Abstract
Lessons Learned
Updated survival data for a phase I/II study of carboplatin plus nab‐paclitaxel and concurrent radiotherapy were collected.
In the group of 58 patients who were enrolled at 14 institutions in Japan, the median overall survival was not reached and the 2‐year overall survival rate was 66.1% (95% confidence interval, 52.1%–76.8%).
Results reveal encouraging feasibility and activity for this regimen.
Background
We report the updated survival data for a phase I/II study of carboplatin plus nab‐paclitaxel (nab‐P/C) and concurrent radiotherapy (CRT) in patients with locally advanced non‐small cell lung cancer (NSCLC).
Methods
Individuals between 20 and 74 years of age with unresectable NSCLC of stage IIIA or IIIB and a performance status of 0 or 1 were eligible for the study. Patients received weekly nab‐paclitaxel at 50 mg/m2 for 6 weeks together with weekly carboplatin at an area under the curve (AUC) of 2 mg/ml/min and concurrent radiotherapy with 60 Gy in 30 fractions. This concurrent phase was followed by a consolidation phase consisting of two 3‐week cycles of nab‐paclitaxel (100 mg/m2 on days 1, 8, and 15) plus carboplatin (AUC of 6 on day 1). After the treatment, patients were observed off therapy. The primary endpoint of the phase II part of the study was progression‐free survival (PFS).
Results
Between October 2014 and November 2016, 58 patients were enrolled at 14 institutions in Japan, with 56 of these individuals being evaluable for treatment efficacy and safety. At the median follow‐up time of 26.0 months (range, 4.0–49.6 months), the median overall survival (OS) was not reached (95% confidence interval [CI], 25.3 months to not reached) and the 2‐year OS rate was 66.1% (95% CI, 52.1%–76.8%). The median PFS was 11.8 months (95% CI, 8.2–21.0 months), and the 2‐year PFS rate was 35.9% (95% CI, 23.1%–48.9%). Subgroup analysis according to tumor histology or patient age revealed no differences in median PFS or OS. Long‐term follow‐up of toxicities did not identify new safety signals, and no treatment‐related deaths occurred during the study period.
Conclusion
Concurrent chemoradiation with nab‐P/C was safe and provided a long‐term survival benefit for patients with locally advanced NSCLC.
Discussion
A recent phase III study thus showed that administration of durvalumab as a consolidative immunotherapy after chemoradiation conferred a significant increase in OS and PFS compared with a placebo control in patients with locally advanced NSCLC 1, 2. Although cytotoxic agents still play an important role in chemoradiation, the optimal concurrent chemotherapy regimen has not been determined. Commonly administered chemotherapy regimens for locally advanced NSCLC include weekly solvent‐based paclitaxel plus carboplatin (sb‐P/C) as a result of its favorable toxicity profile and efficacy 3, 4, 5. Nab‐paclitaxel is a formulation of paclitaxel complexed with albumin, and a phase III study showed a significantly higher objective response rate (ORR) for nab‐P/C compared with sb‐P/C for patients with advanced NSCLC 6. The combination of nab‐P/C and CRT is thus a potential treatment option for locally advanced NSCLC.
We performed the present phase I/II study to determine the recommended dose (RD) and to evaluate the safety and efficacy of this regimen. In the phase I part, one of six patients experienced dose‐limiting toxicity (leukopenia of grade 3 requiring a second consecutive skip in the administration of weekly chemotherapy) with weekly nab‐paclitaxel at 50 mg/m2 and carboplatin at AUC of 2 mg mL−1 min, with this dose of nab‐paclitaxel thus being determined as the RD 7. In the first analysis of the phase II part, the ORR was 76.8% (95% CI, 64.2%–85.9%), median PFS was 11.8 months (60% CI, 10.6–16.2 months; 95% CI, 8.2–20.8 months), and median OS was not reached 7. Common toxicities of grade 3 or 4 during the concurrent chemoradiation included leukopenia (60.7%) and neutropenia (28.6%), and there were no treatment‐related deaths at the time of the first analysis (median follow‐up time, 17.5 months) 7.
