Abstract
Frost weathering of porous materials caused by seasonal temperature changes is a major source of damage to the world’s infrastructure and cultural heritage. Here we investigate poly(vinyl alcohol) (PVA) addition as a means to enhance the freeze–thaw durability of concrete without compromising its structural or mechanical integrity. We evaluate the ice recrystallization inhibition activity of PVA in a cementitious environment and the impact of PVA on key structural and mechanical properties, such as cement hydration (products), microstructure, strength, as well as freeze–thaw resistance. We find that a low amount of PVA significantly reduces the surface scaling of concrete and displays excellent ice recrystallization inhibition in the saturated Ca(OH)2 solution, which has a similar pH value as cement pore solution, while it does not affect cement hydration, microstructure, nor its mechanical properties. These findings contribute to new insights on the freeze–thaw damage mechanism, and more importantly, we disclose a new direction for the design of concrete with excellent freeze–thaw resistance.
1. Introduction
Ice is one of the few substances on Earth that expands upon solidification. This expansion can be destructive to porous materials that take up water such as cement and concrete exposed to freeze–thaw cycles.1 Cement and concrete are the most used man-made materials with global annual consumptions of 4 billion and 25 billion tonnes, respectively.2 Seasonal temperature changes induce freezing–thawing cycles in many landscapes across the globe and are responsible for visible deterioration of concrete structures, costing $18–21 billion annually for repairs in the U.S. alone.3 This has spurred great interest in effective strategies applicable for industrially relevant scales to reduce frost damage.
The first studies conducted on the mechanism of frost damage in concrete hypothesized that the hydraulic pressure generated by the volume expansion of freezing water is the driving force for the damage.4,5 This hydraulic pressure theory argues that ice formation inside the concrete matrix forces unfrozen water to flow away from the ice growth site, which generates a pressure gradient resulting in microcracks and spalling once the local tensile strength of the concrete (capillaries) is exceeded. Decades later, several researchers reported experimental evidence for the importance of crystallization pressure.6,7 This pressure is exerted by a growing ice crystal on its constraining concrete matrix if there is a thin supercooled water film between the growing ice crystal and the pore wall.8 More recent studies by Scherer and co-workers show that both the hydraulic and crystallization pressures damage the concrete in freeze–thaw cycles, and one may dominate the other under different conditions.9
Conventional methods used to counter freeze–thaw deterioration in cementitious materials include entrainment of 5–10% air in the cement mixture and application of deicing salts and/or coatings on the concrete surface. Introducing air voids protects the concrete matrix from frost damage as these offer space for the expansion of ice inside the material.5 Air entrainment is effective up to a certain extent but disadvantageously facilitates the penetration of aggressive fluids into the concrete and reduces its mechanical properties.10,11 Deicing salts lower the freezing point of water on concrete surfaces and in the porous structure but result in chloride-induced leaching of calcium hydroxide and other hydration products, leading to corrosion of the steel reinforcements and scaling. More importantly, saturation by deicing salts will induce more damage.12−14 Surface treatment with suitable coatings can decrease water transport into the concrete matrix, but current strategies usually suffer from an increased probability of crack propagation and elevated costs.15 For lack of a better alternative, the above methods to mitigate frost damage to concrete have been used for over 70 years, despite their disadvantages.
We set out to develop a simple method to improve the freeze–thaw durability of cementitious materials, using small quantities of a suitable additive that reduces ice formation and growth without sacrificing the mechanical properties of the final material. For this purpose, we selected poly(vinyl alcohol) (PVA), which is a commodity polymer known for its ability to drastically slow ice crystal growth by recrystallization processes.16 PVA is not only the most potent synthetic ice recrystallization inhibitor by far but also also relatively easy and inexpensive to synthesize and abundantly available.17−21 Its ice recrystallization inhibition ability has been related to the good match between the conformation of atactic PVA and the ice lattice on the primary and secondary prism planes.22,23 In the present study we investigate whether PVA improves the freeze–thaw durability of Portland cement concrete. We further evaluate the ice recrystallization inhibition (IRI) activity of PVA in a cementitious alkaline environment and the impact of PVA on key structural and mechanical properties of cement mortar. Gratifyingly, we find that <0.1 wt % of PVA displays excellent ice recrystallization inhibition in the saturated Ca(OH)2 solution (pH = 12.5, similar to the pore solution of concrete) and significantly reduces the surface scaling of concrete, while it does not affect cement hydration, microstructure, nor its mechanical properties. These findings disclose a promising route toward concrete with excellent freeze–thaw resistance with great potential for large-scale industrial application.
