Abstract
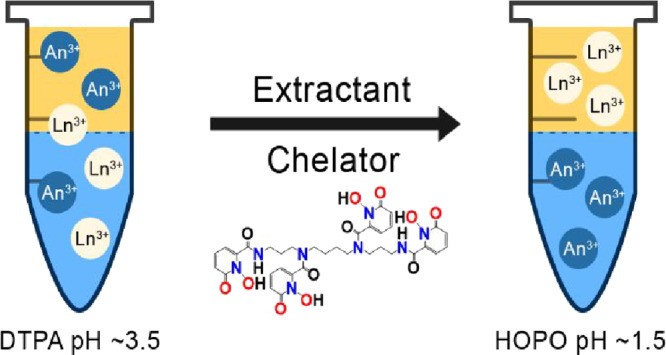
Separation of lanthanides (Ln) from actinides (An) is unanimously challenging in reprocessing used nuclear fuel despite of much dedicated efforts over the past several decades. The TALSPEAK process is the current reference method in the United States for Ln3+/An3+ separation but suffers from several limitations, such as a narrow working pH window (3.5–4.0), costly pH buffers, and slow extraction kinetics. Studies aiming at improving TALSPEAK have so far focused on polyaminocarboxylates hold-back reagents. Here, a new class of water-soluble ligands comprising hydroxypyridinone metal-binding units are evaluated for Ln3+/An3+ separation. The model octadentate chelator 3,4,3-LI(1,2-HOPO) (abbreviated as HOPO) was used in combination with several industry-relevant organic extractants to separate Gd from four transplutonium elements (Am, Cm, Bk, and Cf). Cyanex 301 GN and HDEHP worked best in combination with HOPO, whereas HEH[EHP], Cyanex 572, and ACORGA M5640 did not yield practical Ln3+/An3+ separation. Separation factors between Gd3+ and Am3+ reach about 50 with the HOPO/Cyanex 301 GN system and 30 with the HOPO/HDEHP system. The results using HDEHP (SFGd/Am = 30, SFGd/Cm = 8.5, and SFGd/Cf = 773) are high enough for industrial applications, and the proposed system works at pH values as low as 1.5, which simplifies the process by eliminating the need for pH buffers. In contrast to previously proposed methods, the HOPO-based process is also highly selective at separating Bk from Ln3+ (SFGd/Bk = 273) owing to in situ, spontaneous oxidation of Bk(III) to Bk(IV) by HOPO. The optimal pH in the case of HOPO/Cyanex 301 GN is 3.6 (SFAm/Gd = 50, SFCm/Gd = 23, SFBk/Gd = 1.4, and SFCf/Gd = 3.2), but this system has the advantage of extracting An ions into the organic phase while keeping Ln ions in the aqueous phase, which is opposite to the conventional TALSPEAK process. This study represents the first optimization of a TALSPEAK-like Ln/An separation method using a HOPO chelator and paves the avenue for further developments of analytical science and reprocessing of used nuclear fuel.
Introduction
High-level liquid waste (HLLW), such as PUREX (plutonium uranium redox extraction) raffinate, generates much heat and remains highly radiotoxic for thousands of years mainly due to the presence of long-lived fission products (some of which are lanthanides, Ln) and minor actinides (minor An, mainly consisting of Np, Am, and Cm).1 On the one hand, partitioning and transmutation of minor An can effectively reduce the heat load,2 long-term radiotoxicity, and consequently the vitrified volume for HLLW repositories. On the other hand, some Ln isotopes have higher neutron capture cross sections than minor An and are problematic for transmutation and closing the nuclear fuel cycle. Therefore, among many different groups of radionuclides existing in used nuclear fuel, separation of Ln from minor An is of particular interest. The separation of Am3+ and Cm3+ from Ln3+ is very challenging because they typically exhibit same oxidation state, comparable charge densities, and similar hydrated ionic radii and, as a consequence, very similar solution chemistry behaviors.3,4 Discrimination primarily relies on Ln3+ ions being slightly harder than An3+ ions. One approach for separating Am3+ and Cm3+ from Ln3+ is using water-soluble soft donor chelators to preferentially hold back Am3+ and Cm3+ in the aqueous phase, while Ln3+ ions are selectively extracted into the organic phase by hard donor extractants. A different approach consists of using soft donor extractants to selectively extract Am3+ and Cm3+ while leaving Ln3+ in the aqueous phase.
Many separation processes have been studied based on the first approach. One of the most studied processes in the United States is the so-called trivalent actinide lanthanide separation with phosphorus-reagent extraction from aqueous komplexes (TALSPEAK) process.4 In its standard version, it adopts di-(2-ethylhexyl)phosphoric acid (HDEHP) as the extractant, 1,4-di-isopropylbenzene or n-dodecane as the diluent, lactic acid/lactate as the pH buffer, and diethylenetriaminepentaacetic acid (DTPA) as the An3+ hold-back reagent. The working pH range lies between 3.0 and 4.5.4 It suffers from two major flaws, slow phase-transfer kinetics and high pH dependence due to the pH sensitivity of DTPA and lactate species. Other limitations also include the expensive lactate buffer and lack of compatibility with the acidic feed solutions requiring a pH-adjustment step prior to the actual separation. Below pH 3, DTPA does not bind trivalent metal ions M3+, and above pH 4, the extraction fraction decreases for all M3+ ions as the apparent chelating strength of DTPA becomes stronger and the extraction of lactate by HDEHP starts competing with the extraction of the metal ions. Many studies have been dedicated to the modification of TALSPEAK, especially in improving the separation of Eu3+ and Gd3+ from Am3+ and Cm3+. For instance, extensive work by Shafer et al. proposed the replacement of HDEHP by 2-ethylhexylphosphonic acid mono-2-ethylhexyl ester (HEH[EHP]), and as a consequence, DTPA had to be replaced by the lower strength chelator, ethylenediaminetetraacetic acid (HEDTA). The HEH[EHP]/HEDTA combination limits undesirable extraction of water and lactate and exhibits faster phase-transfer kinetics.3 The separation factors (SF) of Eu3+ versus Am3+ (SFEu/Am) and Gd3+ versus Am3+ (SFGd/Am) increased from 30–60 in the conventional TALSPEAK process to 50–100 using the HEH[EHP]/HEDTA combination at pH 3.6.3
