Abstract
Background:
The Contrast Avoidance Model postulates that individuals with generalized anxiety disorder (GAD) fear a sharp spike in negative emotion, and thus, prefer to worry to maintain their negative affect rather than being in a more euthymic state, such as relaxation. Relaxation induced anxiety (RIA) is a paradoxical phenomenon wherein people experience a spike in their anxiety during relaxation training. Because these phenomena may be related and may also operate among individuals with major depressive disorder (MDD), we attempted to test whether negative contrast sensitivity was a mediator of GAD or MDD in the prediction of RIA.
Methods:
Individuals with GAD (n = 32), MDD (n = 34), and healthy controls (n = 30) were exposed to a negative emotional contrast by engaging with relaxation practice and then watching a negative emotional video. This was followed by the assessment of their negative contrast sensitivity. After this, participants engaged again with relaxation and RIA was measured. We examined mediation effects of negative contrast on the relationship between diagnostic status and RIA.
Results:
Negative contrast sensitivity fully mediated GAD and partially mediated MDD in predicting RIA.
Conclusions:
Our findings support the hypotheses that negative contrast sensitivity is the mediator of both GAD and MDD in predicting RIA. This may have implications for assessment and treatment of GAD and MDD.
Keywords: Relaxation induced anxiety, Contrast Avoidance Model, Generalized anxiety disorder, Major depressive disorder, Mediation analysis
1. Introduction
Generalized anxiety disorder (GAD) is characterized by excessive and uncontrollable worry and anxiety about a broad range of negative events (Newman et al., 2013). Due to transdiagnostic characteristics of worry, GAD has been viewed as a basic disorder, which may be a gateway of other anxiety disorders (Ruscio et al., 2007). However, cognitive-behavioral therapy (CBT) for GAD has been reported to be less efficacious than CBT for other anxiety disorders (Brown et al., 1994; Hofmann and Smits, 2008).
Applied relaxation (AR; Öst, 1987) is often integrated as one crucial component of CBT for GAD (Chambless and Ollendick, 2001). The major purpose of AR is to teach people coping skills that help them relax rapidly to help reduce anxiety responses. AR usually begins by teaching people how to progressively tense and release various muscle groups in a quiet comfortable place and this technique is sometimes combined with diaphragmatic breathing (slowed paced breathing through the diaphragm). Such relaxation is practiced twice a day. Over time, patients are taught ways to achieve relaxation more rapidly and to use it as a portable coping strategy in response to anxiety triggers in their environment. A benefit of AR is that it decreases anxiety and physiological tension. In addition, if anxious individuals engage in AR at the first cues of worry or tension, it is thought to curtail the spirals of anxiety and worry that are characteristic of GAD (Borkovec and Costello, 1993; Öst, 1987). When compared to no treatment, AR yielded significantly more improvement in anxiety symptoms and demonstrated efficacy comparable to cognitive therapy or combined CBT packages (Barlow et al., 1992; Bolognesi et al., 2014; Borkovec and Costello, 1993; Öst and Breitholtz, 2000; Siev and Chambless, 2007; Tarrier and Main, 1986).
Nonetheless, AR does not always lead to a relaxed state. On the contrary, AR may increase anxiety among some individuals. This paradoxical increase is called, “Relaxation Induced Anxiety” (RIA; Heide and Borkovec, 1983). RIA has been operationalized as a spike in anxiety, muscle tension, or anxious thoughts and images when an individual is attempting to engage in relaxation (Heide and Borkovec, 1983, 1984). There are two ironies involved with this phenomenon. The first irony is that anxiety as an outcome of relaxation is totally contradictory to the purpose of the treatment. Another irony is that individuals who are more vulnerable to this unexpected anxiety response are those with anxiety disorders, even though they need to decrease it more than those without anxiety disorders.
Unfortunately, there has been little attention paid to the RIA phenomenon. Heide and Borkovec (1983) were among the first to study it. In participants with chronic tension, they found that 30.8% who engaged with progressive muscle relaxation (PMR) and 53.8% who engaged with mantra meditation experienced RIA. Similarly, higher within-session RIA was associated with higher levels of cognitive anxiety, greater state anxiety, and higher heart rate (Norton et al., 1985). Another study found that individuals with higher RIA had greater fear of becoming anxious and losing control of their anxiety responses than those with lower RIA (Braith et al., 1988).
Because RIA hinders clients from experiencing a relaxed state, it may impede treatment of GAD in a number of ways. First, it may lead to a reduced likelihood of ongoing perceptions of controllability over anxiety reduction. Second, repeated exposure to RIA may condition a belief that “relaxation is dangerous.” As a result, patients could develop resistance to treatment efforts and avoid any activities that could make them relaxed.
RIA has also been found to predict negative effects of treatment. It predicted less reduction in anxiety and depression at 12-month follow-up from treatments containing AR (Borkovec et al., 1987; Borkovec and Costello, 1993). Also, a more recent study compared GAD patients with high peak RIA to those with low peak RIA throughout 14 sessions of CBT and BT (Newman et al., 2018). Patients with lower peak RIA had better post-treatment outcome than those with higher peak RIA. This suggests that there could be person-specific differences in experiencing RIA, establishing a need to uncover a third variable that affects these individual differences.
Nonetheless, there is insufficient data on the mechanism of RIA. At the same time, data suggests some meaningful hypotheses. One hypothesis is that relaxation directs people to focus on their physiological state and internal cues and this could make them more sensitive to their bodily tension. Consequently, relaxation may bring about greater arousal induced anxiety, or fear of fear in some people (Braith et al., 1988; Heide and Borkovec, 1983, 1984; Norton et al., 1985; Reiss, 1987). Another hypothesis is that RIA may be an outcome of anxious individuals’ fear that they will not be able to control negative emotions (i.e., internal locus of control, and fear of losing control; Braith et al., 1988; Heide and Borkovec, 1983, 1984; Norton et al., 1985).
Explaining relaxation as a potential contributor to anxiety (i.e., the first notion) and fear of losing control as a potential cause of RIA (i.e., the second notion) are similar to the notion of the Contrast Avoidance Model of Worry (CAM; Newman and Llera, 2011; Newman et al., 2013). Whereas prior theories of worry have suggested that worry dampens anxiety (see Newman and Llera, 2011 for a full review), CAM suggests that worry increases negative affect and that individuals with anxiety prefer to sustain this heightened negative emotion as protection against a sudden sharp increase in negative affect (e.g., “If I am already worried and feeling bad, there will not be as much of an increase in my negative affect, when I encounter a bad event”). Similar to the notion of RIA, the model also suggests that being in a relaxed state makes individuals more likely to experience a sharp surge in negative emotion if they encounter a stressful or negative event. Consequently, due to the fear of losing control, anxious individuals would rather keep feeling anxious during the process of relaxation (i.e., similar to the second notion of RIA). In this sense, RIA might be due to fear of a negative contrast and a desire to maintain negative emotional constancy (Llera and Newman, 2014; Newman et al., 2018).