2. Experimental Section
Materials
Saturated Ca(OH)2 solution was prepared by dissolving calcium hydroxide (Aldrich, ≥95%) in ultrapure water (18.2 MΩ). The pH of the solution is 12.5, which is close to the value of pore solution of concrete. Atactic poly(vinyl alcohol) (Aldrich), with a weight-average molar mass of Mw = 146 000–186 000 g mol–1 and a degree of hydrolysis of >99%, was added at 4 × 10–3 M monomer concentration (≈0.18 g L–1) to the saturated Ca(OH)2 solution as well as to the mortar specimens. This concentration was previously shown to be sufficiently high to inhibit ice recrystallization;20 however, it has not been proven to be active in a saturated Ca(OH)2 solution. Sucrose (BioUltra, ≥99.5%) was added in 30 wt % to the pore solutions to perform sucrose sandwich IRI assays.23,24 Cover slides (24 × 24 mm Menzel glasses, ThermoScientific) were purchased from VWR and cleaned by sonication in ultrapure water and technical grade acetone (Aldrich) for 10 min, followed by drying with N2 flow. For mortar specimen preparation, the PVA was added to tap water (hardness ∼1.1 mmol L–1, pH ∼ 7.7) and dissolved by stirring the mixture at 90 °C for 2 h. Mortar specimens were prepared with a water/cement ratio of 0.5, applying CEM III/A 52.5 N and standard sand, with and without the addition of 0.018 wt % (4 × 10–3 M monomer concentration) of PVA to the water (Table 1).
Table 1. Recipe of the Mixture for Mortar Specimen Preparation [kg/m3].
| sample | cement (kg/m3) | sand (kg/m3) | water (kg/m3) | PVA (kg/m3) | air content (%) |
|---|---|---|---|---|---|
| reference | 450 | 1350 | 225 | 0 | 1.8 |
| PVA-modified | 450 | 1350 | 225 | 0 | 1.7 |
Methods
Ice recrystallization inhibition assays were performed using a Nikon ECLIPSE Ci-Pol optical microscope controlling the temperature with a Linkam LTS 420 stage, following the sucrose sandwich assay as described elsewhere.25 A home-built analysis script in ImageJ was used to determine the area of each ice crystal, and an equivalent ice crystal radius is calculated. To quantify the ice recrystallization driven by Ostwald ripening, the rate of ice recrystallization (kd) was calculated by plotting the cubic number-average radius (⟨r⟩3) versus time as described previously.26
Mortar was prepared by mixing cement, sand, and water in a laboratory mixer at a low speed for 30 s and rested for 30 s before another 120 s of mixing at a medium speed. Here, PVA was first mixed with water prior to the mortar preparation. The fresh mortar was cast into molds with dimensions of 40 mm × 40 mm × 160 mm. The prisms were demolded 24 h after casting and cured at 100% RH at about 21 °C. After curing for 7 and 28 days, the flexural and compressive strengths of the specimens were tested according to EN 196-1.27 At least three specimens for the flexural strength and six for the compressive strength were tested at each age to compute the average strength and standard deviation.
The air content of the mortar samples is determined according to EN 12350-728 by the air content pressure method. The pore size distribution was measured using mercury intrusion porosimetry (MIP, Autopore IV, Micromeretics), with a maximum applied mercury pressure of 228 MPa, contact angle of 130°, and equilibration time of 20 s. The pore size range was set to 0.0063–900 μm. X-ray diffraction (XRD) was performed on diffractometry equipped with a Cu tube (40 kV, 30 mA) with a scanning range from 5° to 65° 2θ, applying a step 0.02° and 5 s/step measuring time. The qualitative analysis was carried out using the Diffracplus Software (Bruker AXS) and the PDF database of ICDD. Isothermal calorimetry tests were conducted at 20 °C in a TAM AIR calorimeter following CEM III/A 52.5 N.
The surface scaling freeze–thaw test was performed following CEN/TS 12390-9.29 The mortar specimens were cast in PVC tubes with a diameter of 100 mm and height of 60 mm. After 1 day, the mortar was demolded and cured at 100% RH for 27 days. Three specimens were tested for each mix, resulting in a total exposed surface area of 0.024 m2. After saturation, the freeze–thaw test was carried out with a 3 mm layer of demineralized water poured on the top surface. The temperature profile in the climate chamber followed the standard recommendations (Figure 1).29 The water level on the surface of the samples was adjusted regularly. In total, 56 freeze–thaw cycles were applied, during which the surface scaling was measured weekly.