Combinations of extractants have also been investigated. For example, in the minor actinide lanthanide separation process (ALSEP), a synergistic mixture of HEH[EHP] and N,N,N′,N′-tetraoctyl diglycolamide (TODGA) or N,N,N′,N′-tetra-2-ethylhexyl diglycolamide (T2EHDGA) has been proposed by Lumetta et al., and the SFGd/Am values at pH 2–4 reached 120–400 using DTPA as the chelator5 and 70–140 using HEDTA as the chelator.6 The SFEu/Am values decrease slightly, about 50–70 at pH 3.4–4.2, using the same extractant mixture and chelator.7 Gelis et al. used mixed extractants of HEH[EHP] and T2EHDGA and increased the SFEu/Am values to nearly 100 at pH 2 and 3, respectively, using DTPA and HEDTA.8 Another option of extractant mixture is HEH[EHP] and Cyanex 923. The working pH range was 2.0–3.5, and the SFEu/Am values reached respective maxima of ∼65 and ∼50 using triethylenetetraminehexaacetic acid (TTHA) and HEDTA as the chelator.2 Although HDEHP mixed with TODGA or T2EHDGA has also been studied, corresponding separations are not as efficient as those using HEH[EHP].7,9 All these studies focusing on HEH[EHP] in combination with HEDTA have shown results that surpassed the conventional TALSPEAK process; however, a relatively high pH range is still needed (between 3 and 4), which requires a pH buffer and poses implementation challenges in industrial settings, where the upstream acidity is very high (on the order of molars of HNO3) and downstream salts are undesirable. More recently, several structural modifications of acetic acid derivatives have been made to explore their applicability to more acidic conditions;10−12 resulting separation factors decreased considerably from 50–80 at pH 3–4 to <30 at pH < 2. However, in the past 50 years, nearly all efforts have concentrated on only one class of hold-back reagents, the polyaminocarboxylates, especially DTPA,3,4,7,13 HEDTA,2,3 TTHA,2 or DPA (dipicolinic acid).14
While all these TALSPEAK-related studies are based on extracting Ln ions, the reverse TALSPEAK approach should be stressed. Ln are predominant over minor An in used nuclear fuel; it might be more tempting, from the engineering standpoint, to extract minor An into the organic phase for transmutation while keeping Ln in the aqueous phase. Many studies of direct An extraction have been accomplished primarily in Europe, with the diamide extraction (DIAMEX) process that aims at trivalent Ln and An co-extraction,15 the selective actinide extraction (SANEX) process that aims at trivalent Ln/An separation,16,17 and the group actinide extraction (GANEX) process that aims at Pu and minor An separation.18,19 Remarkably, SANEX uses either dithiophosphinic acids (cation exchangers) or 2,6-bis(1,2,4-triazin-3-yl)pyridine (BTP) derivatives (neutral solvating agents) as the extractants.13 A notable BTP derivative, n-propyl-BTP, has been hot-tested on genuine HLLW by the French Atomic Energy Commission (CEA) and the German Institute for Transuranium Elements (ITU), with resulting separation factors comparable to those obtained through the TALSPEAK process. However, BTP derivatives have not yet been adopted in larger-scale industrial processes, in part due to their extreme susceptibility toward radiolysis attributed to the relatively polarizable molecular orbitals encountered in pyridine-based reagents.13
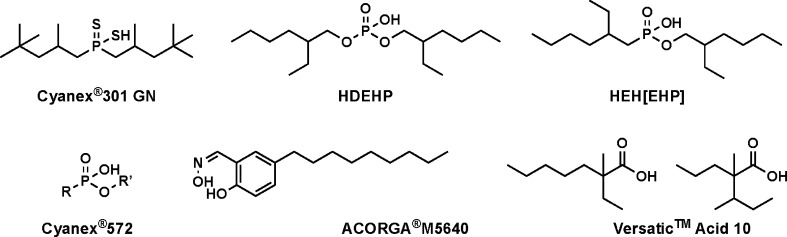
The present study evaluates an alternative family of aqueous chelators, the hydroxypyridinones, for potential use in Ln3+/An3+ separation processes, in lieu of common chelators such as the polyaminocarboxylates. The model compound for this family, 3,4,3-LI(1,2-HOPO) (referred to as HOPO; Table 1), was originally developed for the decorporation of radionuclides from contaminated human bodies owing to its low toxicity and high affinity for An elements.20 The coordination chemistry of this bio-inspired chelator has been studied with several f-elements (e.g., Ce4+/3+,20 Th4+,20,21 UO22+, Pu4+,22 Am3+, Cm3+, Bk4+, Cf3+, and Es3+23), toxic heavy transition metals (e.g., Pd2+, Cd2+, and Sn4+),24 and other transition metals relevant to nuclear medical applications (e.g., Ti4+, Zr4+, and Hf4+).25 Solution thermodynamic data on HOPO show that it has a higher affinity for trivalent An relative to the trivalent Ln.20,21,26 However, its use in Ln3+/An3+ separation has not yet been investigated thoroughly. Because HOPO is more acidic than DTPA (Table 1), it is expected to yield a viable TALSPEAK-like process at a lower pH, making it a promising hold-back chelator that warrants investigation. Meanwhile, several extractants were also studied to narrow down an optimal combination with HOPO. To that end, we sought to test a wide variety of commercially available structures offering different chemical functionalities (phosphoric acid, thiophosphorous acid, oxime, and beta-diketone) that may play a role in extraction capability (Scheme 1). Hence, the main goal of this study was to determine the promise of HOPO-type chelators for efficient trivalent Ln/An separation processes.
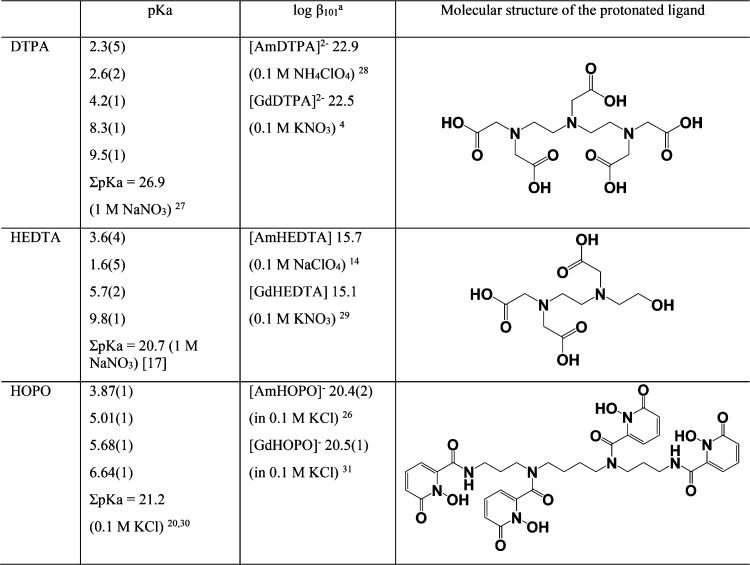
Table 1. Molecular Structures and Solution Thermodynamic Parameters of TALSPEAK-Relevant DTPA, HEDTA, and Proposed Alternative HOPO4,14,17,20,26−31.
βmhl is the stability
constant for the reaction mM + hH + lL↔MmHhLl, 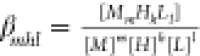 .
.
Scheme 1. Names and Molecular Structures of Commercial Extractants Used in This Study (Detailed Compositions of Certain Extractants Are Considered as Trade Secrets and Thus Not Revealed).
