Abstract
Background
Immunotherapy with immune checkpoint inhibitors (ICIs) is highly effective in microsatellite instability–high (MSI‐H) metastatic colorectal cancer (mCRC); however, specific predictive biomarkers are lacking.
Patients and Methods
Data and samples from 85 patients with MSI‐H mCRC treated with ICIs were gathered. Tumor infiltrating lymphocytes (TILs) and tumor mutational burden (TMB) were analyzed in an exploratory cohort of “super” responders and “clearly” refractory patients; TILs were then evaluated in the whole cohort of patients. Primary objectives were the correlation between the number of TILs and TMB and their role as biomarkers of ICI efficacy. Main endpoints included response rate (RR), progression‐free survival (PFS), and overall survival (OS).
Results
In the exploratory cohort, an increasing number of TILs correlated to higher TMB (Pearson's test, p = .0429). In the whole cohort, median number of TILs was 3.6 in responders compared with 1.8 in nonresponders (Mann‐Whitney test, p = .0448). RR was 70.6% in patients with high number of TILs (TILs‐H) compared with 42.9% in patients with low number of TILs (odds ratio = 3.20, p = .0291). Survival outcomes differed significantly in favor of TILs‐H (PFS: hazard ratio [HR] = 0.42, p = .0278; OS: HR = 0.41, p = .0463).
Conclusion
A significant correlation between higher TMB and increased number of TILs was shown. A significantly higher activity and better PFS and OS with ICI in MSI‐H mCRC were reported in cases with high number of TILs, thus supporting further studies of TIL count as predictive biomarker of ICI efficacy.
Implications for Practice
Microsatellite instability is the result of mismatch repair protein deficiency, caused by germline mutations or somatic modifications in mismatch repair genes. In metastatic colorectal cancer (mCRC), immunotherapy (with immune checkpoint inhibitors [ICIs]) demonstrated remarkable clinical benefit in microsatellite instability–high (MSI‐H) patients. ICI primary resistance has been observed in approximately 25% of patients with MSI‐H mCRC, underlining the need for predictive biomarkers. In this study, tumor mutational burden (TMB) and tumor infiltrating lymphocyte (TIL) analyses were performed in an exploratory cohort of patients with MSI‐H mCRC treated with ICIs, demonstrating a significant correlation between higher TMB and increased number of TILs. Results also demonstrated a significant correlation between high number of TILs and clinical responses and survival benefit in a large data set of patients with MSI‐H mCRC treated with ICI. TMB and TILs could represent predictive biomarkers of ICI efficacy in MSI‐H mCRC and should be incorporated in future trials testing checkpoint inhibitors in colorectal cancer.
Keywords: Colorectal cancer, Microsatellite instability, Tumor mutational burden, Tumor infiltrating lymphocytes, Predictive biomarker
Short abstract
This article explores the role of tumor mutational burden and number of tumor infiltrating lymphocytes in determining response and outcome of patients with microsatellite high metastatic colorectal cancer treated with immune checkpoint inhibitors.
Introduction
High microsatellite instability results from defective DNA mismatch repair (dMMR) and is observed in approximately 3%–5% of metastatic colorectal cancers (mCRCs) 1, 2.
Recently, immunotherapy with immune checkpoint inhibitors (ICIs) demonstrated activity and efficacy in chemorefractory microsatellite instability–high (MSI‐H) mCRC, with over 30% and 50% objective response rates with nivolumab and nivolumab plus ipilimumab, respectively. There was long‐lasting disease control and a 1‐year progression‐free survival (PFS) rate over 50% with nivolumab and over 70% with nivolumab plus ipilimumab 3, 4. On this basis, the U.S. Food and Drug Administration approved checkpoint inhibitors nivolumab ± ipilimumab and pembrolizumab for patients with refractory MSI‐H mCRC.
Despite these extraordinary results, up to 25% of MSI‐H patients do not respond to ICI, and nearly 10% of initially responding patients develop resistance, underlining the need for predictive biomarkers 3, 4.
Currently, tumor mutational burden (TMB) and tumor infiltrating lymphocytes (TILs) are in the spotlight as potential biomarkers for ICI 5.
High TMB, defined as an increased total number of nonsynonymous mutations in the coding regions of genes, predicted benefit from ICI in “hot” tumors such as non‐small cell lung cancer 6, 7, 8, melanoma 9, 10, unselected dMMR tumors 11, and bladder cancers 12. MSI‐H colorectal cancers (CRCs) are globally considered “hot” tumors and display high TMB 13; initial data on TMB and ICI activity in this specific subset were recently reported on a small cohort of 22 patients 14.
Recently, accumulating evidence demonstrated an association between high number of TILs and prognosis in colorectal cancer (CRC) 15, 16, 17, suggesting that host immune response to tumor cells is essential to determine outcomes of patients with CRC 18.
