Abstract
We previously showed that commercially available rice peptide Oryza Peptide-P60 (OP60) increased the intracellular glutathione levels. This study aimed to evaluate the antioxidant potential of this peptide and assess its mechanism of action. Pretreatment of HepG2 cells with OP60 reduced the cytotoxicity caused by H2O2 or acetaminophen (APAP) (47.7 ± 1.3% or 12.2 ± 1.3% of the cytotoxicity for 5 mg/mL OP60 pretreatment compared to that in H2O2- or APAP-treated groups, respectively; p < 0.01) through the restoration of glutathione homeostasis. Moreover, OP60 elevated the mRNA level of genes encoding heavy and light subunits of γ-glutamylcysteine synthetase (γ-GCS) by 2.9 ± 0.1-fold and 2.7 ± 0.2-fold (p < 0.001), respectively, at 8 h and also increased the level of mRNA encoding other antioxidant enzymes. Besides, OP60 promoted Nrf2 nuclear translocation by 2.2 ± 0.3-fold (p < 0.05) after 8 h. Conversely, knockdown of Nrf2 inhibited the increase of the intracellular glutathione levels and suppressed the induction of antioxidant enzyme expression by OP60. In animal studies, OP60 prevented APAP-induced liver injury by suppressing glutathione depletion (from 0.19 ± 0.02 mmol/mg protein to 0.90 ± 0.02 mmol/mg protein; p < 0.01, by pretreatment with 500 mg/kg OP60) and increasing heavy subunit of γ-GCS and heme oxygenase-1 expression in the liver. Our results indicated that OP60 exhibits a cytoprotective effect via the Nrf2 signaling pathway and is one of the few peptides with excellent antioxidant properties.
1. Introduction
Aerobic organisms constitutively produce reactive oxygen species (ROS) as a natural by-product of oxygen metabolism. Excessive ROS production and impaired antioxidant defense potential cause an imbalance in ROS metabolism, which leads to a pro-oxidative state termed as oxidative stress. Oxidative stress is known to cause oxidative damage to biological molecules, such as proteins, lipids, and DNA, compromising cellular functions, thus playing an essential role in the development of various pathological conditions, including cancer,1 diabetes,2 cardiovascular diseases,3 and neurodegenerative disorders.4 In order to prevent these physiological conditions, maintenance of balance between cellular ROS generation and antioxidant defense mechanisms is important.5
In mammalian cells, antioxidant molecules and antioxidant/detoxifying enzymes act as defense systems in order to detoxify ROS or prevent its excess production. Glutathione (GSH) is the most abundant and important non-protein thiol-based antioxidant molecule in cells. GSH alone, or together with GSH peroxidase, can remove H2O2, lipid peroxides, and free radicals. Moreover, it plays a vital role in xenobiotic detoxification through direct thiol conjugation. Thus, the ability of cells to maintain the GSH level is essential to protect cellular function and integrity.6 Biosynthesis of GSH occurs by two reactions, which are adenosine triphosphate (ATP)-dependent.7 The first step, a rate-limiting enzyme in GSH biosynthesis, is catalyzed by γ-glutamylcysteine synthetase (γ-GCS), and its expression is mainly regulated by nuclear factor erythroid 2-related factor 2 (Nrf2).8
Nrf2 also regulates the expression of antioxidant and detoxifying enzymes, such as heme oxygenase-1 (HO-1), NAD(P)H quinone oxidoreductase1 (NQO1), and glutathione reductase (GR). Therefore, Nrf2 acts as a master regulator of cellular responses against environmental stresses. Under basal conditions, Nrf2 is bound to Kelch-like ECH associated protein 1 (Keap1) in the cytoplasm, which facilitates its degradation. In response to oxidative stress, Nrf2 dissociates from Keap1, translocates into the nucleus, and binds to antioxidant response elements (AREs) in the nucleus, resulting in the upregulation of its target genes.9,10 The activation of Nrf2 is expected to protect cells from oxidative damage via the upregulation of antioxidant and detoxifying enzymes.
In addition to vitamins, dietary fibers, polyphenols, and hydrolysates derived from dietary proteins exhibit health-promoting effects, such as antioxidant, antihypertensive, antidiabetic, anti-melanogenic, and immunomodulating activities.11,12 Recently, antioxidant peptides from dietary sources have drawn significant attention. Protein hydrolysates enzymatically produced from proteins of fish, milk, egg, soybean, whey, among others, have been reported to act as direct antioxidants by scavenging ROS and free radicals or sequestering pro-oxidant metals through chelation.13,14 Although the actions of these peptides as direct antioxidants are well-known, the current understanding of how peptides act as indirect antioxidants and activate the Keap1-Nrf2 pathway is limited. Fish skin gelatin-derived protein hydrolysates15 and a tripeptide from Chinese Baijiu16 reportedly induce GSH synthesis through Nrf2 pathway activation. We reported that rice bran protein hydrolysate increases intracellular GSH levels in the HepG2 human hepatoblastoma cells.17 Additionally, rice-derived peptides and sake lees hydrolysate are hepatoprotective in the case of acetaminophen (APAP)-induced liver injury.18,19 These antioxidative effects were suggested to be mediated through the Nrf2 antioxidant pathway. However, only a few peptides have been shown to act as indirect antioxidants by regulating the oxidative defense systems. Besides, peptides derived from eggshell membrane and chickpeas protein hydrolysates were shown to upregulate antioxidant enzymes.20,21 However, the mechanisms of the antioxidant action of these peptides are yet to be fully determined.
In our previous study, we have shown that a commercially available rice peptide Oryza Peptide-P60 (OP60) increased intracellular GSH levels.22 However, its protective effects against oxidative stress and the underlying antioxidant mechanism are yet to be investigated. In this study, the cytoprotective effect of OP60 against H2O2 and APAP-induced oxidative stress in HepG2 cells was determined. The OP60 antioxidant mechanism was assessed through the activation of Nrf2, which is a pivotal regulator of the expressions of several antioxidant enzymes. Furthermore, the protective effect of OP60 against APAP-induced hepatic injury in mice was evaluated. This study provided information about the molecular mechanisms underlying the indirect antioxidative effects of OP60.