Evidence supports these aspects of CAM. In laboratory studies, compared to worry, relaxation immediately preceding negative emotion inducing videos, increased the probability of a sharp surge in negative emotion. These effects were present in both GAD and nonanxious participants with no significant difference between them (Llera and Newman, 2014, 2010). However, Llera and Newman (2014) found that when individuals with GAD relaxed prior to watching the video, they were significantly more likely than nonanxious individuals to report a sensitivity to and discomfort with the negative contrast experience (that both groups exhibited). In addition, Llera and Newman (2017) found that compared to nonanxious controls, individuals with GAD were more likely to report a preference for a negative mood over feeling good. They also reported actively trying to sustain their negative mood because feeling good or euthymic made them more vulnerable to a negative emotional contrast. However, no previous study has directly examined the relationship between RIA and negative contrast sensitivity. Furthermore, RIA and negative contrast sensitivity have only been tested in anxious populations and their correlation with other disorders is unknown.
Major depression (MDD) is one of the most frequently comorbid disorders with GAD (Moffitt et al., 2007; Wittchen et al., 2000). MDD and GAD share commonalities. Similar to worry which is a repetitive thought about future negative outcomes (Borkovec et al., 1983), MDD is characterized by rumination, perseverative thoughts about one’s depressive symptoms and their possible causes and negative consequences (Nolen-Hoeksema, 1991). Similar to findings on GAD, MDD was positively correlated with fear of losing control during the experience of depressed mood (Liverant et al., 2008). In addition, similar to worry, rumination increased sadness from the baseline and this allowed avoidance of sharp surge in sadness during a subsequent sad video exposure (Kim and Newman, 2016). These findings raise the possibility that individuals with GAD and MDD may both experience negative contrast sensitivity.
Furthermore, although it has not been as frequently applied as in GAD, AR has demonstrated some efficacy in the treatment of chronic depression (Lolak et al., 2008; Murphy et al., 1995; Reynolds and Coats, 1986). As indicated above, however, we are not aware of any studies that have examined the relationship between depression and RIA. However, examining RIA based on the framework of the Contrast Avoidance Model may be helpful for a deeper understanding of MDD and its treatment.
In order to better understand the RIA phenomenon and its interface with GAD and MDD, we conducted an experimental study that tested the mediation effects of negative contrast sensitivity. In the current study, we recruited pure GAD (non-MDD), MDD (non-GAD) and non-GAD & MDD controls to avoid potential bias from multicollinearity that can affect the predictions of independent variables (Shieh and Fouladi, 2003; Yu et al., 2015). For cleaner assessments of negative contrast sensitivity and RIA and to ensure the independence of the two variables, we measured them at two different time points rather than measuring them concurrently. Negative contrast sensitivity was measured in the first trial of the study by having all participants first relax and then watch a fear inducing video which has been found in previous studies to amplify the experience of a negative contrast. RIA was then assessed separately following a second relaxation practice.
We made two predictions: first, compared to controls, negative contrast sensitivity would mediate GAD status predicting greater RIA when the covariance of the MDD group was taken into account; second, compared to controls, negative contrast sensitivity would mediate the MDD group predicting greater RIA, when the covariance of the GAD group was taken into account.
2. Methods
2.1. Participants
Ninety-six students taking introductory psychology classes were recruited at a rural state university. As compensation for their participation, partial course credits were given. Of these, 64 participants were female and 32 participants were male. Age ranged from 18 to 34 (Mage = 18.69, SDage = 1.88), and ethnic distribution was 75% White, 13.5% Asian, 8.3% African American, 2.1% Hispanic, and 1.1% other.
Participants were selected based on their responses to the Generalized Anxiety Disorder Questionnaire-IV (GAD-Q-IV; Newman et al., 2002) and the Beck Depression Inventory-II (BDI-II; Beck et al., 1996). They were assigned to the GAD group if they met full diagnostic criteria on the GAD-Q-IV and scored below 14 on the BDI-II. They were assigned to the MDD group if they scored above 19 on the BDI-II but did not meet diagnostic criteria on the GAD-Q-IV. The non-GAD & MDD controls were those who did not meet diagnostic criteria on the GAD-Q-IV and scored below 14 on the BDI-II. This resulted in 32 participants in the GAD group, 34 participants in the MDD group, and 30 participants in the non-GAD & MDD control group. Preliminary data analyses showed no significant demographic differences across the three groups in age, gender, or ethnicity.
2.2. Screening measures
Generalized Anxiety Disorder Questionnaire-IV (GAD-Q-IV;Newman et al., 2002).
This nine-item self-report measure covers the complete symptomatology of GAD as delineated by the Diagnostic and Statistical Manual of Mental Disorders (American Psychiatric Association, 1994, 2013). Two-week retest-reliability was high (κ = 0.64), and it showed good convergent and discriminant validity, as well as good agreement with a diagnostic interview (ADIS-IV; Brown et al., 1994). Penn State Worry Questionnaire (PSWQ; Meyer et al., 1990) scores of undergraduates identified as having GAD with the GAD-Q-IV were not significantly different from PSWQ scores in a treatment seeking community sample (see Newman et al., 2002). Although the GAD-Q-IV can be scored using a dimensional system, the criterion-based scoring system was used in this study requiring that participants met full GAD criteria. When using criterion-based scoring, Newman et al. (2002) found 96% specificity and 67% sensitivity in detecting GAD. Also, in a primary care therapy-seeking sample, criterion scoring was the optimal strategy for identifying GAD (i.e., with a sensitivity of 0.89 and specificity of 0.82; Moore et al., 2014). Participants who met GAD criteria but scored lower than or equal to 13 on the BDI-II (minimal or no depression) were included in the GAD group.
Beck Depression Inventory-II (BDI-II; Beck et al., 1996).
This 21-item scale tests the presence and severity of depression. A recent meta analytic review demonstrated strong internal consistency (Cronbach’s α = 0.83–0.96), and retest-reliability (Pearson’s r = 0.73–0.96). Also, it showed good convergent validity with other depression measures (Pearson’s r = 0.57–0.84) (Wang and Gorenstein, 2013). In a medical sample, a cutoff score of 18 on this scale yielded a sensitivity of 94% and a specificity of 92%, with an overall correct classification rate of 92% (Arnau et al., 2001). As suggested by the developers of the measure (Beck et al., 1996), participants were determined to have MDD when their total scores were greater than or equal to 20 (high depression). Those meeting this cutoff, who did not also meet GAD criteria were assigned to the MDD group.
2.3. Experiment measures
Contrast Avoidance Questionnaire-Experimental Assessment (CAQ-E; Llera and Newman, 2014).
The CAQ-E contains five items rated on a 9-point Likert scale that assess the extent to which prior states such as relaxation made it difficult for participants to cope with emotions elicited by an emotional video. As with a previous study (Llera and Newman, 2014), participants rated their coping scores immediately after watching emotional videos. In this study, the CAQ-E was administered at the end of the first trial. For the purpose of the current study, we coded items so that higher total scores of the CAQ-E indicated greater sensitivity to a negative emotional contrast (e.g., greater discomfort with the emotional contrast from a negative mood exposure immediately following relaxation). On the other hand, lower total scores indicated that relaxation was more helpful in coping with the subsequent negative emotions, which demonstrated less vulnerability to a negative emotional contrast. In a previous study, internal consistency was high (Cronbach’s α = 0.73) (Llera and Newman, 2014). The CAQ-E demonstrated similar internal consistency (Cronbach’s α = 0.70) in the current study.
Response to Relaxation Session (RRS; Heide and Borkovec, 1983).