Results and Discussion
Influence of pH
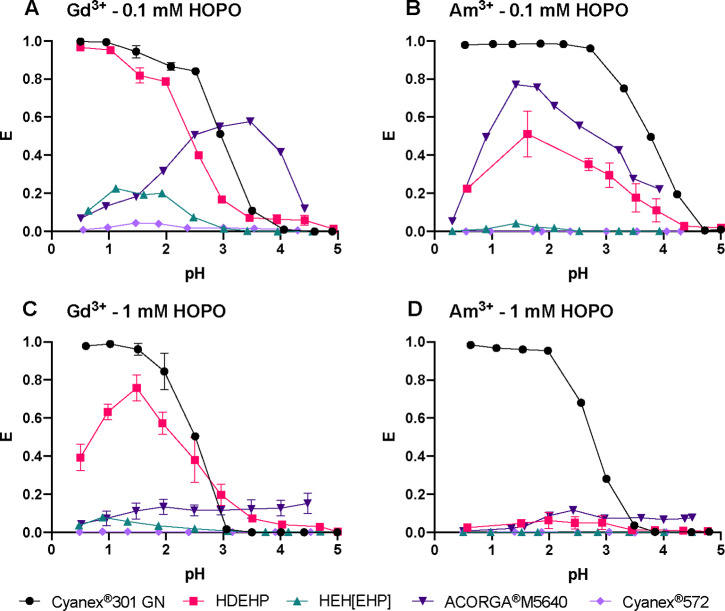
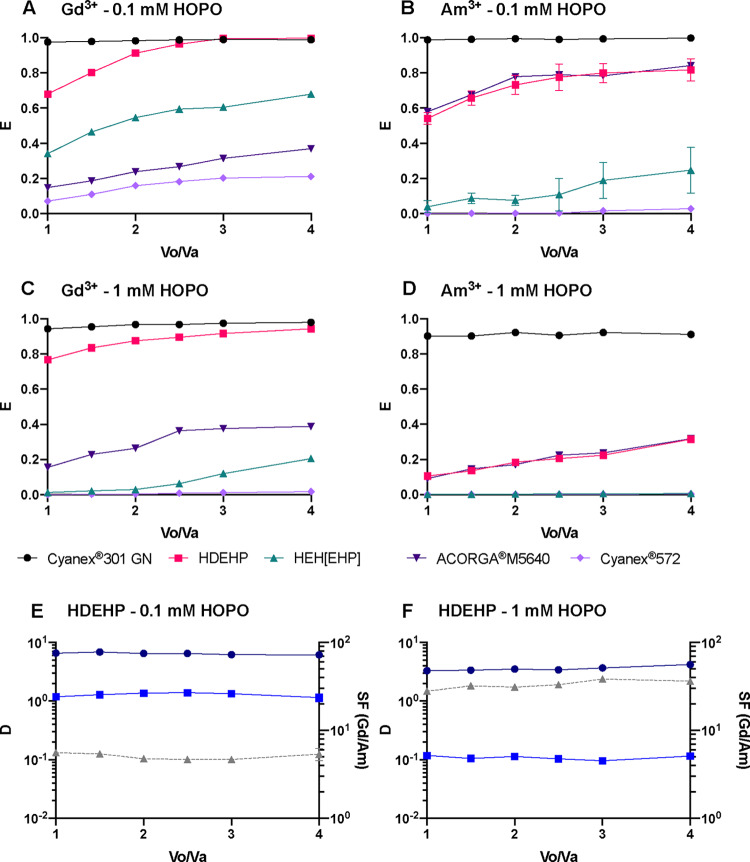
One of the most important industry-relevant parameters for separation processes is the working pH range. In a first series of experiments illustrated in Figures 1 and 2, the extraction capability of tested extractants in the presence of HOPO, between pH 1 and 5, was determined to follow the order: Cyanex 301 GN > HDEHP ≈ ACORGA M5640 > HEH[EHP] > Cyanex 572. While HDEHP, HEH[EHP], and Cyanex 572 show lower affinities to Am3+ than Gd3+, Cyanex 301 GN and ACORGA M5640 favor extracting Am3+ over Gd3+, as shown in Figure 1. This different behavior provides the flexibility to extract either Ln3+ or An3+ into the organic phase while keeping the other in the aqueous phase. Although ACORGA M5640, HEH[EHP], and Cyanex 572 can differentiate the two radiotracers, their respective extraction power is too low to be applicable for industrial separations. Hence, only Cyanex 301 GN and HDEHP are stressed herein.
Figure 1.
Extraction of (A, C) Gd3+ and (B, D) Am3+ into a kerosene organic phase as a function of pH in the aqueous phase, with two different HOPO concentrations (0.1 mM in panels (A) and (B) or 1 mM in panels (C) and (D)) and five different extractants (Cyanex 301 GN (black circles), HDEHP (pink squares), HEH[EHP] (cyan upward triangles), ACORGA M5640 (dark purple downward triangles), or Cyanex 572 (light purple diamonds)). [Extractant] = 0.5 M in kerosene; Vo/Va = 1; I = 1 M.
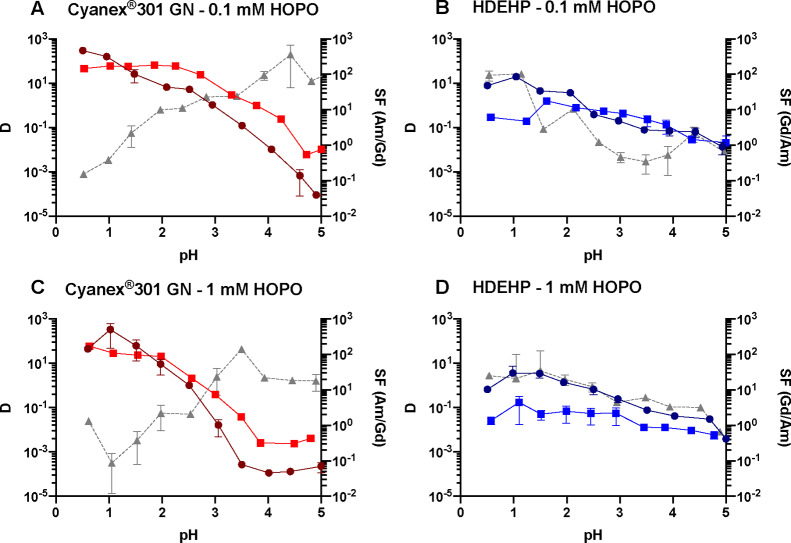
Figure 2.
Distribution ratio for Gd3+ (round solid line) and Am3+ (square solid line) and corresponding separation factors (triangle dashed line) as a function of pH in the aqueous phase, with two different HOPO concentrations (0.1 mM in panels (A) and (B) or 1 mM in panels (C) and (D)) and two different extractants (Cyanex 301 GN (panels (A) and (C)) or HDEHP (panels (B) and (D)). [Extractant] = 0.5 M in kerosene; Vo/Va = 1; I = 1 M.
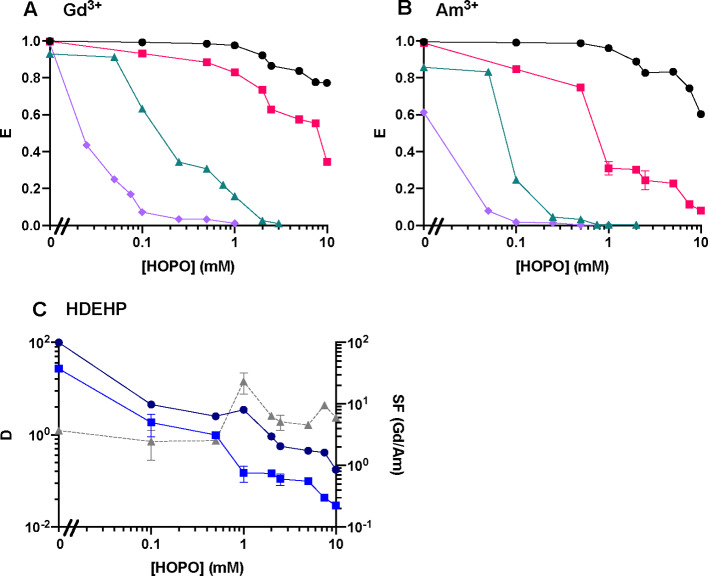
Very few results have been reported on the separation of Am3+ from Ln3+ by Cyanex 301 GN and mostly focused on Eu3+/Am3+.32−35 Cyanex 301 GN quantitatively extracts both f-elements at a low pH range but does not discriminate them at high acidity. This extractant therefore seems suitable for Ln-An co-extraction, but it is not selective under these experimental conditions. It is also worth noting that this study was designed to evaluate HOPO as a new aqueous chelator. We therefore focused on using commercially available extractants and did not perform any purification of these extractants prior to extraction experiments. The impurities present in commercial Cyanex 301 GN may have a significant effect on our results, which will need to be investigated further by scaling up metal concentrations. Above pH 2.5, HOPO starts to deprotonate and binds Am3+ with a slightly higher affinity than that for Ln3+.26 Good separation can be achieved at pH values above 3.5 with Cyanex 301 GN in the presence of 0.1 mM HOPO (Figure 2A). The optimal pH range for the combination HOPO/Cyanex 301 GN is 3.5–4.5, independent of the excess of HOPO (Figure 2A,C).