2. Results
2.1. Protective Effects of OP60 against H2O2- or APAP-Induced Cytotoxicity in HepG2 Cells
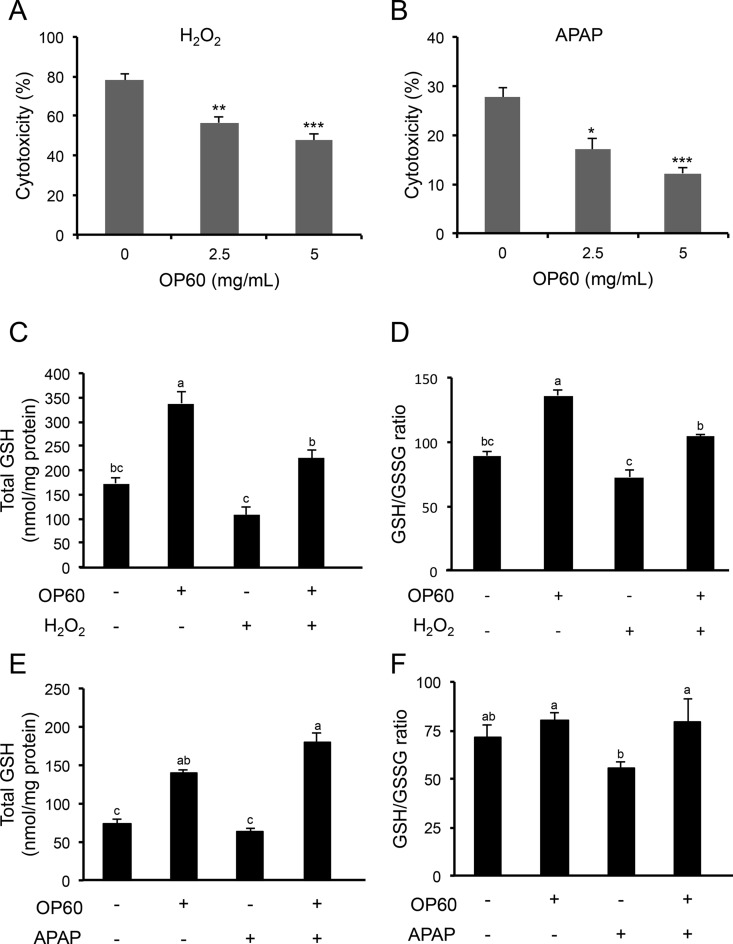
Cytotoxicity was evaluated by measuring the activity of released LDH from damaged cells in the medium. The cells were treated with or without OP60 followed by exposure to 200 μM H2O2 or 10 mM APAP. An overdose of APAP is known to cause an increase in the level of N-acetyl-p-benzoquinone imine (NAPQI), which is a reactive metabolite formed from APAP by cytochrome P450. At low amounts, NAPQI is efficiently detoxified via conjugation with GSH. However, excess NAPQI causes oxidative stress by binding to cellular macromolecules and depleting GSH, ultimately leading to apoptosis and hepatic necrosis.23,24 The treatment of control cells with H2O2 resulted in cytotoxicity of 77.9 ± 3.0%. However, pretreatment with OP60 effectively protected the cells in a dose-dependent manner (Figure 1A). Similarly, treatment with APAP caused 27.6 ± 2.0% cytotoxicity, which was significantly reduced in cells pretreated with OP60 in a dose-dependent manner (Figure 1B). Since OP60 has been previously observed to increase the intracellular GSH levels, total GSH levels were determined under the condition of H2O2- and APAP-induced oxidative stress. Treatment with H2O2 alone decreased the GSH levels after 2 h (Figure 1C). In cells, the ratio of GSH/GSSG tended to decrease, indicating an imbalance of the intracellular redox status (Figure 1D). Pretreatment with OP60 restored the decreased in the total GSH levels and GSH/GSSG ratio to the control levels (Figure 1C,D). The intracellular GSH level in cells exposed to APAP alone was 0.86 ± 0.05-fold of the control, although significant depletion of the intracellular GSH level was not observed in cells exposed to APAP. APAP added to OP60 significantly increased the intracellular GSH level compared to OP60 treatment alone (Figure 1E). Similarly, in APAP-treated cells, the decreased GSH/GSSG ratio was significantly increased by OP60 pretreatment (Figure 1F). These results suggested that OP60 protected the cells from oxidative stress induced by H2O2 and APAP mainly by maintaining the intracellular redox homeostasis.
Figure 1.
Protective effect of OP60 against cell damage caused by oxidative stress in HepG2 cells. (A) Cells were pretreated with 5 mg/mL OP60 for 24 h and then exposed to 200 μM H2O2 for 24 h. (B) Cells were pretreated with 5 mg/mL OP60 for 24 h and then exposed to 10 mM APAP for 24 h. The cytotoxicity was determined by measurement of LDH activity released from damaged cells into the medium. HepG2 cells were treated with 5 mg/mL OP60 for 24 h and then treated with or without 200 μM H2O2/10 mM APAP for 2 h and harvested for measurement of (C, E) total GSH and (D, F) GSH/GSSG ratio. Data shown represent the mean values of three experiments ± SEM. *p < 0.05, **p < 0.01, and ***p < 0.001 vs control group. Values with the same letter are not significantly different (p < 0.05) according to Tukey’s multiple test.
2.2. Effect of OP60 on Antioxidant Enzymes Expression
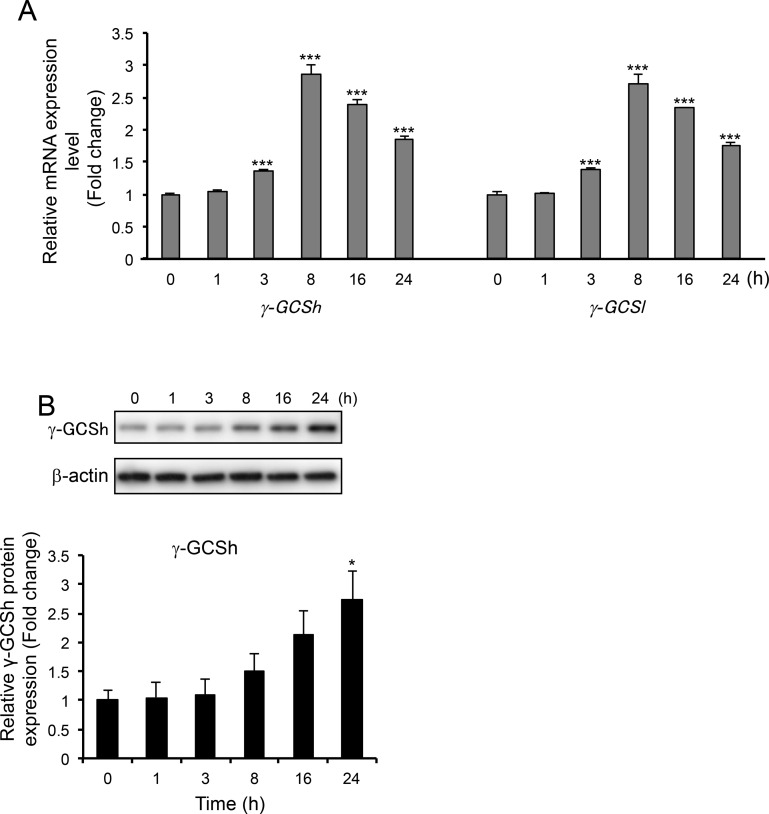
Since the protective effects of OP60 appeared to be related to its antioxidant potential, the mRNA levels of γ-GCSh and γ-GCSl genes, which encode heavy and light subunits of the rate-limiting enzyme in GSH synthesis, were determined. As expected, the expression of both genes began to increase at 3 h and reached maximum levels (2.9 ± 0.1-fold and 2.7 ± 0.2-fold, respectively) at 8 h after the addition of OP60 (Figure 2A). Moreover, following OP60 treatment, the protein level of γ-GCSh was found to be increased at 8 h with a significantly higher increase of 2.7 ± 0.5-fold at 24 h (Figure 2B).
Figure 2.
Effect of OP60 on γ-GCS expression. (A) Real-time PCR analyses of heavy (γ-GCSh) and light (γ-GCSl) subunits of γ-GCS. (B) Western blot analysis of γ-GCSh in HepG2 cells treated with 5 mg/mL OP60 for the indicated periods. Data shown represent the mean values of three experiments ± SEM. *p < 0.05 and ***p < 0.001 vs control group.
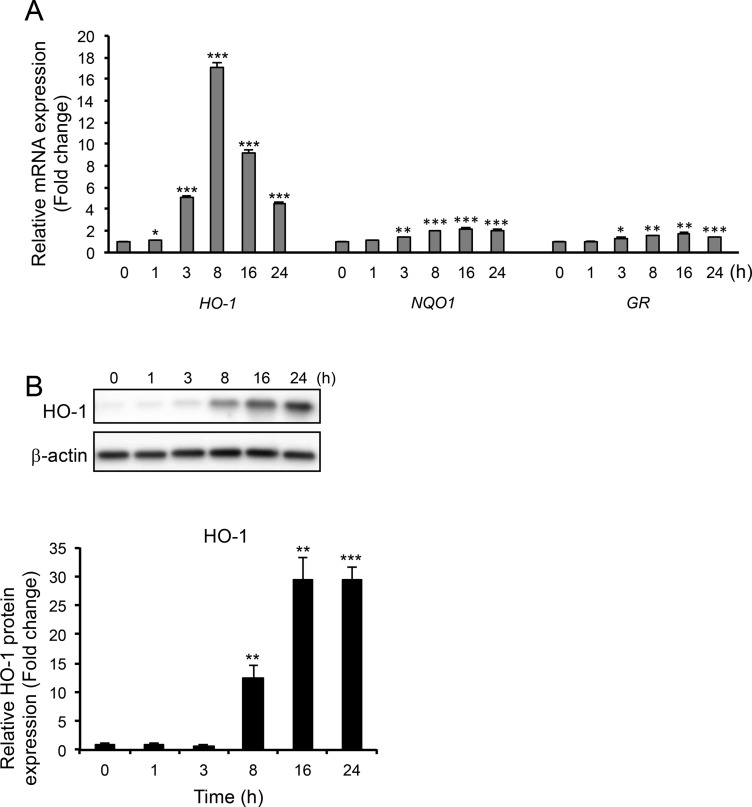
Expression of HO-1, NQO1, and GR genes, which are mainly regulated by Nrf2, was also checked. It was observed that, among the three genes, HO-1 was the most strongly induced. Its mRNA expression was induced at 1 h after treatment with OP60 and significantly increased by 17.0 ± 0.6-fold at 8 h, whereas NQO1 and GR mRNAs levels were induced at 3 h after OP60 exposure, and at 16 h, the expression of these genes increased by 2.2 ± 0.1-fold and 1.7 ± 0.1-fold, respectively (Figure 3A). Similarly, treatment with OP60 strongly induced HO-1 protein expression, which was significantly increased by 30-fold at 16 and 24 h (Figure 3B).