We measured RIA using the RRS, which was used in previous RIA studies (Borkovec and Costello, 1993; Heide and Borkovec, 1983; Newman et al., 2018). In the current study, participants completed this measure in the second trial immediately after relaxation practice. The RRS contains two items which measure (1) changes in anxiety during relaxation (i.e., “How much did you notice an increase in anxiety or nervousness during the relaxation session?”) and (2) levels of distraction due to anxious cognitions (i.e., “How frequently was your attention distracted by unpleasant or negative thoughts or images?”). Each of the items was measured based on a 9-point Likert scale. The RRS demonstrated good retest-reliability when it was measured twice across 3 weeks (ritem1 = 0.73, ritem2 = 0.78) (Newman et al., 2018). In the current study, internal consistency of the RRS was high (Cronbach’s α = 0.80).
2.4. Experimental manipulations
Applied relaxation (AR).
After the baseline assessment, participants were told that they would learn two different types of relaxation techniques. Following guidelines from previous studies (Bernstein and Borkovec, 1973; Borkovec and Costello, 1993), we trained participants in both (1) progressive muscle relaxation and (2) diaphragmatic breathing. During the training, visual aids and written instructions depicting specifics of each posture were provided along with the investigator’s verbal and physical demonstration. In order to standardize the experimental process, we played audiotaped instructions recorded by a professional voice artist, so that each participant engaged in AR for the same 10 min and followed the audio instructions. For the progressive relaxation, participants were asked to tense each of the five muscle groups (i.e., neck and shoulders, back, abdomen, hips and legs, and toes and feet) and then immediately release the tension. For the diaphragmatic breathing, participants inhaled through their noses and slowly exhaled through their mouths. While they were breathing, they placed their hands on their chest and abdomen to ensure they were breathing via their abdomen. In previous studies, AR successfully induced relaxation significantly greater than other inductions such as worry and rumination (Kim and Newman, 2016; Llera and Newman, 2014, 2010).
Video stimuli for negative emotional elicitation.
For the elicitation of negative affect, we used two video clips developed and validated by Gross and Levenson (1995). For the sad exposure, we showed a scene from “the Champ” (Lovell and Zeffirelli, 1979) for 2 min and 51 s, wherein a boy cries at his father’s death due to a brutal boxing match. For the fear exposure, participants watched a scene from the horror movie, “the Shining” (Kubrick, 1980) for 1 min and 22 s. The latter scene depicts a boy playing with toys in an ominous hallway. In the previous validation study, these videos were reliable in eliciting their target emotions. We counter-balanced the order of video exposures so that participants could watch either the fearful or sad video clip during the first and second trials of the experiment. Previous evidence demonstrated strong efficacy in eliciting their target emotions (Converse et al., 2008; Cryder et al., 2008; Inbar and Gilovich, 2011; Tiedens and Linton, 2001).
2.5. Procedure
We used data collected as part of a larger research project investigating contrast avoidance in GAD and MDD (Kim and Newman, 2016). Here, we only report on procedures relevant to the current study. The current experiment consisted of two consecutive steps. In the first step, we exposed participants to a negative contrast and measured their negative contrast sensitivity (i.e., on the CAQ-E). Previous studies showed that engagement with relaxation immediately prior to watching a negative emotion inducing video created the subjective and physiological experience of a negative emotional contrast (Llera and Newman, 2010;2014). However, compared to controls, those with GAD reported significantly more sensitivity to that experience (Llera and Newman, 2014). Thus, the goal of the first relaxation immediately prior to watching the negative emotion inducing video was to create a negative emotional contrast experience and assess participants’ sensitivity to that negative contrast. In the second step, we had participants engage in relaxation and we immediately assessed RIA (i.e., using the RRS). This was based on prior studies (e.g., Heide and Borkovec, 1983, 1984; Newman et al., 2018), which assessed RIA by having participants engage in formal relaxation practice and then complete the RRS. The goal of the second relaxation was to have a “clean” assessment of RIA separate from the initial negative contrast assessment. Instructions for all experimental tasks and video stimuli were presented in a standardized manner to minimize any potential experimenter bias. After providing consent, participants were seated in front of a computer display (i.e., Dell E2317H 23-inch LED computer monitor, Full HD with 1920 × 1080 pixels at 60 Hz). Next, they spent 5 min becoming acclimated to the laboratory environment. Participants than received instructions on the relaxation procedure, which entailed the use of both progressive muscle relaxation and diaphragmatic breathing. After experimenters were certain that participants understood how to implement the two relaxation techniques, participants engaged with the relaxation induction for 10 min while being guided by audio instructions. Next, they watched one of the two movie clips validated to induce negative affect (i.e., either fear or sadness video). Then, using the CAQ-E, participants reported the degree to which the prior relaxation task hindered them from coping with the negative affect induced by the video clip exposure. Following this, they were again auditorily guided to repeat the 10-minute relaxation induction. After the completion of the second relaxation task, they reported their RIA on the RRS. After completion of the experiment, participants were fully debriefed and the purpose of the study was explained along with resources for any potential psychological harm. No participants complained about psychological difficulties due to the experimental manipulations. The current experiment was programmed using E-prime software (Psychology Software Tools Inc., 2002).
2.6. Statistical analysis methods
Outlier detection.
In order to identify multivariate outliers, we calculated a Mahalanobis distance for each group by entering negative contrast sensitivity scores as the mediator and RIA total as the dependent variable (Tabachnick and Fidell, 2007). Participants whose Mahalanobis distance exceeded 3.84 were determined to be outliers (df = 1, p < .05). Based on this, five participants were excluded from the data analysis (i.e., three participants from the GAD group, one participant from the MDD group, and one participant from the non-GAD & MDD controls). In addition, two participants who failed to complete the second trial due to a computer error were also removed (i.e., listwise deletion) from the data analyses (i.e., one person from the GAD group, and one person from the MDD group). This left 89 participants who were included in the data analyses: GAD group = 28; MDD group = 32; non-GAD & MDD controls = 29.
Mediation analysis.
For this analysis, we used the RRS score from the second trial as the dependent variable and the preceding CAQ score as the mediator with diagnostic status as the independent variable. For the main data analysis, we used the PROCESS Macro for SPSS (Hayes, 2013). Given that the independent variable of the current study consisted of three different groups (i.e., a multi-categorical construct), we first conducted a dummy code transformation (i.e., indicator coding) to create two dummy variables, as suggested by Hayes and Preacher (2014) (see Fig. 1). With the two dummy variables, we examined mediation paths based on bias-corrected bootstrap confidence intervals which were derived from 10,000 bootstrap samples (Hayes and Preacher, 2014; Hayes, 2013; Preacher and Hayes, 2008). We tested the first hypothesis by comparing effects of the GAD group to controls taking into account the covariance of MDD. For the second hypothesis, we compared effects of the MDD group to controls controlling for the effects of GAD. In each analysis, we tested direct and indirect effects of each group (independent variables) on RIA (dependent variable) via negative contrast sensitivity (the mediator). Along with mediation analyses, participants’ descriptive statistics and correlations across the variables were also examined. All analyses were conducted using SPSS version 24.
Fig. 1.
