Abstract
Physical activity decreases the risk of a network of diseases, and exercise may be prescribed as medicine for lifestyle-related disorders such as type 2 diabetes, dementia, cardiovascular diseases, and cancer. During the past couple of decades, it has been apparent that skeletal muscle works as an endocrine organ, which can produce and secrete hundreds of myokines that exert their effects in either autocrine, paracrine, or endocrine manners. Recent advances show that skeletal muscle produces myokines in response to exercise, which allow for crosstalk between the muscle and other organs, including brain, adipose tissue, bone, liver, gut, pancreas, vascular bed, and skin, as well as communication within the muscle itself. Although only few myokines have been allocated to a specific function in humans, it has been identified that the biological roles of myokines include effects on, for example, cognition, lipid and glucose metabolism, browning of white fat, bone formation, endothelial cell function, hypertrophy, skin structure, and tumor growth. This suggests that myokines may be useful biomarkers for monitoring exercise prescription for people with, for example, cancer, diabetes, or neurodegenerative diseases.
Keywords: metabolism, cytokines, exercise, physical activity, diabetes, cancer
Graphical Abstract
Graphical Abstract.
Essential Points.
Myokines are defined as cytokines and other peptides that are produced, expressed and released by muscle fibers and exert either autocrine, paracrine, or endocrine effects
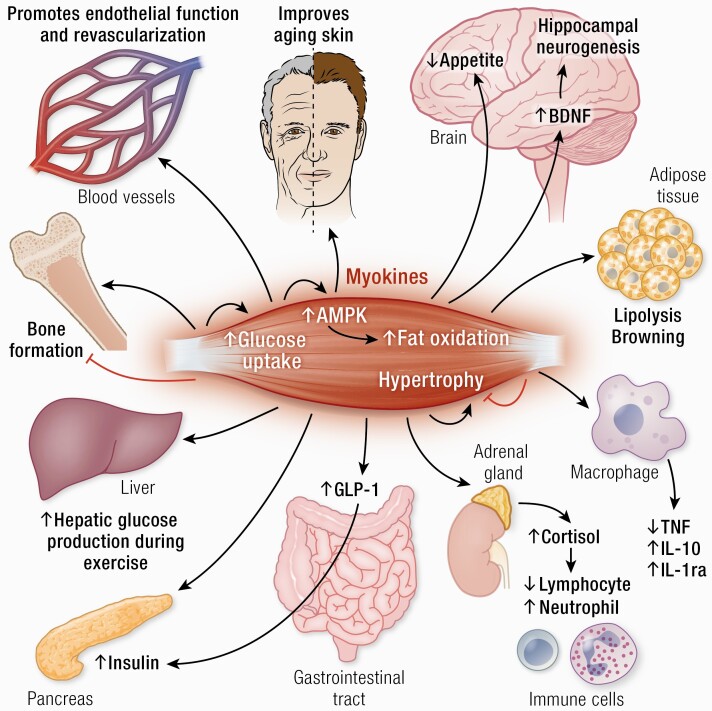
Myokines mediate communication between muscle and other organs, including brain, adipose tissue, bone, liver, gut, pancreas, vascular bed, and skin, as well as within the muscle itself
Myokines exert their effects on, for example, cognition, lipid and glucose metabolism, browning of white fat, bone formation, endothelial cell function, hypertrophy, skin structure, and tumor growth
The myokine IL-6 mediates the exercise-associated anti-inflammatory effects both acutely with each bout of exercise and as a consequence of training adaptation, including reduction in abdominal adiposity.
The identification of new myokines and their specific roles may lead to novel therapeutic targets
Myokines can be useful biomarkers for monitoring the type and amount of exercise that are required for the prescription of exercise for people with, for example, cancer, diabetes, or neurodegenerative diseases
Within the society of human integrative physiology, the awareness of an exercise factor that is able to mediate exercise-induced changes in other organs such as liver and adipose tissue dates back more than 50 years. It was clear that signaling pathways from exercising skeletal muscle to other organs were not solely mediated via the nervous system, since electrical stimulation of paralyzed muscles in patients with no efferent or afferent nerve impulses induced the same types of physiological changes as were found in healthy human beings (1, 2). Thus, it was obvious that 1 or several humoral factors had to be released from contracting muscles to the blood (3).
Before such factors were identified, they were referred to as the “work factor” or the “exercise factor” (4). Our finding in 2000 that skeletal muscle produced and released interleukin-6 (IL-6) into the circulation (5) as well as research during the subsequent years, demonstrating that IL-6 has multiple metabolic effects in other parts of the body (6), identified IL-6 as an exercise factor and skeletal muscle as a secretory organ with endocrine functions.
Given the multiple physiological, metabolic, and immunological effects of exercise, it was obvious that more than 1 exercise factor was likely to be found. In 2003, we introduced the term “myokines” (4) and suggested that “cytokines and other peptides that are produced, expressed and released by muscle fibers and exert either autocrine, paracrine or endocrine effects should be classified as myokines” (4, 7).
Following the identification of muscle-derived IL-6, it soon became clear that muscles were able to secrete hundreds of peptides. Although the biological function has been described for only 5% of all known myokines, the identification of the myokinome has provided a new paradigm and a conceptual basis for understanding by which mechanisms muscles communicate with other organs. It has been proposed that the total sum of all exercise-induced factors (such as peptides and nucleic acids) released from muscle and other organs into the blood should be named “exerkines” (8, 9). Exerkines may be released within extracellular vesicles known as exosomes (10), which may contain nucleic acids, peptides, messenger ribonucleic acid (mRNA), microRNA and mitochondrial deoxyribonucleic acid. Although there is an overlap between myokines and exerkines, the present review focuses on myokines.
The role of myokines has previously been reviewed (7, 11-37), identifying more than 650 myokines (38). Some myokines are responsible for mediating energy supply in relation to acute bouts of exercise. Myokines are also involved in muscle proliferation, differentiation, and regeneration independent of exercise (39, 40). During exercise, myokines signal within the muscle and mediate muscle–organ crosstalk to the brain, adipose tissue, bone, liver, gut, pancreas, vascular bed, and skin (7, 29, 30). In addition, myokines with anticancer effects have been recognized (41, 42). The aim of the present review is to provide an update of recent advances within the myokine field.
Muscle–Muscle Crosstalk
Myogenesis
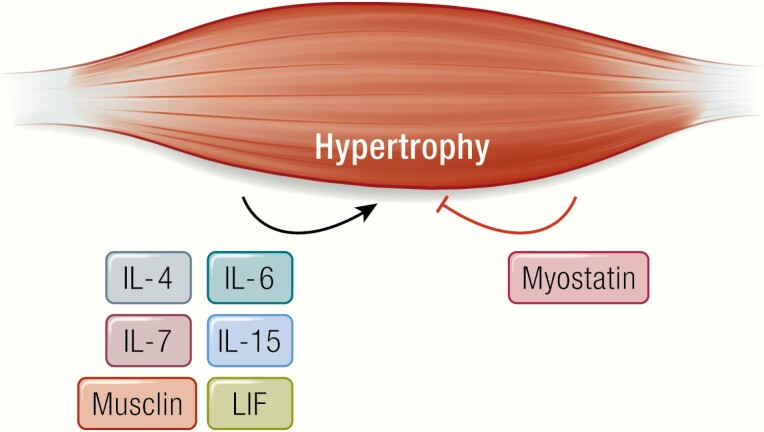
Some myokines exert their effect within skeletal muscle itself and are involved in the regulation of muscle mass (14) (Fig. 1).
Figure 1.
Musclin, LIF, IL-4, IL-6, IL-7, and IL-15 promote muscle hypertrophy. Myostatin inhibits muscle hypertrophy.
Myostatin was the first identified muscle-derived factor that fulfills the myokine criteria as outlined above (43). Myostatin is a member of the transforming growth factor β (TGF-β) superfamily and negatively regulates myogenesis in an autocrine manner (43). Massive muscle hypertrophy is seen in myostatin knockout mice, cattle, sheep, and dogs (43-45) that demonstrate an increase in fiber cross-sectional area and in fiber number.
Decorin has been identified as a myokine that is regulated by exercise and acts as an antagonist to myostatin (46). Circulating levels of decorin are increased in response to exercise in humans (46), whereas exercise training reduces the levels of myostatin within muscles and blood (47, 48).
Although the myokine IL-6 is mostly recognized for its regulatory effects in lipid and glucose metabolism, IL-6 also plays important roles in myogenesis. Muñoz-Cánoves and her team identified IL-6 as an anabolic factor in preclinical models. Genetic loss of IL-6 impaired muscle hypertrophy in vivo, whereas myotube-produced IL-6 stimulated muscle cell proliferation in a paracrine fashion (49).
Leukemia inhibitory factor (LIF) is a member of the IL‑6 cytokine superfamily and has multiple biological functions. LIF protein has been shown to be secreted from human cultured myotubes; when electrically stimulated (50) LIF stimulates satellite cell proliferation (51). It has further been shown that both IL-6 and LIF activate myotube mTORC1 signaling in a time- and dose-dependent fashion (52).
A number of other myokines, including IL‑15 (53) and IL-7 (54) have further been demonstrated to possess anabolic features in rodent models.
Metabolic actions
While IL-6 is characterized as a myokine with endocrine effects, it also works in a paracrine manner exerting metabolic effects within the muscle itself (6, 7).
Physical inactivity is associated with high circulating basal levels of IL-6 in humans (55). Moreover, the acute exercise-induced rise in systemic levels of IL-6 and muscular IL-6 mRNA are diminished by training in humans (56). In contrast, the muscular expression of the IL-6 receptor (IL-6R) is elevated in trained human muscle (57), suggesting that muscular sensitivity to IL-6 is increased by training adaptation. IL-6 signaling within the muscle can affect both glucose uptake and fat oxidation.
It is well documented that IL-6 increases both basal glucose uptake and glucose transporter GLUT4 translocation (58). In addition, IL-6 increases insulin-stimulated glucose uptake in vitro and in healthy humans in vivo. Thus, when recombinant human IL-6 (rhIL-6) was infused into healthy humans together with a hyperinsulinemic, euglycemic clamp, it improved peripheral insulin-stimulated glucose uptake. The effects of IL-6 on glucose uptake in vitro was shown to be mediated by activation of adenosine 5′-monophosphate-activated protein kinase (AMPK) (58). Several other studies have described that IL-6 can increase intramyocellular (58-60) or whole body (61) fatty acid oxidation via AMPK activation (58, 62).
Brain-derived neurotrophic factor (BDNF) is also expressed in human skeletal muscles, but BDNF is not released into the circulation and does not work in an endocrine way. In contrast, BDNF is identified as a myokine capable of enhancing AMPK activation and hence lipid oxidation in an autocrine or paracrine manner (63).
Musclin has been identified as an exercise-induced factor (64) promoting skeletal muscle mitochondrial biogenesis in mice (65). Recent evidence shows that musclin abolishes muscle atrophy related with cancer in mice (66).
Muscle–Brain Crosstalk
Evidence is accumulating that physical exercise has positive health effects on cognitive function and brain health (67, 68). Physical activity and exercise training decrease the risk of dementia (69-71) and appear to play a role in the treatment of this disease (72). In general, it is found that physical activity decreases the rate of cognitive decline in healthy people and in people with neurodegenerative disorders across the life span (73). Moreover, physical exercise has a positive impact on stress, anxiety, and depression (72). Other studies have shown that an active lifestyle is associated with learning and memory (74), executive functions (75), language and reaction time (76), academic achievement in children, and intelligence in adolescents (77). Physical activity has also beneficial effects on appetite (78), sleep (79), and mood (80).
Exercise has been shown to influence the hippocampus more than any other part of the brain. Studies in rodents (81) and humans (82) have shown that exercise increases hippocampus volume and the blood flow to this part of the brain (81). In particular, exercise has been shown to influence neurogenesis in the dentate gyrus (67, 68) and to increase synapse plasticity (67, 68).