We aimed to explore the role of TMB and number of TILs in determining response and outcome of patients with MSI‐H mCRC treated with ICIs.
Subjects, Materials, and Methods
Selection Criteria
Data from consecutive patients with MSI‐H mCRC treated with ICI (i.e., anti–PD‐1 pembrolizumab or nivolumab ± anti‐CTLA4 ipilimumab) at three Italian oncology units (Veneto Institute of Oncology, Istituto di Ricovero e Cura a Carattere Scientifico [IRCCS], Padua; Candiolo Cancer Institute, Fondazione del Piemonte per l'Oncologia [FPO]–IRCCS; Candiolo and University Hospital of Modena, Modena) were gathered.
The main inclusion criteria were age at least 18 years, histologically proven diagnosis of CRC, metastatic and measurable disease according to Response Evaluation Criteria In Solid Tumors (RECIST) version 1.1, availability of detailed clinical data and of a tumor tissue specimen, and locally detected dMMR by means of immunohistochemistry (i.e., MLH1, PMS2, MSH2, MSH6; Dako, Glostrup, Denmark) 19 or MSI‐H by means of polymerase chain reaction (PCR) and sequencing (Titano kit, Diatech Pharmacogenetics, Jesi, Italy) testing either the primary tumor or a metastatic sample.
Representative formalin‐fixed paraffin‐embedded blocks from archival primary CRC tumors or metastatic lesions were centralized at the Surgical Pathology Unit of the Padua University Hospital. Hematoxylin and eosin–stained slides were reviewed by a gastrointestinal pathologist blinded to patients' outcomes. Grading and histologic tumor type were reassessed according to the World Health Organization Classification of Tumours of the Digestive System, 2010 20.
The start date for data collection was June 2015, and data cutoff was February 2019. For each patient we collected data on demographics (age and sex), tumor characteristics (primary tumor location [i.e., right‐sided, from caecum up to transverse colon, vs. left‐sided, from splenic flexure to rectum], mucinous histology, time to metastatic presentation [synchronous vs. metachronous], type and number of metastatic sites, tumor grading, and routine molecular analysis [RAS and V600E BRAF mutations]), systemic treatment, response, and survival.
Study Design
This was a prospectively planned observational study. All molecular and pathological analyses were conducted post hoc after completion of accrual at the time of data cutoff. The study was approved by local ethics committees. All patients gave their consent for data collection and molecular analyses.
Microsatellite instasbility status was centrally confirmed at the Unit of Surgical Pathology of Padua, by means of PCR and sequencing for the whole cohort. A selected exploratory cohort of “super” responders and “clearly” refractory patients was identified to conduct initial comprehensive next‐generation sequencing (NGS) analyses. “Super” responders were defined as patients achieving complete or partial response to ICI who remained progression free at 6 months from treatment initiation; “clearly” refractory patients were defined as patients receiving at least 8 weeks of treatment, not interrupted for toxicities, with progressive disease including appearance of new lesions at first evaluation. Number of TILs was subsequently assessed in the whole cohort of gathered patients.
TMB Analysis and TIL Analysis
TMB was assessed by means of NGS (GPS Cancer, Nantomics, Culver City, CA) 21 in samples from the exploratory cohort of super responders and clearly refractory patients.
As previously reported 22, the number of TILs was defined as the mean value of five random observations and count at high‐power fields (40×) of tumor‐enriched areas composed of >60% of neoplastic cells. In paucicellular tumors, such as mucinous adenocarcinomas, the analysis was performed within fields with highest tumor cell density. Only tumor epithelium infiltrating lymphocytes were retained for scoring. Based on previous data 22, tumors showing an average number of TILs of <2.0 were defined as “low number of TILs” (TILs‐L), whereas ≥2.0 TILs were defined as “high number of TILs” (TILs‐H). All samples were jointly evaluated by two gastrointestinal pathologists who were unaware of any clinical information.
Statistical Analysis
The primary objectives of this study were to explore the correlation between the number of TILs and TMB and their role as biomarkers of ICI activity and efficacy. Main endpoints included best overall response (BOR), PFS, and overall survival (OS). BOR was defined as the best response obtained according to RECIST version 1.1 criteria between the dates of first dose administration and progression. PFS was defined as the time from ICI start to the date of disease progression, or the date of death without documented progression, or the date of last follow‐up information for living patients without progression. OS was defined as the time from diagnosis of metastatic disease to death from any cause, or the date of last follow‐up information for living patients. OS and PFS curves were calculated by means of the Kaplan‐Meier method, and groups were compared using the log‐rank test. Statistical significance was set at p = .05 for a bilateral test. Fisher's exact test was used to compare BOR according to number of TILs. Exploratory analyses were conducted in a selected cohort of ICI responders and refractory patients using Pearson's correlation test to measures the linear dependence between TILs and TMB. Given the exploratory nature of the study, no formal calculations for sample size were elaborated. All analyses were carried out by means of IBM SPSS Statistics Standard version 22.0 and GraphPad Prism version 8.