Figure 3.
Effect of OP60 on the expression of antioxidant enzymes. (A) Real-time PCR analyses of HO-1, NQO1, and GR. (B) Western blot analysis of HO-1 in HepG2 cells treated with 5 mg/mL OP60 for the indicated periods. Data shown represent the mean values of three experiments ± SEM. *p < 0.05, **p < 0.01, and ***p < 0.001 vs control group.
2.3. Effects of OP60 on Nrf2 Expression
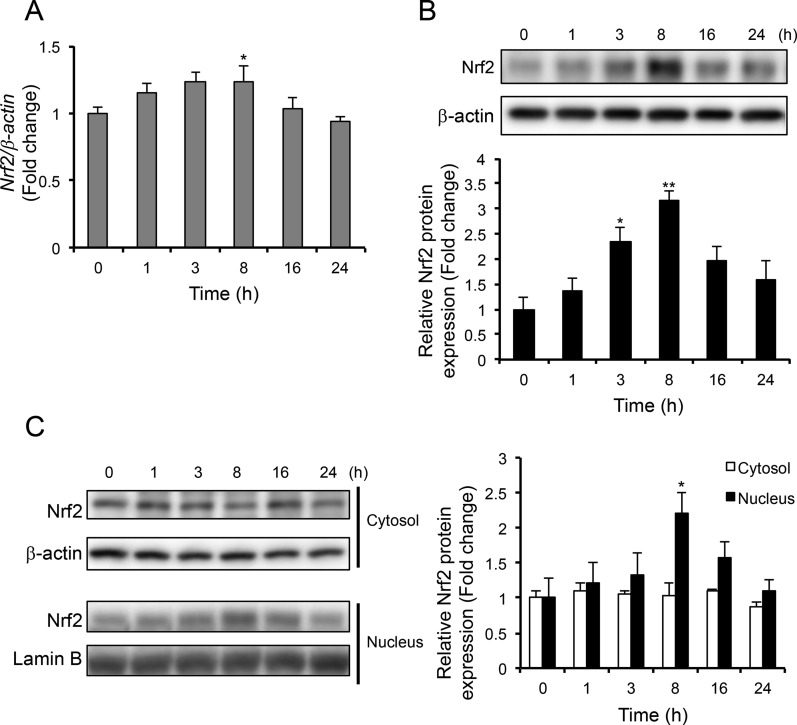
Since the mRNA and protein expression of antioxidant enzymes was induced by OP60, the expression levels of Nrf2, which is a crucial regulator for antioxidant enzymes, were determined. Nrf2 mRNA expression was observed to be induced at 1 h and increased significantly by 1.2 ± 0.1-fold at 8 h, which was followed by a gradual decrease to its basal levels (Figure 4A). In the total cell lysate, Nrf2 protein levels started to increase at 1 h and further increased by 3.2 ± 0.2-fold at 8 h (Figure 4B). Similarly, nuclear Nrf2 protein expression was induced at 1 h followed by its significant increase by 2.2 ± 0.3 fold at 8 h (Figure 4C). Conversely, the levels of Nrf2 in the cytosolic fraction remained unchanged. These observations indicated that OP60 treatment stabilized Nrf2 and induced its translocation into the nucleus.
Figure 4.
Effect of OP60 on Nrf2 expression. (A) Real-time PCR analysis of Nrf2 and its western blot analysis from (B) cell lysate and (C) the cytosolic or nuclear fraction of HepG2 cells treated with 5 mg/mL OP60 for the indicated periods. Data shown represent the mean values of three experiments ± SEM. *p < 0.05 and **p < 0.01 vs control group.
2.4. Role of Nrf2 in OP60-Mediated Increase in Intracellular GSH and Antioxidant Enzymes
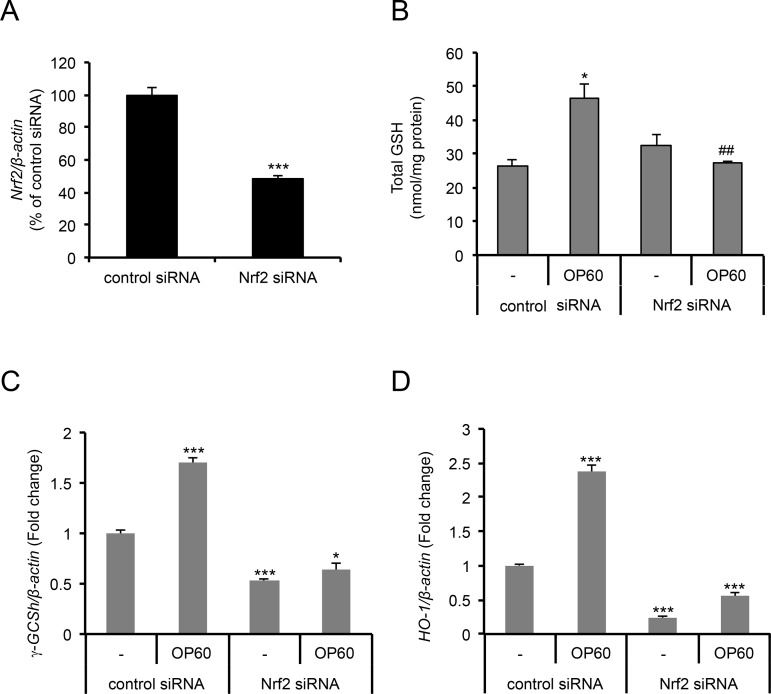
To determine whether the OP60-mediated increase in the levels of GSH and antioxidant enzymes occurs via Nrf2 activation, Nrf2 or control siRNAs were transiently transfected HepG2 cells. The Nrf2 mRNA level was effectively reduced to less than 50% in cells transfected with Nrf2 siRNA (Figure 5A). Knockdown of the Nrf2 gene significantly suppressed the levels of OP60-induced intracellular GSH (Figure 5B) and γ-GCSh and HO-1 mRNA expressions (Figure 5C,D). These results suggested that the induction of GSH synthesis and antioxidant enzymes occurs through a mechanism by which the Nrf2 pathway is consecutively activated.
Figure 5.
Effect of Nrf2 knockdown on the induction of intracellular GSH levels and antioxidant enzymes expression. Cells were transfected with control or Nrf2 siRNAs and incubated for 48 h. After further incubation in fresh medium with or without 5 mg/mL OP60 for 24 h, cells were harvested for real-time PCR in order to evaluate (A) Nrf2, (C) γ-GCSh, and (D) HO-1 expressions and (B) intracellular GSH levels. Data shown represent the mean values of three experiments ± SEM. **p < 0.01 and ***p < 0.001 vs control siRNA group treated with OP60, ##p < 0.01 vs Nrf2 siRNA group without OP60.