Figure 1.
Temperature profile for the 24 h long freeze–thaw cycle, following the CEN standard.
3. Results and Discussion
Ice Recrystallization of a Saturated Ca(OH)2 (Artificial Cement Pore Solution) Solution
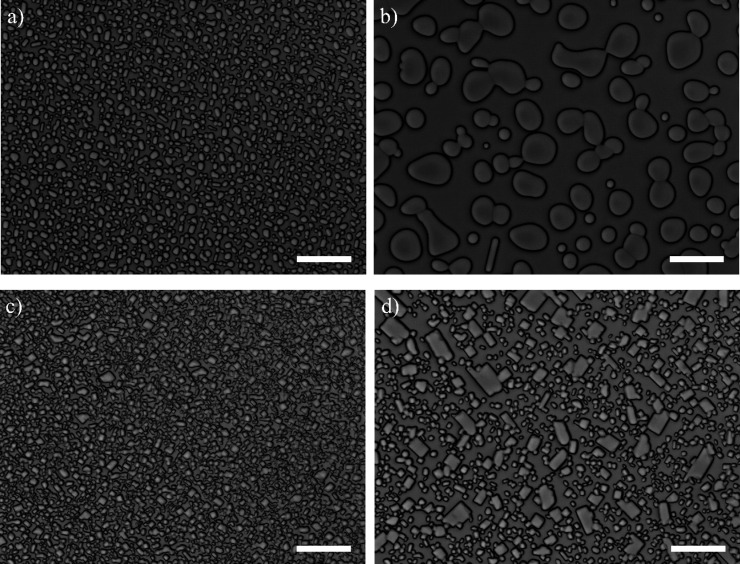
To probe whether PVA hampers ice recrystallization in a saturated Ca(OH)2 solution, we monitor the process with and without 0.018 wt % PVA in the presence of 30 wt % sucrose to warrant a low ice volume fraction. As expected,26 large ice crystals grow at the expense of small ones in both samples during 60 min annealing at −7.0 °C (Figure 2). Over time, the mean ice crystal size increases, while the total ice crystal number density decreases. Noticeable differences in mean crystal size and shape become more pronounced over time. The ice crystals in the PVA-containing solution remain small and are shaped into rectangular morphologies with sharper edges compared to the larger crystals formed in the solutions without PVA, which have relatively round shapes.
Figure 2.
Microphotographs of samples annealed at −7.0 °C in a saturated Ca(OH)2 solution without (a,b) and with 0.018 wt % PVA (c, d), at t = 0 min for (a) and (c) and t = 60 min for (b) and (d). The scale bars represent 50 μm.
We use the Lifshitz, Slyozov, and Wagner (LSW) theory of Ostwald ripening to quantify the observed differences in ice crystal growth kinetics caused predominantly by differential migratory recrystallization rates.26 It states that the temporal increase in cubic mean radius at constant volume fraction, temperature, and pressure can be expressed as
| 1 |
Here, ⟨r⟩(0) (μm) is the initial mean radius at time t = 0 min, and kd (μm3 min–1) is the observed rate constant of recrystallization. This rate constant is thus extracted from the slope of the cubed number-average mean radius versus time (Figure 3). In the pore solution (Figure 2a and b), small ice crystals of around one micrometer in radius grow into larger crystals of tens of micrometers within 60 min. Addition of PVA hampers this process (Figure 2c and 2d), lowering the ice growth rate constant by more than 99% from kd = 15 μm3 min–1 to kd < 0.1 μm3 min–1 upon addition of just 0.18 g L–1 of the polymer (Figure 3). PVA is thus highly IRI-active in the alkaline cement pore solution. As a comparison, we also report the ice growth rates as determined in water at neutral pH, in the absence and presence of the same concentration of PVA. The rate constant obtained in water, kd = 3.5 μm3 min–1, is significantly lower than that in the pore solution, which we attribute to the absence of salts in this sample, compared to the saturated solution. The higher ice growth rate in the pore solution, compared to pure water, further stresses the need for IRI-active additives, as the presence of the salt also increases the recrystallization rates. For the polymer solution at neutral pH, we find kd = 0.17 μm3 min–1, which is slightly higher than that found in the calcium hydroxide solution. This result is in line with our expectation, as a higher pH has been shown before to increase the IRI activity of PVA, probably due to promotion of hydrogen bonding between the polymer and the ice crystal lattice.30
Figure 3.