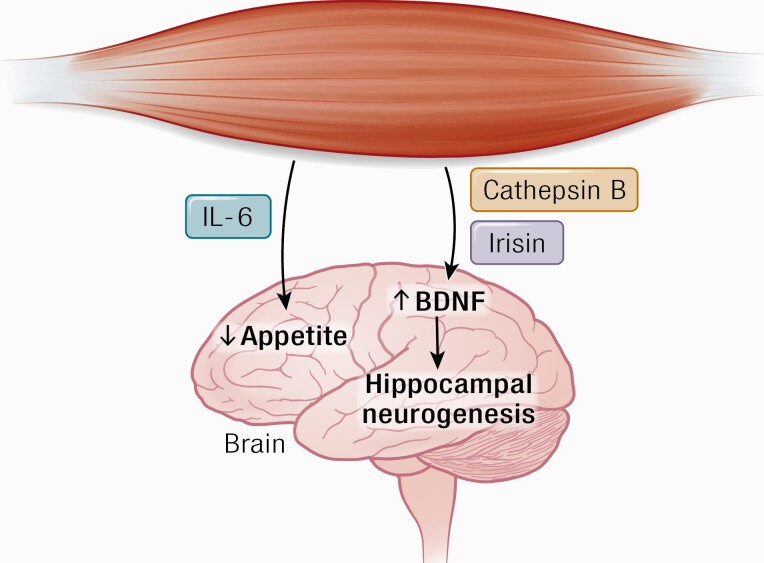
The finding that muscle contraction is sensed by the brain suggests that peripheral factors induced by exercise may be involved in direct crosstalk between working muscle and brain function (7, 29, 30, 83) (Fig. 2).
Figure 2.
Cathepsin B and irisin cross the blood–brain barrier and stimulate BDNF production, which leads to hippocampal neurogenesis. IL-6 stimulates appetite. Abbreviations: BDNF, brain-derived neurotrophic factor.
Cognition, hippocampal neurogenesis, and learning
Recent findings suggest that a muscle–brain endocrine loop exists, which at least in part may be mediated by myokine signaling. Other possible mediators include various metabolites (84), noncoding RNAs (85), hormonal responses, and muscular enzymes with impact on circulating compounds (30). BDNF appears to play a dominant role in mediating the effects of exercise on hippocampus (86). Rodent studies demonstrate increased BDNF mRNA and BDNF protein within the hippocampus in response to wheel running for 1-8 weeks (87-92). Furthermore, BDNF has been shown to be mechanistically linked to exercise-induced improvement in cognitive functions, such as memory and learning (93, 94).
Studies in humans show that BDNF is released from the brain during a bout of bicycle exercise (95, 96), and aerobic exercise training for 3 months increases the volume of the hippocampus in healthy individuals by 12% and by 16% in patients with schizophrenia (97). BDNF is a growth factor for the hippocampus and involved in, for example, cell survival and learning (98). The finding that BDNF is also expressed in human skeletal muscle during exercise is interesting; however, muscle-derived BDNF has not been shown to be released from muscle into the blood stream, thereby mediating a direct muscle–brain interaction (63).
A couple of interesting studies propose that the myokines cathepsin-B and irisin may pass the blood–brain barrier and provoke an increase in BDNF. Moon et al. (99) recently identified a novel myokine, cathepsin B (CTSB) (99) and demonstrated in a series of elegant studies that exercise leads to elevated systemic levels of CTSB, which promote expression of BDNF in the hippocampus and stimulate neurogenesis. Running led to an increased muscular expression of the CTSB gene in mice and an increase in CTSB in plasma from mice, rhesus monkeys, and in humans following treadmill running for 4 months. CTSB was furthermore shown to pass the blood–brain barrier in mice. Moon et al. (99) also performed studies in CTSB knockout mice and showed that mice lacking CTSB were resistant to an effect of voluntary exercise as regards hippocampal growth and improved cognition. It is not known if the myokine CTSB mediates enhanced cognitive functions in humans in response to exercise training (99, 100).
The PGC-1α-dependent myokine irisin, known for its browning effects (101), may also be involved in mediating effects of physical activity on the brain (98). When irisin is overexpressed in primary cortical neurons, it leads to an increase in BDNF expression, whereas RNAi-mediated knockdown of FNDC5 is followed by a reduction of BDNF. Moreover, systemic levels of irisin is elevated when irisin is delivered to the murine liver via adenoviral vectors, which leads to increased levels of BDNF in the hippocampus. It is controversial whether exercise raises plasma concentrations of irisin in humans (102, 103), and whether irisin is involved in a muscle–brain endocrine loop.
Appetite
Elevated levels of IL-6 accompany, for example, obesity and type 2 diabetes (7), and IL-6 is often linked with the metabolic syndrome, not least in animal models (104, 105). However, IL-6 has also been shown to affect metabolic actions beneficially. IL-6-deficient mice gain weight and develop whole-body insulin resistance (106, 107). Other rodent studies show that IL-6 triggers proliferation of pancreatic alpha cells in the obese state (108) and stimulates the production of glucagon-like peptide (GLP)-1 and hence insulin secretion (108). Studies in murine macrophages and hepatocytes show that IL-6 improves glucose homeostasis (109, 110).
Human studies demonstrate that physiological levels of IL-6 have many positive effects, including an enhancement of both insulin-stimulated glucose uptake (58) and lipolysis and fat oxidation (61). IL-6 also delays gastric emptying and thereby exerts effects on postprandial glucose control (111). Infusion of IL-6 to humans stimulates the production of IL-1ra and IL-10 (112) and inhibits endotoxin-induced tumor necrosis factor (TNF) production (113), thereby inducing anti-inflammatory effects.
During muscle work, IL-6 is produced by human contracting skeletal muscle and released into the blood (114) in a TNF-independent fashion (115). The release of IL-6 leads to an exponential rise in circulating concentrations of IL-6 in humans. Systemic IL-6 knockout mice accumulate adipose tissue (106, 107), whereas central overexpression of IL-6 (116, 117) leads to a decrease in body weight, indicating that IL-6 is a player in body weight control. Another murine study demonstrated that lack of muscular IL-6 led to a decrease in body weight and food consumption in response to leptin (118).
A study showed that IL-6 improves glucose tolerance and suppresses feeding when it is applied centrally in mice, but not intraperitoneally at the same dose (119). However, a 4-fold higher IL-6 concentration injected peripherally significantly reduced food intake. This finding suggests that high systemic concentrations of IL-6 can pass the blood–brain barrier and exert central effects on appetite. Thus, it is likely that muscle-derived IL-6, elicited by exercise of long duration and high intensity, may inhibit appetite.
Muscle–Adipose Crosstalk
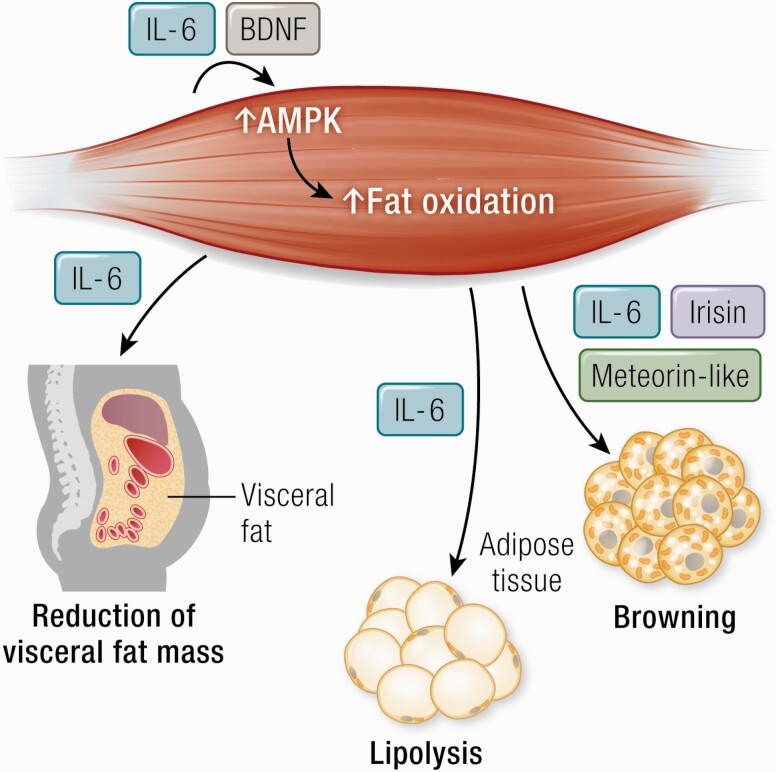
Myokines are involved in the regulation of lipid metabolism in relation to exercise and recent evidence suggests that some myokines may also have the capacity to induce browning of white adipose tissue (Fig. 3).
Figure 3.
IL-6 stimulates lipolysis decreases visceral fat mass. Irisin, meteorin-like, and IL-6 have a role in “browning” of white adipose tissue. IL-6 and BDNF stimulate AMPK activation. Abbreviations: AMPK, 5′-AMP-activated protein kinase; BDNF, brain-derived neurotrophic factor.
Lipolysis
The effect of exercise-induced IL-6 on fat metabolism is one of the most well supported findings (120, 121). In vitro studies and studies in rodents show that IL-6 can enhance lipolysis and fat oxidation, via a mechanism that involves AMPK activation (6). In vivo studies show that rhIL-6 enhances lipolysis and fat oxidation in healthy young and elderly humans (60, 61) and IL-6 autoantibodies appear to be involved in the pathogenesis of a subset of type 2 diabetes (122).
Abdominal adiposity is associated with type 2 diabetes (123), cardiovascular disease (124), dementia (125), colon cancer (126), and breast cancer (127). Abdominal adiposity is also associated with all‐cause mortality, both in obese people and in people with a normal body weight (128). Epidemiological studies clearly show that an association exists between abdominal adiposity and low fitness (129, 130) as well as between abdominal adiposity and low-grade inflammation (129-132). Intervention studies show that physical inactivity promotes an increase in the amount of visceral adipose tissue (29, 133), whereas exercise training diminishes visceral adipose tissue mass (134, 135).
It was, however, not until recently that a mechanism underlying the link between exercise and abdominal fat was established (136). Abdominally obese humans were randomized to tocilizumab (IL-6 receptor antibody) or placebo during an intervention of 12 weeks with either aerobic exercise or no exercise (136, 137). As expected, exercise training led to a reduction in visceral adipose tissue mass. However, this effect was abolished by IL-6 receptor blockade (136). Moreover, IL-6 receptor blockade abolished the exercise-induced loss of cardiac fat (138).
Browning
Brown fat expresses a set of proteins, such as uncoupling protein 1 (UCP1). The fact that white adipose tissue can shift into a brown-like phenotype, the discovery of brown fat in humans, and the potentially beneficial effects of these depots have stimulated a number of studies to explore whether lifestyle, such as exercise, can contribute to induce browning of white fat (12, 17, 139).
In 2012, irisin was reported as a myokine with the ability to brown white adipose tissue in mice. It was shown that muscular PGC1-α expression stimulates an increase in the expression of the membrane FNDC5 that is cleaved and secreted as irisin. Cell culture studies demonstrated that irisin stimulates UCP1 expression and other brown fat-like genes (101). However, while evidence exists that irisin is released from rodent muscle and has browning effects, it is debated whether exercise leads to an increase in plasma irisin levels in humans. The controversy is mainly based on the fact that previous studies have used commercial enzyme-linked immunosorbent assay kits for irisin, which seems to be unspecific (102, 140).
A couple of other exercise-induced myokines with browning effects have been identified. In 2014, Spiegelman’s group (141) identified meteorin-like (Metrnl), a circulating muscle-derived factor, that is induced in muscle after exercise. Metrnl stimulates the expression of genes associated with beige fat thermogenesis, it further stimulates energy expenditure and improves glucose tolerance. Yet, the role of Metrnl in humans remains to be identified.
A world of literature has proven that IL-6 is released from contracting human muscle cells into the circulation and that it contributes to the exponential increase in plasma IL-6 in relation to exercise, reviewed in 29, 120, 142-145. Studies suggest that IL-6 can induce browning of white adipose tissue. Daily intraperitoneal injections of IL-6 to mice for 1 week increased UCP1 mRNA in inguinal white adipose tissue (146).
A study by Kristóf et al. (147) found that IL-6 was mainly produced by fully differentiated adipocytes. When the IL-6 receptor was blocked during differentiation, brown marker genes were downregulated, suggesting that beige adipocytes regulate IL-6 production to enhance browning in an autocrine manner. It remains to be shown that the physiological concentrations of IL-6, released during exercise, have browning effects.
There are a few other circulating factors during exercise, which have the potential to induce browning. β-Aminoisobutyric acid is a small molecule, a nonprotein beta-amino acid, not classified as a myokine, but secreted from myocytes (148, 149). Moreover, β-aminoisobutyric acid has browning effects on human adipocytes (148, 149). In addition, 2 hepatokines appear to play a role in exercise-induced browning of white adipose tissue. The Fibroblast growth factor 21 (FGF-21) (150) and Follistatin (151) are released from human liver during exercise and this release is controlled by the glucagon-to-insulin ratio (152). Evidence exists that both Follistatin (153) and FGF-21 (154) can induce browning of white adipose tissue cells.
The finding that circulating factors during exercise may induce browning of white adipose tissue has so far largely been restricted to rodents and has not been consistently demonstrated in humans (155, 156).