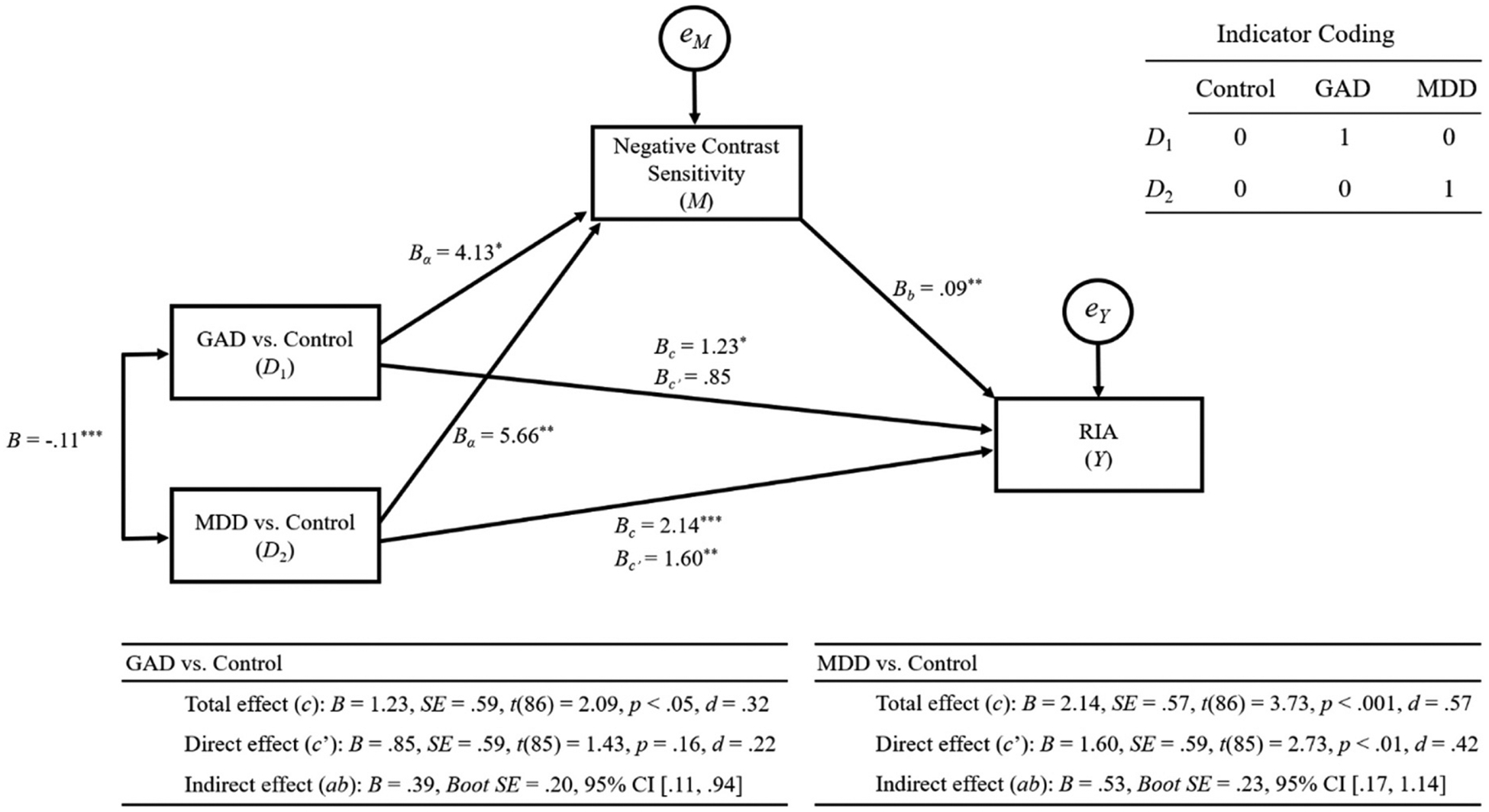
Results from mediation analyses.
3. Results
Descriptive statistics for each of the groups can be seen in Table 1. In addition to the descriptive statistics test, a one-way between-subject ANOVA was conducted to ensure we had formed distinct groups of GAD, MDD and the non-GAD & MDD controls using continuous scores on both measures in these analyses. Results indicated that there were significant differences across the groups on the two target screening scores, FGAD-Q-IV (2, 86) = 96.75, p < .001; FBDI-II (2, 86) = 248.92, p < .001. Post-hoc Bonferroni comparisons indicated that the mean continuous GAD-Q-IV score for the GAD group (M = 9.51, SD = 1.12) was significantly higher (p < .001 for all comparisons) than the MDD group (M = 5.65, SD = 3.31), and the non-GAD & MDD controls (M = 1.22, SD = 1.49). In addition, the MDD group’s GAD-Q-IV scores were higher than those of the control group (p < .001). As we intended in the screening, results of the BDI-II also showed that the MDD group (M = 26.50, SD = 5.86) was significantly higher (p < .001 for all comparisons) than the GAD group (M = 7.89, SD = 3.32), and the non-GAD & MDD controls (M = 2.79, SD = 3.23). Also, we found a significant difference between the GAD group and controls on the BDI-II (p < .001). These results indicated that the two screening measures used in the current study appropriately discriminated the groups on the presence of GAD and MDD.
Table 1.
Means and standard deviations for study variables by groups.
| Full sample (N = 89) M (SD) | GAD (N = 28) M (SD) | MDD (N = 32) M (SD) | Controls (N = 29) M (SD) | |
|---|---|---|---|---|
| GAD-Q-IV (Cont.) | 5.42 (4.02) | 9.51 (1.12) | 5.65 (3.31) | 1.22 (1.49) |
| BDI-II | 12.92 (11.30) | 7.89 (3.32) | 26.50 (5.86) | 2.79 (3.23) |
| CAQ-E | 22.99 (6.92) | 23.79 (6.48) | 25.31 (7.50) | 19.66 (5.43) |
| RRS | 3.71 (2.38) | 3.79 (2.47) | 4.69 (2.83) | 2.55 (0.74) |
Note. Multivariate outliers were excluded from this analysis. GAD = generalized anxiety disorder group, MDD = major depressive disorder group, Controls = non-GAD & MDD Controls; GAD-Q-IV (Cont.) = The Generalized Anxiety Disorder Questionnaire (Continuous scoring), BDI-II = The Beck Depression Inventory-II, CAQ-E = The Contrast Avoidance Questionnaire-Experiment Version, RRS = The Response to Relaxation Session.
We tested the first hypothesis (i.e., “Compared to controls, negative contrast sensitivity would mediate GAD status predicting greater RIA when the covariance of the MDD group was taken into account”). For effect size, we calculated Cohen’s d statistics based on the algorithm, d = t(2/n)1/2 (Dunlap et al., 1996). Results are provided in Fig. 1. In this model, GAD (D1) significantly predicted negative contrast sensitivity (M): a1, B = 4.13, SE = 1.74, t(86) = 2.38, p < .05, d = 0.36. Also, negative contrast sensitivity (M) significantly predicted RIA (Y): b, B = 0.09, SE = 0.04, t(85) = 2.65, p < .01, d = 0.41. When the mediation effect of negative contrast sensitivity (M) was not considered, GAD (D1) significantly predicted RIA (Y): c, B = 1.23, SE = 0.59, t(86) = 2.09, p < .05, d = 0.32. However, when GAD (D1) and negative contrast sensitivity (M) were simultaneously entered into the model, GAD (D1) no longer significantly predicted RIA (Y): c’, B = 0.85, SE = 0.59, t(85) = 1.43, p = .16, d = 0.22. Analysis from the biascorrected bootstrap showed that the confidence interval did not include zero, indicating that the indirect effect of GAD (D1) on RIA (Y) via negative contrast sensitivity (M) was significant: a × b, B = 0.39, Boot SE = 0.20, 95% CI [.11, 0.94]. Consequently, GAD (D1) was fully mediated by negative contrast sensitivity (M) in predicting RIA (Y).