2.5. Effect of OP60 Treatment against APAP-Induced Liver Injury in Mice
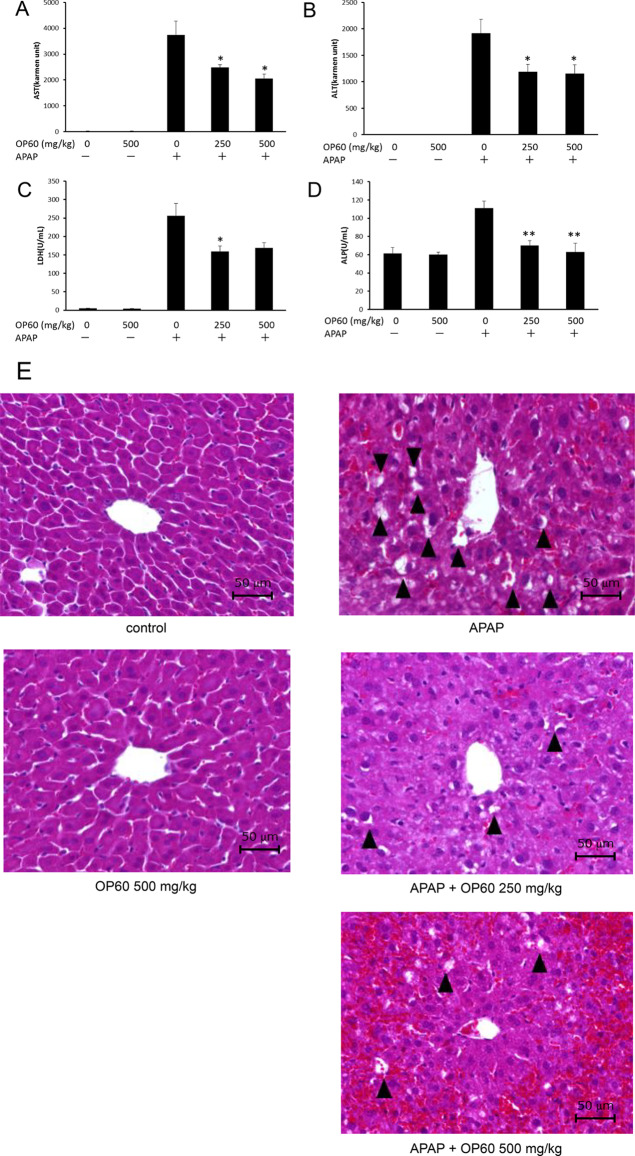
The cytoprotective effect of OP60 in HepG2 cells was further assessed in vivo using an APAP-induced liver injury model. No significant changes were observed in body weight between the groups during the 7 days of treatment (data not shown). APAP overdose significantly increased the serum levels of AST and ALT from 28.1 ± 0.8 and 4.4 ± 0.5 Karmen units to 3745.3 ± 535.1 and 1917.0 ± 262.9 Karmen units, respectively, which indicated liver injury. However, the administration of OP60 (500 mg/kg) effectively reduced the serum levels of these hepatic marker enzymes to 2058.6 ± 165.5 (for AST) and 1154.1 ± 166.5 (for ALT) Karmen units, respectively (Figure 6A,B). Additionally, APAP increased the LDH and ALP levels (256.2 ± 33.6 U/mL and 111.1 ± 7.8 U/mL, respectively) compared to those in the control group (4.8 ± 0.6 U/mL and 61.5 ± 6.4 U/mL, respectively). OP60 administration decreased the levels of LDH and ALP to 169.0 ± 14.5 U/mL and 63.0 ± 9.8 U/mL, respectively (Figure 6C,D). The levels of serum marker enzymes did not change in mice treated with OP60 alone.
Figure 6.
Protective effect of OP60 against APAP-induced liver injury in mice. The serum levels of (A) AST, (B) ALT, (C) LDH, and (D) ALP were determined in mice with APAP-induced liver injury. Data shown represent the mean values of six mice per group ± SEM. *p < 0.05 and **p < 0.01 vs APAP-treated group. (E) Representative images of H&E staining of liver sections from the control group, OP60-treated (500 mg/kg) group, APAP-treated group, and OP60 (250 and 500 mg/kg) plus APAP-treated groups (original magnification, 400×). Arrowhead: multiple and extensive areas of hepatocellular vacuolation.
Furthermore, histological examination of liver sections obtained from the mice in different groups indicated the protective effect of OP60 against APAP-induced liver injury (Figure 6E). The liver from the control and OP60-treated mice showed a typical lobular architecture and cell structure. However, APAP treatment induced multiple extensive areas of hepatocellular vacuolation, which were randomly distributed throughout the parenchyma, whereas OP60 pretreatment ameliorated the APAP-induced liver damage.
APAP overdose is also known to induce the depletion of GSH in the liver. Pretreatment with OP60 significantly recovered APAP-induced glutathione depletion 6 h after APAP administration (Table 1). These results indicated that OP60 effectively improved APAP-induced liver injury.
Table 1. Effect of OP60 on Hepatic GSH Levels in Mice with APAP-Induced Liver Injurya.
| groups | total GSH (nmol/mg protein) |
|---|---|
| control | 34.9 ± 0.8 |
| OP60 (500 mg/kg) | 39.0 ± 0.8 |
| APAP | 0.19 ± 0.02## |
| APAP + OP60 (250 mg/kg) | 0.52 ± 0.10**, ## |
| APAP + OP60 (500 mg/kg) | 0.90 ± 0.11**, ## |
Data shown represent the mean values of six experiments ±SEM. ##p < 0.01 vs control group. **p < 0.01 vs APAP-treated group.
2.6. Effect of OP60 Treatment on γ-GCS and HO-1 Protein Expression Levels in the Liver of APAP-Exposed Mice
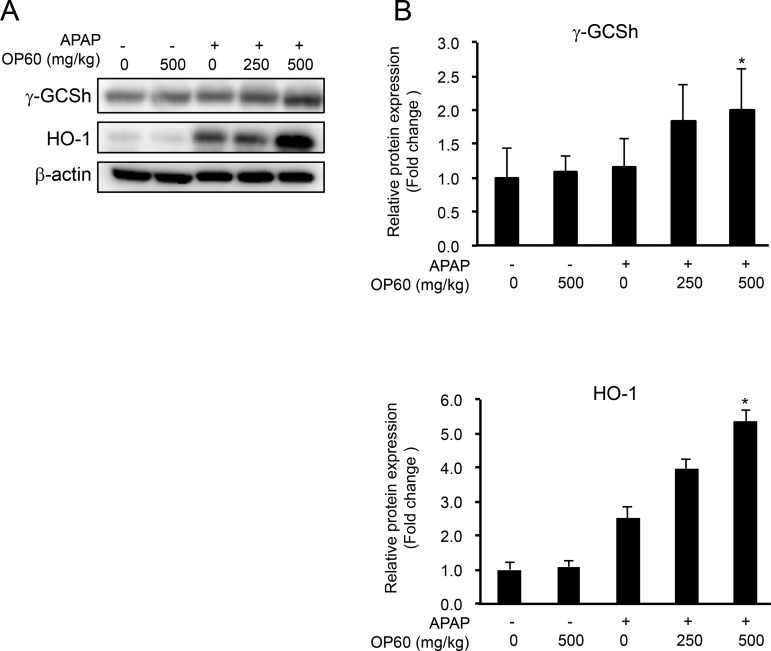
Since OP60 increased the intracellular GSH levels via activating the Nrf2 pathway in HepG2 cells, we predicted that the protective effect of OP60 against APAP-induced liver injury is associated with Nrf2. Thus, to test this prediction, the protein expression of γ-GCSh and HO-1 was determined by western blotting. It was observed that the protein expression of γ-GCSh was not affected by OP60 and APAP treatments separately, but an overdose of APAP after the administration of OP60 increased expression by 2.0 ± 0.6-fold. HO-1 protein expression was induced by APAP alone and further increased by 5.4 ± 0.3-fold by pretreatment with OP60. APAP treatment in addition to OP60 increased the expression levels of these proteins. These results suggested that OP60 exerted protective effects on APAP-induced liver injury by attenuating the depletion of GSH through the upregulation of γ-GCS expression and the induction of HO-1 expression, which is known to be a cytoprotective protein.