Muscle–Bone Crosstalk
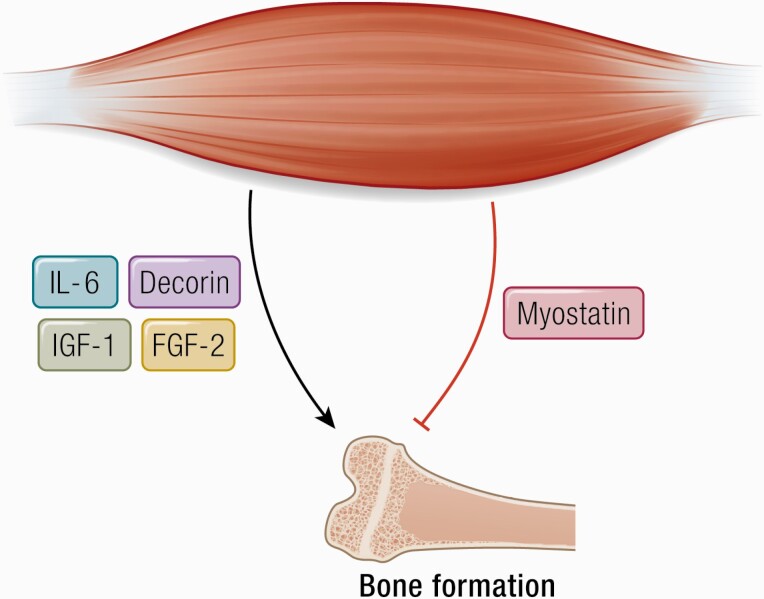
Muscle and bone are closely related during development growth (157), and muscle disuse and/or muscle atrophy result in osteoporosis (158). As pointed out by Guo et al. (159), muscle mass, measured as lean body mass, can only explain up to 20% of the variety in bone mineral density (158) and decreased mechanical loading, as seen with muscle atrophy alone, is not likely to fully explain the loss of bone mass. It is obvious that bone mass could also be regulated by muscle-derived biochemical factors such as myokines (160) (Fig. 4).
Figure 4.
Decorin, IL-6, IGF-1, and FGF-2 positively regulate bone formation. Abbreviations: FGF-2, fibroblast growth factor 2; IGF-1, insulin-like growth factor I.
Studies in mice show that inhibition of the myokine myostatin pathway leads to an increase in bone mass, whereas (161) myostatin reduces osteoclast formation and bone destruction in a TNF-α transgenic mouse model of rheumatoid arthritis (162). Thus, whereas myostatin is a negative regulator of bone, it is a positive regulator of bone resorption.
Overexpression of IL-6 in IL-6 transgenic mice resulted in increased osteoclastogenesis (163). IL-6 appears to induce bone resorption through receptor activator of nuclear factor kappa-Β ligand (RANKL) -dependent enhanced osteoclastogenesis/osteoclast differentiation (164, 165) as well as via osteoblast-derived prostaglandin E2 (PGE2)-dependent osteoclast activation (166-168).
Given that trained people have low circulating basal levels of IL-6, whereas IL-6 increases with each bout of exercise, the interpretation of the findings above only makes sense if it is the chronic basal levels of IL-6 that modulate bone, rather than the acute peaks in IL-6 levels as also pointed out by Banfi (169).
Insulin-like growth factor 1 (IGF-1) has been shown to have a positive effect on bone formation (170). Muscle-derived IGF-1 can act on local osteoblasts that express the IGF-1 receptor and thereby promote bone formation (171).
Osteoglycin is a myokine (172) that appears to inhibit myoblast migration during myogenesis (173). Other myokines have been shown to affect bone metabolism, either positively (IGF-I, FGF-2, IL-15), or negatively (eg, TGF-β) (12, 174).
Muscle–Liver Crosstalk
In order to maintain glucose homeostasis during exercise, glucose uptake in muscle is accompanied by increased glucose production from the liver (175). Mediators of endogenous glucose production include an increase in the portal venous glucagon-to-insulin ratio, epinephrine, and norepinephrine, but these factors cannot alone account for the rapid increase in glucose production (reviewed in, eg, (176)). In 1961, Goldstein (3) suggested that muscle cells might be able to produce a “humoral” component that could contribute to hepatic glucose production.
We infused rhIL-6 into resting human subjects and showed that acute administration of physiological concentrations of rhIL-6 did not influence whole-body glucose disposal, glucose uptake, or endogenous glucose production (177). However, in 2004, we published a study showing that during bicycle exercise IL-6 contributes to the increase in endogenous glucose production. Healthy young males underwent 2 hours of bicycle exercise on 3 separate occasions at (1) a relatively high intensity; (2) a low intensity, and (3) a low intensity + infusion of IL-6 to mimic the plasma levels of IL-6 observed during high-intensity exercise. The study showed the existence of direct muscle–liver crosstalk. Muscle-derived IL-6 plays a role in triggering glucose output from the liver during exercise in humans (176).
A murine study from 2018 showed that IL-6 treatment enhances AKT signaling and reduces gluconeogenic gene expression in livers from low and high fat fed mice, demonstrating that the beneficial effects of IL-6 on glucose and insulin homeostasis, in vivo, are maintained in obesity (178).
Muscle–Gut Crosstalk
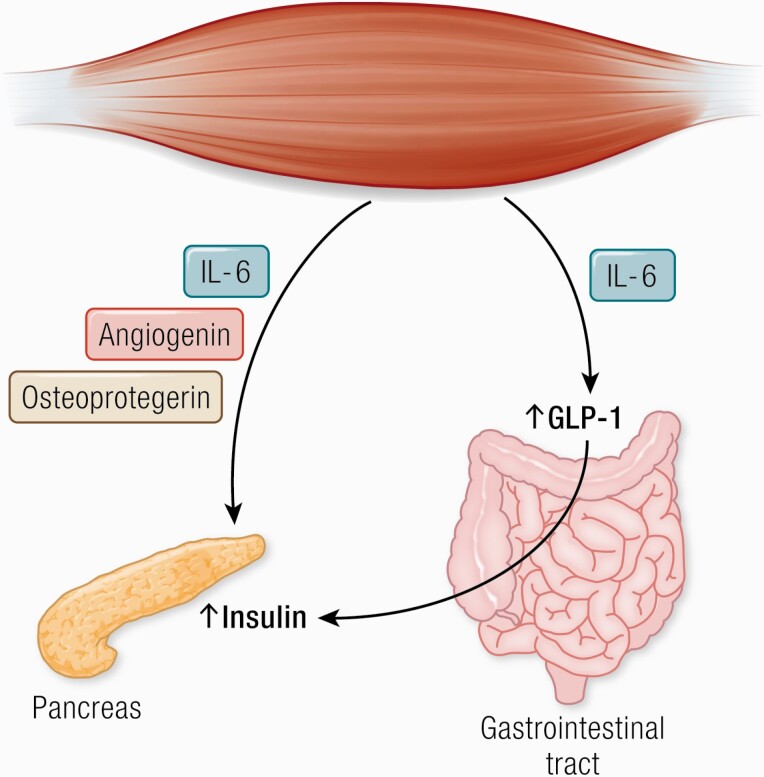
A classic study by Ellingsgaard et al. (108) elegantly showed that acute elevations in IL-6 stimulates GLP-1 secretion from both intestinal L‐cells and pancreatic β‐cells, leading to improved secretion of insulin. This finding implicates IL‐6 in a beneficial regulation of insulin secretion and suggests that IL‐6 is involved in an endocrine loop that may protect against impaired glucose homeostasis (Fig. 5).
Figure 5.
Angiogenin, osteoprotegerin, and IL-6 possess pancreatic β-cell protective actions against proinflammatory cytokines. IL-6 increases insulin secretion by inducing the expression of GLP-1 by the L cells of the intestine. Abbreviations: GLP-1, glucagon-like peptide 1.
A recent study from our group (111) looked at the effects of IL-6 on postprandial glycemia and insulin secretion in humans and found that IL-6 delays the rate of gastric emptying, which is the most significant regulator of postprandial glucose (179). The study identifies a new role of human IL-6 being involved in gastric emptying and sparing insulin in a postprandial situation.
Muscle–β-Cell Crosstalk
It is well established that exercise can enhance insulin sensitivity, whereas it is less clear whether exercise can improve insulin secretion and whether a communication exists between insulin-resistant skeletal muscle and pancreatic β-cells.
It has previously been shown that excessive concentrations of TNF-α induce insulin resistance in humans in vivo (180). We used TNF-α to induce insulin resistance in human myotubes. Conditioned media from muscle cells incubate with and without TNF-α were added to human and rat primary β-cells. The study identified a link between skeletal muscle and β-cells that is influenced by the insulin resistant state of the muscle (181).
Studying primary muscle cell cultures established from triceps brachii, soleus, and quadriceps led to the identification of angiogenin and osteoprotegerin, which were shown to be triceps-specific myokines that could mediate anti-inflammatory actions and protect β-cell survival (182). These results indicate that type I and type II muscles impact insulin secretion differentially in type 2 diabetes via specific myokines secretion.
Whereas TNF-α may inhibit β‐cell function indirectly, IL‐1β has been identified as a direct key player in β‐cell damage (183-189), although IL-1β inhibition with canakinumab did not reduce the incidence of diabetes (190). It has clearly been shown that IL‐6 positively regulates β‐cell mass in vivo by stimulating β‐cell proliferation and preventing apoptosis induced by metabolic stress (191). Therefore, exercise‐induced increase in IL‐6 production may be involved in protecting pancreatic β‐cell mass and function.
Muscle–Vascular Bed Crosstalk
By stimulating the in vivo growth of functional type II muscle fibers, the Walsh group identified novel muscle-secreted factors (192). Follistatin-like 1 (FSTL1) was shown to be produced by both skeletal and cardiac muscle cells and is also termed a cardiokine (193).
FSTL1 has been shown to possess cardioprotective effects, promoting endothelial cell function and thereby revascularization in animal models of cardiac injury through a mechanism that includes nitric oxide synthase (194, 195). Circulating levels of FSTL1 may work as a biomarker as high concentrations of FSTL1 are seen in patients with systolic and diastolic heart failure (196, 197), and as FSTL1 levels exhibit prognostic significance in the acute coronary syndrome (198). Using a dog model, it was recently shown that FSTL1 can positively regulate myocardial substrate metabolism, in vivo (199).
Muscle–Skin Crosstalk
Aging is associated with numerous alterations, including changes of the skin. Tarnopolsky and colleagues (200) demonstrated that endurance exercise improves age-associated skin changes in both mice and humans. They showed that exercise regulates muscular IL-15 expression via skeletal muscle AMPK. Elimination of muscle AMPK led to a weakening of skin structure, whereas IL-15 injections mimic some of the anti-aging effects of exercise on murine skin. The study supports the idea that exercise retards skin aging via a mechanism that involves muscle-derived IL-15.
Muscle–Immune Inflammation Crosstalk
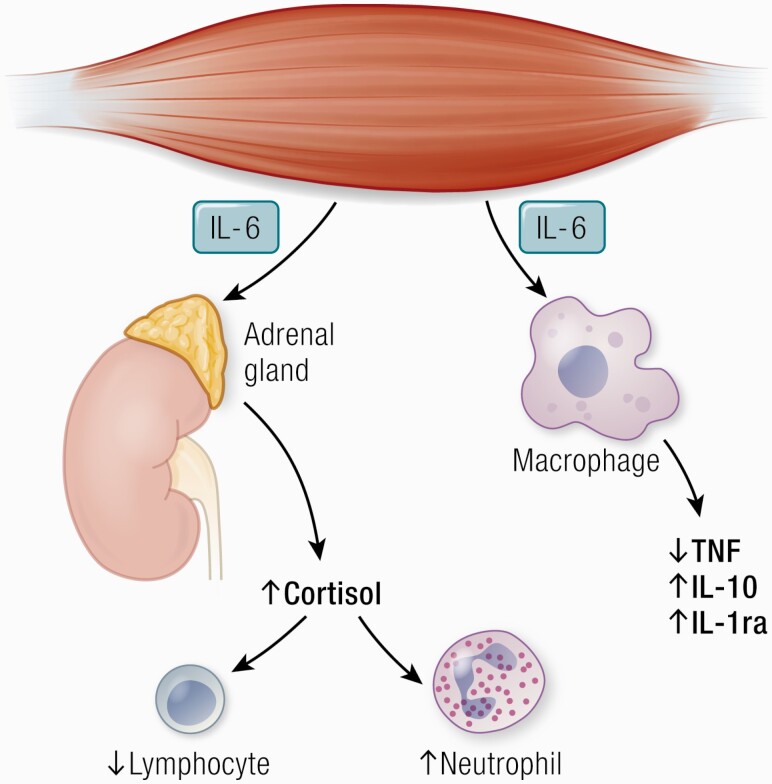
During exercise, muscle works as an immunoregulatory organ with impact on leukocyte subset trafficking and inflammation (201) (Fig. 6).