Next, we tested the second hypothesis (i.e., “Compared to controls, negative contrast sensitivity would mediate the MDD group predicting greater RIA, when the covariance of the GAD group was taken into account”). Results indicated that MDD (D2) was a significant predictor of negative contrast sensitivity (M): a1, B = 5.66, SE = 1.68, t(86) = 3.36, p < .01, d = 0.51. In addition, negative contrast sensitivity (M) significantly predicted RIA (Y): b, B = 0.09, SE = 0.04, t(85) = 2.65, p < .01, d = 0.41. When the mediation effect of negative contrast sensitivity (M) was not taken into account, MDD (D2) significantly predicted RIA (Y): c, B = 2.14, SE = 0.57, t(86) = 3.73, p < .001, d = 0.57. With the mediation effect of negative contrast sensitivity (M) partialed, MDD (D2) still significantly predicted RIA (Y) but with decreased strength: c’, B = 1.60, SE = 0.59, t(85) = 2.73, p < .01, d = 0.42. Results from the bias-corrected bootstrapping showed that the confidence interval did not straddle zero within its range, confirming the significance of the indirect effect of MDD (D2) and negative contrast sensitivity (M) on RIA (Y): a × b, B = 0.53, Boot SE = 0.23, 95% CI [.17, 1.14]. These results demonstrate that MDD (D2) was partially mediated by negative contrast sensitivity (M) in predicting RIA (Y).
The two models accounted for 21% of the total variance of RIA (Y), F(3, 85) = 7.31, R2 = 0.21, p < .001. In order to compare the contribution of the two indirect effects in predicting RIA (Y), we calculated the ratio of indirect to total effect (Ditlevsen et al., 2005; Freedman, 2001). The ratio of indirect to total effect of the GAD (D1) model was 0.32, and that of the MDD (D2) model was 0.25, showing that negative contrast sensitivity (M) served a more important role in GAD (D1) than in MDD (D2), in predicting RIA (Y). This result is consistent with the results indicating the full mediation of the GAD (D1) model as opposed to the partial mediation of the MDD (D2) model.
In order to test the heterogeneity of the two indirect effects, we conducted a series of follow-up analyses. First, we examined the confidence intervals of the GAD (D1) and MDD (D2) models. Confidence intervals of the GAD (D1) model: a × b, B = 0.39, Boot SE = 0.20, 95% CI [.11, 0.94], overlapped with that of the MDD (D2) model: a × b, B = 0.53, Boot SE = 0.23, 95% CI [.17, 1.14], which indicated that the two indirect effects were not independent from each other. In addition, we re-coded the dummy variables: Control, D ‘1 = 0, D ‘2 = 1; GAD, D ‘1 = 1, D ‘2 = 0; MDD, D ‘1 = 0, D ‘2 = 0, so that the GAD and Control groups were entered as variables of interest and MDD as a control variable: D ‘1, GAD vs. MDD; D ‘2, Controls vs. MDD. Using the recoded model (D ‘1) which controlled the effect of D ‘2, we tested the indirect effects of GAD (D1’) and negative contrast sensitivity (M) in predicting RIA (Y). The results showed that there was a non-significant effect of GAD in predicting negative contrast sensitivity (M): a1, B = −1.53, SE = 1.70, t(86) = −0.90, p = .37, d = −0.14, and RIA (Y) via negative contrast sensitivity (M): a × b, B = −0.14, Boot SE = 0.18, 95% CI [−0.61, 0.14], which indicated that effects of GAD and MDD groups were not different from each other. These results are consistent with results from the first follow-up test.
4. Discussion
Previous studies have suggested that individuals with anxiety may have specific traits associated with RIA, such as a higher sensitivity to anxiety, and a desire to have control over their emotion. However, it has not been clear how these traits were related to RIA. Connecting the dots, the current study sought to test RIA in GAD accounting for negative contrast sensitivity. Given commonalities between GAD and MDD, we also tested RIA among individuals with MDD as well. Results aligned with our predictions. Negative contrast sensitivity mediated the effects of both GAD and MDD in predicting RIA. These findings indicate that negative contrast sensitivity and RIA might be transdiagnostic processes that operate in the two disorders.
Although there have not been any studies that tested RIA among depressed individuals or in the context of the Contrast Avoidance Model, previous evidence has shown that depression may also be relevant to avoidance of a negative emotional shift. Similar to those with high anxiety, depressed individuals were more likely to hold positive beliefs about rumination (Papageorgiou and Wells, 2003; Watkins et al., 2005; Watkins and Moulds, 2005), which is a common feature of depression. Rumination was also associated with a more intense and prolonged dysphoric mood (McLaughlin et al., 2007; Moberly and Watkins, 2008; Nolen-Hoeksema and Morrow, 1993). In addition, similar to worry, rumination was positively associated with physiological arousal and subjective discomfort (Ottaviani et al., 2011; Ottaviani et al., 2016). Thus, rumination may also be motivated by its ability to avoid a negative emotional contrast.
Another experimental study which tested relaxation versus worry and rumination in GAD and MDD found supportive evidence (Kim and Newman, 2016). In this study, both worry and rumination, in individuals with GAD or MDD, increased negative emotion from baseline (whereas relaxation did not). Also, whereas prior relaxation led to an enhanced emotional contrast, worry and rumination reduced the likelihood of experiencing a negative emotional contrast in both disorders, upon exposure to negative emotional videos.
Thus far, repeated application of AR has been proposed for the improvement of RIA (Cohen et al., 1985). However, one study only found a significant negative correlation between the frequency of relaxation training and the levels of RIA at 6-month follow-up but not at 1 year follow-up (Borkovec and Costello, 1993). Lack of consistency in treatment outcome suggests a need to consider another intervening variable.
Although the current study cannot draw any causal inferences, it is possible that negative contrast sensitivity causes RIA. If this is the case, an intervention targeting negative contrast sensitivity might be considered as a treatment method for RIA. As proposed in Newman et al. (2018), the treatment of negative contrast sensitivity could entail both cognitive and behavioral intervention methods. The cognitive modality could be implemented by modifying patients’ fear of negative emotional contrast and their positive beliefs about worry/tension. In addition, by repeatedly administering negative contrast exposure (i.e., engaging in long sessions of AR prior to negative imaginal exposure), patients could desensitize their aversion toward an abrupt negative emotional shift. While applying AR, therapists should also make sure to assess patients’ levels of RIA. Any application of AR should not be abbreviated or stopped but it should be continued until the anxiety has decreased. This would be crucial to capitalize on the treatment effects of negative contrast exposure (by maximizing the contrast between the relaxed state and the later discomfort) and to prevent the intensification of RIA over the course of AR treatment. Improvement during the course of the treatment can also be monitored in order to make sure there is within and between-session habituation of negative contrast sensitivity and RIA.
Also, it should be noted that there were differences between findings for GAD and MDD in the current study. Unlike the full mediation in the GAD model, negative contrast sensitivity partially mediated MDD. This indicates that MDD may have an additional mediator of RIA. Anhedonia may be one such mediator. Anhedonia is a hallmark symptom of MDD that is characterized by an inability to feel pleasure and low interest in positive activities. Such anhedonia might contribute to feeling a lack of pleasure from relaxation and thus exacerbate RIA. Therefore, on top of measuring negative contrast sensitivity, future studies should assess anhedonia as a possible mediator of RIA.