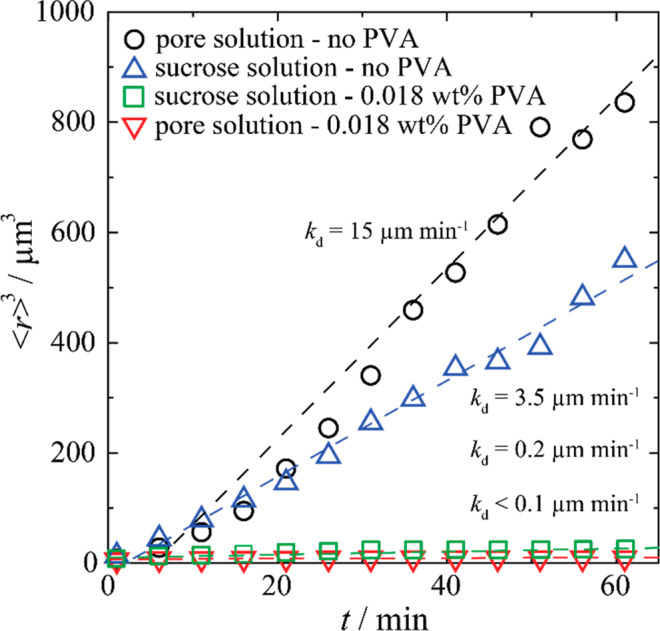
Ice growth rate constant (kd) is determined from the slope of the cubic mean crystal radius (⟨r⟩3) versus time for saturated Ca(OH)2 solution without (black circles) and with 0.018 wt % PVA (red trianlges) containing 30 wt % sucrose. As a comparison, the ice growth rate is shown for sucrose solution at neutral pH without (blue triangles) and with the same PVA concentration (green squares).
Hydration Kinetics and Reaction Products
Adding extra compounds to the concrete mixture can affect its curing process. Therefore, it is important to evaluate the impact of PVA addition on the hydration rate and products of the cement mixture. Isothermal calorimetry gives insight into the reaction kinetics by monitoring the heat release per solid mass over 40 h (Figure 4a and b). The calorimetric peaks of the initial dissolution stage with significant higher heat flow during the first few minutes are shown in the inset of Figure 4a, corresponding to wetting and dissolution of raw materials. It can be seen that the addition of PVA has little influence on this process as the two curves present very similar shapes. Both the reference and PVA-modified mixtures display four typical reaction stages (Figure 4a): initial dissolution (I), induction (II), acceleration (III), and a deceleration period (IV), in accordance with a previous study.31,32 These stages correspond to the initial wetting and dissolution of the raw materials (I), the formation of a protective phase inhibiting dissolution or delayed nucleation and growth (II), the massive formation of reaction products (III), and a reaction that was slowed down by forming a diffusion barrier at the surface of reaction products and water deficiency (IV). Evidently, PVA addition does not significantly impact the hydration kinetics as the calorimetric profiles of the PVA-modified and reference samples are virtually identical within the experimental error. An acceleration peak at the same time point of equal magnitude indicates that reaction products appear in similar quantities in stage III after approximately 10 h in both samples. X-ray diffraction profiles further reveal that the composition and phase state of the hydrated cement are also unaltered by PVA addition (Figure 4c). The XRD results of the paste samples show typical reaction products of hydrated cement paste. The peaks at 2θ = 18° and 34° are portlandite, and the peaks at 2θ = 29° are silicate hydrate (CSH).
Figure 4.
(a) Heat evolution (initial profile shown in the inset), (b) cumulative heat evolution, and (c) XRD patterns of the reference (dashed black lines) and PVA-modified (red solid lines) cement mixtures. Peaks correspond to ettringite, calcium aluminate hydrate, portlandite (CH), melilite, quartz, calcium silicate hydrate (C–S–H), dicalcium silicate (C2S), and tricalcium silicate (C3S).
Structural Integrity
To critically assess whether it is advantageous to add PVA to enhance the freeze–thaw resistance of cementitious materials, we next evaluated by mercury intrusion porosimetry and mechanical testing if the required PVA dosage does not deteriorate the structural integrity and mechanical properties of the concrete. The differential intrusion curves of reference and PVA-modified samples demonstrate a pore size distribution with two main peaks belonging to the capillary pores (Figure 5a). The first and second peaks are located around 10–40 nm and 0.05–0.1 μm, respectively, which all are remnants of the original water-filled space between the cement particles. The striking similarity in the pore size distribution curves implies a preservation in total porosity and rules out differences in effective volume upon the addition of low dosages of PVA.
Figure 5.