Figure 6.
IL-6 has anti-inflammatory effects as it inhibits TNF production and stimulates the production of IL-1ra and IL-10. IL-6 stimulates cortisol production and thereby induces neutrocytosis and lymphopenia. Abbreviations: IL-1ra, IL-1 receptor antagonist; TNF, tumor necrosis factor.
Lymphocyte and neutrophil trafficking
During exercise, lymphocytes and neutrophils are mobilized to the blood. Following long-duration exercise of high intensity, the concentration of lymphocytes falls below pre-exercise values whereas the neutrophil number continues to increase (202, 203). The acute exercise effect on lymphocytes and neutrophils is mediated by adrenaline, but the post-exercise reduction in lymphocyte number and the continuous increase in neutrophil number are mediated by both adrenaline and cortisol.
There are some indications that the exercise-induced rise in cortisol is mediated by IL-6. The infusion of IL-6 to mimic the effects of exercise led to an increase in cortisol and, consequently, a decrease in the lymphocyte number accompanied by an increase in neutrophil number (112).
Two other studies payed some support to a possible link between IL-6, lymphocyte number and cortisol. Carbohydrate ingestion during exercise blunted the exercise-induced IL-6 response, the increase in lymphocyte number as well as the cortisol (204, 205). Moreover, antioxidant supplementation totally inhibited the release of IL-6 from exercising human muscle as well as the exercise-induced increase in systemic levels of cortisol (206).
The anti-inflammatory effects of exercise
Physical inactivity is associated with low-grade chronic inflammation, not least when a physical inactive lifestyle is associated with obesity (29, 142, 144, 145, 207-210).
In humans, exercise training can induce anti-inflammatory effects both acutely with each bout of exercise and via long-term training adaptation including reduction in abdominal adiposity. The exercise-induced acute increase in IL‐6 stimulates an anti-inflammatory systemic environment. Thus, IL-6 promotes an increase in the production of the anti-inflammatory cytokines, IL‐1 receptor antagonist (IL‐1ra) and IL‐10 (112). IL‐1ra inhibits IL‐1β signal transduction (211) and IL‐10 inhibits synthesis of TNF‐α (212).
We infused a very low dose of Escherichia coli endotoxin to healthy subjects, who were randomized to either rest or exercise prior to the endotoxin administration (113). Exercise prior to endotoxin totally blunted the increase in circulating levels of TNF‐α that was observed during a resting situation.
Previous studies in cultured human monocytes have shown that IL‐6 prevents endotoxin-induced TNF‐α production (213). Moreover, it was shown that IL‐6‐deficient knockout mice have elevated levels of TNF‐α (214). It was therefore expected that an infusion of rhIL-6 prior to endotoxin administration would also blunt the TNF‐α response in humans and this was in fact what was found (113).
Together these data show that an acute bout of exercise induces anti‐inflammatory effects that may at least partially be mediated by IL‐6, not excluding other anti-inflammatory factors such as adrenaline and cortisol, as previously discussed (142).
A recent murine study suggested that IL-6 may induce either pro- or anti-inflammatory actions depending on cell source (215). Using a mouse model with conditional expression of the Il6 gene, it was found that IL6 derived from adipocytes increased, while IL6 derived from myeloid cells and muscle suppressed, macrophage infiltration of adipose tissue. The finding of opposite actions of IL-6, depending of the cell source, appeared to be due to a switch of IL6 signaling from a canonical mode (myeloid cells) to a noncanonical trans-signaling mode (adipocytes and muscle) which involved increased expression of the ADAM10/17 metalloprotease that enhances trans-signaling via the soluble IL6 receptor α (215).
Long-term anti-inflammatory effects are facilitated via exercise-training reduction in abdominal fat (216). In fact, an association has been established between physical inactivity and visceral fat in both rodents (217) and humans (133, 218-220). Accumulation of visceral fat, which is more inflamed than subcutaneous fat, leads to chronic systemic inflammation that predisposes to atherosclerosis, elevated blood lipids, insulin resistance, neurodegeneration, muscle waste, and anemia, factors that are likely to lead to decreased physical activity. Lack of exercise provokes accumulation of more visceral fat and thereby further enhances inflammation and hence a network of chronic diseases. Thereby, a vicious circle of chronic inflammation is established (29).
Exercise training will lead to a decrease in visceral and cardiac fat mass (136, 138, 221) and hence a decrease in circulating inflammatory molecules via a mechanism that involves exercise-induced increase in IL-6 (136), as described above.
Muscle–Cancer Crosstalk
Epidemiological studies suggest that physical activity in leisure time reduces the risk of at least 13 different cancer types (41, 42, 222, 223). People who are physically active after a diagnosis of prostate cancer, colorectal cancer, and breast cancer have a higher survival rate than physically inactive people suffering from the same cancer types (121).
It is obvious, that many cancers are accompanied by systemic low-grade chronic inflammation and that such inflammation may drive tumor progression. Therefore, the anti-inflammatory effects of physical training may mediate some of the protective effects of exercise on cancer development (41).
Pernille Hojman and her team explored the effect of exercise on tumor growth in preclinical models (42, 222). She first established a B16F10 melanoma model and randomized tumor-bearing mice to voluntary wheel running or control. Running mice demonstrated a marked reduction in tumor volume and incidence across 6 different tumor models. The effects of exercise on cancer growth were mediated via a direct regulation of natural killer cells by a mechanism that involved epinephrine-dependent mobilization of natural killer cells to the circulation and an IL-6-dependent redistribution to tumors. Blocking IL-6 signaling during exercise abolished the exercise-induced inhibition of tumor growth. The findings in mice indicate that IL-6 may have a role in mediating anti-cancer effects.
A few mechanistic studies have demonstrated a potential role of other myokines, including Oncostatin M, irisin, and SPARC in the suppression of breast and colon cancer growth (41, 224-227).
Myokines and Other “Kines”: Adipokines, Hepatokines, and Batokines
The views on organ crosstalk in health and disease have changed over the past 30 years.
It all began with the work from the Spiegelman and Flier laboratories in 1987 (228) that defined adipose tissue as an endocrine organ by the identification of a secretory protein, called adipsin. This was followed by a landmark finding by Friedman and his team (229), who identified leptin. Since then the list of adipokines have included, for example, adiponectin, resistin, and visfatin (230, 231).
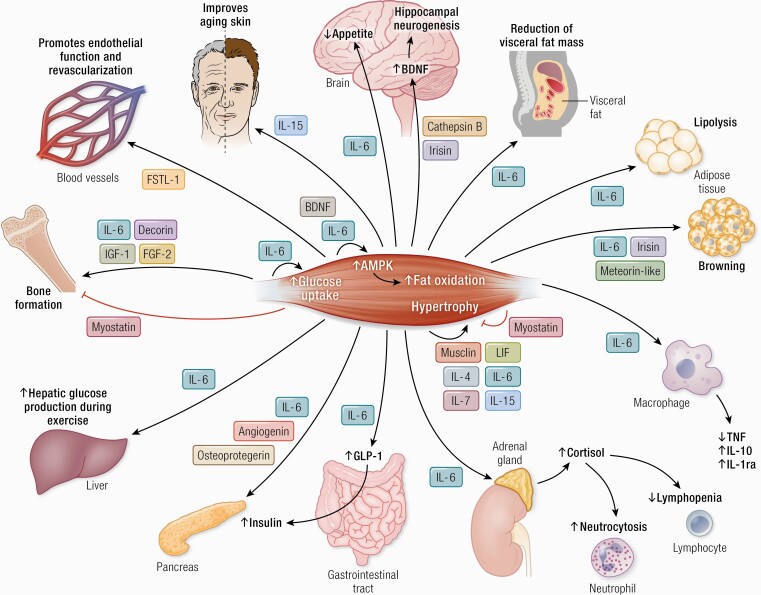
The identification of muscle as a secretory organ began with the finding of muscle-derived IL-6 in 2000 (5) and the subsequent definition of myokines in 2003 (4), and led via the work of many research groups later to the identification of hundreds of myokines. The present review identifies crosstalk between muscle and several other organs, including brain, adipose tissue, bone, liver, gut, pancreas, vascular bed, and skin. Moreover, several myokines signal within the muscle itself (Fig. 7).
Figure 7.
Cathepsin B and irisin cross the blood–brain barrier and stimulate BDNF production and hippocampal neurogenesis. IL-6 stimulates appetite and lipolysis and decreases visceral fat mass. Irisin, meteorin-like, and IL-6 have a role in “browning” of white adipose tissue. IL-15 improves aging skin. Decorin, IL-6, IGF-1 and FGF-2 positively regulate bone formation. Myostatin negatively regulate bone formation. Musclin, LIF, IL-4, IL-6, IL-7, and IL-15 promote muscle hypertrophy. Myostatin inhibits muscle hypertrophy. BDNF and IL-6 are involved in AMPK-mediated fat oxidation. IL-6 enhances insulin-stimulated glucose uptake and stimulates glucose output from the liver, but only during exercise. IL-6 increases insulin secretion by inducing the expression of GLP-1 by the L cells of the intestine. IL-6 has anti-inflammatory effects as it inhibits TNF production and stimulates the production of IL-1ra and IL-10. IL-6 stimulates cortisol production and thereby induces neutrocytosis and lymphopenia. FSTL-1 improves endothelial function and revascularization of ischemic blood vessels. Angiogenin, osteoprotegerin and IL-6 possess pancreatic β-cell protective actions against proinflammatory cytokines. Abbreviations: AMPK, 5′-AMP-activated protein kinase; BDNF, brain-derived neurotrophic factor; FGF-2, fibroblast growth factor 2; FGF-21, fibroblast growth factor 21; FSTL-1, follistatin-related protein 1; GLP-1, glucagon-like peptide 1; IGF-1, insulin-like growth factor I; IL-1ra, IL-1 receptor antagonist; LIF, leukemia inhibitory factor; TGF-β, transforming growth factor β; TNF, tumor necrosis factor.
Recently, a novel group of liver-derived exercise factors has been identified. Hepatokines include FGF-21, follistatin, angiopoietin-like protein 4, heat shock protein 72, and IGF binding protein, which are all released from the liver during or immediately after an exercise bout (232). These hepatokines increase in the circulation after muscle work and appear to be involved in mediating some of the metabolic effects of exercise.
The latest news regarding other “kines” started with the identification of classic brown adipose tissue in adult humans (233), which led to the batokine concept. Most recently, 101 proteins were exclusively quantified into brown and not white adipocyte tissue by proteomic-based identification (234).
However, among the “kines,” focus is still primarily on myokines and hepatokines when it comes to mediating exercise-induced communication between muscle and other organs. Lack of physical activity is associated with a large network of diseases, including type 2 diabetes, cardiovascular diseases, cancer, dementia, and osteoporosis (72, 121), and it is likely that the detrimental effects of lack of exercise to some degree is mediated by a lack of myokine release and/or resistance to the effects of myokines. The identification of new myokines and their specific roles will likely lead to novel therapeutic targets for lifestyle-related diseases. However, the biological identification of several myokines has turned these molecules into useful biomarkers for monitoring the amount, intensity, and mode of exercise that is sufficient to induce specific physiological and metabolic responses for people with, for example, cancer, diabetes, or neurodegenerative diseases.
Acknowledgments
Financial Support: The Centre for Physical Activity Research (CFAS) is supported by a grant from TrygFonden. Grant supporting the writing of the paper: TrygFonden should be acknowledged.
Glossary
Abbreviations
- AMPK
adenosine 5′-monophosphate-activated protein kinase
- BDNF
brain-derived neurotrophic factor
- CTSB
cathepsin B
- FGF
Fibroblast growth factor
- FSTL1
follistatin-like 1
- GLP
glucagon-like peptide
- IGF-1
insulin-like growth factor 1
- IL
interleukin
- LIF
leukemia inhibitory factor
- Metrnl
meteorin-like
- mRNA
messenger ribonucleic acid
- TGF-β
transforming growth factor β
- TNF
tumor necrosis factor
- UCP1
uncoupling protein 1
Additional Information
Disclosure Summary: We have nothing to disclose.