It is important to note several limitations of the current study. First, even though we measured negative contrast sensitivity and RIA at two separate time-points, we cannot completely ensure the independence of the two measures, since they were measured within the same experimental study across a relatively short time period. It was not our intention to condition a sensitivity to relaxation or create RIA where none existed although aspects of our experimental procedure might have done this. We were instead trying to capture natural reactions to the emotional contrast and the second relaxation practice. At the same time, if participants did have a sensitivity to a negative contrast and it was a mediator of RIA, we expected that having participants relax after the experience of a negative contrast would increase the probability that it would trigger their already present RIA.
Also, although we included state measures of both negative contrast sensitivity and RIA, we made assumptions that these reflected trait levels of these variables. On the one hand, inducing a negative contrast and then measuring participants’ sensitivity to it and/or engaging participants in relaxation and measuring their anxiety response are probably more valid ways of measuring these things than getting participants to report on their typical negative contrast sensitivity or RIA tendencies. On the other hand, it is possible that some aspects of our experimental design (i.e., having participants relax immediately after experiencing a negative emotional contrast from a prior relaxation practice) might have gone beyond eliciting already present responses that might or might not generalize across situations and overall tendencies. Therefore, it might be interesting to also measure trait levels of these variables and examine whether our mediational processes held. Furthermore, the relationship between trait and state measures of these variables should be examined directly in future studies.
In addition, there may be other limits to the generalizability of our findings. Participants recruited for this study were undergraduate students who were highly homogeneous in their demographics such as age, gender, and ethnicity. In addition, although the participants had clinical levels of symptoms of depression and anxiety (except for healthy controls), they were not treatment seeking. For better generalizability, the current variables should be tested with a more diverse population.
Other limitations are related to potential measurement and design issues. Our study relied solely on subjective responses and there is a possibility that anxious or depressed individuals had a tendency to exaggerate their RIA. In order to minimize this, future studies might consider using non-subjective measures, such as skin conductance and heart rate variability. At the same time, prior studies found that subjective measures detected contrast sensitivity in individuals with GAD whereas physiological measures showed no differences across groups in the actual degree of a negative contrast between relaxation and an emotional video. One could argue that subjective views of both contrast sensitivity and RIA are more important than physiological measures (even if exaggerated) because they could drive individuals to avoid relaxation practice and to actively try to maintain chronic anxiety states. Thus, it is also possible that perception of contrast sensitivity and perception of anxiety in response to relaxation are more important than objective measures of physiology. In addition, we used clips from iconic movies released in the late 1970s and early 1980s and easily available on streaming television. It may be the case that participants who were previously exposed to the clips responded differently compared to those not previously exposed. Future studies should assess whether participants have viewed these clips and test whether prior viewing of them predicts reduced emotion elicitation.
In the current study, we administered AR by combining two different relaxation skills, which were diaphragmatic breathing and progressive muscle relaxation. Given that the two relaxation techniques are quite distinct in their ways of enhancing a relaxed state, and they involve different physiological experiences (i.e., a deep and slow phased respiration vs. relief of tension in muscle segments), there may be differences between the two methods in eliciting RIA. Therefore, in future studies, researchers may consider administering the two relaxation techniques separately, and compare their differential effects on RIA in the same individuals. This comparison may allow a more efficient application of AR. However, given that diaphragmatic breathing and progressive muscle relaxation are very commonly combined in CBT, our study still offers valuable insights into current clinical practice.
Even though our focus was on the comparison between pure effects of GAD and MDD, it would be of interest to test the effects of the shared variance between the two diagnostic conditions. Considering the high comorbidity between anxiety and depression, testing these effects is expected to help further elucidate the transdiagnostic aspect of the RIA process. Despite the absence of this feature, our study revealed that both GAD and MDD predicted RIA when controlling for the other one and as mediated by negative contrast sensitivity.
Future research could also test the interface between RIA and negative contrast sensitivity in other disorders. Given findings showing RIA among individuals with panic disorder (Cohen et al., 1985; Ley, 1988; Wells, 1990), there is a possibility that negative contrast sensitivity may operate in this disorder as well. In addition, given that persistent depressive disorder (dysthymia) shares many commonalities with MDD, further research can be undertaken to examine the interplay between negative contrast sensitivity and dysthymia. Also, as noted above, measuring third variables such as anhedonia may be a worth while addition to elucidate the process of RIA among depressed individuals.
Our study showed that AR may not be a panacea for everyone suffering from anxiety and depression. If AR is applied without considering one’s sensitivity to a negative emotional contrast, it could be a poisoned chalice to those in need of help. Our findings might contribute to a more precise application of AR and CBT.
Acknowledgments
We thank the department head, Dr. Melvin Mark and research fellowship committee of the Department of Psychology at Penn State for the research fellowship. We also thank undergraduate research assistants for their help in data collection and data entry.
Role of the funding source
This research was supported in part by the Bruce V. Moore Graduate Fellowship in Psychology from the Department of Psychology at Penn State.
Footnotes
Declaration of Competing Interest
None.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jad.2019.08.045.
References
- American Psychiatric Association, 1994. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). American Psychiatry Press, Washington, DC. [Google Scholar]
- American Psychiatric Association, A. P. A., 2013. Diagnostic and Statistical Manual of Mental Disorders, 5th ed American Psychiatric Association, Arlington, Virginia: 10.1176/appi.books.9780890425596. [DOI] [Google Scholar]