Given that the goal of concurrent chemoradiation in locally advanced NSCLC is to effect a cure, long‐term PFS and OS data are important. We therefore now provide updated results for OS in this trial after a median follow‐up time of 26.0 months. The updated analysis of our study has revealed that weekly nab‐P/C with CRT yielded an OS rate at 2 years of 66.1% (95% CI, 52.1%–76.8%) and a PFS rate at 2 years of 35.9% (95% CI, 23.1%–48.9 %) and median OS was not reached (95% CI, 25.3 months to not reached) in patients with locally advanced NSCLC (Fig. 1A, 1B). The OS rate at 2 years for weekly sb‐P/C combined with CRT was up to 40% 3, 4, 5, and the PFS rate at 2 years was up to 20% 3. Although the two regimens are not directly comparable, weekly nab‐P/C with CRT showed promising survival data compared with weekly sb‐P/C with CRT. Regarding the late toxicity, no new safety signals were identified. Our results reveal encouraging feasibility and activity for this regimen.
Figure 1.
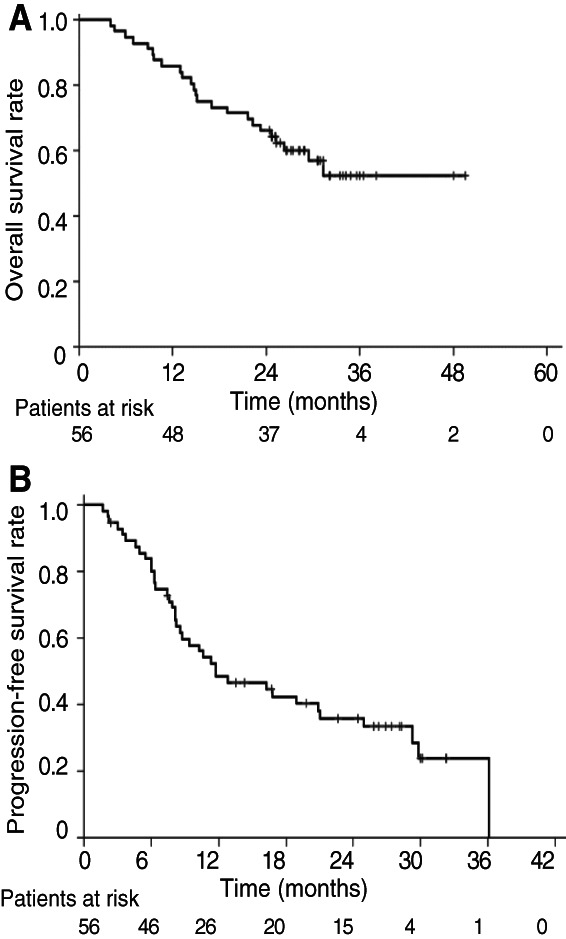
Kaplan‐Meier curves for overall survival (A) and progression‐free survival (B) in the study patients.