In the presence of HOPO, HDEHP is more suitable than the other three extractants tested (ACORGA M5640, HEH[EHP], and Cyanex 572) to achieve high extraction fractions at a low pH and simultaneous discrimination of Gd3+ and Am3+. This remains true when HOPO increases from 0.1 mM (Figure 2B) to 1 mM (Figure 2D), and the separation improves with a SFGd/Am value of ∼70 (Figure 2D). The HOPO/HDEHP combination presents an extraction maximum for Gd3+ at pH 1.5 (Figure 1C) that is likely due to the protonation of HDEHP at a low pH (corresponding to HDEHP’s pKa value of ∼1.5).36 At the extractant concentration used in the present study, the extraction of Am3+ by HDEHP is completely suppressed by 1 mM HOPO over the pH range of 1–5, offering a robust Gd3+/Am3+ separation. Above pH 1.5, HOPO starts complexing Ln3+ ions, and their extraction is hindered, thus yielding an extraction maximum peak around pH 1.5 for Gd3+. A working pH at 1.5 is chosen considering both high extraction and efficient separation. The HOPO/HEH[EHP] combination exhibits a behavior similar to that of HOPO/HDEHP, but the extraction fractions are much lower. Indeed, HOPO seems too strong for HEH[EHP] whose extraction efficiency is known to be lower than that of HDEHP.3 Thus, other compounds of the HOPO family that are selective but weaker metal binders, such as the hexadentate ligand TREN-HOPO,37 could be more suitable in combination with HEH[EHP]. A system with a higher HEH[EHP] concentration relative to HDEHP for a given HOPO concentration is also likely to yield efficient Ln/An separation.
Influence of Phase Volume Ratio (Vo/Va)
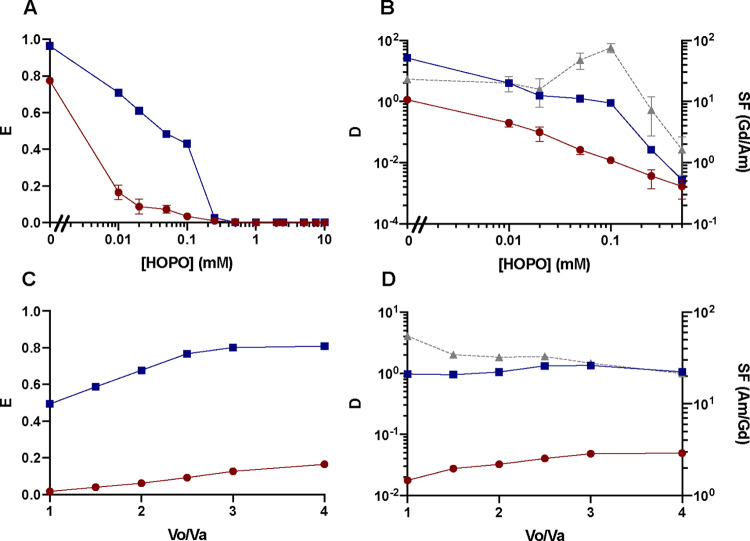
For the sake of comparison, extraction data at various phase volume ratios and at pH 1.5 were collected. The extraction results displayed in Figure 3 are congruous with the results from the pH-dependence studies, and the extractant efficacy follows the order: Cyanex 301 GN > HDEHP > HEH[EHP] > Cyanex 572. The high affinity of HOPO for trivalent f-elements therefore allows comparing the strength of a broad variety of extractants, including the most efficient ones like Cyanex 301 GN (Figures 1 and 3). In combination with 0.1 mM HOPO at pH 1.5, Cyanex 301 GN almost fully extracts both Gd3+ and Am3+ even when the phase volume ratio is as low as 1 (Figure 3). HDEHP also quantitatively extracts Gd3+ (Figure 3A) and more than 50% of Am3+ at Vo/Va ≥ 1 (Figure 3B). ACORGA M5640 and Cyanex 572 exhibit low separation factors or poor extraction capability in the presence of HOPO even at a high Vo/Va. All the extraction curves, except those at extremities, i.e., when E approaches 0 and 1, increase fast in the beginning and gradually reaches a plateau, which agrees with the mathematical expression of E. All tested extractants show reduced extraction of Am3+ relative to Gd3+ except Cyanex 301 GN that outcompetes HOPO at pH 1.5 (Figure 3). Interestingly, the extraction of Gd3+ by ACORGA M5640 at pH 1.5 is not influenced by the presence of HOPO (Figures 3A and 3C), whereas the extraction of Am3+ is hindered as the concentration of HOPO increases (Figure 3B and 3D). This is in line with previous solution thermodynamic studies that demonstrated the higher affinity of HOPO toward 5f elements relative to the 4f series at a low pH.26,31 Although HEH[EHP] can differentiate Gd3+ from Am3+, the separation is impractical as D values for both M3+ ions are much lower than 1 (Supplementary Figure S1) under the studied conditions. For the HDEHP/HOPO combination, a Vo/Va value of 1 and a HOPO concentration of 1 mM HOPO are optimal (Figure 3E,F). The optimal Vo/Va value for Cyanex 301 GN will be shown later since the data for Cyanex 301 GN at pH 1.5 (Supplementary Figure S1) does not demonstrate good separation. The optimal Vo/Va condition at the working pH range will be provided in the Optimal Conditions for Separation section.
Figure 3.
Extraction of (A, C) Gd3+ and (B, D) Am3+ into a kerosene organic phase as a function of phase volume ratio, with two different HOPO concentrations (0.1 mM in panels (A) and (B) or 1 mM in panels (C) and (D)) and five different extractants (Cyanex 301 GN (black circles), HDEHP (pink squares), HEH[EHP] (cyan upward triangles), ACORGA M5640 (dark purple downward triangles), or Cyanex 572 (light purple diamonds)). [Extractant] = 0.5 M; pH = 1.50 ± 0.02; I = 1 M; corresponding distribution ratios for Gd3+ (round solid line) and Am3+ (square solid line) and separation factors (triangle dashed line) with HDEHP, at (E) 0.1 mM HOPO or (F) 1 mM HOPO.
