanced echo imaging, cardiac biomarkers, and advanced heart failure therapies. Clin Med Insights Cardiol 2014;8(Suppl. 1):67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44).Saner FH, Neumann T, Canbay A, Treckmann JW, Hartmann M, Goerlinger K, et al. High brain-natriuretic peptide level predicts cirrhotic cardiomyopathy in liver transplant patients. Transpl Int 2011;24:425–432. [DOI] [PubMed] [Google Scholar]
- 45).Wiese S, Mortensen C, Gotze JP, Christensen E, Andersen O, Bendtsen F, et al. Cardiac and proinflammatory markers predict prognosis in cirrhosis. Liver Int 2014;34:e19–e30. [DOI] [PubMed] [Google Scholar]
- 46).Pimenta J, Paulo C, Mascarenhas J, Gomes A, Azevedo A, Rocha-Goncalves F, et al. BNP at discharge in acute heart failure patients: is it all about volemia? A study using impedance cardiography to assess fluid and hemodynamic status. Int J Cardiol 2010;145:209–214. [DOI] [PubMed] [Google Scholar]
- 47).Coss E, Watt KD, Pedersen R, Dierkhising R, Heimbach JK, Charlton MR. Predictors of cardiovascular events after liver transplantation: a role for pretransplant serum troponin levels. Liver Transpl 2011;17:23–31. [DOI] [PubMed] [Google Scholar]
- 48).Safadi A, Homsi M, Maskoun W, Lane KA, Singh I, Sawada SG, et al. Perioperative risk predictors of cardiac outcomes in patients undergoing liver transplantation surgery. Circulation 2009;120:1189–1194. [DOI] [PubMed] [Google Scholar]
- 49).Watt KD, Coss E, Pedersen RA, Dierkhising R, Heimbach JK, Charlton MR. Pretransplant serum troponin levels are highly predictive of patient and graft survival following liver transplantation. Liver Transpl 2010;16:990–998. [DOI] [PubMed] [Google Scholar]
- 50).Antman EM, Tanasijevic MJ, Thompson B, Schactman M, McCabe CH, Cannon CP, et al. Cardiac-specific troponin I levels to predict the risk of mortality in patients with acute coronary syndromes. N Engl J Med 1996;335:1342–1349. [DOI] [PubMed] [Google Scholar]
- 51).Nakamura H, Niwano S, Fukaya H, Murakami M, Kishihara J, Satoh A, et al. Cardiac troponin T as a predictor of cardiac death in patients with left ventricular dysfunction. J Arrhythm 2017;33:463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52).Shah RV, Chen-Tournoux AA, Picard MH, van Kimmenade RR, Januzzi JL. Galectin-3, cardiac structure and function, and long-term mortality in patients with acutely decompensated heart failure. Eur J Heart Fail 2010;12:826–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53).Emdin M, Aimo A, Vergaro G, Bayes-Genis A, Lupon J, Latini R, et al. sST2 predicts outcome in chronic heart failure beyond NT-proBNP and high-sensitivity troponin T. J Am Coll Cardiol 2018;72:2309–2320. [DOI] [PubMed] [Google Scholar]
- 54).Pejnovic N, Jeftic I, Jovicic N, Arsenijevic N, Lukic ML. Galectin-3 and IL-33/ST2 axis roles and interplay in diet-induced steatohepatitis. World J Gastroenterol 2016;22:9706–9717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55).Zimmermann HW, Koch A, Seidler S, Trautwein C, Tacke F. Circulating soluble urokinase plasminogen activator is elevated in patients with chronic liver disease, discriminates stage and aetiology of cirrhosis and predicts prognosis. Liver Int 2012;32:500–509. [DOI] [PubMed] [Google Scholar]
- 56).Izzy M, Oh J, Watt KD. Cirrhotic cardiomyopathy after transplantation: neither the transient nor innocent bystander. Hepatology 2018;68:2008–2015. [DOI] [PubMed] [Google Scholar]
- 57).Salgado AA, Barbosa PRB, Ferreira AG, Reis C, Terra C. Prognostic value of a new marker of ventricular repolarization in cirrhotic patients. Arq Bras Cardiol 2016;107:523–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58).Huang WA, Dunipace EA, Sorg JM, Vaseghi M. Liver disease as a predictor of new-onset atrial fibrillation. J Am Heart Assoc 2018;7:e008703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59).Chokesuwattanaskul R, Thongprayoon C, Bathini T, Ungprasert P, Sharma K, Wijampreecha K, et al. Liver transplantation and atrial fibrillation: a meta-analysis. World J Hepatol 2018;10:761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60).VanWagner LB, Serper M, Kang R, Levitsky J, Hohmann cardiovascular events after liver transplantation among a national sample. Am J Transplant 2016;16:2684–2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61).Wiese S, Hove J, Mo S, Mookerjee RP, Petersen CL, VesterAndersen MK, et al. Myocardial extracellular volume quantified by magnetic resonance is increased in cirrhosis and related to poor outcome. Liver Int 2018;38:1614–1623. [DOI] [PubMed] [Google Scholar]
- 62).Lewin SM, Kallianos K, Nevah MI, Zhao S, Fix OK, Brooks GC, et al. Cardiac MRI T2* in liver transplant candidates: application and performance of a novel imaging technique to identify patients at risk for poor posttransplant cardiac outcomes. Transplant Direct 2018;4:e363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63).Sampaio F, Lamata P, Bettencourt N, Alt SC, Ferreira N, Kowallick JT, et al. Assessment of cardiovascular physiology using dobutamine stress cardiovascular magnetic resonance reveals impaired contractile reserve in patients with cirrhotic cardiomyopathy. J Cardiovasc Magn Reson 2015;17:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64).Almuntaser I, Mahmud A, Brown A, Murphy R, King G, Crean P, et al. Blood pressure control determines improvement in diastolic dysfunction in early hypertension. Am J Hypertens 2009;22:1227–1231. [DOI] [PubMed] [Google Scholar]
- 65).Watt KD, Pedersen RA, Kremers WK, Heimbach JK, Charlton MR. Evolution of causes and risk factors for mortality post-liver transplant: results of the NIDDK long-term follow-up study. Am J Transplant 2010;10:1420–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]