(a) Differential (with symbols) and cumulative (lines) pore volume distribution curves for the reference (dashed black line) and PVA-modified (solid red line) mortar specimens. (b) Flexural and compressive strengths of mortar specimens at three different ages show no significant influence of 0.018 wt % PVA addition (red triangle) compared to the reference (black circle). Dashed line and displaced data points for visual aid. Error bars indicate standard deviation between specimens.
It is equally important that PVA addition does not weaken the mechanical properties of concrete. Gratifyingly, measurements of the flexural and compressive strength of reference and PVA-modified samples show the same trend of increasing resistance to flexural and compressive stresses upon aging (Figure 5b). No significant differences were observed between samples. We ascribe this to the almost identical porosity of the samples since the amount of air entrainment in cement is one of the key determinants of its mechanical properties of cement. Our findings contrast with previous studies which reported that the addition of PVA to concrete mixtures decreased their mechanical properties due to generation of more pores after hydration of the cement.33 We do not observe such an adverse effect, due to the much lower amount of PVA used in this study (0.018 wt %) compared to the dosage of 2 wt % in the previous study.33 Our findings thus confirm that PVA can be applied at sufficiently low concentrations to strongly inhibit ice recrystallization without any decline in mechanical strength, which is of high importance for cementitious materials.
Freeze–Thaw Resistance
Having confirmed the IRI efficacy of a low PVA dosage in pore solutions without compromising the hydration process (Figure 4), porosity, and mechanical properties of the cement (Figure 5), we study its effect on the stability of concrete in freezing environments. Freeze–thaw tests were performed for 56 cycles of 24 h, during which the surface scaling of concrete was measured every 7 days. The weight loss of the samples in the surface scaling test is shown in Table 2.
Table 2. Mass Loss of the Samples in the Surface Scaling Test [g].
| freeze–thaw cycles | 0 | 1 | 7 | 14 | 21 | 28 | 35 | 42 | 49 | 56 |
|---|---|---|---|---|---|---|---|---|---|---|
| ref | 0 | 0.01 | 0.13 | 0.25 | 0.53 | 0.83 | 1.15 | 1.78 | 2.30 | 3.06 |
| PVA-modified | 0 | 0.01 | 0.04 | 0.11 | 0.19 | 0.33 | 0.49 | 0.68 | 0.94 | 1.23 |
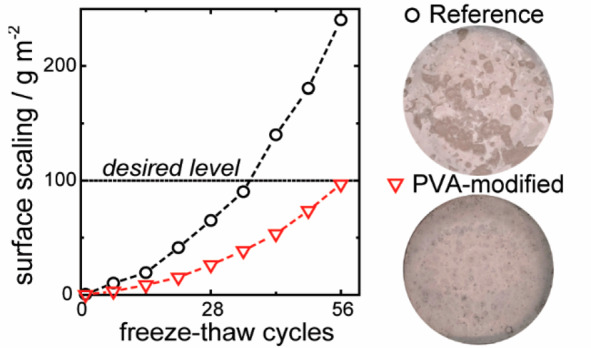
The total surface scaling measured for the reference specimen after 56 cycles is 241 g m–2, while that for the PVA-modified sample is only 99 g m–2 as shown in Figure 6. A small amount of PVA is thus sufficient to reduce surface scaling by nearly 60%, which is below 100 g m–1 and thus classified as “very good” in terms of freeze–thaw stable concrete using the Borås method.34,35 A close relationship between the pore structure and the freeze–thaw durability of concrete was proposed previously.36 However, in this study, we find no such relation. The pore structure and the air content of the concrete are unaffected by the low PVA dosage, while the freeze–thaw durability is enhanced more than 2-fold. This means that the reduced surface scaling must originate from another characteristic of PVA. We attribute its impact on freeze–thaw resistance to the observed ice recrystallization inhibition activity of PVA in the pore solution (Figure 2).
Figure 6.