3. Discussion
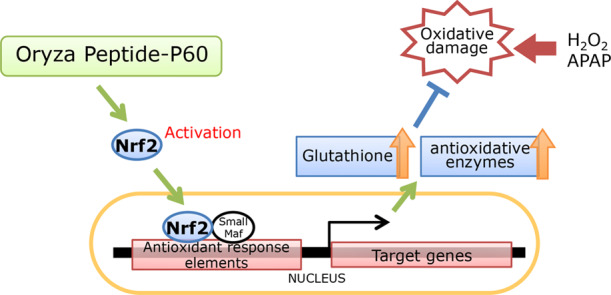
GSH is the most abundant non-protein thiol in cells and plays a vital role in the antioxidant defense mechanism. We previously demonstrated that OP60, a rice-derived peptide, increased intracellular GSH levels.22 To evaluate the effect of OP60 on cytotoxicity induced by oxidative stress, HepG2 cells were treated with H2O2 and APAP as oxidizing agents. Pretreatment of cells with OP60 increased the viability of HepG2 cells against oxidative stress-induced by H2O2 or APAP (Figure 1A,B). By determining GSH levels and its redox status, it was observed that oxidative stress-mediated reductions in GSH levels or GSH/GSSG ratio were restored by OP60 treatment, suggesting that OP60 pretreatment can attenuate oxidative stress (Figure 1C–F).
Next, the mechanism underlying the antioxidant action of OP60 was explored. OP60 was observed to induce the expression of γ-GCS, which is the rate-limiting enzyme in GSH biosynthesis. Variation in the mRNA expression pattern of genes encoding both γ-GCS subunits (γ-GCSh and γ-GCSl) was similar at different time points (Figure 2A). In addition to γ-GCS genes, the expression of genes encoding antioxidant enzymes, such as HO-1, NQO1, and GR, which are known to be positively regulated by Nrf2 were induced by OP60 (Figure 3A,B). Moreover, Nrf2 levels were slightly elevated in whole-cell lysates and nuclear extracts at 1 h after OP60 addition, reaching a maximum level at 8 h, which corresponded to the expression of antioxidant enzymes (Figure 4A–C). These results suggested that OP60 was able to stabilize the Nrf2 protein and induce its nuclear translocation in order to exert antioxidant effect by upregulating the expression of its target genes, including γ-GCS, HO-1, NQO1, and GR. The response of HO-1 mRNA expression was faster and stronger than the responses of the other genes of antioxidative enzymes. The HO-1 gene has been reported to directly regulate stress-responsive transcription factors, heat shock factor (HSF), nuclear factor−κB (NF-κB), and activator protein–1 (AP-1) in addition to Nrf2.25 The response of HO-1 mRNA expression may be related to regulation by other transcription factors.
In addition, knockdown of the Nrf2 gene suppressed the increase of the intracellular GSH levels (Figure 5B) and the expressions of γ -GCSh and HO-1 that were induced by OP60 treatment (Figure 5C,D). These results strongly suggested that OP60 upregulated GSH biosynthesis and increased the expression of antioxidant enzymes via activation of the Nrf2 pathway.
Although many compounds derived from natural products, such as polyphenols, isothiocyanates, and resveratrol, have been reported to be activators of the Nrf2 pathway,26,27 very few peptides have been shown to induce an Nrf2-mediated antioxidant response. Recently, bioactive peptides of fish skin gelatin hydrolysate (FSGHF3) were reported to be translocated into cells by Pept1, an oligopeptide transporter, and exert antioxidant effects through activation of the Nrf2 pathway.15 Regarding the mechanism, FSGHF3 increased Nrf2-mediated expression of antioxidant enzymes through p62, an upstream regulator of Nrf2.28 A tripeptide from Chinese Baijiu (Pro-His-Pro, PHP) was shown to protect the cells against oxidative stress via the Nrf2 signaling pathway and was considered to decrease the affinity of Nrf2 and Keap1.16 Although the interaction target might be different between FSGHF3 and PHP, it was indicated that the expression level of Keap1 protein is reduced when the expression of antioxidant enzymes including Nrf2 is induced by the peptide. In this study, OP60 was also demonstrated to exhibit an antioxidative effect via the Nrf2 signaling pathway. However, we still need to evaluate the changes of Keap1, a critical factor, and Nrf2 with respect to the oxidative response upon their exposure to OP60 and how the active peptides in OP60 leads to the activation of the Nrf2 pathway.
OP60 showed an antioxidative effect at a concentration of 2.5 or 5 mg/mL in HepG2 cells. Protein hydrolysates from rice bran,17 rice,18 eggshell membrane,20 chickpea,21 and FSGHF3,15 which are the mixtures of peptides, have been reported to increase the intracellular GSH levels and upregulate antioxidative enzymes in cells at a range from 0.1 to 5 mg/mL. For example, treatment with 5 mg/mL rice bran-hydrolysate for 24 h increased intracellular GSH levels in HepG2 cells by ca. 2-fold.17 A 12 h treatment with 2.5 or 5 mg/mL FSGHF3 increased mRNA levels of γ-GCSh by ca. 2.3-fold and intracellular GSH levels by 1.3-fold in IPEC-J2 cells.15 OP60 showed similar bioactivity to these protein hydrolysates. In our previous study, we identified a peptide (Pep3, YQQQFQQFLPEGQSQSQK) from OP60 that restores the activity of the serotonin N-acetyltransferase enzyme after it is inactivated by H2O2 treatment.22 In the same study, Pep3 was shown to increase the intracellular GSH levels in HepG2 cells at 10 μg/mL. Preliminary results showed that Pept3 induces mRNA expression of γ-GCSh but not that of HO-1 (data not shown), whereas OP60 itself markedly induced the HO-1 expression (Figure 3A,B). These observations suggest that OP60 contains other bioactive peptides. We are attempting to identify the active peptides by evaluating both the activation of NQO1 activity and an increase in intracellular GSH level in HepG2 cells. The activity was recovered in 40% acetonitrile elution fraction upon performing a C18 reversed-phase cartridge (data not shown). Thus, further study is needed to identify the sequences of active peptides of OP60, which can exert antioxidative activity at lower concentrations such as observed with Pep3, and to better understand the mechanism through which Nrf2 is activated in response to OP60 treatment.
The antioxidant and hepatoprotective effect of OP60 was also demonstrated in mice with APAP-induced hepatic injury. The increased serum levels of AST, ALT, LDH, and ALP induced by APAP overdose were reduced in mice administered OP60 (Figure 6A–D). hematoxylin and eosin (H&E) staining of liver sections showed that OP60 decreased hepatic vacuolation (Figure 6E). These in vivo results indicated the protective effect of OP60 against APAP-induced liver injury. Furthermore, the administration of OP60 to mice significantly inhibited the APAP-induced GSH depletion (Table 3) and induced γ-GCS and HO-1 expression compared to those in mice treated with APAP alone (Figure 7). HO-1 converts heme into carbon monoxide, free iron, and biliverdin, which is rapidly reduced to bilirubin, a potent antioxidant.29 Some natural products, such as sulforaphane and caffeic acid, have been shown to exert protective effects against APAP-induced liver injury based on their ability to induce antioxidant responses mediated by the upregulation of the expression of Nrf2-target genes such as HO-1 in addition to the inhibition of GSH depletion.30,31 Our results suggested that OP60 attenuates liver injury induced by APAP through activation of the Nrf2-mediated antioxidant response. Indeed, there is a report on the high sensitivity of Nrf2-knockout mice to APAP-induced hepatotoxicity.32
Table 3. Primer Sequences Used for Real-Time PCR.
| gene | forward primer | reverse primer |
|---|---|---|
| γ-GCSh | TGCTGTCTCCAGGTGACATTC | CCCAGCGACAATCAATGTCT |
| γ-GCSl | TCCAGTTCCTGCACATCTACCA | TCATCGCCCCACTTGAGAA |
| HO-1 | GCAACCCGACAGCATGC | TGCGGTGCAGCTCTTCTG |
| NQO1 | CATGAATGTCATTCTCTGGCCA | CTGGAGTGTGCCCAATGCTA |
| GR | ATGATCAGCACCAACTGCAC | CGACAAAGTCTTTTTAACCTCCTT |
| Nrf2 | TGCTTTATAGCGTGCAAACCTCGC | ATCCATGTCCCTTGACAGCACAGA |
| β-actin | CCTGGCACCCAGCACAAT | GCCGATCCACACGGAGTACT |
Figure 7.