Influence of 3,4,3-LI(1,2-HOPO) Concentration
For the sake of comparison, extraction data at various HOPO concentrations (at pH 1.5 and Vo/Va = 3) were measured for HDEHP, HEH[EHP], Cyanex 301 GN, and Cyanex 572. Figure 4A,B shows that HOPO is a strong chelator for both Ln3+ and An3+ ions since the extraction curves of all extractants decrease as the HOPO concentration increases. At pH 1.5, Cyanex 301 GN is the strongest extractant for both Gd3+ and Am3+ and is slightly influenced by HOPO complexation with the metal ions. In contrast, HEH[EHP] and Cyanex 572 are most sensitive to the competing hold-back complexation reactions as extraction fractions plummet even at a low HOPO concentration. Both E and SF values are low for Cyanex 572, and it appears to be not suitable for Ln3+/An3+ separation. Although HEH[EHP] is efficient and exhibits the highest SF of ∼60 (Supplementary Figure S2), the D values of both M3+ ions used to calculate the SF value are very low (DGd = 0.09 and DAm = 0.0016) (Supplementary Figure S2), rendering the HOPO/HEH[EHP] combination impractical at this pH. HDEHP is slightly weaker than Cyanex 301 GN in extracting the two elements, being the second least influenced by an excess of HOPO. The extraction capability is congruent with the results from the pH- and Vo/Va-dependence studies and follows the order: Cyanex 301 GN > HDEHP > HEH[EHP] > Cyanex 572. Hence, HOPO competition with the extractants follows the order: Cyanex 301 GN < HDEHP < HEH[EHP] < Cyanex 572. The results obtained here show that HOPO has a higher affinity for An3+ ions than Ln3+ ions as most extraction fractions of Am3+ are lower than those of Gd3+ (expect for the Cyanex 301/HOPO combination due to the specificity of this extractant, vide infra). In the absence of HOPO, nearly all trivalent ions are extracted by the five tested extractants, and thus no practical separation is observed under the tested conditions (Figure 4). Once HOPO is added to the liquid–liquid extraction system, the SF values increases even for Cyanex 301, while this pH is not optimal for this particular extractant (Supplementary Figure S1). The SF values with HDEHP/HOPO combination gradually increase as the HOPO concentration increases (Figure 4C). A HOPO concentration of 1 mM was selected for HDEHP to ensure optimal separation, while that of Cyanex 301 GN is discussed hereafter since its optimal pH range is 3.5–4 instead of 1.5.
Figure 4.
Extraction of (A) Gd3+ and (B) Am3+ into a kerosene organic phase as a function of HOPO concentration in the aqueous phase, with four different extractants: Cyanex 301 GN (black circles), HDEHP (pink squares), HEH[EHP] (cyan upward triangles), or Cyanex 572 (light purple diamonds). [Extractant] = 0.5 M; Vo/Va = 3; pH = 1.50 ± 0.03; I = 1 M; (C) corresponding distribution ratio for Gd3+ (round solid line) and Am3+ (square solid line) and separation factors (triangle dashed line).
Optimal Conditions for Ln3+/An3+ Separation
For a robust separation process, the SF should be no smaller than 10,7 and the results described above show that both Cyanex 301 GN and HDEHP yield SF values greater than 10 in the presence of HOPO. It should be noted that 3,4,3-LI(1,2-HOPO) is just a model compound of the HOPO family, and it was not initially developed for separation purposes; thus, even more selective HOPO derivatives are likely to be developed in the future. For the combination HDEHP/HOPO, as detailed above (Figures 1–4), the best separation performances are obtained at pH 1.5 with 1 mM HOPO and for Vo/Va = 1. For Cyanex 301 GN, the Gd3+/Am3+ separation has been optimized at pH 3.6. The influence of the HOPO concentration and the phase volume ratio Vo/Va on the extraction of Gd3+ and Am3+ in the system HOPO/Cyanex 301 GN are displayed in Figure 5. As mentioned above, this extraction formulation allows for the preferential extraction of Am3+ over Gd3+ under all the conditions tested. The HOPO/Cyanex 301 GN system therefore offers a remarkable opportunity to develop the reverse-TALSPEAK processes. The optimal conditions for this system are 0.1 mM HOPO and Vo/Va = 1.
Figure 5.
Influence of the HOPO concentration in (A) the aqueous phase and (C) the phase volume ratio on Cyanex 301 GN extraction of Gd3+ (round solid line) and Am3+ (square solid line); (B, D) corresponding distribution ratios and separation factors (triangle dashed line). [Cyanex 301 GN] = 0.5 M in kerosene; Vo/Va = 1; pH = 3.55 ± 0.05; I = 1 M.
Separation of Gd from Am, Cm, Bk, and Cf
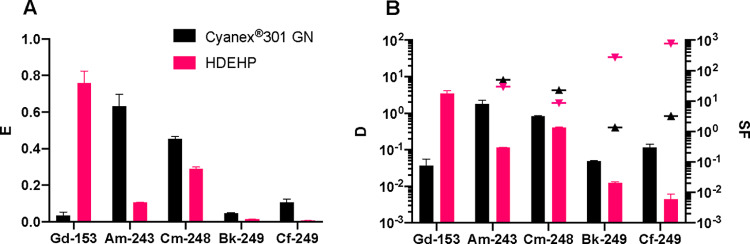
The optimized conditions determined above were used to test the performance of the HOPO/Cyanex 301 GN and HOPO/HDEHP extraction systems for the separation of Gd from Am, Cm, Bk, and Cf. As shown in Figure 6, for both HOPO/extractant systems, the extraction trend along the actinide series is as follows: Am ≈ Cm > Bk ≈ Cf. The decrease in extraction fraction from Am3+ and Cm3+ to Cf3+ is consistent with recently reported DFT-calculated free energies of complexation of [AmHOPO]−, [CmHOPO]−, and [CfHOPO]− that showed almost identical stability of the Am3+ and Cm3+ complexes and a slightly higher stability of the Cf3+ complex.38 Extraction fractions for Bk were far lower than for Am3+ and Cm3+. This is consistent with oxidation of Bk3+ to Bk4+ under the tested conditions and in line with our recent discovery of the HOPO-mediated stabilization of Bk4+ in aqueous solutions due to the extreme affinity and selectivity of this chelator for tetravalent cations.23,39 The Cyanex 301 GN/HOPO formulation is selective for Am3+ and Cm3+ against Gd3+ but does not segregate Gd3+ from Bk4+ and Cf3+ because of the low extraction yields for Bk4+ and Cf3+. Of note, the SF values between Am3+ and Cm3+ and between Bk4+ and Cf3+ are relatively low, highlighting the difficulty of separating adjacent transplutonium elements under those conditions (SFAm/Cm = 2.2 and SFCf/Bk = 2.4). The HDEHP/HOPO combination was also found to be selective in Gd3+/Am3+ separation (Figure 6), with a resulting SFGd/Am value slightly lower than that of the TALSPEAK process but at a higher acidity (pH 1.5) and therefore appealing for industrial applications.
Figure 6.
(A) Extraction fraction of Gd3+ and An3+ using Cyanex 301 GN (black bars, [Cyanex 301 GN] = 0.5 M in kerosene, pH = 3.6, [HOPO] = 0.1 mM, Vo/Va = 1, I = 1 M) and HDEHP (pink bars, [HDEHP] = 0.5 M in kerosene pH = 1.5, [HOPO] = 1 mM, Vo/Va = 1, I = 1 M); (B) corresponding distribution ratios (bars) and separation factors between Gd3+ and An3+ (triangle solid points) using Cyanex 301 GN (black) and HDEHP (pink).
Conclusions
A new class of water-soluble ligands with hydroxypyridinone binding units have been evaluated for Ln/An separation in TALSPEAK-like configurations. The model compound 3,4,3-LI(1,2-HOPO) was coupled with several industry-relevant organic extractants for the separation of Gd and four transplutonium elements (Am, Cm, Bk, and Cf). Two extractants with the highest separation performance were selected, and three physicochemical parameters (pH, HOPO concentration, and phase volume ratio) were optimized to give the best separation. The Cyanex 301 GN/HOPO and HDEHP/HOPO combinations have opposite separation behaviors. Cyanex 301 GN/HOPO preferentially extracts the actinides into the organic phase, while HDEHP/HOPO preferentially extracts Gd3+, providing two different approaches for Ln/An separation. The optimized separation factors attained between Gd3+ and Am3+ are 50 and 30, respectively, using Cyanex 301 GN and HDEHP. The results using HDEHP are comparable to those of the TALSPEAK process but at a much lower pH (1.5 instead of 3.0–4.5), which is preferable since it eliminates the need for a pH buffer and is more compatible with the highly acidic upstream steps. The separation factors between other actinides and Gd3+ have also been measured with SFAm/Gd = 50, SFCm/Gd = 23, SFBk/Gd = 1.4, and SFCf/Gd = 3.2 for Cyanex 301 GN and SFGd/Am = 30, SFGd/Cm = 8.5, SFGd/Bk = 273, and SFGd/Cf = 773 for HDEHP. This first investigation shows that HOPO chelators are promising candidates for further developments in actinide/lanthanide separation. Future studies are therefore warranted and will aim at characterizing these systems more in depth by probing a variety of phenomena and parameters such as extraction kinetics, influence of organic extractant concentration and purity, influence of nitrate ion concentration, susceptibility of HOPO ligands toward radiolysis, differences among cations from the whole lanthanide series, system behavior as metal concentrations are increased, etc.