- Arnau RC, Meagher MW, Norris MP, Bramson R, 2001. Psychometric evaluation of the beck depression inventory-II with primary care medical patients. Health Psychol. 20, 112–119. 10.1037/0278-6133.20.2.112. [DOI] [PubMed] [Google Scholar]
- Barlow DH, Rapee RM, Brown TA, 1992. Behavioral treatment of generalized anxiety disorder. Behav. Ther 23, 551–570. 10.1016/S0005-7894(05)80221-7. [DOI] [Google Scholar]
- Beck AT, Steer RA, Brown GK, 1996. Manual for the Beck Depression Inventory – II, 2nd ed The Psychological Corporation, San Antonio, TX. [Google Scholar]
- Bernstein DA, Borkovec TD, 1973. Progressive Relaxation training: A manual for the Helping Professions 66 Research Press, Champaign, IL. [Google Scholar]
- Bolognesi F, Baldwin DS, Ruini C, 2014. Psychological interventions in the treatment of generalized anxiety disorder: a structured review. J. Psychopathol 20, 111–126. [Google Scholar]
- Borkovec TD, Mathews AM, Chambers A, Ebrahimi S, Lytle R, Nelson R, 1987. The effects of relaxation training with cognitive or nondirective therapy and the role of relaxation-induced anxiety in the treatment of generalized anxiety. J. Consult. Clin. Psychol 55, 883–888. 10.1037/0022-006X.55.6.883. [DOI] [PubMed] [Google Scholar]
- Borkovec TD, Costello E, 1993. Efficacy of applied relaxation and cognitive-behavioral therapy in the treatment of generalized anxiety disorder. J. Consult. Clin. Psychol 61, 611–619. 10.1037/0022-006X.61.4.611. [DOI] [PubMed] [Google Scholar]
- Borkovec TD, Robinson E, Pruzinsky T, DePree JA, 1983. Preliminary exploration of worry: some characteristics and processes. Behav. Res. Ther 21, 9–16. 10.1016/0005-7967(83)90121-3. [DOI] [PubMed] [Google Scholar]
- Braith JA, McCullough JP, Bush JP, 1988. Relaxation-induced anxiety in a subclinical sample of chronically anxious subjects. J. Behav. Ther. Exp. Psychiatry 19, 193–198. 10.1016/0005-7916(88)90040-7. [DOI] [PubMed] [Google Scholar]
- Brown TA, Barlow DH, Liebowitz MR, 1994Aa. The empirical basis of generalized anxiety disorder. Am. J. Psychiatry 151, 1272–1280. 10.1176/ajp.151.9.1272. [DOI] [PubMed] [Google Scholar]
- Brown TA, Di Nardo PA, & Barlow DH (1994). Anxiety disorders interview schedule for DSM-IV. New York: Oxford University Press. [Google Scholar]
- Chambless DL, Ollendick TH, 2001. Empirically supported psychological interventions: controversies and evidence. Annu. Rev. Psychol 52, 685–716. 10.1146/annurev.psych.52.1.685. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Barlow DH, Blanchard EB, 1985. Psychophysiology of relaxation-associated panic attacks. J. Abnorm. Psychol 94, 96–101. 10.1037/0021-843X.94.1.96. [DOI] [PubMed] [Google Scholar]
- Converse BA, Lin S, Keysar B, Epley N, 2008. In the mood to get over yourself: mood affects theory-of-mind use. Emotion 8, 725 10.1037/a0013283. [DOI] [PubMed] [Google Scholar]
- Cryder CE, Lerner JS, Gross JJ, Dahl RE, 2008. Misery is not miserly: sad and self focused individuals spend more. Psychol. Sci 19, 525–530. 10.1111/j.1467-9280.2008.02118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditlevsen S, Christensen U, Lynch J, Damsgaard MT, Keiding N, 2005. The mediation proportion: a structural equation approach for estimating the proportion of exposure effect on outcome explained by an intermediate variable. Epidemiology 16, 114–120. 10.1097/01.ede.0000147107.76079.07. [DOI] [PubMed] [Google Scholar]
- Dunlap WP, Cortina JM, Vaslow JB, Burke MJ, 1996. Meta-analysis of experiments with matched groups or repeated measures designs. Psychol. Methods 1, 170–177. 10.1037/1082-989x.1.2.170. [DOI] [Google Scholar]
- Freedman LS, 2001. Confidence intervals and statistical power of the ‘Validation’ ratio for surrogate or intermediate endpoints. J. Stat. Plan. Inference 96, 143–153. 10.1016/S0378-3758(00)00330-X. [DOI] [Google Scholar]
- Gross JJ, Levenson RW, 1995. Emotion elicitation using films. Cogn. Emot 9, 87–108. 10.1080/02699939508408966. [DOI] [Google Scholar]
- Hayes AF, Preacher KJ, 2014. Statistical mediation analysis with a multicategorical independent variable. Br. J. Math. Stat. Psychol 67, 451–470. 10.1111/bmsp.12028. [DOI] [PubMed] [Google Scholar]
- Hayes AF (2013). PROCESS SPSS macro [Computer software and manual].
- Heide FJ, Borkovec TD, 1983. Relaxation-induced anxiety: paradoxical anxiety enhancement due to relaxation training. J. Consult. Clin. Psychol 51, 171–182. 10.1037/0022-006X.51.2.171. [DOI] [PubMed] [Google Scholar]
- Heide FJ, Borkovec TD, 1984. Relaxation-induced anxiety: mechanisms and theoretical implications. Behav. Res. Ther 22, 1–12. 10.1016/0005-7967(84)90027-5. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Smits JAJ, 2008. Cognitive-behavioral therapy for adult anxiety disorders: a meta-analysis of randomized placebo-controlled trials. J. Clin. Psychiatry 69, 621–632. 10.4088/JCP.v69n0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inbar Y, Gilovich T, 2011. Angry (or disgusted), but adjusting? The effect of specific emotions on adjustment from self-generated anchors. Soc Psychol Personal Sci 2, 563–569. 10.1177/1948550611401426. [DOI] [Google Scholar]
- Kim H, Newman MG, 2016. Emotional contrast avoidance in generalized anxiety disorder and major depressive disorder: a comparison between the perseveration processes of worry and rumination. Paper presented at the 50th Annual Meeting of the Association for Behavioral and Cognitive Therapies, New York, NY. [Google Scholar]
- Kubrick S, 1980. The Shining. Warner Brothers, Burbank, CA: [Film]. [Google Scholar]
- Ley R, 1988. Panic attacks during relaxation and relaxation-induced anxiety: a hyperventilation interpretation. J. Behav. Ther. Exp. Psychiatry 19, 253–259. 10.1016/0005-7916(88)90054-7. [DOI] [PubMed] [Google Scholar]
- Liverant GI, Brown TA, Barlow DH, Roemer L, 2008. Emotion regulation in unipolar depression: the effects of acceptance and suppression of subjective emotional experience on the intensity and duration of sadness and negative affect. Behav. Res. Ther 46, 1201–1209. 10.1016/j.brat.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Llera SJ, Newman MG, 2014. Rethinking the role of worry in generalized anxiety disorder: evidence supporting a model of emotional contrast avoidance. Behav. Ther 45, 283–299. 10.1016/j.beth.2013.12.011. [DOI] [PubMed] [Google Scholar]
- Llera SJ, Newman MG, 2010. Effects of worry on physiological and subjective reactivity to emotional stimuli in generalized anxiety disorder and nonanxious control participants. Emotion 10, 640–650. 10.1037/a0019351. [DOI] [PubMed] [Google Scholar]
- Llera SJ, Newman MG, 2017. Development and validation of two measures of emotional contrast avoidance: the contrast avoidance questionnaires. J. Anxiety Disord 49, 114–127. 10.1016/j.janxdis.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lolak S, Connors GL, Sheridan MJ, Wise TN, 2008. Effects of progressive muscle relaxation training on anxiety and depression in patients enrolled in an outpatient pulmonary rehabilitation program. Psychother. Psychosom 77, 119–125. 10.1159/000112889. [DOI] [PubMed] [Google Scholar]
- Lovell D, Zeffirelli F, 1979. The Champ. MGM/Pathe Home Video, Culver City, CA: [Film]. [Google Scholar]