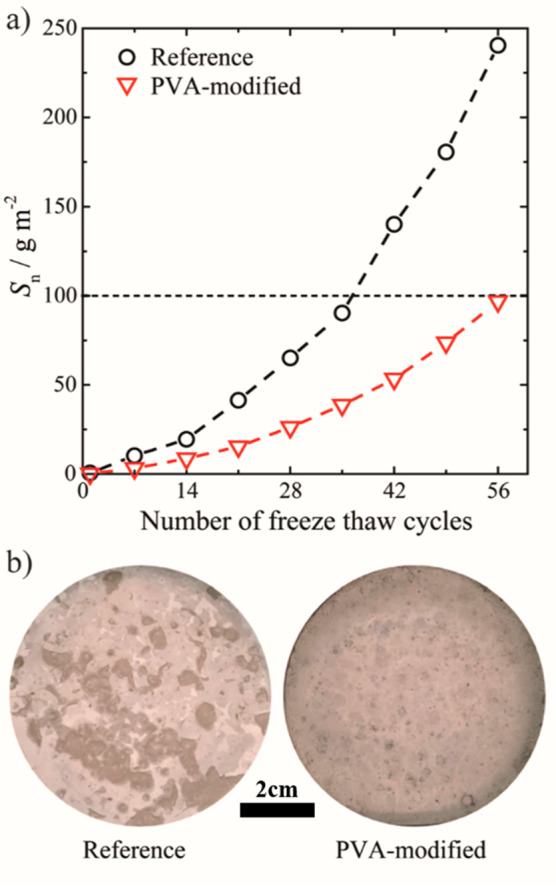
(a) Cumulative surface scaling (Sn in g m–2) measured weekly for the duration of 56 freeze–thaw cycles for reference (black circles) and 0.04 kg m–3 PVA-modified (red triangles) mortar specimens. The low dosage of PVA reduced the total surface scaling by more than 2-fold. (b) Reference and PVA-modified specimen surface showing a distinct difference in surface scaling after 56 days.
4. Conclusions
Ice recrystallization in concrete pore solution is hypothesized to cause deterioration to cementitious materials upon exposure to freeze–thaw cycles. This study shows the effect of PVA addition on the freeze–thaw resistance of concrete. The ice recrystallization process of saturated Ca(OH)2 solution is assessed, and the poly(vinyl alcohol) (PVA) is shown to reduce the ice crystal growth rate by over 99% at a monomer concentration of only 4 × 10–3 M (≈0.18 g L–1). The structural and mechanical integrity of PVA-modified specimens were investigated to rule out that this advantage is offset by an adverse impact of polymer addition on, e.g., the porosity, flexural, and compressive strengths of the concrete. Our results show that neither the cement hydration kinetics and products nor the pore structure of the cement matrix is affected by PVA addition because of the low dosage. Furthermore, the mechanical strength of the modified concrete remains unaltered, indicating a high engineering application potential. Most notably, PVA addition (0.04 kg m–3) significantly improved the freeze–thaw resistance of concrete, as the surface scaling of the prepared concrete after 56 freeze–thaw cycles decreased dramatically from 241 g m–2 to 99 g m–2 compared to the reference sample. This is attributed to the ability of PVA to inhibit ice recrystallization in the pore solution. We hypothesize that when water enters the pores and starts to crystallize upon cooling, the added PVA will adsorb to embryonic crystals, blocking their growth and helping to keep the pore structure intact. Our results thus disclose great potential for PVA as an additive to improve the freeze–thaw stability of cementitious materials.
Acknowledgments
Z.Y.Q. is grateful to the China Scholarship Council (20160695006) for its financial support. I.K.V. is grateful to the European Union (ERC-2014-StG Contract No. 635928), the Dutch Science Foundation (NWO ECHO Grant No. 712.016.0002), and the Dutch Ministry of Education, Culture and Science (Gravity Program 024.001.035) for their financial support.
Author Contributions
Z.Y.Q. and S.Q.G. conceived and initiated the project. Z.Y.Q., S.Q.G., C.C.M.S., R.S.V., and I.K.V. designed the experimental work. Z.Y.Q., S.Q.G., C.C.M.S., and R.S.V. performed the experimental work. S.Q.G. and I.K.V. supervised the research tasks. All authors analyzed experimental results. Z.Y.Q. and S.Q.G. wrote the initial draft of the manuscript. C.C.M.S., R.S.V., Q.L.Y., and I.K.V. coedited the manuscript. All authors reviewed the manuscript before submission.
The authors declare no competing financial interest.
References
- Sun Z.Mechanism of Frost Damage to Concrete; Princeton University, 2010. [Google Scholar]
- Portland Cement Association . 2016 U.S. Cement Industry Annual Yearbook, 2016. [Google Scholar]
- ICRI . Vision 2020: A Vision for the Concrete Repair Protection and Strengthening Industry; 2006; pp 1–25. [Google Scholar]
- Powers T. C. A Working Hypothesis for Further Studies of Frost Resistance of Concrete. Journal Proceedings 1945, 41, 245–272. [Google Scholar]
- Powers T. C.; Willis T. F.. The Air Requirement of Frost Resistant Concrete. Highway Research Board Proceedings 1950; Vol. 29.