Effect of OP60 on the expression levels of γ-GCS and HO-1 in the liver of mice. (A) Western blot analysis of the protein expression of γ-GCSh and HO-1 after 6 h of APAP treatment. Lane 1: Control; Lane 2: OP60 (500 mg/kg); Lane 3: APAP; Lane 4: APAP + OP60 (250 mg/kg); and Lane 5: APAP + OP60 (500 mg/kg). (B) Densitometric analysis of western blots. Data shown represent the mean values of six mice per group ± SEM. *p < 0.05 and **p < 0.01 vs APAP-treated group.
It is widely known that an overdose of APAP induces severe hepatotoxicity by the formation of large amounts of NAPQI, which can bind to cellular proteins and induce GSH depletion.23,24 In the current study, GSH levels in the mouse liver were depleted by an overdose of APAP (Table 3), but even cytotoxic doses of APAP did not alter the intracellular GSH levels in HepG2 cells (Figure 1E). Recently, Behrends et al. reported that intracellular GSH was not depleted in HepG2 cells treated with 5 mM APAP for 24 h, whereas cell death was induced.33 They suggested that any redox stress subsequent to APAP exposure might be due to the reduced production of NADPH rather than the consumption of reducing equivalents by reactive species. It was reported that the NADPH production pathway is also activated by Nrf2.34 Restoring NADPH levels by OP60 might contribute to the attenuation of cytotoxicity in HepG2 cells. Unexpectedly, treatment with APAP for 2 h in addition to OP60 for 24 h increased the intracellular GSH level higher than that of only OP60 treatment (Figure 1E). It was reported that NAPQI could activate the Nrf2 signaling pathway via the direct modification of cysteine residues in Keap1.35 Activation of the Nrf2 signaling pathway by NAPQI might contribute to the increase in intracellular GSH level in addition to activation by OP60.
4. Conclusions
In summary, OP60 induced GSH synthesis and antioxidant enzymes through activation of the Nrf2 pathway, leading to the suppression of oxidative stress-induced hepatotoxicity in vitro and in vivo. This study suggested that OP60 contained crucial peptides with substantial antioxidant activities. Thus, it could be potentially served as a functional food for the prevention of diseases associated with oxidative stress.
5. Materials and Methods
5.1. Chemicals
The commercial rice peptide OP60 was a gift from Oryza Oil and Fat Chemical Co., Ltd. (Aichi, Japan). OP60 is a water-soluble rice peptide produced by the enzymatic decomposition of rice protein. The peptide content in OP60 is 68.5%, along with 26.7% carbohydrate, 1.0% fiber, 0.1% fat, and 4.6% ash. The tripeptide content of OP60 was estimated to be approximately 50%. Amino acid composition, which was determined based on ion-exchange chromatography followed by post-column derivatization with ninhydrin, is shown Table 2.
Table 2. Amino Acid Composition of OP60.
| amino acid | amino acid content (g/100 g) |
|---|---|
| arginine | 6.73 |
| lysine | 2.14 |
| histidine | 1.81 |
| phenylalanine | 3.09 |
| tyrosine | 2.49 |
| leucine | 4.45 |
| isoleucine | 2.16 |
| methionine | 0.88 |
| valine | 3.33 |
| alanine | 3.31 |
| glycine | 2.77 |
| proline | 2.79 |
| glutamic acid | 11.20 |
| serine | 3.11 |
| threonine | 2.08 |
| aspartic acid | 5.85 |
| tryptophan | 0.39 |
| cystine | 0.79 |
Acetaminophen was purchased from Sigma (St. Louis, MO, USA). 5,5′-Dithiobis-(2-nitrobenzoic acid) (DTNB) was procured from Fujifilm Wako Pure Chemical (Osaka, Japan). NADPH and yeast GR were purchased from Oriental Yeast Co., Ltd. (Tokyo, Japan). 7-Benzo-2-oxa-1,3-diazole-4-sulfonic acid (SBD-F) and 4-(aminosulfonyl)-7-fluoro-2,1,3-benzoxadiazole (ABD-F) were obtained from Dojindo Labs (Kumamoto, Japan). Primary antibodies against γ-GCSh, Nrf2, and Lamin B2 were purchased from Santa Cruz Biotechnology (Dallas, TX, USA). Primary antibodies against HO-1 and β-actin were obtained from Enzo Life Sciences (Farmingdale, NY) and Sigma (St. Louis, MO, USA), respectively. The protein assay kit was obtained from Bio-Rad Laboratories Inc. (Hercules, CA, USA).
5.2. Cell Culture
HepG2 cells were purchased from the RIKEN Cell Bank (Tsukuba, Japan) and were maintained in Eagle’s minimum essential medium (MEM) supplemented with 10% fetal bovine serum (FBS), 100 μg/mL streptomycin, 100 μg/mL penicillin, and 0.56 μg/mL amphotericin B in a humidified atmosphere containing 5% CO2 at 37 °C. Cells were seeded into 6-well plates (Thermo Fisher Scientific, Waltham, MA, USA) at a density of 1.5 × 105 cells/well and were treated with 5 mg/mL OP60 for the indicated periods. After incubation, cells were harvested for western blot analysis and total RNA preparation.
5.3. Lactate Dehydrogenase (LDH) Cytotoxicity Assay
The activity of LDH released from damaged cells into the medium was measured using a Cytotoxic Detection Kit (Roche Applied Science, Mannheim, Germany). HepG2 cells were seeded in 96-well microplates at a density of 1.5 × 104 cells/well and were treated with 2.5 or 5 mg/mL OP60 for 24 h. The medium was then replaced with FBS-free medium containing 200 μM H2O2 or 10 mM APAP followed by incubation for 24 h. The culture medium was then collected and used for the measurement of released LDH activity (LDHsample). The maximum amount of releasable LDH enzyme activity in the culture (LDHhigh control) was determined by lysing cells in 1% Triton X-100, whereas the LDH activity from the medium of untreated cells was defined as LDHlow control. After subtracting background absorbance from all other values, the cytotoxicity was calculated as follows:
5.4. Real-Time PCR Analysis
Total RNA from HepG2 cells was extracted using an RNeasy Plus Mini Kit (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions. One microgram of total RNA was then reverse-transcribed into cDNA using a PrimeScript RT-PCR kit (Takara, Tokyo, Japan). Real-time PCR was performed using SYBR Premix EX Taq (Takara) on a QuantStudio 3 thermal cycler (Thermo Fisher Scientific, Waltham, MA, USA). The cDNA levels of tested genes were normalized to the housekeeping gene β-actin mRNA expression. The sequences of the primers are given in Table 3.