Results
A total of 85 eligible patients with mCRC were included. Among them, 30 patients met all the criteria for entering the exploratory cohort (15 super responders and 15 clearly refractory patients). Five patients (5.9%) were centrally identified by means of PCR and sequencing as having a microsatellite stable (MSS) tumor (further confirmed with NGS) and were excluded from subsequent analyses. Interestingly, all excluded patients belonged to the subset of clearly refractory patients.
The median age of the final analysis cohort (n = 80) was 60 years (range 17–85 years), and 45 patients (56.2%) were male. Fifty‐six (70.0%) had a right‐sided primary tumor, 67 (83.7%) had a poorly differentiated tumor, and 36 (45.0%) had mucinous histology. Peritoneal metastases occurred in 30 (37.5%) cases, liver metastases in 25 (31.3%), and lung metastases in 7 (8.8%) cases. V600E BRAF mutation was detected in 28 tumors (35%) and RAS mutations in 27 (33.8%). The median number of treatment lines for metastatic disease was two (range one to nine). Twenty‐two patients (27.5%) received ICI as first‐line treatment, 27 (33.8%) as second‐line treatment, and 31 (38.7%) in subsequent lines. Forty patients (50%) were treated with nivolumab or pembrolizumab monotherapy, whereas 40 patients (50%) were treated with the combination of nivolumab plus ipilimumab. Detailed patients’ characteristics are reported in Tables 1 and 2 and in supplemental online Table 1.
Table 1.
Tumor pathology and molecular characteristics
| Characteristic | Total (n = 80), n (%) | Response to ICIsa | PFSHR (95% CI), p valueb | OSHR (95% CI), p valueb | ||
|---|---|---|---|---|---|---|
| CR and PR (n = 48), n (%) | SD and PD (n = 31), n (%) | p valueb | ||||
| Primary tumor location | ||||||
| a. Right | 56 (70.0) | 32 (66.7) | 24 (77.4) | .187 |
1.07 (0.44–2.62), .882 |
0.68 (0.27–1.72), .410 |
| b. Left / Rectum | 22 (27.5) | 16 (33.3) | 5 (16.1) | |||
| Missing | 2 (2.5) | 2 (6.5) | ||||
| Mucinous histology | ||||||
| a. Yes | 36 (45.0) | 18 (37.5) | 18 (58.1) | .105 |
2.17 (0.95–4.96), .067 |
1.41 (0.58–3.47), .458 |
| b. No | 44 (55.0) | 30 (62.5) | 13 (41.9) | |||
| Lymphovascular invasion | ||||||
| a. Yes | 44 (55.0) | 30 (62.5) | 14 (45.2) | .3383 |
0.43 (0.16–1.17), .099 |
0.41 (0.14–1.19), .100 |
| b. No | 15 (18.8) | 7 (14.6) | 7 (22.6) | |||
| Missing | 21 (26.2) | 11 (22.9) | 10 (32.2) | |||
| Grading | ||||||
| a. High grade | 67 (83.7) | 39 (81.3) | 27 (87.1) | .5517 |
2.48 (0.58–10.63), .222 |
1.80 (0.42–7.84), .430 |
| b. Low grade | 13 (16.3) | 9 (18.7) | 4 (12.9) | |||
| RAS mutation | ||||||
| a. Mutation | 27 (32.5) | 17 (33.3) | 9 (29.0) | .8059 |
0.98 (0.41–2.33), .967 |
1.34 (0.56–3.48), .474 |
| b. WT | 53 (67.5) | 31 (66.7) | 22 (71.0) | |||
| BRAF mutation | ||||||
| a. Mutation | 28 (35.0) | 16 (33.3) | 12 (38.7) | .6386 |
1.26 (0.55–2.91), .588 |
1.52 (0.61–3.78), .369 |
| b. WT | 52 (65.0) | 32 (66.7) | 19 (61.3) | |||
| Number of TILs | ||||||
| a. High | 52 (65.0) | 36 (75.0) | 15 (48.4) | .029 |
0.42 (0.15–0.88), .028 |
0.41 (0.14–0.98), .046 |
| b. Low | 28 (35.0) | 12 (25.0) | 16 (51.6) | |||
One patient not evaluable for response to ICIs.
All comparisons made for variable a versus b. Fisher's exact test was adopted for response analyses, log‐rank test for PFS and OS analyses. Significant p values in bold.
Abbreviations: CI, confidence interval; CR, complete response; HR, hazard ratio; ICI, immune checkpoint inhibititor; PD, progressive disease; PFS, progression‐free survival; PR, partial response; SD, stable disease; TIL, tumor infiltrating lymphocyte; WT, wild type.