Data Availability: Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Kjaer M, Secher NH, Bangsbo J, et al. Hormonal and metabolic responses to electrically induced cycling during epidural anesthesia in humans. J Appl Physiol (1985). 1996;80(6):2156-2162. [DOI] [PubMed] [Google Scholar]
- 2. Mohr T, Andersen JL, Biering-Sørensen F, et al. Long-term adaptation to electrically induced cycle training in severe spinal cord injured individuals. Spinal Cord. 1997;35(1):1-16. [DOI] [PubMed] [Google Scholar]
- 3. Goldstein M. Humoral nature of hypoglycemia in muscular activity. Am J Physiol. 1961;200:67-70. [Google Scholar]
- 4. Pedersen BK, Steensberg A, Fischer C, et al. Searching for the exercise factor: is IL-6 a candidate? J Muscle Res Cell Motil. 2003;24(2-3):113-119. [DOI] [PubMed] [Google Scholar]
- 5. Steensberg A, van Hall G, Osada T, Sacchetti M, Saltin B, Klarlund Pedersen B. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J Physiol. 2000;529(Pt 1):237-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev. 2008;88(4):1379-1406. [DOI] [PubMed] [Google Scholar]
- 7. Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8(8):457-465. [DOI] [PubMed] [Google Scholar]
- 8. Safdar A, Tarnopolsky MA. Exosomes as mediators of the systemic adaptations to endurance exercise. Cold Spring Harb Perspect Med. 2018;8(3):a029827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Safdar A, Saleem A, Tarnopolsky MA. The potential of endurance exercise-derived exosomes to treat metabolic diseases. Nat Rev Endocrinol. 2016;12(9):504-517. [DOI] [PubMed] [Google Scholar]
- 10. Whitham M, Parker BL, Friedrichsen M, et al. Extracellular vesicles provide a means for tissue crosstalk during exercise. Cell Metab. 2018;27(1):237-251.e4. [DOI] [PubMed] [Google Scholar]
- 11. Das DK, Graham ZA, Cardozo CP. Myokines in skeletal muscle physiology and metabolism: recent advances and future perspectives. Acta Physiol (Oxf). 2020;228(2):e13367. [DOI] [PubMed] [Google Scholar]
- 12. Eckel J. Myokines in metabolic homeostasis and diabetes. Diabetologia. 2019;62(9):1523-1528. [DOI] [PubMed] [Google Scholar]
- 13. Garneau L, Aguer C. Role of myokines in the development of skeletal muscle insulin resistance and related metabolic defects in type 2 diabetes. Diabetes Metab. 2019;45(6):505-516. [DOI] [PubMed] [Google Scholar]
- 14. Lee JH, Jun HS. Role of myokines in regulating skeletal muscle mass and function. Front Physiol. 2019;10:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Díaz BB, González DA, Gannar F, Pérez MCR, de León AC. Myokines, physical activity, insulin resistance and autoimmune diseases. Immunol Lett. 2018;203:1-5. [DOI] [PubMed] [Google Scholar]
- 16. Hoffmann C, Weigert C. Skeletal muscle as an endocrine organ: the role of myokines in exercise adaptations. Cold Spring Harb Perspect Med. 2017;7(11):a029793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rodríguez A, Becerril S, Ezquerro S, Méndez-Giménez L, Frühbeck G. Crosstalk between adipokines and myokines in fat browning. Acta Physiol (Oxf). 2017;219(2):362-381. [DOI] [PubMed] [Google Scholar]
- 18. Schnyder S, Handschin C. Skeletal muscle as an endocrine organ: PGC-1α, myokines and exercise. Bone. 2015;80:115-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ahima RS, Park HK. Connecting myokines and metabolism. Endocrinol Metab (Seoul). 2015;30(3):235-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Raschke S, Eckel J. Adipo-myokines: two sides of the same coin–mediators of inflammation and mediators of exercise. Mediators Inflamm. 2013;2013:320724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pedersen BK. Exercise-induced myokines and their role in chronic diseases. Brain Behav Immun. 2011;25(5):811-816. [DOI] [PubMed] [Google Scholar]
- 22. Trayhurn P, Drevon CA, Eckel J. Secreted proteins from adipose tissue and skeletal muscle - adipokines, myokines and adipose/muscle cross-talk. Arch Physiol Biochem. 2011;117(2):47-56. [DOI] [PubMed] [Google Scholar]
- 23. Hamrick MW. A role for myokines in muscle-bone interactions. Exerc Sport Sci Rev. 2011;39(1):43-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brandt C, Pedersen BK. The role of exercise-induced myokines in muscle homeostasis and the defense against chronic diseases. J Biomed Biotechnol. 2010;2010:520258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Arnold AS, Egger A, Handschin C. PGC-1α and myokines in the aging muscle - a mini-review. Gerontology. 2011;57(1):37-43. [DOI] [PubMed] [Google Scholar]
- 26. Pedersen BK. The diseasome of physical inactivity- and the role of myokines in muscle-fat cross talk. J Physiol. 2009;587(Pt 23):5559-5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Walsh K. Adipokines, myokines and cardiovascular disease. Circ J. 2009;73(1):13-18. [DOI] [PubMed] [Google Scholar]
- 28. Pedersen BK, Akerström TC, Nielsen AR, Fischer CP. Role of myokines in exercise and metabolism. J Appl Physiol (1985). 2007;103(3):1093-1098. [DOI] [PubMed] [Google Scholar]
- 29. Benatti FB, Pedersen BK. Exercise as an anti-inflammatory therapy for rheumatic diseases-myokine regulation. Nat Rev Rheumatol. 2015;11(2):86-97. [DOI] [PubMed] [Google Scholar]
- 30. Pedersen BK. Physical activity and muscle-brain crosstalk. Nat Rev Endocrinol. 2019;15(7):383-392. [DOI] [PubMed] [Google Scholar]
- 31. Whitham M, Febbraio MA. The ever-expanding myokinome: discovery challenges and therapeutic implications. Nat Rev Drug Discov. 2016;15(10):719-729. [DOI] [PubMed] [Google Scholar]
- 32. Pal M, Febbraio MA, Whitham M. From cytokine to myokine: the emerging role of interleukin-6 in metabolic regulation. Immunol Cell Biol. 2014;92(4):331-339. [DOI] [PubMed] [Google Scholar]
- 33. Ruiz-Casado A, Martín-Ruiz A, Pérez LM, Provencio M, Fiuza-Luces C, Lucia A. Exercise and the hallmarks of cancer. Trends Cancer. 2017;3(6):423-441. [DOI] [PubMed] [Google Scholar]
- 34. Fiuza-Luces C, Santos-Lozano A, Joyner M, et al. Exercise benefits in cardiovascular disease: beyond attenuation of traditional risk factors. Nat Rev Cardiol. 2018;15(12):731-743. [DOI] [PubMed] [Google Scholar]
- 35. Indrakusuma I, Sell H, Eckel J. Novel mediators of adipose tissue and muscle crosstalk. Curr Obes Rep. 2015;4(4):411-417. [DOI] [PubMed] [Google Scholar]
- 36. Graf C, Ferrari N. Metabolic health-the role of adipo-myokines. Int J Mol Sci. 2019;20(24):6159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Coelho-Junior HJ, Picca A, Calvani R, Uchida MC, Marzetti E. If my muscle could talk: myokines as a biomarker of frailty. Exp Gerontol. 2019;127:110715. [DOI] [PubMed] [Google Scholar]
- 38. Khan SU, Ghafoor S. Myokines: discovery challenges and therapeutic impediments. J Pak Med Assoc. 2019;69(7):1014-1017. [PubMed] [Google Scholar]
- 39. Henningsen J, Pedersen BK, Kratchmarova I. Quantitative analysis of the secretion of the MCP family of chemokines by muscle cells. Mol Biosyst. 2011;7(2):311-321. [DOI] [PubMed] [Google Scholar]
- 40. Henningsen J, Rigbolt KT, Blagoev B, Pedersen BK, Kratchmarova I. Dynamics of the skeletal muscle secretome during myoblast differentiation. Mol Cell Proteomics. 2010;9(11):2482-2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hojman P, Gehl J, Christensen JF, Pedersen BK. Molecular mechanisms linking exercise to cancer prevention and treatment. Cell Metab. 2018;27(1):10-21. [DOI] [PubMed] [Google Scholar]
- 42. Pedersen L, Idorn M, Olofsson GH, et al. Voluntary running suppresses tumor growth through epinephrine- and IL-6-dependent NK cell mobilization and redistribution. Cell Metab. 2016;23(3):554-562. [DOI] [PubMed] [Google Scholar]
- 43. McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387(6628):83-90. [DOI] [PubMed] [Google Scholar]
- 44. Mosher DS, Quignon P, Bustamante CD, et al. A mutation in the myostatin gene increases muscle mass and enhances racing performance in heterozygote dogs. PLoS Genet. 2007;3(5):e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Grobet L, Martin LJ, Poncelet D, et al. A deletion in the bovine myostatin gene causes the double-muscled phenotype in cattle. Nat Genet. 1997;17(1):71-74. [DOI] [PubMed] [Google Scholar]
- 46. Kanzleiter T, Rath M, Görgens SW, et al. The myokine decorin is regulated by contraction and involved in muscle hypertrophy. Biochem Biophys Res Commun. 2014;450(2):1089-1094. [DOI] [PubMed] [Google Scholar]
- 47. Saremi A, Gharakhanloo R, Sharghi S, Gharaati MR, Larijani B, Omidfar K. Effects of oral creatine and resistance training on serum myostatin and GASP-1. Mol Cell Endocrinol. 2010;317(1-2):25-30. [DOI] [PubMed] [Google Scholar]
- 48. Hittel DS, Axelson M, Sarna N, Shearer J, Huffman KM, Kraus WE. Myostatin decreases with aerobic exercise and associates with insulin resistance. Med Sci Sports Exerc. 2010;42(11):2023-2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Serrano AL, Baeza-Raja B, Perdiguero E, Jardí M, Muñoz-Cánoves P. Interleukin-6 is an essential regulator of satellite cell-mediated skeletal muscle hypertrophy. Cell Metab. 2008;7(1):33-44. [DOI] [PubMed] [Google Scholar]
- 50. Broholm C, Mortensen OH, Nielsen S, et al. Exercise induces expression of leukaemia inhibitory factor in human skeletal muscle. J Physiol. 2008;586(8):2195-2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Broholm C, Pedersen BK. Leukaemia inhibitory factor–an exercise-induced myokine. Exerc Immunol Rev. 2010;16:77-85. [PubMed] [Google Scholar]
- 52. Gao S, Durstine JL, Koh HJ, Carver WE, Frizzell N, Carson JA. Acute myotube protein synthesis regulation by IL-6-related cytokines. Am J Physiol Cell Physiol. 2017;313(5):C487-C500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nielsen AR, Mounier R, Plomgaard P, et al. Expression of interleukin-15 in human skeletal muscle effect of exercise and muscle fibre type composition. J Physiol. 2007;584(Pt 1):305-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Haugen F, Norheim F, Lian H, et al. IL-7 is expressed and secreted by human skeletal muscle cells. Am J Physiol Cell Physiol. 2010;298(4):C807-C816. [DOI] [PubMed] [Google Scholar]
- 55. Fischer CP. Interleukin-6 in acute exercise and training: what is the biological relevance? Exerc Immunol Rev. 2006;12:6-33. [PubMed] [Google Scholar]
- 56. Fischer CP, Plomgaard P, Hansen AK, Pilegaard H, Saltin B, Pedersen BK. Endurance training reduces the contraction-induced interleukin-6 mRNA expression in human skeletal muscle. Am J Physiol Endocrinol Metab. 2004;287(6):E1189-E1194. [DOI] [PubMed] [Google Scholar]
- 57. Keller C, Steensberg A, Hansen AK, Fischer CP, Plomgaard P, Pedersen BK. Effect of exercise, training, and glycogen availability on IL-6 receptor expression in human skeletal muscle. J Appl Physiol (1985). 2005;99(6):2075-2079. [DOI] [PubMed] [Google Scholar]
- 58. Carey AL, Steinberg GR, Macaulay SL, et al. Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase. Diabetes. 2006;55(10):2688-2697. [DOI] [PubMed] [Google Scholar]
- 59. Bruce CR, Dyck DJ. Cytokine regulation of skeletal muscle fatty acid metabolism: effect of interleukin-6 and tumor necrosis factor-alpha. Am J Physiol Endocrinol Metab. 2004;287(4):E616-E621. [DOI] [PubMed] [Google Scholar]
- 60. Petersen EW, Carey AL, Sacchetti M, et al. Acute IL-6 treatment increases fatty acid turnover in elderly humans in vivo and in tissue culture in vitro: evidence that IL-6 acts independently of lipolytic hormones. Am J Physiol. 2005;288(1):E155-E162. [DOI] [PubMed] [Google Scholar]
- 61. van Hall G, Steensberg A, Sacchetti M, et al. Interleukin-6 stimulates lipolysis and fat oxidation in humans. J Clin Endocrinol Metab. 2003;88(7):3005-3010. [DOI] [PubMed] [Google Scholar]