5.5. Western Blotting Analysis
For the preparation of protein extracts, HepG2 cells or liver tissues were lysed using the RIPA Lysis Buffer System (Santa Cruz Biotechnology) for 30 min on ice. Lysates were then centrifuged (13,000g, 15 min, 4 °C), and supernatants were collected as cell lysates. Nuclear extracts from HepG2 cells were prepared as described previously.36 Briefly, harvested cells were suspended in extraction buffer containing 10 mM HEPES, pH 7.5, 150 mM NaCl, 0.6% Nonidet P-40, 1 mM EDTA, and 5 mM DTT supplemented with a proteinase-inhibitor cocktail (Roche Applied Science). After a 20 min incubation on ice, nuclei were pelleted by centrifugation at 13,000g for 15 min at 4 °C. The pellets were extracted with nuclear extraction buffer containing 10 mM HEPES, pH 7.9, 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, and 5 mM DTT with a proteinase-inhibitor cocktail. The extracts were then centrifuged (13,000g, 15 min, 4 °C), and supernatants were collected as nuclear extracts. The protein concentration of each extract was determined by performing a Bio-Rad protein assay. Extracted proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto a polyvinylidene difluoride (PVDF) membrane (Bio-Rad) electrophoretically. The membranes were then blocked with PVDF Blocking Reagent (Toyobo, Osaka, Japan) and probed with primary antibodies against γ-GCSh, Nrf2, HO-1, Lamin B2, or β-actin followed by incubation with horseradish peroxidase-linked secondary antibodies. The immunoreactive bands on the membranes were visualized using Amersham ECL Prime Western Blotting Detection Reagent (GE Healthcare, Chicago, IL, USA) and detected with an Amersham Imager 600 (GE Healthcare). Band intensities were then quantified using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
5.6. Measurement of Intracellular GSH Levels
After treatment with or without OP60, cells were rinsed twice with phosphate-buffered saline (PBS), harvested, homogenized in 0.1 M HCl containing 1 mM BAPS, and deproteinized with sodium perchlorate. The concentrations of reduced and oxidized glutathione (GSH and GSSG, respectively) were determined simultaneously by HPLC with fluorescence detection followed by labeling with ABD-F and SBD-F, respectively.37 Total GSH was measured via an enzymatic recycling method using GR and DTNB.38
5.7. Transfection with siRNA
Silencer Select Pre-designed siRNA for Nrf2 and negative control siRNA were obtained from Ambion (Life Technologies, Carlsbad, CA, USA). The target sequences for the Nrf2 siRNAs were 5′-GAAUGGUCCUAAAACACCATT-3′ (sense) and 5′-UGGUGUUUUAGGACCAUUCTG-3′ (antisense). Reverse transfections of siRNAs into HepG2 cells were performed using Lipofectamine RNAiMAX (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. In brief, HepG2 cells were maintained in antibiotic-free medium. Each siRNA was diluted with Opti-MEM I Reduced Serum Medium (Gibco, Carlsbad, CA, USA) and added along with Lipofectamine RNAiMAX to a single well of a 6-well plate. After 20 min of incubation at room temperature, a suspension of 2.5 × 105 HepG2 cells was added to obtain a 2 nM final siRNA concentration. After 48 h of incubation, the medium was replaced with fresh medium with or without OP60, and cells were further incubated for 24 h. Cells were then harvested for the measurement of GSH and total RNA preparation. The efficiency of siRNA knockdown of Nrf2 was determined by real-time PCR.
5.8. Animals
Four-week-old male ICR mice were purchased from Japan SLC (Shizuoka, Japan). All the animals were fed a standard diet and distilled water ad libitum and housed at 24 ± 1 °C under a 12 h light/dark cycle for 1 week before the experiment. All protocols for animal experimentation were approved by the Animal Experimentation Committee of Shujitsu University (no. 028-002), and the study was conducted according to the Animal Experimentation Guidelines of Shujitsu University. The procedures were performed as described in our previous publications.18,19 Mice were randomly divided into five groups. The rice peptide groups were orally administered OP60 (250 or 500 mg/kg body weight) via a stomach tube daily for 7 days, whereas the control and APAP groups were administrated saline. All mice were then fasted for 18 h before the intraperitoneal injection of APAP (700 mg/kg) and sacrificed 6 h after APAP administration. Blood samples were collected, and the serum was separated by centrifugation (750g for 10 min at 4 °C) for biochemical assays, whereas liver samples were extracted for western blot and histopathological analyses.
5.9. Serum Biochemical Analysis
Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels in the serum were measured using a Transaminase CII Test Wako kit (Wako Pure Chemical Industries Ltd., Osaka, Japan), and the results are expressed in Karmen units. LDH and alkaline phosphatase (ALP) were measured using a Cytotoxicity Detection KitPLUS (Roche Applied Science) and LabAssay ALP (Wako Pure Chemical Industries Ltd.), respectively.
5.10. Histopathological Examination
Liver tissues were fixed in 10% phosphate-buffered neutral formalin, dehydrated through a graded series of alcohol (50–100%), and embedded in paraffin. Thin sections (6 μm) were cut and stained with H&E stain for pathological assessment via photomicroscopy (BZ-9000; Keyence Corporation, Osaka, Japan).
5.11. Statistical Analysis
All data are expressed as the mean ± standard error of the mean (SEM) of at least three independent experiments. Data were analyzed by one-way analysis of variance (ANOVA) followed by a Student’s t-test or Dunnett’s test to determine the significance between the groups. Data in Figure 1C–F were analyzed by two-way ANOVA followed by Tukey’s multiple test to check the statistical significance between different groups. p values less than 0.05 were considered statistically significant.
Acknowledgments
This project was supported by the Iijima Memorial Foundation for the Promotion of Food Science and Technology (project no. 2018–35).
The authors declare no competing financial interest.
References
- Trachootham D.; Alexandre J.; Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach?. Nat. Rev. Drug Discovery 2009, 8, 579–591. 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- Evans J. L.; Goldfine I. D.; Maddux B. A.; Grodsky G. M. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr. Rev. 2002, 23, 599–622. 10.1210/er.2001-0039. [DOI] [PubMed] [Google Scholar]
- Lefer D. J.; Granger D. N. Oxidative stress and cardiac disease. Am. J. Med. 2000, 109, 315–323. 10.1016/S0002-9343(00)00467-8. [DOI] [PubMed] [Google Scholar]
- Shukla V.; Mishra S. K.; Pant H. C. Oxidative stress in neurodegeneration. Adv. Pharmacol. Sci. 2011, 2011, 572634. 10.1155/2011/572634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birben E.; Sahiner U. M.; Sackesen C.; Erzurum S.; Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S. C. Regulation of hepatic glutathione synthesis: Current concepts and controversies. FASEB J. 1999, 13, 1169–1183. 10.1096/fasebj.13.10.1169. [DOI] [PubMed] [Google Scholar]
- Griffith O. W.; Mulcahy R. T. The enzymes of glutathione synthesis: γ-glutamylcysteine synthetase. Adv. Enzymol. Relat. Areas Mol. Biol. 1999, 73, 209–267. 10.1002/9780470123195.ch7. [DOI] [PubMed] [Google Scholar]
- Wild A. C.; Moinova H. R.; Mulcahy R. T. Regulation of γ-glutamylcysteine synthetase subunit gene expression by the transcription factor Nrf2. J. Biol. Chem. 1999, 274, 33627–33636. 10.1074/jbc.274.47.33627. [DOI] [PubMed] [Google Scholar]
- Itoh K.; Tong K. I.; Yamamoto M. Molecular mechanism activating Nrf2-Keap1 pathway in regulation of adaptive response to electrophiles. Free Radical Biol. Med. 2004, 36, 1208–1213. 10.1016/j.freeradbiomed.2004.02.075. [DOI] [PubMed] [Google Scholar]