- McLaughlin KA, Borkovec TD, Sibrava NJ, 2007. The effects of worry and rumination on affect states and cognitive activity. Behav. Ther 38, 23–38. 10.1016/j.beth.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Meyer TJ, Miller ML, Metzger RL, Borkovec TD, 1990. Development and validation of the penn state worry questionnaire. Behav. Res. Ther 28, 487–495. 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- Moberly NJ, Watkins ER, 2008. Ruminative self-focus, negative life events, and negative affect. Behav. Res. Ther 46, 1034–1039. 10.1016/j.brat.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt TE, Harrington H, Caspi A, Kim-Cohen J, Goldberg D, Gregory AM,Poulton R, 2007. Depression and generalized anxiety disorder: cumulative and sequential comorbidity in a birth cohort followed prospectivity to age 32 years. Arch. Gen. Psychiatry 64, 651–660. 10.1001/archpsyc.64.6.651. [DOI] [PubMed] [Google Scholar]
- Moore MT, Anderson NL, Barnes JM, Haigh EAP, Fresco DM, 2014. Using the GAD-Q-IV to identify generalized anxiety disorder in psychiatric treatment seeking and primary care medical samples. J. Anxiety Disord 28, 25–30. 10.1016/j.janxdis.2013.10.009. [DOI] [PubMed] [Google Scholar]
- Murphy GE, Carney RM, Knesevich MA, Wetzel RD, Whitworth P, 1995. Cognitive behavior therapy, relaxation training, and tricyclic antidepressant medication in the treatment of depression. Psychol. Rep 77, 403–420. 10.2466/pr0.1995.77.2.403. [DOI] [PubMed] [Google Scholar]
- Newman MG, Zuellig AR, Kachin KE, Constantino MJ, Przeworski A, Erickson T, Cashman-McGrath L, 2002. Preliminary reliability and validity of the generalized anxiety disorder questionnaire-iv: a revised self-report diagnostic measure of generalized anxiety disorder. Behav. Ther 33, 215–233. 10.1016/S0005-7894(02)80026-0. [DOI] [Google Scholar]
- Newman MG, Llera SJ, 2011. A novel theory of experiential avoidance in generalized anxiety disorder: a review and synthesis of research supporting a contrast avoidance model of worry. Clin. Psychol. Rev 31, 371–382. 10.1016/j.cpr.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman MG, Lafreniere LS, Jacobson NC, 2018. Relaxation-induced anxiety: effects of peak and trajectories of change on treatment outcome for generalized anxiety disorder. Psychother. Res 28, 616–629. 10.1080/10503307.2016.1253891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman MG, Llera SJ, Erickson TM, Przeworski A, Castonguay LG, 2013. Worry and generalized anxiety disorder: a review and theoretical synthesis of research on nature, etiology, and treatment. Annu. Rev. Clin. Psychol 9, 275–297. 10.1146/annurev-clinpsy-050212-185544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, 1991. Responses to depression and their effects on the duration of depressive episodes. J. Abnorm. Psychol 100, 569–582. 10.1037/0021-843X.100.4.569. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Morrow J, 1993. Effects of rumination and distraction on naturally occurring depressed mood. Cogn. Emot 7, 561–570. 10.1080/02699939308409206. [DOI] [Google Scholar]
- Norton GR, Rhodes L, Hauch J, Kaprowy E, 1985. Characteristics of subjects experiencing relaxation and relaxation-induced anxiety. J. Behav. Ther. Exp. Psychiatry 16, 211–216. 10.1016/0005-7916(85)90065-5. [DOI] [PubMed] [Google Scholar]
- Öst LG, 1987. Applied relaxation: description of a coping technique and review of controlled studies. Behav. Res. Ther 25, 397–409. 10.1016/0005-7967(87)90017-9. [DOI] [PubMed] [Google Scholar]
- Öst LG, Breitholtz E, 2000. Applied relaxation vs. cognitive therapy in the treatment of generalized anxiety disorder. Behav. Res. Ther 38, 777–790. 10.1016/S0005-7967(99)00095-9. [DOI] [PubMed] [Google Scholar]
- Ottaviani C, Shapiro D, Fitzgerald L, 2011. Rumination in the laboratory: what happens when you go back to everyday life? Psychophysiology 48, 453–461. 10.1111/j.1469-8986.2010.01122.x. [DOI] [PubMed] [Google Scholar]
- Ottaviani C, Thayer JF, Verkuil B, Lonigro A, Medea B, Couyoumdjian A, Brosschot JF, 2016. Physiological concomitants of perseverative cognition: a systematic review and meta-analysis. Psychol. Bull 142, 231 10.1037/bul0000036. [DOI] [PubMed] [Google Scholar]
- Papageorgiou C, Wells A, 2003. An empirical test of a clinical metacognitive model of rumination and depression. Cognit. Ther. Res 27, 261–273. 10.1023/A:1023962332399. [DOI] [Google Scholar]
- Preacher KJ, Hayes AF, 2008. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav. Res. Methods 40, 879–891. 10.3758/BRM.40.3.879. [DOI] [PubMed] [Google Scholar]
- Psychology Software Tools Inc. (2002). E-Prime 2.0 Pittsburgh, PA, USA. [Google Scholar]
- Reiss S, 1987. Theoretical perspectives on the fear of anxiety. Clin. Psychol. Rev 7, 585–596. 10.1016/0272-7358(87)90007-9. [DOI] [Google Scholar]
- Reynolds WM, Coats KI, 1986. A comparison of cognitive-behavioral therapy and relaxation training for the treatment of depression in adolescents. J. Consult. Clin. Psychol 54, 653 10.1037/0022-006X.54.5.653. [DOI] [PubMed] [Google Scholar]
- Ruscio AM, Chiu WT, Roy-Byrne P, Stang PE, Stein DJ, Wittchen HU, Kessler RC, 2007. Broadening the definition of generalized anxiety disorder: effects on prevalence and associations with other disorders in the national comorbidity survey replication. J. Anxiety Disord 21, 662–676. 10.1016/j.janxdis.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh Y−Y, Fouladi RT, 2003. The effect of multicollinearity on multilevel modeling parameter estimates and standard errors. Educ. Psychol. Meas 63, 951–985. 10.1177/0013164403258402. [DOI] [Google Scholar]
- Siev J, Chambless DL, 2007. Specificity of treatment effects: cognitive therapy and relaxation for generalized anxiety and panic disorders. J. Consult. Clin. Psychol 75, 513–522. 10.1037/0022-006X.75.4.513. [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS, 2007. Using Multivariate Statistics. Allyn & Bacon/Pearson Education. [Google Scholar]
- Tarrier N, Main CJ, 1986. Applied relaxation training for generalised anxiety and panic attacks: the efficacy of a learnt coping strategy on subjective reports. Br. J. Psychiatry 149, 330–336. 10.1192/bjp.149.3.330. [DOI] [PubMed] [Google Scholar]
- Tiedens LZ, Linton S, 2001. Judgment under emotional certainty and uncertainty: the effects of specific emotions on information processing. J. Pers. Soc. Psychol 81, 973 10.1037/0022-3514.81.6.973. [DOI] [PubMed] [Google Scholar]
- Wang Y−P, Gorenstein C, 2013. Psychometric properties of the beck depression inventory-II: a comprehensive review. Revista Brasileira de Psiquiatria 35, 416–431. 10.1590/1516-4446-2012-1048. [DOI] [PubMed] [Google Scholar]
- Watkins E, Moulds M, Mackintosh B, 2005. Comparisons between rumination and worry in a non-clinical population. Behav. Res. Ther 43, 1577–1585. 10.1016/j.brat.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Watkins E, Moulds M, 2005. Distinct modes of ruminative self-focus: impact of abstract versus concrete rumination on problem solving in depression. Emotion 5, 319 10.1037/1528-3542.5.3.319. [DOI] [PubMed] [Google Scholar]
- Wells A, 1990. Panic disorder in association with relaxation induced anxiety: an at tentional training approach to treatment. Behav. Ther 21, 273–280. 10.1016/S0005-7894(05)80330-2. [DOI] [Google Scholar]
- Wittchen HU, Fuetsch M, Sonntag H, Müller N, Liebowitz M, 2000. Disability and quality of life in pure and comorbid social phobia: findings from a controlled study. Eur. Psychiatry 15, 46–58. 10.1016/S0924-9338(00)00211-X. [DOI] [PubMed] [Google Scholar]
- Yu H, Jiang S, Land KC, 2015. Multicollinearity in hierarchical linear models. Soc. Sci. Res 53, 118–136. 10.1016/j.ssresearch.2015.04.008. [DOI] [PubMed] [Google Scholar]