- Beaudoin J. J.; MacInnis C. The Mechanism of Frost Damage in Hardened Cement Paste. Cem. Concr. Res. 1974, 4 (2), 139–147. 10.1016/0008-8846(74)90128-8. [DOI] [Google Scholar]
- Scherer G. W. Crystallization in Pores. Cem. Concr. Res. 1999, 29 (8), 1347–1358. 10.1016/S0008-8846(99)00002-2. [DOI] [Google Scholar]
- Yang R.; Lemarchand E.; Fen-Chong T.; Azouni A. A Micromechanics Model for Partial Freezing in Porous Media. Int. J. Solids Struct. 2015, 75, 109–121. 10.1016/j.ijsolstr.2015.08.005. [DOI] [Google Scholar]
- Sun Z.; Scherer G. W. Effect of Air Voids on Salt Scaling and Internal Freezing. Cem. Concr. Res. 2010, 40 (2), 260–270. 10.1016/j.cemconres.2009.09.027. [DOI] [Google Scholar]
- Yang Q.; Zhu P.; Wu X.; Huang S. Properties of Concrete with a New Type of Saponin Air-Entraining Agent. Cem. Concr. Res. 2000, 30 (8), 1313–1317. 10.1016/S0008-8846(00)00340-9. [DOI] [Google Scholar]
- Spiesz P.; Yu Q. L.; Brouwers H. J. H. Development of Cement-Based Lightweight Composites - Part 2: Durability-Related Properties. Cem. Concr. Compos. 2013, 44, 30–40. 10.1016/j.cemconcomp.2013.03.029. [DOI] [Google Scholar]
- Litvan G. G. Frost Action in Cement in the Presence of De-Icers. Cem. Concr. Res. 1976, 6 (3), 351–356. 10.1016/0008-8846(76)90097-1. [DOI] [Google Scholar]
- Wang K.; Nelsen D. E.; Nixon W. A. Damaging Effects of Deicing Chemicals on Concrete Materials. Cem. Concr. Compos. 2006, 28 (2), 173–188. 10.1016/j.cemconcomp.2005.07.006. [DOI] [Google Scholar]
- Khan M. U.; Ahmad S.; Al-Gahtani H. J. Chloride-Induced Corrosion of Steel in Concrete: An Overview on Chloride Diffusion and Prediction of Corrosion Initiation Time. Int. J. Corros. 2017, 2017, 1. 10.1155/2017/5819202. [DOI] [Google Scholar]
- Liu Z.; Hansen W. Effect of Hydrophobic Surface Treatment on Freeze-Thaw Durability of Concrete. Cem. Concr. Compos. 2016, 69, 49–60. 10.1016/j.cemconcomp.2016.03.001. [DOI] [Google Scholar]
- Voets I. K. From Ice-Binding Proteins to Bio-Inspired Antifreeze Materials. Soft Matter 2017, 13 (28), 4808–4823. 10.1039/C6SM02867E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congdon T.; Notman R.; Gibson M. I. Antifreeze (Glyco) Protein Mimetic Behavior of Poly (Vinyl Alcohol): Detailed Structure Ice Recrystallization Inhibition Activity Study. Biomacromolecules 2013, 14 (5), 1578–1586. 10.1021/bm400217j. [DOI] [PubMed] [Google Scholar]
- Budke C.; Dreyer A.; Jaeger J.; Gimpel K.; Berkemeier T.; Bonin A. S.; Nagel L.; Plattner C.; DeVries A. L.; Sewald N. Quantitative Efficacy Classification of Ice Recrystallization Inhibition Agents. Cryst. Growth Des. 2014, 14 (9), 4285–4294. 10.1021/cg5003308. [DOI] [Google Scholar]
- Olijve L. L. C.; Hendrix M. M. R. M.; Voets I. K. Influence of Polymer Chain Architecture of Poly (Vinyl Alcohol) on the Inhibition of Ice Recrystallization. Macromol. Chem. Phys. 2016, 217 (8), 951–958. 10.1002/macp.201500497. [DOI] [Google Scholar]
- Biggs C. I.; Bailey T. L.; Stubbs C.; Fayter A.; Gibson M. I. Polymer Mimics of Biomacromolecular Antifreezes. Nat. Commun. 2017, 8 (1), 1546. 10.1038/s41467-017-01421-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sproncken C. C. M.; Surís-Valls R.; Cingil H. E.; Detrembleur C.; Voets I. K. Complex Coacervate Core Micelles Containing Poly (Vinyl Alcohol) Inhibit Ice Recrystallization. Macromol. Rapid Commun. 2018, 39, 1700814. 10.1002/marc.201700814. [DOI] [PubMed] [Google Scholar]