Experimental Methods
Caution!
All isotopes used in this study, 153Gd (t1/2 = 240.4 d, 3.5 × 103 Ci/g), 243Am (t1/2 = 7388 y, 0.2 Ci/g), 248Cm (t1/2 = 3.49 × 105 y, 4.2 × 10–3 Ci/g), 249Bk (t1/2 = 330 d, 1.6 × 103 Ci/g), and 249Cf (t1/2 = 351 y, 4.1 Ci/g), are hazardous and radioactive materials with high specific radioactivities and should be handled only in specifically designated facilities in accordance with appropriate safety controls.
Chemicals
HOPO was procured from Ash Stevens, Inc. (Detroit, MI). 153Gd was acquired as GdCl3 in 1 M HCl from Eckert and Ziegler Isotope Products (Valencia, CA). An 243Am3+ stock solution was prepared by dissolving 243Am2O3 (Lawrence Berkeley National Laboratory inventory, Berkeley, CA) in 1 M HNO3; 248CmCl3 (95.78% 248Cm, 4.12% 246Cm, 0.06% 245Cm, and 0.02% 244Cm/247Cm isotopic distribution by atom percentage), 249BkCl3, and 249CfCl3 were purchased from the National Isotope Development Center at Oak Ridge National Laboratory (Oak Ridge, TN) and dissolved in 0.1 M HCl. HNO3 (6 M) was purchased from VWR Chemicals BDH, HNO3 (0.1 M) from EMD Millipore Corp. (Billerica, MA), HCOOH from Sigma-Aldrich Co. LLC (St. Louis, MO), NaNO3 with >99% purity from Alfa Aesar (Ward Hill, MA), NaOH with ≥97% purity from Sigma-Aldrich Co. LLC (St. Louis, MO), kerosene from Alfa Aesar (Heysham, U.K.), and Ultima Gold from Perkin Elmer Inc. (Waltham, MA). All extractants detailed below were used as received. HDEHP with ≥95% purity was purchased from EMD Millipore Corp. (Billerica, MA) and HEH[EHP] with 97% purity from Sigma-Aldrich Co. LLC (St. Louis, MO). Cyanex 301 GN (68–72% R2PSSH, 18–22% petro distillate, 5–7% R3PS, and 1–2% R2P(=S)OH), Cyanex 572 (30–60% (2-ethylhexyl)-ester phosphonic acid, and 40–70% organophosphorus), and ACORGA M5640 (50% 5-nonylsalicylaldoxime, modified with 2,2,4-trimethyl-1,3-pentanediol diisobutyrate (TXIB)) were kindly supplied as test samples by Cytec Industry Inc. (Princeton, NJ). Two other extractants, Versatic Acid 10 (carboxylic acids) and 2-thenoyltrifluoroacetone (TTA), were also tested. The preliminary results (not shown) indicated that Versatic Acid 10 has virtually no extraction capability at the working pH range and TTA has severe salting-out effects, making them impractical for this study, and thus no further discussion will be presented on them.
Liquid–Liquid Extraction Procedures
The following conditions were kept consistent for all experiments except where otherwise noted: (i) all extractants were fixed at 0.5 M in kerosene; (ii) sodium nitrate (1 M) was used to keep the ionic strength constant; (iii) formic acid (50 mM) was used as the buffer for pH 3–4 and acetic acid (50 mM) for pH 4.5–5; (iv) all experiments were performed at ambient temperature (25 °C); (v) contact time was 1 h; and (vi) experiments were done in triplicate. Each extraction consisted of the following three steps: (i) Conditioning: the organic phase was contacted with the aqueous phase (phase volume ratio, defined as the organic-to-aqueous volume ratio, Vo/Va = 1 for pH-dependence studies and Vo/Va = 0.5 for the rest studies), which contained every component but metal ions, and shaken to reach equilibrium. (ii) Extraction: a volume of 2 μL (maintained at this level to minimize the possible effects of extractant loading) radiotracer was pipetted into 398 μL of aqueous phase, the pH was determined, and 400 μL of conditioned organic phase was contacted with the aqueous phase and shaken for 60 min. Several tests were performed to investigate the influences of pH, phase volume ratio, and HOPO concentration; and (iii) Counting: the contacted two phases were separated by centrifugation (5 min at 3000 rpm), and a volume of 100 μL was aliquoted out of each phase into a scintillation vial with 10 mL of scintillation cocktail (Ultima Gold, Perkin Elmer, Shelton, CT) for liquid scintillation analysis (Packard Tri-Carb model B4430, Perkin Elmer).
Data Interpretation
Three parameters were used to characterize the extraction performance, distribution ratio (D, eq 1) in terms of radioactivity (A) with the unit of counts per minute (cpm), extraction fraction (E, eq 2) calculated from D and the phase volume ratio Vo/Va, and separation factor (SF, eq 3):
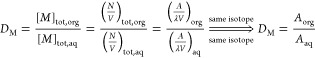 |
1 |
| 2 |
| 3 |
Acknowledgments
This work was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, Chemical Sciences, Geosciences, and Biosciences Division at the Lawrence Berkeley National Laboratory under Contract DE-AC02-05CH11231. R.J.A. also acknowledges support from the Nuclear Regulatory Commission under Faculty Development Grant NRC-HQ-84-14-G-0052.
Glossary
Abbreviations
- An
actinides
- ALSEP
minor actinide lanthanide separation process (ALSEP)
- BTP
2,6-bis(1,2,4-triazin-3-yl)pyridine
- CEA
French Atomic Energy Commission
- DIAMEX
diamide extraction
- DTPA
diethylenetriaminepentaacetic acid
- DPA
dipicolinic acid
- GANEX
group actinide extraction
- HEDEHP
di-(2-ethylhexyl)phosphoric acid
- HEDTA
ethylenediaminetetraacetic acid
- HEH[EHP]
2-ethylhexylphosphonic acid mono-2-ethylhexyl ester
- HLLW
high-level liquid waste
- HOPO
3,4,3-LI(1,2-HOPO)
- ITU
Institute for Transuranium Elements
- Ln
lanthanides
- PUREX
plutonium uranium redox extraction
- SANEX
selective actinide extraction
- TALSPEAK
trivalent actinide lanthanide separation with phosphorus-reagent extraction from aqueous komplexes
- TODGA
N,N,N′,N′-tetraoctyl diglycolamide
- TTHA
triethylenetetraminehexaacetic acid
- T2EHDGA
N,N,N′,N′-tetra-2-ethylhexyl diglycolamide
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c00873.