- 62. Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1(1):15-25. [DOI] [PubMed] [Google Scholar]
- 63. Matthews VB, Aström MB, Chan MH, et al. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia. 2009;52(7):1409-1418. [DOI] [PubMed] [Google Scholar]
- 64. Nishizawa H, Matsuda M, Yamada Y, et al. Musclin, a novel skeletal muscle-derived secretory factor. J Biol Chem. 2004;279(19):19391-19395. [DOI] [PubMed] [Google Scholar]
- 65. Subbotina E, Sierra A, Zhu Z, et al. Musclin is an activity-stimulated myokine that enhances physical endurance. Proc Natl Acad Sci U S A. 2015;112(52):16042-16047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Re Cecconi AD, Forti M, Chiappa M, et al. Myokine induced by aerobic exercise, retards muscle atrophy during cancer cachexia in mice. Cancers. 2019;11(10):1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30(9):464-472. [DOI] [PubMed] [Google Scholar]
- 68. Mattson MP. Energy intake and exercise as determinants of brain health and vulnerability to injury and disease. Cell Metab. 2012;16(6): 706-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Aarsland D, Sardahaee FS, Anderssen S, Ballard C; Alzheimer’s Society Systematic Review group . Is physical activity a potential preventive factor for vascular dementia? A systematic review. Aging Ment Health. 2010;14(4):386-395. [DOI] [PubMed] [Google Scholar]
- 70. Williams JW, Plassman BL, Burke J, Benjamin S. Preventing Alzheimer’s disease and cognitive decline. Evid Rep Technol Assess (Full Rep). 2010;(193):1-727. [PMC free article] [PubMed] [Google Scholar]
- 71. Santos-Lozano A, Pareja-Galeano H, Sanchis-Gomar F, et al. Physical activity and Alzheimer disease: a protective association. Mayo Clin Proc. 2016;91(8):999-1020. [DOI] [PubMed] [Google Scholar]
- 72. Pedersen BK, Saltin B. Exercise as medicine - evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports. 2015;25 Suppl(3):1-72. [DOI] [PubMed] [Google Scholar]
- 73. Voss MW, Nagamatsu LS, Liu-Ambrose T, Kramer AF. Exercise, brain, and cognition across the life span. J Appl Physiol (1985). 2011;111(5):1505-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25(6):295-301. [DOI] [PubMed] [Google Scholar]
- 75. Smith PJ, Blumenthal JA, Hoffman BM, et al. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med. 2010;72(3):239-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Snowden M, Steinman L, Mochan K, et al. Effect of exercise on cognitive performance in community-dwelling older adults: review of intervention trials and recommendations for public health practice and research. J Am Geriatr Soc. 2011;59(4):704-716. [DOI] [PubMed] [Google Scholar]
- 77. Aberg MA, Pedersen NL, Torén K, et al. Cardiovascular fitness is associated with cognition in young adulthood. Proc Natl Acad Sci U S A. 2009;106(49):20906-20911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Blundell JE, Gibbons C, Caudwell P, Finlayson G, Hopkins M. Appetite control and energy balance: impact of exercise. Obes Rev. 2015;16 Suppl (1):67-76. [DOI] [PubMed] [Google Scholar]
- 79. Kelley GA, Kelley KS. Exercise and sleep: a systematic review of previous meta-analyses. J Evid Based Med. 2017;10(1):26-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Crush EA, Frith E, Loprinzi PD. Experimental effects of acute exercise duration and exercise recovery on mood state. J Affect Disord. 2018;229:282-287. [DOI] [PubMed] [Google Scholar]
- 81. Kobilo T, Liu QR, Gandhi K, Mughal M, Shaham Y, van Praag H. Running is the neurogenic and neurotrophic stimulus in environmental enrichment. Learn Mem. 2011;18(9):605-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Erickson KI, Voss MW, Prakash RS, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108(7):3017-3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Leardini-Tristao M, Charles AL, Lejay A, et al. Beneficial effect of exercise on cognitive function during peripheral arterial disease: potential involvement of myokines and microglial anti-inflammatory phenotype enhancement. J Clin Med. 2019;8(5):653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Rai M, Demontis F. Systemic nutrient and stress signaling via myokines and myometabolites. Annu Rev Physiol. 2016;78:85-107. [DOI] [PubMed] [Google Scholar]
- 85. Makarova JA, Maltseva DV, Galatenko VV, et al. Exercise immunology meets MiRNAs. Exerc Immunol Rev. 2014;20:135-164. [PubMed] [Google Scholar]
- 86. Loprinzi PD, Frith E. A brief primer on the mediational role of BDNF in the exercise-memory link. Clin Physiol Funct Imaging. 2019;39(1):9-14. [DOI] [PubMed] [Google Scholar]
- 87. Neeper SA, Gómez-Pinilla F, Choi J, Cotman C. Exercise and brain neurotrophins. Nature. 1995;373(6510):109. [DOI] [PubMed] [Google Scholar]
- 88. Liu PZ, Nusslock R. Exercise-mediated neurogenesis in the hippocampus via BDNF. Front Neurosci. 2018;12:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Oliff HS, Berchtold NC, Isackson P, Cotman CW. Exercise-induced regulation of brain-derived neurotrophic factor (BDNF) transcripts in the rat hippocampus. Brain Res Mol Brain Res. 1998;61(1-2):147-153. [DOI] [PubMed] [Google Scholar]
- 90. Van Hoomissen JD, Chambliss HO, Holmes PV, Dishman RK. Effects of chronic exercise and imipramine on mRNA for BDNF after olfactory bulbectomy in rat. Brain Res. 2003;974(1-2):228-235. [DOI] [PubMed] [Google Scholar]
- 91. Farmer J, Zhao X, van Praag H, Wodtke K, Gage FH, Christie BR. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience. 2004;124(1):71-79. [DOI] [PubMed] [Google Scholar]
- 92. Adlard PA, Perreau VM, Cotman CW. The exercise-induced expression of BDNF within the hippocampus varies across life-span. Neurobiol Aging. 2005;26(4):511-520. [DOI] [PubMed] [Google Scholar]
- 93. Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20(10):2580-2590. [DOI] [PubMed] [Google Scholar]
- 94. Vaynman S, Ying Z, Gómez-Pinilla F. Exercise induces BDNF and synapsin I to specific hippocampal subfields. J Neurosci Res. 2004;76(3):356-362. [DOI] [PubMed] [Google Scholar]
- 95. Rasmussen P, Brassard P, Adser H, et al. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Exp Physiol. 2009;94(10):1062-1069. [DOI] [PubMed] [Google Scholar]
- 96. Seifert T, Brassard P, Wissenberg M, et al. Endurance training enhances BDNF release from the human brain. Am J Physiol Regul Integr Comp Physiol. 2010;298(2):R372-R377. [DOI] [PubMed] [Google Scholar]
- 97. Pajonk FG, Wobrock T, Gruber O, et al. Hippocampal plasticity in response to exercise in schizophrenia. Arch Gen Psychiatry. 2010;67(2):133-143. [DOI] [PubMed] [Google Scholar]
- 98. Wrann CD, White JP, Salogiannnis J, et al. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab. 2013;18(5):649-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Moon HY, Becke A, Berron D, et al. Running-induced systemic cathepsin b secretion is associated with memory function. Cell Metab. 2016;24(2):332-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Suzuki WA. How body affects brain. Cell Metab. 2016;24(2):192-193. [DOI] [PubMed] [Google Scholar]
- 101. Boström P, Wu J, Jedrychowski MP, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481(7382):463-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Albrecht E, Norheim F, Thiede B, et al. Irisin - a myth rather than an exercise-inducible myokine. Sci Rep. 2015;5:8889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Wrann CD. FNDC5/irisin - their role in the nervous system and as a mediator for beneficial effects of exercise on the brain. Brain Plast. 2015;1(1):55-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kim HJ, Higashimori T, Park SY, et al. Differential effects of interleukin-6 and -10 on skeletal muscle and liver insulin action in vivo. Diabetes. 2004;53(4):1060-1067. [DOI] [PubMed] [Google Scholar]
- 105. Cai D, Yuan M, Frantz DF, et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11(2):183-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Matthews VB, Allen TL, Risis S, et al. Interleukin-6-deficient mice develop hepatic inflammation and systemic insulin resistance. Diabetologia. 2010;53(11):2431-2441. [DOI] [PubMed] [Google Scholar]
- 107. Wallenius V, Wallenius K, Ahrén B, et al. Interleukin-6-deficient mice develop mature-onset obesity. Nat Med. 2002;8(1):75-79. [DOI] [PubMed] [Google Scholar]
- 108. Ellingsgaard H, Hauselmann I, Schuler B, et al. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat Med. 2011;17(11):1481-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Mauer J, Chaurasia B, Goldau J, et al. Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin. Nat Immunol. 2014;15(5):423-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Mauer J, Denson JL, Brüning JC. Versatile functions for IL-6 in metabolism and cancer. Trends Immunol. 2015;36(2):92-101. [DOI] [PubMed] [Google Scholar]
- 111. Lang Lehrskov L, Lyngbaek MP, Soederlund L, et al. Interleukin-6 delays gastric emptying in humans with direct effects on glycemic control. Cell Metab. 2018;27(6):1201-1211.e3. [DOI] [PubMed] [Google Scholar]
- 112. Steensberg A, Fischer CP, Keller C, Møller K, Pedersen BK. IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am J Physiol Endocrinol Metab. 2003;285(2):E433-E437. [DOI] [PubMed] [Google Scholar]
- 113. Starkie R, Ostrowski SR, Jauffred S, Febbraio M, Pedersen BK. Exercise and IL-6 infusion inhibit endotoxin-induced TNF-alpha production in humans. FASEB J. 2003;17(8):884-886. [DOI] [PubMed] [Google Scholar]
- 114. Febbraio MA, Pedersen BK. Muscle-derived interleukin-6: mechanisms for activation and possible biological roles. FASEB J. 2002;16(11):1335-1347. [DOI] [PubMed] [Google Scholar]
- 115. Keller C, Hellsten Y, Steensberg A, Pedersen BK. Differential regulation of IL-6 and TNF-alpha via calcineurin in human skeletal muscle cells. Cytokine. 2006;36(3-4):141-147. [DOI] [PubMed] [Google Scholar]
- 116. Hidalgo J, Florit S, Giralt M, Ferrer B, Keller C, Pilegaard H. Transgenic mice with astrocyte-targeted production of interleukin-6 are resistant to high-fat diet-induced increases in body weight and body fat. Brain Behav Immun. 2010;24(1):119-126. [DOI] [PubMed] [Google Scholar]
- 117. Señarís RM, Trujillo ML, Navia B, et al. Interleukin-6 regulates the expression of hypothalamic neuropeptides involved in body weight in a gender-dependent way. J Neuroendocrinol. 2011;23(8):675-686. [DOI] [PubMed] [Google Scholar]
- 118. Molinero A, Fernandez-Perez A, Mogas A, et al. Role of muscle IL-6 in gender-specific metabolism in mice. PLoS One. 2017;12(3):e0173675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Timper K, Denson JL, Steculorum SM, et al. IL-6 improves energy and glucose homeostasis in obesity via enhanced central IL-6 trans-signaling. Cell Rep. 2017;19(2):267-280. [DOI] [PubMed] [Google Scholar]
- 120. Pedersen BK. Muscle as a secretory organ. Compr Physiol. 2013;3(3):1337-1362. [DOI] [PubMed] [Google Scholar]
- 121. Pedersen BK. The physiology of optimizing health with a focus on exercise as medicine. Annu Rev Physiol. 2019;81:607-627. [DOI] [PubMed] [Google Scholar]
- 122. Fosgerau K, Galle P, Hansen T, et al. Interleukin-6 autoantibodies are involved in the pathogenesis of a subset of type 2 diabetes. J Endocrinol. 2010;204(3):265-273. [DOI] [PubMed] [Google Scholar]
- 123. Bays HE. “Sick fat,” metabolic disease, and atherosclerosis. Am J Med. 2009;122(1 Suppl):S26-S37. [DOI] [PubMed] [Google Scholar]
- 124. Haffner SM. Abdominal adiposity and cardiometabolic risk: do we have all the answers? Am J Med. 2007;120(9 Suppl 1):S10-S16; discussion S16. [DOI] [PubMed] [Google Scholar]
- 125. Whitmer RA, Gustafson DR, Barrett-Connor E, Haan MN, Gunderson EP, Yaffe K. Central obesity and increased risk of dementia more than three decades later. Neurology. 2008;71(14):1057-1064. [DOI] [PubMed] [Google Scholar]