- Kobayashi M.; Yamamoto M. Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv. Enzyme Regul. 2006, 46, 113–140. 10.1016/j.advenzreg.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Saadi S.; Saari N.; Anwar F.; Abdul Hamid A.; Ghazali H. M. Recent advances in food biopeptides: production, biological functionalities and therapeutic applications. Biotechnol. Adv. 2015, 33, 80–116. 10.1016/j.biotechadv.2014.12.003. [DOI] [PubMed] [Google Scholar]
- Walther B.; Sieber R. Bioactive proteins and peptides in foods. Int. J. Vitam. Nutr. Res. 2011, 81, 181–192. 10.1024/0300-9831/a000054. [DOI] [PubMed] [Google Scholar]
- Samaranayaka A. G. P.; Li-Chan E. C. Y. Food-derived peptidic antioxidants: A review of their production, assessment, and potential applications. J. Funct. Foods 2011, 3, 229–254. 10.1016/j.jff.2011.05.006. [DOI] [Google Scholar]
- Dinkova-Kostova A. T.; Talalay P. Direct and indirect antioxidant properties of inducers of cytoprotective proteins. Mol. Nutr. Food Res. 2008, 52, S128–S138. 10.1002/mnfr.200700195. [DOI] [PubMed] [Google Scholar]
- Zheng L.; Wei H.; Yu H.; Xing Q.; Zou Y.; Zhou Y.; Peng J. Fish skin gelatin hydrolysate production by ginger powder induces glutathione synthesis to prevent hydrogen peroxide induced intestinal oxidative stress via the Pept1-p62-Nrf2 cascade. J. Agric. Food Chem. 2018, 66, 11601–11611. 10.1021/acs.jafc.8b02840. [DOI] [PubMed] [Google Scholar]
- Wu J.; Sun B.; Luo X.; Zhao M.; Zheng F.; Sun J.; Li H.; Sun X.; Huang M. Cytoprotective effects of a tripeptide from Chinese Baijiu against AAPH-induced oxidative stress in HepG2 cells via Nrf2 signaling. RSC Adv. 2018, 8, 10898–10906. 10.1039/C8RA01162A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritani C.; Kawakami K.; Fujita A.; Kawakami K.; Hatanaka T.; Tsuboi S. Anti-oxidative activity of hydrolysate from rice bran protein in HepG2 cells. Biol. Pharm. Bull. 2017, 40, 984–991. 10.1248/bpb.b16-00971. [DOI] [PubMed] [Google Scholar]
- Kawakami K.; Moritani C.; Uraji M.; Fujita A.; Kawakami K.; Hatanaka T.; Suzaki E.; Tsuboi S. Hepatoprotective effects of rice-derived peptides against acetaminophen-induced damage in mice. J. Clin. Biochem. Nutr. 2017, 60, 115–120. 10.3164/jcbn.16-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami K.; Moritani C.; Uraji M.; Fujita A.; Kawakami K.; Hatanaka T.; Suzaki E.; Tsuboi S. Sake lees hydrolysate protects against acetaminophen-induced hepatotoxicity via activation of the Nrf2 antioxidant pathway. J. Clin. Biochem. Nutr. 2017, 61, 203–209. 10.3164/jcbn.17-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y.; Kovacs-Nolan J.; Jiang B.; Tsao R.; Mine Y. Peptides derived from eggshell membrane improve antioxidant enzyme activity and glutathione synthesis against oxidative damage in Caco-2 cells. J. Funct. Foods 2014, 11, 571–580. 10.1016/j.jff.2014.08.017. [DOI] [Google Scholar]
- Guo Y.; Zhang T.; Jiang B.; Miao M.; Mu W. The effects of an antioxidative pentapeptide derived from chickpea protein hydrolysates on oxidative stress in Caco-2 and HT-29 cell lines. J. Funct. Foods 2014, 7, 719–726. 10.1016/j.jff.2013.12.013. [DOI] [Google Scholar]
- Moritani C.; Kawakami K.; Fujita A.; Kawakami K.; Shimoda H.; Hatanaka T.; Tsuboi S. Isolation of activating factors of serotonin N-acetyltransferase from rice peptides. J. Funct. Foods 2018, 41, 148–154. 10.1016/j.jff.2017.12.051. [DOI] [Google Scholar]
- Laskin D. L.; Pilaro A. M. Potential role of activated macrophages in acetaminophen hepatotoxicity. I. Isolation and characterization of activated macrophages from rat liver. Toxicol. Appl. Pharmacol. 1986, 86, 204–215. 10.1016/0041-008x(86)90051-7. [DOI] [PubMed] [Google Scholar]
- Hazai E.; Vereczkey L.; Monostory K. Reduction of toxic metabolite formation of acetaminophen. Biochem. Biophys. Res. Commun. 2002, 291, 1089–1094. 10.1006/bbrc.2002.6541. [DOI] [PubMed] [Google Scholar]
- Alam J.; Cook J. L. How many transcription factors does it take to turn on the heme oxygenase-1 gene?. Am. J. Respir. Cell. Mol. Biol. 2007, 36, 166–174. 10.1165/rcmb.2006-0340TR. [DOI] [PubMed] [Google Scholar]
- Kumar H.; Kim I.-S.; More S. V.; Kim B.-W.; Choi D.-K. Natural product-derived pharmacological modulators of Nrf2/ARE pathway for chronic diseases. Nat. Prod. Rep. 2014, 31, 109–139. 10.1039/C3NP70065H. [DOI] [PubMed] [Google Scholar]
- Kode A.; Rajendrasozhan S.; Caito S.; Yang S.-R.; Megson I. L.; Rahman I. Resveratrol induces glutathione synthesis by activation of Nrf2 and protects against cigarette smoke-mediated oxidative stress in human lung epithelial cells. Am. J. Physiol.: Lung Cell. Mol. Physiol. 2008, 294, L478–L488. 10.1152/ajplung.00361.2007. [DOI] [PubMed] [Google Scholar]
- Katsuragi Y.; Ichimura Y.; Komatsu M. Regulation of the Keap1–Nrf2 pathway by p62/SQSTM1. Curr. Opin. Toxicol. 2016, 1, 54–61. 10.1016/j.cotox.2016.09.005. [DOI] [Google Scholar]
- Ryter S. W.; Otterbein L. E.; Morse D.; Choi A. M. K. Heme oxygenase/carbon monoxide signaling pathways, regulation and functional significance. Mol. Cell. Biochem. 2002, 234/235, 249–263. 10.1023/A:1015957026924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh J.-R.; Kim Y.-H.; Hwang J. H.; Choi D.-H.; Kim K.-S.; Oh W.-K.; Lee C.-H. Sulforaphane protects against acetaminophen-induced hepatotoxicity. Food Chem. Toxicol. 2015, 80, 193–200. 10.1016/j.fct.2015.03.020. [DOI] [PubMed] [Google Scholar]
- Wang L.; Zhang S.; Cheng H.; Lv H.; Cheng G.; Ci X. Nrf2-mediated liver protection by esculentoside A against acetaminophen toxicity through the AMPK/Akt/GSK3β pathway. Free Radical Biol. Med. 2016, 101, 401–412. 10.1016/j.freeradbiomed.2016.11.009. [DOI] [PubMed] [Google Scholar]
- Enomoto A.; Itoh K.; Nagayoshi E.; Haruta J.; Kimura T.; O’Connor T.; Harada T.; Yamamoto M. High sensitivity of Nrf2 knockout mice to acetaminophen hepatotoxicity associated with decreased expression of ARE-regulated drug metabolizing enzymes and antioxidant genes. Toxicol. Sci. 2001, 59, 169–177. 10.1093/toxsci/59.1.169. [DOI] [PubMed] [Google Scholar]
- Behrends V.; Giskeo̷degård G. F.; Bravo-Santano N.; Letek M.; Keun H. C. Acetaminophen cytotoxicity in HepG2 cells is associated with a decoupling of glycolysis from the TCA cycle, loss of NADPH production, and suppression of anabolism. Arch. Toxicol. 2019, 93, 341–353. 10.1007/s00204-018-2371-0. [DOI] [PubMed] [Google Scholar]
- Mitsuishi Y.; Taguchi K.; Kawatani Y.; Shibata T.; Nukiwa T.; Aburatani H.; Yamamoto M.; Motohashi H. Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer Cell 2012, 22, 66–79. 10.1016/j.ccr.2012.05.016. [DOI] [PubMed] [Google Scholar]
- Copple I. M.; Goldring C. E.; Jenkins R. E.; Chia A. J. L.; Randle L. E.; Hayes J. D.; Kitteringham N. R.; Park B. K. The hepatotoxic metabolite of acetaminophen directly activates the Keap1-Nrf2 cell defense system. Hepatology 2008, 48, 1292–1301. 10.1002/hep.22472. [DOI] [PubMed] [Google Scholar]
- Ramyaa P.; Krishnaswamy R.; Padma V. V. Quercetin modulates OTA-induced oxidative stress and redox signalling in HepG2 cells - up regulation of Nrf2 expression and down regulation of NF-κB and COX-2. Biochim. Biophys. Acta, Gen. Subj. 2014, 1840, 681–692. 10.1016/j.bbagen.2013.10.024. [DOI] [PubMed] [Google Scholar]
- Toyo’oka T.; Uchiyama S.; Saito Y.; Imai K. Simultaneous determination of thiols and disulfides by high-performance liquid chromatography with fluorescence detection. Anal. Chim. Acta 1988, 205, 29–41. 10.1016/S0003-2670(00)82313-2. [DOI] [Google Scholar]
- Matsumoto S.; Teshigawara M.; Tsuboi S.; Ohmori S. Determination of glutathione and glutathione disulfide in biological samples using acrylonitrile as a thiol-blocking reagent. Anal. Sci. 1996, 12, 91–95. 10.2116/analsci.12.91. [DOI] [Google Scholar]