Distribution ratios for Gd3+ and Am3+ and corresponding separation factors as a function of phase volume ratio and HOPO concentration for Cyanex 301 GN and HEH[EHP] extraction (PDF)
Author Present Address
⊥ Glenn T. Seaborg Institute, Physical and Life Sciences, Lawrence Livermore National Laboratory, Livermore, California 94550, United States (Gauthier J.-P. Deblonde)
Author Contributions
§ Y.W. and G.J.-P.D. contributed equally. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Geist A.; Müllich U.; Magnusson D.; Kaden P.; Modolo G.; Wilden A.; Zevaco T. Actinide(III)/Lanthanide(III) Separation Via Selective Aqueous Complexation of Actinides(III) Using a Hydrophilic 2,6-Bis(1,2,4-Triazin-3-Yl)-Pyridine in Nitric Acid. Solvent Extr. Ion Exch. 2012, 30, 433–444. 10.1080/07366299.2012.671111. [DOI] [Google Scholar]
- Johnson A. T.; Nash K. L. Mixed Monofunctional Extractants for Trivalent Actinide/Lanthanide Separations: TALSPEAK-MME. Solvent Extraction and Ion Exchange Solvent Extr. Ion Exch. 2015, 33, 642–655. 10.1080/07366299.2015.1085225. [DOI] [Google Scholar]
- Braley J. C.; Grimes T. S.; Nash K. L. Alternatives to HDEHP and DTPA for Simplified TALSPEAK Separations. Ind. Eng. Chem. Res. 2011, 51, 629–638. 10.1021/ie200285r. [DOI] [Google Scholar]
- Nash K. L. The Chemistry of TALSPEAK: A Review of the Science. Solvent Extr. Ion Exch. 2015, 33, 1–55. 10.1080/07366299.2014.985912. [DOI] [Google Scholar]
- Lumetta G. J.; Gelis A. V.; Carter J. C.; Niver C. M.; Smoot M. R. The Actinide-Lanthanide Separation Concept. Solvent Extr. Ion Exch. 2014, 32, 333–347. 10.1080/07366299.2014.895638. [DOI] [Google Scholar]
- Lumetta G. J.; Casella A. J.; Rapko B. M.; Levitskaia T. G.; Pence N. K.; Carter J. C.; Niver C. M.; Smoot M. R. An Advanced TALSPEAK Concept Using 2-Ethylhexylphosphonic Acid Mono-2-Ethylhexyl Ester as the Extractant. Solvent Extr. Ion Exch. 2014, 33, 211–223. 10.1080/07366299.2014.985920. [DOI] [Google Scholar]
- Gelis A. V.; Lumetta G. J. Actinide Lanthanide Separation Process—ALSEP. Ind. Eng. Chem. Res. 2014, 53, 1624–1631. 10.1021/ie403569e. [DOI] [Google Scholar]
- Gelis A. V.; Kozak P.; Breshears A. T.; Brown M. A.; Launiere C.; Campbell E. L.; Hall G. B.; Levitskaia T. G.; Holfeltz V. E.; Lumetta G. J. Closing the Nuclear Fuel Cycle with a Simplified Minor Actinide Lanthanide Separation Process (ALSEP) and Additive Manufacturing. Sci. Rep. 2019, 9, 12842. 10.1038/s41598-019-48619-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullekson B. J.; Brown M. A.; Paulenova A.; Gelis A. V. Speciation of Select F-Elements with Lipophilic Phosphorus Acids and Diglycol Amides in the ALSEP Backward-Extraction Regime. Ind. Eng. Chem. Res. 2017, 56, 12174–12183. 10.1021/acs.iecr.7b02379. [DOI] [Google Scholar]
- Heathman C. R.; Grimes T. S.; Jansone-Popova S.; Roy S.; Bryantsev V. S.; Zalupski P. R. Influence of a Pre-Organized N-Donor Group on the Coordination of Trivalent Actinides and Lanthanides by an Aminopolycarboxylate Complexant. Chem. – Eur. J. 2019, 25, 2545–2555. 10.1002/chem.201804723. [DOI] [PubMed] [Google Scholar]
- Grimes T. S.; Heathman C. R.; Jansone-Popova S.; Ivanov A. S.; Roy S.; Bryantsev V. S.; Zalupski P. R. Influence of a Heterocyclic Nitrogen-Donor Group on the Coordination of Trivalent Actinides and Lanthanides by Aminopolycarboxylate Complexants. Inorg. Chem. 2018, 57, 1373–1385. 10.1021/acs.inorgchem.7b02792. [DOI] [PubMed] [Google Scholar]
- Heathman C. R.; Grimes T. S.; Jansone-Popova S.; Ivanov A. S.; Bryantsev V. S.; Zalupski P. R. Synthesis and Characterization of a Novel Aminopolycarboxylate Complexant for Efficient Trivalent F-Element Differentiation: N-Butyl-2-Acetamide-Diethylenetriamine-N,N′,N″,N′′-Tetraacetic Acid. Dalton Trans. 2018, 47, 1092–1105. 10.1039/C7DT04104G. [DOI] [PubMed] [Google Scholar]
- Modolo G.; Geist A.; Miguirditchian M.10 - Minor Actinide Separations in the Reprocessing of Spent Nuclear Fuels: Recent Advances in Europe. In Reprocessing and Recycling of Spent Nuclear Fuel; Taylor R., Ed.; Woodhead Publishing Series in Energy; Woodhead Publishing: Oxford, 2015; pp 245–287. [Google Scholar]
- Heathman C. R.; Nash K. L. Characterization of Europium and Americium Dipicolinate Complexes. Sep. Sci. Technol. 2012, 47, 2029–2037. 10.1080/01496395.2012.704225. [DOI] [Google Scholar]
- Modolo G.; Vijgen H.; Serrano-Purroy D.; Christiansen B.; Malmbeck R.; Sorel C.; Baron P. DIAMEX Counter-Current Extraction Process for Recovery of Trivalent Actinides from Simulated High Active Concentrate. Sep. Sci. Technol. 2007, 42, 439–452. 10.1080/01496390601120763. [DOI] [Google Scholar]
- Modolo G.; Wilden A.; Daniels H.; Geist A.; Magnusson D.; Malmbeck R. Development and Demonstration of a New SANEX Partitioning Process for Selective Actinide(III)/Lanthanide(III) Separation Using a Mixture of CyMe4BTBP and TODGA. Radiochim. Acta 2013, 101, 155–162. 10.1524/ract.2013.2016. [DOI] [Google Scholar]
- Modolo G.; Wilden A.; Kaufholz P.; Bosbach D.; Geist A. Development and Demonstration of Innovative Partitioning Processes (i-SANEX and 1-Cycle SANEX) for Actinide Partitioning. Prog. Nucl. Energy 2014, 72, 107–114. 10.1016/j.pnucene.2013.07.021. [DOI] [Google Scholar]
- Bell K.; Carpentier C.; Carrott M.; Geist A.; Gregson C.; Hérès X.; Magnusson D.; Malmbeck R.; McLachlan F.; Modolo G.; Müllich U.; Sypula M.; Taylor R.; Wilden A. Progress Towards the Development of a New GANEX Process. Procedia Chem. 2012, 7, 392–397. 10.1016/j.proche.2012.10.061. [DOI] [Google Scholar]
- Carrott M.; Bell K.; Brown J.; Geist A.; Gregson C.; Hères X.; Maher C.; Malmbeck R.; Mason C.; Modolo G.; Müllich U.; Sarsfield M.; Wilden A.; Taylor R. Development of a New Flowsheet for Co-Separating the Transuranic Actinides: The “EURO-GANEX” Process. Solvent Extr. Ion Exch. 2014, 32, 447–467. 10.1080/07366299.2014.896580. [DOI] [Google Scholar]