Table 2.
Systemic treatments for metastatic disease
| Systemic treatments | Total (n = 80), n (%) |
|---|---|
| Lines of all treatments, median (range) | 2 (1–9) |
| Lines of all treatments | |
| 1 | 22 (27.5) |
| 2 | 26 (32.4) |
| 3 | 18 (22.5) |
| 4 | 7 (8.8) |
| ≥5 | 7 (8.8) |
| Lines of immunotherapy treatments | |
| 1 | 22 (27.5) |
| 2 | 27 (33.8) |
| 3 | 17 (21.2) |
| 4 | 8 (10.0) |
| ≥5 | 6 (7.5) |
| Immunotherapy treatment | |
| Anti–PD‐1 monotherapya | 40 (50.0) |
| Anti–PD‐1 + anti‐CTLA4b | 40 (50.0) |
Either nivolumab or pembrolizumab.
Nivolumab plus ipilimumab.
At the time of data cutoff, the median duration of follow‐up was 22.8 months (range 0.5–50.9). A total of 48 (60%) patients were still receiving ICIs; discontinuations occurred in a total of 32 patients (40%), specifically, because of disease progression in 24 (30%) patients, to treatment related toxicity in 5 (6.2%) patients, to surgery on metastases with curative intent in 1 patient (1.3%), and to patients’ decision in the remaining 2 cases (2.5%).
The overall response rate (ORR) was 60 (n = 48): 6 patients (7.5%) achieved a complete response (CR), and 42 (52.5%) a partial response (PR; supplemental online Table 2). The median PFS was 41.5 months, whereas the median OS was not yet reached.
Exploratory Cohort: TMB Analysis and TILs Correlation
Out of 25 centrally confirmed MSI‐H patients, 20 had adequate tissue specimens to perform both TMB and TILs analyses (13 super responders and 7 clearly refractory patients). A total of 30 samples for the 20 patients were analyzed (i.e., 20 primary tumors and related metastases for 10 cases). Because TMB and TILs results were almost identical in matched primary and metastases, only data from primary tumor specimens were considered.
The median TMB was equal to 68.6 mutations per megabase (mut/Mb) ranging from 11 to 227.9 mut/Mb. Pearson's test for correlation demonstrated the correspondence between an increasing number of TILs and higher TMB (r = 0.4567; 95% confidence interval [CI], 0.01779–0.7481; p = .0429; Fig. 1A).
Figure 1.
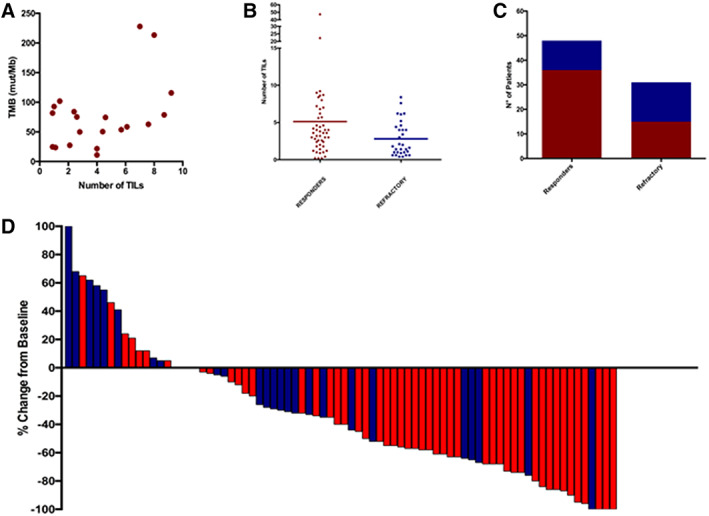
Main TMB, TILs and response correlation findings. (A): Correlation between number of TILs and tumor mutational burden (Pearson's test, p = .0429). (B): Correlation between number of TILs and response to immune checkpoint inhibitors (ICIs; Mann‐Whitney's test, p = .0448). (C): Number of TILs number in responders versus nonresponders to ICIs (Fisher's exact test, p = .0291). (D): Waterfall plot showing best response to ICIs according to number of TILs. Red indicates high number of TILs; blue indicates low number of TILs.Abbreviations: TIL, tumor infiltrating lymphocyte; TMB, tumor mutational burden.
The median TMB in super responders was 78.6 mut/Mb (11–227.9), whereas in clearly refractory patients it was 50.4 mut/Mb (range 23.6–101.8 mut/Mb). The median number of TILs in super responders was 4.0 (range 0.9–9.2), whereas in clearly refractory patients the median number of TILs was 2.1 (range 0.9–7.6; supplemental online Table 3).