- Inada T.; Lu S.-S. Inhibition of Recrystallization of Ice Grains by Adsorption of Poly (Vinyl Alcohol) onto Ice Surfaces. Cryst. Growth Des. 2003, 3 (5), 747–752. 10.1021/cg0340300. [DOI] [Google Scholar]
- Budke C.; Koop T. Ice Recrystallization Inhibition and Molecular Recognition of Ice Faces by Poly (Vinyl Alcohol). ChemPhysChem 2006, 7 (12), 2601–2606. 10.1002/cphc.200600533. [DOI] [PubMed] [Google Scholar]
- Smallwood M.; Worrall D.; Byass L.; Elias L.; Ashford D.; Doucet C. J.; Chris H.; Telford J.; Lillford P.; Bowles D. J. Isolation and Characterization of a Novel Antifreeze Protein from Carrot (Daucus Carota). Biochem. J. 1999, 340 (2), 385–391. 10.1042/0264-6021:3400385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olijve L. L. C.; Oude Vrielink A. S.; Voets I. K. A Simple and Quantitative Method to Evaluate Ice Recrystallization Kinetics Using the Circle Hough Transform Algorithm. Cryst. Growth Des. 2016, 16 (8), 4190–4195. 10.1021/acs.cgd.5b01637. [DOI] [Google Scholar]
- Budke C.; Heggemann C.; Koch M.; Sewald N.; Koop T. Ice Recrystallization Kinetics in the Presence of Synthetic Antifreeze Glycoprotein Analogues Using the Framework of LSW Theory. J. Phys. Chem. B 2009, 113 (9), 2865–2873. 10.1021/jp805726e. [DOI] [PubMed] [Google Scholar]
- BS-EN-196-1. Methods of Testing Cement - Part 1: Determination of Strength. Br. Stand. Institution-BSI CEN Eur. Comm. Stand. 2005. 10.1111/j.1748-720X.1990.tb01123.x. [DOI] [Google Scholar]
- En N. F. 12350-7. Test. Fresh Concr. 7 Air Content—Pressure Methods 2012.
- Testing A.Testing hardened concrete - Part 9: Freeze-thaw resistance – Scaling. CEN/TS 12390-9. CEN (European Committee for Standardization), 2006.
- Burkey A. A.; Riley C. L.; Wang L. K.; Hatridge T. A.; Lynd N. A. Understanding Poly (Vinyl Alcohol)-Mediated Ice Recrystallization Inhibition through Ice Adsorption Measurement and PH Effects. Biomacromolecules 2018, 19 (1), 248–255. 10.1021/acs.biomac.7b01502. [DOI] [PubMed] [Google Scholar]
- Ma S.; Qian Y.; Kawashima S. Experimental and Modeling Study on the Non-Linear Structural Build-up of Fresh Cement Pastes Incorporating Viscosity Modifying Admixtures. Cem. Concr. Res. 2018, 108, 1–9. 10.1016/j.cemconres.2018.02.022. [DOI] [Google Scholar]
- Yuan B.; Yu Q. L.; Brouwers H. J. H. Reaction Kinetics, Reaction Products and Compressive Strength of Ternary Activators Activated Slag Designed by Taguchi Method. Mater. Des. 2015, 86, 878–886. 10.1016/j.matdes.2015.07.077. [DOI] [Google Scholar]
- Kim J.-H.; Robertson R. E.; Naaman A. E. Structure and Properties of Poly (Vinyl Alcohol)-Modified Mortar and Concrete1. Cem. Concr. Res. 1999, 29 (3), 407–415. 10.1016/S0008-8846(98)00246-4. [DOI] [Google Scholar]
- Józwiak-Niedzwiedzka D. Scaling Resistance of High Performance Concretes Containing a Small Portion of Pre-Wetted Lightweight Fine Aggregate. Cem. Concr. Compos. 2005, 27 (6), 709–715. 10.1016/j.cemconcomp.2004.11.001. [DOI] [Google Scholar]
- Józwiak-Niedzwiedzka D. Scaling Resistance of Different Concretes Tested with Borås Method. International Symposium on Non-Traditional Cement & Concrete, Brno 2002, 463–472. [Google Scholar]
- Cai H.; Liu X. Freeze-Thaw Durability of Concrete: Ice Formation Process in Pores. Cem. Concr. Res. 1998, 28 (9), 1281–1287. 10.1016/S0008-8846(98)00103-3. [DOI] [Google Scholar]