- Deblonde G. J.-P.; Sturzbecher-Hoehne M.; Abergel R. J. Solution Thermodynamic Stability of Complexes Formed with the Octadentate Hydroxypyridinonate Ligand 3,4,3-LI(1,2-HOPO): A Critical Feature for Efficient Chelation of Lanthanide(IV) and Actinide(IV) Ions. Inorg. Chem. 2013, 52, 8805–8811. 10.1021/ic4010246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Captain I.; Deblonde G. J.-P.; Rupert P. B.; An D. D.; Illy M.-C.; Rostan E.; Ralston C. Y.; Strong R. K.; Abergel R. J. Engineered Recognition of Tetravalent Zirconium and Thorium by Chelator–Protein Systems: Toward Flexible Radiotherapy and Imaging Platforms. Inorg. Chem. 2016, 55, 11930–11936. 10.1021/acs.inorgchem.6b02041. [DOI] [PubMed] [Google Scholar]
- Durbin P. W.; Kullgren B.; Xu J.; Raymond K. N. Multidentate Hydroxypyridinonate Ligands for Pu(IV) Chelation in Vivo : Comparative Efficacy and Toxicity in Mouse of Ligands Containing 1,2-HOPO or Me-3,2-HOPO. Int. J. Radiat. Biol. 2000, 76, 199–214. 10.1080/095530000138853. [DOI] [PubMed] [Google Scholar]
- Deblonde G. J.-P.; Sturzbecher-Hoehne M.; Rupert P. B.; An D. D.; Illy M.-C.; Ralston C. Y.; Brabec J.; de Jong W. A.; Strong R. K.; Abergel R. J. Chelation and Stabilization of Berkelium in Oxidation State +IV. Nat. Chem. 2017, 9, 843–849. 10.1038/nchem.2759. [DOI] [PubMed] [Google Scholar]
- Deblonde G. J.-P.; Lohrey T. D.; An D. D.; Abergel R. J. Toxic Heavy Metal – Pb, Cd, Sn – Complexation by the Octadentate Hydroxypyridinonate Ligand Archetype 3,4,3-LI(1,2-HOPO). New J. Chem. 2018, 42, 7649–7658. 10.1039/C7NJ04559J. [DOI] [Google Scholar]
- Sturzbecher-Hoehne M.; Choi T. A.; Abergel R. J. Hydroxypyridinonate Complex Stability of Group (IV) Metals and Tetravalent f-Block Elements: The Key to the Next Generation of Chelating Agents for Radiopharmaceuticals. Inorg. Chem. 2015, 54, 3462–3468. 10.1021/acs.inorgchem.5b00033. [DOI] [PubMed] [Google Scholar]
- Sturzbecher-Hoehne M.; Yang P.; D′Aléo A.; Abergel R. J. Intramolecular Sensitization of Americium Luminescence in Solution: Shining Light on Short-Lived Forbidden 5f Transitions. Dalton Trans. 2016, 45, 9912–9919. 10.1039/C6DT00328A. [DOI] [PubMed] [Google Scholar]
- Omoto T.; Wall N. A. Stability Constant Determinations for Technetium (IV) Complexation with Selected Amino Carboxylate Ligands in High Nitrate Solutions. Radiochim. Acta 2017, 105, 621–627. 10.1515/ract-2016-2702. [DOI] [Google Scholar]
- Baybarz R. D. Dissociation Constants of the Transplutonium Element Chelates of Diethylenetriaminepenta-Acetic Acid (DTPA) and the Application of DTPA Chelates to Solvent Extraction Separations of Transplutonium Elements from the Lanthanide Elements. J. Inorg. Nucl. Chem. 1965, 27, 1831–1839. 10.1016/0022-1902(65)80327-X. [DOI] [Google Scholar]
- Powell J. E.; Mackey J. L. Determination of the Rare Earth-HEDTA Stability Constants by the Mercury Electrode Method. Inorg. Chem. 1962, 1, 418–421. 10.1021/ic50002a045. [DOI] [Google Scholar]
- Abergel R. J.; D’Aléo A.; Ng Pak Leung C.; Shuh D. K.; Raymond K. N. Using the Antenna Effect as a Spectroscopic Tool: Photophysics and Solution Thermodynamics of the Model Luminescent Hydroxypyridonate Complex [EuIII(3,4,3-LI(1,2-HOPO))]−. Inorg. Chem. 2009, 48, 10868–10870. 10.1021/ic9013703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturzbecher-Hoehne M.; Leung C. N. P.; D′Aléo A.; Kullgren B.; Prigent A.-L.; Shuh D. K.; Raymond K. N.; Abergel R. J. 3,4,3-LI(1,2-HOPO): In Vitro Formation of Highly Stable Lanthanide Complexes Translates into Efficacious in Vivo Europium Decorporation. Dalton Trans. 2011, 40, 8340–8346. 10.1039/c1dt10840a. [DOI] [PubMed] [Google Scholar]
- Zhu Y.; Chen J. Separation of Americium from Fission Product Lanthanides Using Cyanex 301 Extraction. Tsinghua Sci. Technol. 2001, 6, 151–155. [Google Scholar]
- Zhu Y.; Chen J.; Jiao R. Extraction of Am(III) and Eu(III) from Nitrate Solution with Purified Cyanex 301. Solvent Extr. Ion Exch. 2007, 14, 61–68. 10.1080/07366299608918326. [DOI] [Google Scholar]
- Bhattacharyya A.; Mohapatra P. K.; Manchanda V. K. Extraction of Am(III) and Eu(III) from Nitrate Media with Cyanex®-301 and Neutral “N” Donor Ligands: A Thermodynamic Study. Radiochim. Acta 2010, 98, 141. 10.1524/ract.2010.1699. [DOI] [Google Scholar]
- Zhang W.; He X.; Ye G.; Yi R.; Chen J. Americium(III) Capture Using Phosphonic Acid-Functionalized Silicas with Different Mesoporous Morphologies: Adsorption Behavior Study and Mechanism Investigation by EXAFS/XPS. Environ. Sci. Technol. 2014, 48, 6874–6881. 10.1021/es500563q. [DOI] [PubMed] [Google Scholar]
- Hilal S. H.; Carreira L. A.; Karickhoff S. W.; Politzer P.; Murray J. S.. Theoretical and Computational Chemistry, Quantitative Treatment of Solute/Solvent Interactions. Eds. Politzer P.; Murray J. S; Elsevier Publishers; 1994. [Google Scholar]
- Jocher C. J.; Moore E. G.; Xu J.; Avedano S.; Botta M.; Aime S.; Raymond K. N. 1,2-Hydroxypyridonates as Contrast Agents for Magnetic Resonance Imaging: TREN-1,2-HOPO. Inorg. Chem. 2007, 46, 9182–9191. 10.1021/ic700985j. [DOI] [PubMed] [Google Scholar]
- Kelley M. P.; Deblonde G. J.-P.; Su J.; Booth C. H.; Abergel R. J.; Batista E. R.; Yang P. Bond Covalency and Oxidation State of Actinide Ions Complexed with Therapeutic Chelating Agent 3,4,3-LI(1,2-HOPO). Inorg. Chem. 2018, 57, 5352–5363. 10.1021/acs.inorgchem.8b00345. [DOI] [PubMed] [Google Scholar]
- Deblonde G. J.-P.; Ricano A.; Abergel R. J. Ultra-Selective Ligand-Driven Separation of Strategic Actinides. Nat. Commun. 2019, 10, 2438. 10.1038/s41467-019-10240-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.