Whole Cohort: TILs Density and Outcome Correlations
A total of 52 patients (65%) were defined as TILs‐H, whereas 28 (35%) were defined as TILs‐L (supplemental online Table 4). The median number of TILs was 3.6 in responding patients compared with 1.8 in nonresponders (Mann‐Whitney p = .0448; Fig. 1B). Thirty‐six out of 51 evaluable TILs‐H patients responded (ORR of 70.6%), compared with 12 out of 28 evaluable TILs‐L patients (ORR of 42.9%; odds ratio = 3.20; 95% CI, 1.22–8.37; p = .0291; Fig. 1C).
At the time of data analyses, median PFS was not reached for TILs‐H patients and was 27.8 months for TILs‐L patients (hazard ratio [HR] = 0.42; 95% CI, 0.15–0.88; p = .028; Fig. 2A). The median OS were not reached, but survival outcome differed significantly in favor of TILs‐H patients (HR = 0.41; 95% CI, 0.14–0.98; p = .046; Fig. 2B). Figure 3 shows histologic and radiological imaging of two selected cases, one responder and one refractory patient, respectively.
Figure 2.
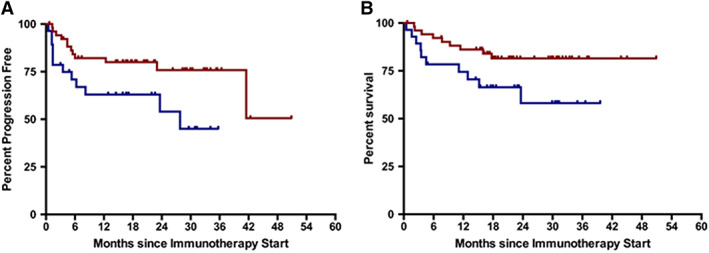
Survival analyses. (A): Progression‐free survival curves according to number of tumor infiltrating lymphocytes (TILs). (B): Overall survival curves according to number of TILs. Red indicates high number of TILs; blue indicates low number of TILs.
Figure 3.
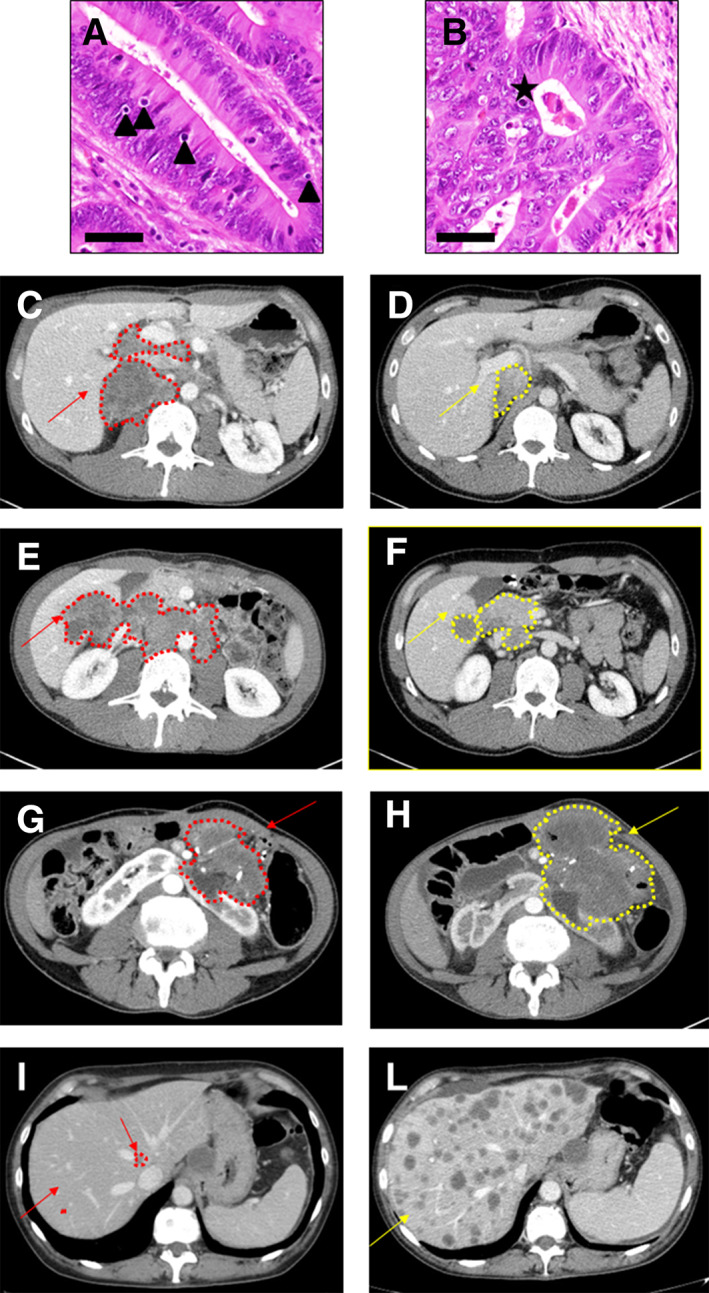
Histologic and radiologic imaging of two representative cases. (A): Example of tumor with high number of tumor infiltrating lymphocytes (TILs); triangles show TILs. Bar scale, 100 μm. (B): Example of tumor with low number of TILs; star shows apoptotic body. Bar scale, 100 μm. (C–F): Baseline (C, E) and best response (D, F) computed tomography (CT) scans of two representative cases with high number of TILs. (G–L): Baseline (G, I) and 8‐weeks first assessment (H, L) CT scans of two representative cases with tumors with low number of TILs showing a clearly progressive disease. Red indicates baseline lesions; yellow indicates best overall response to ICI.