- 126. Giovannucci E. Metabolic syndrome, hyperinsulinemia, and colon cancer: a review. Am J Clin Nutr. 2007;86(3):s836-s842. [DOI] [PubMed] [Google Scholar]
- 127. Xue F, Michels KB. Diabetes, metabolic syndrome, and breast cancer: a review of the current evidence. Am J Clin Nutr. 2007;86(3):s823-s835. [DOI] [PubMed] [Google Scholar]
- 128. Pischon T, Boeing H, Hoffmann K, et al. General and abdominal adiposity and risk of death in Europe. N Engl J Med. 2008;359(20):2105-2120. [DOI] [PubMed] [Google Scholar]
- 129. Wedell-Neergaard AS, Krogh-Madsen R, Petersen GL, et al. Cardiorespiratory fitness and the metabolic syndrome: roles of inflammation and abdominal obesity. PLoS One. 2018;13(3):e0194991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Wedell-Neergaard AS, Eriksen L, Grønbæk M, Pedersen BK, Krogh-Madsen R, Tolstrup J. Low fitness is associated with abdominal adiposity and low-grade inflammation independent of BMI. PLoS One. 2018;13(1):e0190645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Yudkin JS, Eringa E, Stehouwer CD. “Vasocrine” signalling from perivascular fat: a mechanism linking insulin resistance to vascular disease. Lancet. 2005;365(9473):1817-1820. [DOI] [PubMed] [Google Scholar]
- 132. Handschin C, Spiegelman BM. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature. 2008;454(7203):463-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Olsen RH, Krogh-Madsen R, Thomsen C, Booth FW, Pedersen BK. Metabolic responses to reduced daily steps in healthy nonexercising men. JAMA. 2008;299(11):1261-1263. [DOI] [PubMed] [Google Scholar]
- 134. Nordby P, Auerbach PL, Rosenkilde M, et al. Endurance training per se increases metabolic health in young, moderately overweight men. Obesity (Silver Spring). 2012;20(11):2202-2212. [DOI] [PubMed] [Google Scholar]
- 135. Ross R, Dagnone D, Jones PJ, et al. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men. A randomized, controlled trial. Ann Intern Med. 2000;133(2):92-103. [DOI] [PubMed] [Google Scholar]
- 136. Wedell-Neergaard AS, Lang Lehrskov L, Christensen RH, et al. Exercise-induced changes in visceral adipose tissue mass are regulated by IL-6 signaling: a randomized controlled trial. Cell Metab. 2019;29(4):844-855.e3. [DOI] [PubMed] [Google Scholar]
- 137. Christensen RH, Wedell-Neergaard AS, Lehrskov LL, et al. The role of exercise combined with tocilizumab in visceral and epicardial adipose tissue and gastric emptying rate in abdominally obese participants: protocol for a randomised controlled trial. Trials. 2018;19(1):266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Christensen RH, Lehrskov LL, Wedell-Neergaard AS, et al. Aerobic exercise induces cardiac fat loss and alters cardiac muscle mass through an interleukin-6 receptor-dependent mechanism: cardiac analysis of a double-blind randomized controlled clinical trial in abdominally obese humans. Circulation. 2019;140(20):1684-1686. [DOI] [PubMed] [Google Scholar]
- 139. Townsend LK, Wright DC. Looking on the “brite” side exercise-induced browning of white adipose tissue. Pflugers Arch. 2019;471(3):455-465. [DOI] [PubMed] [Google Scholar]
- 140. Dinas PC, Lahart IM, Timmons JA, et al. Effects of physical activity on the link between PGC-1a and FNDC5 in muscle, circulating Ιrisin and UCP1 of white adipocytes in humans: a systematic review. F1000Res. 2017;6:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Rao R, Long J, White J, et al. Meteorin-like is a hormone that regulates immune-adipose interactions to Increase BEige Fat Thermogenesis. Cell. 2014;157(6):1279-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Pedersen BK. Anti-inflammatory effects of exercise: role in diabetes and cardiovascular disease. Eur J Clin Invest. 2017;47(8):600-611. [DOI] [PubMed] [Google Scholar]
- 143. Karstoft K, Pedersen BK. Skeletal muscle as a gene regulatory endocrine organ. Curr Opin Clin Nutr Metab Care. 2016;19(4):270-275. [DOI] [PubMed] [Google Scholar]
- 144. Karstoft K, Pedersen BK. Exercise and type 2 diabetes: focus on metabolism and inflammation. Immunol Cell Biol. 2016;94(2):146-150. [DOI] [PubMed] [Google Scholar]
- 145. Knudsen SH, Pedersen BK. Targeting inflammation through a physical active lifestyle and pharmaceuticals for the treatment of type 2 diabetes. Curr Diab Rep. 2015;15(10):82. [DOI] [PubMed] [Google Scholar]
- 146. Knudsen JG, Murholm M, Carey AL, et al. Role of IL-6 in exercise training- and cold-induced UCP1 expression in subcutaneous white adipose tissue. PLoS One. 2014;9(1):e84910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Kristóf E, Klusóczki Á, Veress R, et al. Interleukin-6 released from differentiating human beige adipocytes improves browning. Exp Cell Res. 2019;377(1-2):47-55. [DOI] [PubMed] [Google Scholar]
- 148. Roberts LD, Boström P, O’Sullivan JF, et al. β-Aminoisobutyric acid induces browning of white fat and hepatic β-oxidation and is inversely correlated with cardiometabolic risk factors. Cell Metab. 2014;19(1):96-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Kammoun HL, Febbraio MA. Come on BAIBA light my fire. Cell Metab. 2014;19(1):1-2. [DOI] [PubMed] [Google Scholar]
- 150. Hansen JS, Clemmesen JO, Secher NH, et al. Glucagon-to-insulin ratio is pivotal for splanchnic regulation of FGF-21 in humans. Mol Metab. 2015;4(8):551-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Hansen JS, Rutti S, Arous C, et al. Circulating follistatin is liver-derived and regulated by the glucagon-to-insulin ratio. J Clin Endocrinol Metab. 2016;101(2):550-560. [DOI] [PubMed] [Google Scholar]
- 152. Hansen JS, Pedersen BK, Xu G, Lehmann R, Weigert C, Plomgaard P. Exercise-induced secretion of FGF21 and follistatin are blocked by pancreatic clamp and impaired in type 2 diabetes. J Clin Endocrinol Metab. 2016;101(7):2816-2825. [DOI] [PubMed] [Google Scholar]
- 153. Singh R, Braga M, Pervin S. Regulation of brown adipocyte metabolism by myostatin/follistatin signaling. Front Cell Dev Biol. 2014;2:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Véniant MM, Sivits G, Helmering J, et al. Pharmacologic effects of FGF21 are independent of the “browning” of white adipose tissue. Cell Metab. 2015;21(5):731-738. [DOI] [PubMed] [Google Scholar]
- 155. Norheim F, Langleite TM, Hjorth M, et al. The effects of acute and chronic exercise on PGC-1α, irisin and browning of subcutaneous adipose tissue in humans. Febs J. 2014;281(3):739-749. [DOI] [PubMed] [Google Scholar]
- 156. Vosselman MJ, Hoeks J, Brans B, et al. Low brown adipose tissue activity in endurance-trained compared with lean sedentary men. Int J Obes (Lond). 2015;39(12):1696-1702. [DOI] [PubMed] [Google Scholar]
- 157. Schipilow JD, Macdonald HM, Liphardt AM, Kan M, Boyd SK. Bone micro-architecture, estimated bone strength, and the muscle-bone interaction in elite athletes: an HR-pQCT study. Bone. 2013;56(2):281-289. [DOI] [PubMed] [Google Scholar]
- 158. Verschueren S, Gielen E, O’Neill TW, et al. Sarcopenia and its relationship with bone mineral density in middle-aged and elderly European men. Osteoporos Int. 2013;24(1):87-98. [DOI] [PubMed] [Google Scholar]
- 159. Guo B, Zhang ZK, Liang C, et al. Molecular communication from skeletal muscle to bone: a review for muscle-derived myokines regulating bone metabolism. Calcif Tissue Int. 2017;100(2):184-192. [DOI] [PubMed] [Google Scholar]
- 160. Gomarasca M, Banfi G, Lombardi G. Myokines: the endocrine coupling of skeletal muscle and bone. Adv Clin Chem. 2020;94:155-218. [DOI] [PubMed] [Google Scholar]
- 161. Bialek P, Parkington J, Li X, et al. A myostatin and activin decoy receptor enhances bone formation in mice. Bone. 2014;60:162-171. [DOI] [PubMed] [Google Scholar]
- 162. Dankbar B, Fennen M, Brunert D, et al. Myostatin is a direct regulator of osteoclast differentiation and its inhibition reduces inflammatory joint destruction in mice. Nat Med. 2015;21(9):1085-1090. [DOI] [PubMed] [Google Scholar]
- 163. De Benedetti F, Rucci N, Del Fattore A, et al. Impaired skeletal development in interleukin-6-transgenic mice: a model for the impact of chronic inflammation on the growing skeletal system. Arthritis Rheum. 2006;54(11):3551-3563. [DOI] [PubMed] [Google Scholar]
- 164. Jilka RL, Hangoc G, Girasole G, et al. Increased osteoclast development after estrogen loss: mediation by interleukin-6. Science. 1992;257(5066):88-91. [DOI] [PubMed] [Google Scholar]
- 165. Le Goff B, Blanchard F, Berthelot JM, Heymann D, Maugars Y. Role for interleukin-6 in structural joint damage and systemic bone loss in rheumatoid arthritis. Joint Bone Spine. 2010;77(3):201-205. [DOI] [PubMed] [Google Scholar]
- 166. Axmann R, Böhm C, Krönke G, Zwerina J, Smolen J, Schett G. Inhibition of interleukin-6 receptor directly blocks osteoclast formation in vitro and in vivo. Arthritis Rheum. 2009;60(9):2747-2756. [DOI] [PubMed] [Google Scholar]
- 167. Palmqvist P, Persson E, Conaway HH, Lerner UH. IL-6, leukemia inhibitory factor, and oncostatin M stimulate bone resorption and regulate the expression of receptor activator of NF-kappa B ligand, osteoprotegerin, and receptor activator of NF-kappa B in mouse calvariae. J Immunol. 2002;169(6):3353-3362. [DOI] [PubMed] [Google Scholar]
- 168. Saidenberg-Kermanac’h N, Cohen-Solal M, Bessis N, De Vernejoul MC, Boissier MC. Role for osteoprotegerin in rheumatoid inflammation. Joint Bone Spine. 2004;71(1):9-13. [DOI] [PubMed] [Google Scholar]
- 169. Lombardi G, Sanchis-Gomar F, Perego S, Sansoni V, Banfi G. Implications of exercise-induced adipo-myokines in bone metabolism. Endocrine. 2016;54(2):284-305. [DOI] [PubMed] [Google Scholar]
- 170. Yakar S, Rosen CJ, Beamer WG, et al. Circulating levels of IGF-1 directly regulate bone growth and density. J Clin Invest. 2002;110(6):771-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Perrini S, Laviola L, Carreira MC, Cignarelli A, Natalicchio A, Giorgino F. The GH/IGF1 axis and signaling pathways in the muscle and bone: mechanisms underlying age-related skeletal muscle wasting and osteoporosis. J Endocrinol. 2010;205(3):201-210. [DOI] [PubMed] [Google Scholar]
- 172. Chan CY, Masui O, Krakovska O, et al. Identification of differentially regulated secretome components during skeletal myogenesis. Mol Cell Proteomics. 2011;10(5):M110.004804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Chan CY, McDermott JC, Siu KW. Secretome analysis of skeletal myogenesis using SILAC and shotgun proteomics. Int J Proteomics. 2011;2011:329467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Kaji H. Effects of myokines on bone. Bonekey Rep. 2016;5:826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Wasserman DH, Lacy DB, Colburn CA, Bracy D, Cherrington AD. Efficiency of compensation for absence of fall in insulin during exercise. Am J Physiol. 1991;261(5 Pt 1):E587-E597. [DOI] [PubMed] [Google Scholar]
- 176. Febbraio MA, Hiscock N, Sacchetti M, Fischer CP, Pedersen BK. Interleukin-6 is a novel factor mediating glucose homeostasis during skeletal muscle contraction. Diabetes. 2004;53(7):1643-1648. [DOI] [PubMed] [Google Scholar]
- 177. Steensberg A, Fischer CP, Sacchetti M, et al. Acute interleukin-6 administration does not impair muscle glucose uptake or whole body glucose disposal in healthy humans. J Physiol. 2003;548(Pt 2):631-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Peppler WT, Townsend LK, Meers GM, et al. Acute administration of IL-6 improves indices of hepatic glucose and insulin homeostasis in lean and obese mice. Am J Physiol Gastrointest Liver Physiol. 2019;316(1):G166-G178. [DOI] [PubMed] [Google Scholar]