Trial Information
| Disease | Lung cancer – NSCLC |
| Disease | Advanced cancer |
| Stage of Disease/Treatment | Metastatic/advanced |
| Prior Therapy | None |
| Type of Study – 1 | Phase II |
| Type of Study – 2 | Single arm |
| Primary Endpoint | Progression‐free survival |
| Secondary Endpoint | Overall response rate |
| Secondary Endpoint | Overall survival |
| Secondary Endpoint | Safety |
| Secondary Endpoint | 2‐year OS rate |
| Additional Details of Endpoints or Study Design | |
| Study Design and Patients | |
| We designed a multicenter, open‐label, single‐arm, prospective, combined phase I dose‐escalation and phase II study, which was conducted as previously described 7. Eligible patients had histologically or cytologically documented unresectable locally advanced (stage III) NSCLC according to the 7th edition of the American Joint Committee on Cancer staging system. They also had to have no prior history of chemotherapy, radiotherapy, or surgery and to be able to undergo radiotherapy according to the study protocol (percentage of normal lung receiving at least 20 Gy [V20] of ≤35%). Other eligibility requirements included an age of 20–74 years, an Eastern Cooperative Oncology Group performance status of 0 or 1, and adequate lung, bone marrow, liver, and kidney function. The study protocol was approved by the institutional review boards at the participating centers, and the study was conducted in accordance with the provisions of the Declaration of Helsinki. All patients provided written informed consent before enrollment. The trial has been registered in the University Hospital Medical Information Network (UMIN) database with the ID UMIN000012719. All data for the phase II study were centrally monitored by the Clinical Research Support Center Kyushu. | |
| All patients were to be treated with nab‐P/C weekly for 6 weeks in the concurrent chemoradiation phase. Carboplatin was administered at a fixed dose of an AUC of 2 mg/ml/min during a 60‐minute intravenous (IV) infusion on day 1, and nab‐paclitaxel was administered at a dose of 50 mg/m2 during a 30‐minute IV infusion on day 1 and was repeated weekly for 6 weeks. The RD of nab‐paclitaxel during concurrent radiotherapy was determined in the phase I part of the study 7. Radiotherapy was delivered with the photon beam of a linear accelerator at 6–10 MV from day 1. The primary tumor and involved nodal disease received 60 Gy in 2‐Gy fractions over 6 weeks. A more detailed description of the radiation regimen was provided previously 7. At 4–8 weeks after the completion of concurrent radiotherapy, two 3‐week cycles of nab‐paclitaxel (100 mg/m2 on days 1, 8, and 15) plus carboplatin (AUC of 6 mg/ml/min on day 1) were administered as consolidation chemotherapy. | |
| Details regarding baseline evaluations, toxicity assessment, and follow‐up were provided in our previous report 7. Toxicity was assessed on the basis of Common Terminology Criteria for Adverse Events version 4.0. Late toxicities were defined as adverse events occurring more than 30 days after the completion of radiotherapy. Patient response was evaluated according to RECIST version 1.1. | |
| Statistical Analysis | |
| The primary endpoint of the phase II part of the study was PFS. The sample size for this part of the study was calculated on the basis of an assumed PFS of 13 months, with 9 months being the lower limit of interest. Given this assumption, a sample size of 50 patients was required to achieve a power of 80% at a one‐sided level of alpha error of 0.20 according to the method of Brookmeyer and Crowley. On the basis of the additional assumption that 10% of patients would not be evaluable for response, we set the accrual goal at 56 patients for the phase II part of the study, including the 6 patients who received treatment at the RD in the phase I part. OS and PFS were estimated with the Kaplan‐Meier method. | |
| Investigator's Analysis | Active and should be pursued further |
Drug Information
| Drug 1 | |
| Generic/Working Name | Nab‐paclitaxel |
| Route | IV |
| Schedule of Administration |
Concurrent chemoradiation phase: 50 mg/m2 on days 1, 8, 15, 22, 29, and 36 Consolidation chemotherapy: 100 mg/m2 on days 1, 8, 15 (every 3 weeks), with the second consolidation cycle starting immediately after the first |