Discussion
The present study examined a large data set of MSI‐H mCRCs treated with ICI, investigating the correlation between TMB, number of TILs, and ICI activity.
Published data supports TMB as a reliable marker of ICI efficacy across multiple tumor types 5, 23, 24. We therefore analyzed TMB and TILs in an exploratory cohort and demonstrated a significant correlation between higher TMB and increased number of TILs. TILs analysis was then performed in our cohort of 80 patients with MSI‐H CRC and demonstrated that patients with higher number of TILs were more likely to achieve CR or PR (odds ratio = 3.20; 95% CI, 1.22–8.37; p = .0291). High number of TILs predicted better survival both in terms of PFS (HR = 0.37; 95% CI, 0.15–0.90; p = .0278) and OS (HR = 0.37; 95% CI, 0.14–0.98; p = .0463). Our results are in line with data published by Schrock et al. 14 at the time of writing the present manuscript, showing an association between higher TMB and higher chances of response and better PFS in 22 patients with mCRC treated with ICI 14.
The major value of our study resides in gathering samples from a large cohort of patients with MSI‐H mCRC treated with ICI with detailed data collection, including patients’ and tumor characteristics, systemic treatment, response, and survival.
Central reassessment ensured that subsequent analyses were performed only in confirmed MSI‐H cases. Our central reassessment revealed that five patients (5.9%) previously categorized as MSI‐H were in fact MSS. These patients demonstrated primary resistance to ICI, in line with recent data published by Cohen et al. 25.
This is the first report on TMB and TILs analyses in a selected cohort of patients with MSI‐H mCRC treated with ICIs. We suggest that number of TILs could represent a surrogate of TMB. Our evidence that high number of TILs is linked with better response, PFS, and OS could be of practical relevance, considering that TILs analysis is a simple and economic test.
Our results are affected by potential bias typical of post hoc analyses. These hurdles are difficult to bypass at the moment given the recent introduction of ICI in the treatment of MSI‐H mCRC. Limitations include the fact that ICI was administered in different lines of treatment and in combination with different drugs (e.g., some PD‐1 inhibitors were administered in combination with anti‐CTLA4 therapy). Our sample size was relatively limited, but considering that MSI‐H prevalence is less than 5% in mCRC, this is the largest series treated with immunotherapy analyzed for TILs and TMB so far. Furthermore, there was no control arm for better describing the predictive versus prognostic significance of PFS and OS data.
Optimal TMB cutoffs for predicting benefit from ICI have not yet been defined. Specifically, in MSI‐H colorectal cancer, given its peculiar high TMB profile, current evidence is extremely limited, and larger studies will be needed in the next future. TILs analysis is not yet universally standardized. Our cutoff to distinguish low or high number of TILs (<2 or ≥ 2) was chosen based on data available in the literature, and its definition has historically been rather arbitrary 26 and not specifically elaborated for MSI‐H tumors. Of note, after analyses completion, all “outliers” (i.e., cases labeled as TILs‐L achieving a response or as TILs‐H but not responding, n = 27) were reviewed on newly cut slides from formalin‐fixed paraffin‐embedded blocks. The case labeled as AC_31, a mucinous adenocarcinoma initially diagnosed as TILs‐L but achieving a clear RECIST response (i.e., 76% reduction in target lesions diameter), at review showed a heterogenous pattern in which the mucinous component previously described (mean number of TILs = 1.8) was actually intermixed with areas of NOS adenocarcinoma characterized by a high prevalence of TILs (mean = 5.4). This underlines how heterogeneity, even if it seems to occur rather rarely, may significantly affect TILs evaluation and should be carefully considered when evaluating small biopsies and/or mucinous tumors.
Our hypothesis is that a more detailed characterization of tumor‐infiltrating subtypes of immune cells could be correlated with benefit from or resistance to ICIs; this area requires further research. Recent innovative research suggests the degree of microsatellite instability, the resultant TMB, and more specifically indel mutational load should be considered as additional determinants of benefit from ICI in MSI‐H patients 27. The correlation of those parameters with number of TILs and subtypes would give precious information for a better understanding of resistance mechanisms to ICIs.