- 179. Woerle HJ, Albrecht M, Linke R, et al. Importance of changes in gastric emptying for postprandial plasma glucose fluxes in healthy humans. Am J Physiol Endocrinol Metab. 2008;294(1): E103-E109. [DOI] [PubMed] [Google Scholar]
- 180. Plomgaard P, Bouzakri K, Krogh-Madsen R, Mittendorfer B, Zierath JR, Pedersen BK. Tumor necrosis factor-alpha induces skeletal muscle insulin resistance in healthy human subjects via inhibition of Akt substrate 160 phosphorylation. Diabetes. 2005;54(10):2939-2945. [DOI] [PubMed] [Google Scholar]
- 181. Bouzakri K, Plomgaard P, Berney T, Donath MY, Pedersen BK, Halban PA. Bimodal effect on pancreatic β-cells of secretory products from normal or insulin-resistant human skeletal muscle. Diabetes. 2011;60(4):1111-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Rutti S, Dusaulcy R, Hansen JS, et al. Angiogenin and Osteoprotegerin are type II muscle specific myokines protecting pancreatic beta-cells against proinflammatory cytokines. Sci Rep. 2018;8(1):10072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11(2):98-107. [DOI] [PubMed] [Google Scholar]
- 184. Eguchi K, Manabe I. Macrophages and islet inflammation in type 2 diabetes. Diabetes Obes Metab. 2013;15 Suppl (3):152-158. [DOI] [PubMed] [Google Scholar]
- 185. Ehses JA, Perren A, Eppler E, et al. Increased number of islet-associated macrophages in type 2 diabetes. Diabetes. 2007;56(9):2356-2370. [DOI] [PubMed] [Google Scholar]
- 186. Westwell-Roper CY, Ehses JA, Verchere CB. Resident macrophages mediate islet amyloid polypeptide-induced islet IL-1β production and β-cell dysfunction. Diabetes. 2014;63(5):1698-1711. [DOI] [PubMed] [Google Scholar]
- 187. Alluisi EA, Beisel WR, Caldwell LS. Effects of sandfly fever on isometric muscular strength, endurance, and recovery. J Mot Behav. 1980;12(1):1-11. [DOI] [PubMed] [Google Scholar]
- 188. Cabrera SM, Wang X, Chen YG, et al. ; Type 1 Diabetes TrialNet Canakinumab Study Group; AIDA Study Group . Interleukin-1 antagonism moderates the inflammatory state associated with Type 1 diabetes during clinical trials conducted at disease onset. Eur J Immunol. 2016;46(4): 1030-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Urwyler SA, Schuetz P, Ebrahimi F, Donath MY, Christ-Crain M. Interleukin-1 antagonism decreases cortisol levels in obese individuals. J Clin Endocrinol Metab. 2017;102(5):1712-1718. [DOI] [PubMed] [Google Scholar]
- 190. Everett BM, Donath MY, Pradhan AD, et al. Anti-inflammatory therapy with canakinumab for the prevention and management of diabetes. J Am Coll Cardiol. 2018;71(21):2392-2401. [DOI] [PubMed] [Google Scholar]
- 191. Ellingsgaard H, Ehses JA, Hammar EB, et al. Interleukin-6 regulates pancreatic alpha-cell mass expansion. Proc Natl Acad Sci U S A. 2008;105(35):13163-13168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Izumiya Y, Hopkins T, Morris C, et al. Fast/Glycolytic muscle fiber growth reduces fat mass and improves metabolic parameters in obese mice. Cell Metab. 2008;7(2):159-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Shimano M, Ouchi N, Walsh K. Cardiokines: recent progress in elucidating the cardiac secretome. Circulation. 2012;126(21):e327-e332. [DOI] [PubMed] [Google Scholar]
- 194. Ouchi N, Oshima Y, Ohashi K, et al. Follistatin-like 1, a secreted muscle protein, promotes endothelial cell function and revascularization in ischemic tissue through a nitric-oxide synthase-dependent mechanism. J Biol Chem. 2008;283(47):32802-32811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Oshima Y, Ouchi N, Sato K, Izumiya Y, Pimentel DR, Walsh K. Follistatin-like 1 is an Akt-regulated cardioprotective factor that is secreted by the heart. Circulation. 2008;117(24):3099-3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. El-Armouche A, Ouchi N, Tanaka K, et al. Follistatin-like 1 in chronic systolic heart failure: a marker of left ventricular remodeling. Circ Heart Fail. 2011;4(5):621-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Tanaka K, Valero-Muñoz M, Wilson RM, et al. Follistatin like 1 regulates hypertrophy in heart failure with preserved ejection fraction. JACC Basic Transl Sci. 2016;1(4):207-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Ogura Y, Ouchi N, Ohashi K, et al. Therapeutic impact of follistatin-like 1 on myocardial ischemic injury in preclinical models. Circulation. 2012;126(14):1728-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Seki M, Powers JC, Maruyama S, et al. Acute and chronic increases of circulating FSTL1 normalize energy substrate metabolism in pacing-induced heart failure. Circ Heart Fail. 2018;11(1):e004486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Crane JD, MacNeil LG, Lally JS, et al. Exercise-stimulated interleukin-15 is controlled by AMPK and regulates skin metabolism and aging. Aging Cell. 2015;14(4):625-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Duggal NA, Niemiro G, Harridge SDR, Simpson RJ, Lord JM. Can physical activity ameliorate immunosenescence and thereby reduce age-related multi-morbidity? Nat Rev Immunol. 2019;19(9):563-572. [DOI] [PubMed] [Google Scholar]
- 202. McCarthy DA, Dale MM. The leucocytosis of exercise. A review and model. Sports Med. 1988;6(6):333-363. [DOI] [PubMed] [Google Scholar]
- 203. Pedersen BK, Hoffman-Goetz L. Exercise and the immune system: regulation, integration, and adaptation. Physiol Rev. 2000;80(3):1055-1081. [DOI] [PubMed] [Google Scholar]
- 204. Nieman DC, Fagoaga OR, Butterworth DE, et al. Carbohydrate supplementation affects blood granulocyte and monocyte trafficking but not function after 2.5 h or running. Am J Clin Nutr. 1997;66(1):153-159. [DOI] [PubMed] [Google Scholar]
- 205. Nehlsen-Cannarella SL, Fagoaga OR, Nieman DC, et al. Carbohydrate and the cytokine response to 2.5 h of running. J Appl Physiol (1985). 1997;82(5):1662-1667. [DOI] [PubMed] [Google Scholar]
- 206. Fischer CP, Hiscock NJ, Penkowa M, et al. Supplementation with vitamins C and E inhibits the release of interleukin-6 from contracting human skeletal muscle. J Physiol. 2004;558(Pt 2):633-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Petersen AM, Pedersen BK. The role of IL-6 in mediating the anti-inflammatory effects of exercise. J Physiol Pharmacol. 2006;57 Suppl (10):43-51. [PubMed] [Google Scholar]
- 208. Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol (1985). 2005;98(4):1154-1162. [DOI] [PubMed] [Google Scholar]
- 209. Pedersen BK, Steensberg A, Keller P, et al. Muscle-derived interleukin-6: lipolytic, anti-inflammatory and immune regulatory effects. Pflugers Arch. 2003;446(1):9-16. [DOI] [PubMed] [Google Scholar]
- 210. Pedersen BK. The anti-inflammatory effect of exercise: its role in diabetes and cardiovascular disease control. Essays Biochem. 2006;42:105-117. [DOI] [PubMed] [Google Scholar]
- 211. Dinarello CA. The interleukin-1 family: 10 years of discovery. Faseb J. 1994;8(15):1314-1325. [PubMed] [Google Scholar]
- 212. Opp MR, Smith EM, Hughes TK Jr. Interleukin-10 (cytokine synthesis inhibitory factor) acts in the central nervous system of rats to reduce sleep. J Neuroimmunol. 1995;60(1-2):165-168. [DOI] [PubMed] [Google Scholar]
- 213. Schindler R, Mancilla J, Endres S, Ghorbani R, Clark SC, Dinarello CA. Correlations and interactions in the production of interleukin-6 (IL-6), IL-1, and tumor necrosis factor (TNF) in human blood mononuclear cells: IL-6 suppresses IL-1 and TNF. Blood. 1990;75(1):40-47. [PubMed] [Google Scholar]
- 214. Mizuhara H, O’Neill E, Seki N, et al. T cell activation-associated hepatic injury: mediation by tumor necrosis factors and protection by interleukin 6. J Exp Med. 1994;179(5):1529-1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Han MS, White A, Perry RJ, et al. Regulation of adipose tissue inflammation by interleukin 6. Proc Natl Acad Sci U S A. 2020;117(6):2751-2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Rosenkilde M, Nordby P, Stallknecht B. Maintenance of improvements in fitness and fatness 1 year after a 3-month lifestyle intervention in overweight men. Eur J Clin Nutr. 2016;70(10):1212-1214. [DOI] [PubMed] [Google Scholar]
- 217. Laye MJ, Thyfault JP, Stump CS, Booth FW. Inactivity induces increases in abdominal fat. J Appl Physiol (1985). 2007;102(4):1341-1347. [DOI] [PubMed] [Google Scholar]
- 218. Krogh-Madsen R, Pedersen M, Solomon TP, et al. Normal physical activity obliterates the deleterious effects of a high-caloric intake. J Appl Physiol (1985). 2014;116(3):231-239. [DOI] [PubMed] [Google Scholar]
- 219. Krogh-Madsen R, Thyfault JP, Broholm C, et al. A 2-wk reduction of ambulatory activity attenuates peripheral insulin sensitivity. J Appl Physiol (1985). 2010;108(5):1034-1040. [DOI] [PubMed] [Google Scholar]
- 220. Harder-Lauridsen NM, Nielsen ST, Mann SP, et al. The effect of alternate-day caloric restriction on the metabolic consequences of 8 days of bed rest in healthy lean men: a randomized trial. J Appl Physiol (1985). 2017;122(2):230-241. [DOI] [PubMed] [Google Scholar]
- 221. Christensen RH, Wedell-Neergaard AS, Lehrskov LL, et al. Effect of aerobic and resistance exercise on cardiac adipose tissues: secondary analyses from a randomized clinical trial. JAMA Cardiol. 2019;4(8):778-787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Moore SC, Lee IM, Weiderpass E, et al. Association of leisure-time physical activity with risk of 26 types of cancer in 1.44 million adults. JAMA Intern Med. 2016;176(6):816-825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Christensen JF, Simonsen C, Hojman P. Exercise training in cancer control and treatment. Compr Physiol. 2018;9(1):165-205. [DOI] [PubMed] [Google Scholar]
- 224. Hojman P, Dethlefsen C, Brandt C, Hansen J, Pedersen L, Pedersen BK. Exercise-induced muscle-derived cytokines inhibit mammary cancer cell growth. Am J Physiol Endocrinol Metab. 2011;301(3):E504-E510. [DOI] [PubMed] [Google Scholar]
- 225. Aoi W, Naito Y, Takagi T, et al. A novel myokine, secreted protein acidic and rich in cysteine (SPARC), suppresses colon tumorigenesis via regular exercise. Gut. 2013;62(6):882-889. [DOI] [PubMed] [Google Scholar]
- 226. Lucia A, Ramírez M. Muscling in on cancer. N Engl J Med. 2016;375(9):892-894. [DOI] [PubMed] [Google Scholar]
- 227. Manole E, Ceafalan LC, Popescu BO, Dumitru C, Bastian AE. Myokines as possible therapeutic targets in cancer cachexia. J Immunol Res. 2018;2018:8260742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Cook KS, Min HY, Johnson D, et al. Adipsin: a circulating serine protease homolog secreted by adipose tissue and sciatic nerve. Science. 1987;237(4813):402-405. [DOI] [PubMed] [Google Scholar]
- 229. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425-432. [DOI] [PubMed] [Google Scholar]
- 230. Scherer PE. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes. 2006;55(6):1537-1545. [DOI] [PubMed] [Google Scholar]
- 231. Funcke JB, Scherer PE. Beyond adiponectin and leptin: adipose tissue-derived mediators of inter-organ communication. J Lipid Res. 2019;60(10):1648-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Weigert C, Hoene M, Plomgaard P. Hepatokines-a novel group of exercise factors. Pflugers Arch. 2019;471(3):383-396. [DOI] [PubMed] [Google Scholar]
- 233. Jespersen NZ, Larsen TJ, Peijs L, et al. A classical brown adipose tissue mRNA signature partly overlaps with brite in the supraclavicular region of adult humans. Cell Metab. 2013;17(5):798-805. [DOI] [PubMed] [Google Scholar]
- 234. Deshmukh AS, Peijs L, Beaudry JL, et al. Proteomics-based comparative mapping of the secretomes of human brown and white adipocytes reveals EPDR1 as a novel batokine. Cell Metab. 2019;30(5):963-975.e7. [DOI] [PubMed] [Google Scholar]