Conclusion
Our analysis is one of the first proofs of potential predictive biomarkers of ICI efficacy in MSI‐H mCRC. These data may potentially guide future patient selection and further translational studies. TMB and TILs assessment should be incorporated in future trials testing ICI in CRC in order to confirm our results and explore questions on methods and cutoffs for routine clinical application.
Author Contributions
Conception/design: Fotios Loupakis, Sara Lonardi, Matteo Fassan
Provision of study material or patients: Fotios Loupakis, Ilaria Depetris, Paola Biason, Rossana Intini, Alessandra Anna Prete, Francesco Leone, Pasquale Lombardi, Roberto Filippi, Andrea Spallanzani, Stefano Cascinu, Luca Reggiani Bonetti, Giulia Maddalena, Nicola Valeri, Andrea Sottoriva, Luis Zapata, Roberta Salmaso, Giada Munari, Massimo Rugge, Angelo Paolo Dei Tos, Justin Golovato, John Z. Sanborn, Andrew Nguyen, Marta Schirripa, Vittorina Zagonel, Sara Lonardi, Matteo Fassan
Collection and/or assembly of data: Fotios Loupakis, Ilaria Depetris, Paola Biason, Rossana Intini, Alessandra Anna Prete, Francesco Leone, Pasquale Lombardi, Roberto Filippi, Andrea Spallanzani, Stefano Cascinu, Luca Reggiani Bonetti, Giulia Maddalena, Nicola Valeri, Andrea Sottoriva, Luis Zapata, Roberta Salmaso, Giada Munari, Massimo Rugge, Angelo Paolo Dei Tos, Justin Golovato, John Z. Sanborn, Andrew Nguyen, Marta Schirripa, Vittorina Zagonel, Sara Lonardi, Matteo Fassan
Data analysis and interpretation: Fotios Loupakis, Ilaria Depetris, Paola Biason, Giulia Maddalena, Nicola Valeri, Andrea Sottoriva, Luis Zapata, Roberta Salmaso, Giada Munari, Justin Golovato, John Z. Sanborn, Andrew Nguyen, Matteo Fassan
Manuscript writing: Fotios Loupakis, Ilaria Depetris, Nicola Valeri, Andrea Sottoriva, Matteo Fassan
Final approval of manuscript: Fotios Loupakis, Ilaria Depetris, Paola Biason, Rossana Intini, Alessandra Anna Prete, Francesco Leone, Pasquale Lombardi, Roberto Filippi, Andrea Spallanzani, Stefano Cascinu, Luca Reggiani Bonetti, Giulia Maddalena, Nicola Valeri, Andrea Sottoriva, Luis Zapata, Roberta Salmaso, Giada Munari, Massimo Rugge, Angelo Paolo Dei Tos, Justin Golovato, John Z. Sanborn, Andrew Nguyen, Marta Schirripa, Vittorina Zagonel, Sara Lonardi, Matteo Fassan
Disclosures
Nicola Valeri: Pfizer, Eli Lilly & Co., Merck (H); John Z. Sanborn: ImmunityBio (E), NantOmics, NantHealth (OI), NantOmics (IP); Andrew Nguyen: ImmunityBio (E); Vittorina Zagonel: Bristol‐Myers Squibb, Merck (C/A), Bayer, Roche, Eli Lilly & Co., AstraZeneca, Bristol‐Myers Squibb, Ipsen, Astellas (RF), Bayer, Roche, Bristol‐Myers Squibb, Servier (other—speakers’ bureau). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Tables
Acknowledgments
We acknowledge Dr. Hazel Lote for critical review of the manuscript. A.S. is supported by the Wellcome Trust (202778/B/16/Z) and Cancer Research UK (A22909). We acknowledge funding from the National Institute of Health (NCI U54 CA217376) to A.S. This work was also supported a Wellcome Trust award to the Centre for Evolution and Cancer (105104/Z/14/Z). L.Z. is supported by the European Union's Horizon 2020 research and innovation programme under the Marie Sklodowska‐Curie Research Fellowship scheme (846614).
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Koopman M, Kortman GAM, Mekenkamp L et al. Deficient mismatch repair system in patients with sporadic advanced colorectal cancer. Br J Cancer 2009;100:266–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Venderbosch S, Nagtegaal ID, Maughan TS et al. Mismatch repair status and BRAF mutation status in metastatic colorectal cancer patients: A pooled analysis of the CAIRO, CAIRO2, COIN, and FOCUS studies. Clin Cancer Res 2014;20:5322–5330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Overman MJ, McDermott R, Leach JL et al. Nivolumab in patients with metastatic DNA mismatch repair‐deficient or microsatellite instability‐high colorectal cancer (CheckMate 142): An open‐label, multicentre, phase 2 study. Lancet Oncol 2017;18:1182–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Overman MJ, Lonardi S, Wong KYM et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair–deficient/microsatellite instability–high metastatic colorectal cancer. J Clin Oncol 2018;36:773–779. [DOI] [PubMed] [Google Scholar]
- 5. Chan TA, Yarchoan M, Jaffee E et al. Development of tumor mutation burden as an immunotherapy biomarker: Utility for the oncology clinic. Ann Oncol 2019;30:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hellmann MD, Nathanson T, Rizvi H et al. Genomic features of response to combination immunotherapy in patients with advanced non‐small‐cell lung cancer. Cancer Cell 2018;33:843–852.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Berland L, Heeke S, Humbert O et al. Current views on tumor mutational burden in patients with non‐small cell lung cancer treated by immune checkpoint inhibitors. J Thorac Dis 2019;11(suppl 1):S71–S80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hellmann MD, Ciuleanu TE, Pluzanski A et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med 2018;378:2093–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Snyder A, Makarov V, Merghoub T et al. Genetic basis for clinical response to CTLA‐4 blockade in melanoma. N Engl J Med 2014;371:2189–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Van Allen EM, Miao D, Schilling B et al. Genomic correlates of response to CTLA‐4 blockade in metastatic melanoma. Science 2015;350:207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Le DT, Uram JN, Wang H et al. PD‐1 blockade in tumors with mismatch‐repair deficiency. N Engl J Med 2015;372:2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rosenberg JE, Hoffman‐Censits J, Powles T et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum‐based chemotherapy: A single‐arm, multicentre, phase 2 trial. Lancet 2016;387:1909–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maleki Vareki S. High and low mutational burden tumors versus immunologically hot and cold tumors and response to immune checkpoint inhibitors. J Immunother Cancer 2018;6:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schrock AB, Ouyang C, Sandhu J et al. Tumor mutational burden is predictive of response to immune checkpoint inhibitors in MSI‐high metastatic colorectal cancer. Ann Oncol 2019;30:1096–1103. [DOI] [PubMed] [Google Scholar]
- 15. Huh JW, Lee JH, Kim HR. Prognostic significance of tumor‐infiltrating lymphocytes for patients with colorectal cancer. Arch Surg 2012;147:366–372. [DOI] [PubMed] [Google Scholar]
- 16. Rozek LS, Schmit SL, Greenson JK et al. Tumor‐infiltrating lymphocytes, Crohn's‐like lymphoid reaction, and survival from colorectal cancer. J Natl Cancer Inst 2016;108:djw027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kwak Y, Koh J, Kim DW et al. Immunoscore encompassing CD3+ and CD8+ T cell densities in distant metastasis is a robust prognostic marker for advanced colorectal cancer. Oncotarget 2016;7:81778–81790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pagès F, Berger A, Camus M et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med 2005;353:2654–2666. [DOI] [PubMed] [Google Scholar]
- 19. Remo A, Fassan M, Lanza G. Immunohistochemical evaluation of mismatch repair proteins in colorectal carcinoma: The AIFEG/GIPAD proposal. Pathologica 2016;108:104–109. [PubMed] [Google Scholar]
- 20. Bosman FT, World Health Organization, International Agency for Research on Cancer, eds. WHO Classification of Tumours of the Digestive System. 4th ed. Lyon, France: International Agency for Research on Cancer, 2010. [Google Scholar]
- 21. North JP, Golovato J, Vaske CJ et al. Cell of origin and mutation pattern define three clinically distinct classes of sebaceous carcinoma. Nat Commun 2018;9:1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Williams DS, Mouradov D, Jorissen RN et al. Lymphocytic response to tumour and deficient DNA mismatch repair identify subtypes of stage II/III colorectal cancer associated with patient outcomes. Gut 2018. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 23. Goodman AM, Kato S, Bazhenova L et al. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther 2017;16:2598–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Samstein RM, Lee CH, Shoushtari AN et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet 2019;51:202–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cohen R, Hain E, Buhard O et al. Association of primary resistance to immune checkpoint inhibitors in metastatic colorectal cancer with misdiagnosis of microsatellite instability or mismatch repair deficiency status. JAMA Oncol 2019;5:551–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Iseki Y, Shibutani M, Maeda K et al. A new method for evaluating tumor‐infiltrating lymphocytes (TILs) in colorectal cancer using hematoxylin and eosin (H‐E)‐stained tumor sections. PLoS One 2018;13:e0192744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mandal R, Samstein RM, Lee KW et al. Genetic diversity of tumors with mismatch repair deficiency influences anti‐PD‐1 immunotherapy response. Science 2019;364:485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Tables