| Drug 2 | |
| Generic/Working Name | Carboplatin |
| Route | IV |
| Schedule of Administration |
Concurrent chemoradiation phase: AUC of 2 mg/ml/min on days 1, 8, 15, 22, 29, and 36 Consolidation chemotherapy: AUC of 6 mg/ml/min on day 1 (every 3 weeks) |
Patient Characteristics
| Number of Patients, Male | 48 |
| Number of Patients, Female | 8 |
| Stage | IIIA, 30; IIIB, 26 |
| Age | Median (range): 66 (45–74) |
| Number of Prior Systemic Therapies | Median (range): 0 |
| Performance Status: ECOG |
0 — 26 1 — 30 2 — 0 3 — 0 Unknown — 0 |
| Other |
Smoking status: Never, 6; Former 50 EGFR mutation status: Negative, 32; Positive, 6; Unknown, 18 ALK fusion status: Negative, 25; Positive, 1; Unknown, 30 |
| Cancer Types or Histologic Subtypes |
Adenocarcinoma, 30 Squamous cell carcinoma, 23 Other, 3 |
Primary Assessment Method
| Title | PFS |
| Number of Patients Screened | 58 |
| Number of Patients Enrolled | 58 |
| Number of Patients Evaluable for Toxicity | 56 |
| Number of Patients Evaluated for Efficacy | 56 |
| Evaluation Method | RECIST 1.1 |
| Response Assessment CR | n = 1 (1.8%) |
| Response Assessment PR | n = 42 (75%) |
| Response Assessment SD | n = 10 (17.9%) |
| Response Assessment PD | n = 3 (5.4%) |
| Response Assessment OTHER | n = 0 (0%) |
| (Median) Duration Assessments PFS | 11.8 months, CI: 95% CI, 8.2–21.0 months |
| Outcome Notes | |
| Efficacy | |
| As of November 30, 2018, which was the data cutoff date for this analysis, 24 (42.9%) of the 56 patients had died. At the median follow‐up time of 26.0 months (range, 4.0–49.6 months), the median OS was not reached (95% CI, 25.3 months to not reached) and the 2‐year OS rate for all treated patients was 66.1% (95% CI, 52.1%–76.8 %; Fig. 1A). The median PFS was 11.8 months (95% CI, 8.2–21.0 months), and the 2‐year PFS rate was 35.9% (95% CI, 23.1%–48.9%; Fig. 1B). | |
| We also evaluated efficacy according to histology. The median OS was not reached (95% CI, 23.2 months to not reached) for patients with nonsquamous cell histology (n = 33) and 29.4 months (95% CI, 19.0 months to not reached) for those with squamous cell histology (n = 23; Fig. 2A). The median PFS was 16.8 months (95% CI, 8.1–29.8 months) in patients with nonsquamous cell histology and 8.7 months (95% CI, 6.3–20.8 months) in those with squamous cell histology (Fig. 2B). The ORR was 75.7% (95% CI, 57.7%–88.9%) in patients with nonsquamous cell histology and 78.3% (95% CI, 56.3%–92.5%) in patients with squamous cell histology. | |
| With regard to age, the median OS was 31.3 months (95% CI, 21.6 months to not reached) in patients younger than 70 years (n = 41) and not reached (95% CI, 19.0 months to not reached) in those aged 70–74 years (n = 15; Fig. 2C). The median PFS was 11.3 months (95% CI, 7.6–21.0 months) in patients younger than age 70 and 12.8 months (95% CI, 8.5 months to not reached) in those aged 70–74 years (Fig. 2D). The ORR was 82.9% (95% CI, 68.7%–91.5%) in patients younger than 70 years and 60.0% (95% CI, 35.7%–80.2%) in those aged 70–74 years. | |
| Pattern of Recurrence | |
| Among the 42 patients with recurrence, local relapse alone was observed in 16 patients (38.1%), distant metastasis alone in 24 patients (57.1%), and both local relapse and distant metastasis in 2 patients (4.8%). Eight (14.3%) of the total of 56 patients showed brain metastasis as the initial failure site, with the brain being the most common site for distant metastasis. | |
| Postdiscontinuation Therapy | |
| Thirty‐seven (88.1%) of the 42 patients with recurrence received disease‐related anticancer therapy after discontinuation of the study treatment. A total of 32 patients (76.2 %) received systemic therapy (19 patients, cytotoxic chemotherapy; 20 patients, immunotherapy; 6 patients, an epidermal growth factor receptor tyrosine kinase inhibitor; 1 patient, an anaplastic lymphoma kinase tyrosine kinase inhibitor). Thirteen patients (31.0%) received radiotherapy (12 patients, brain or bone metastasis; 1 patient, thoracic radiotherapy), and 3 patients (7.1%) underwent surgery (2 patients, bone metastasis; 1 patient, brain metastasis). | |
Figure 2.
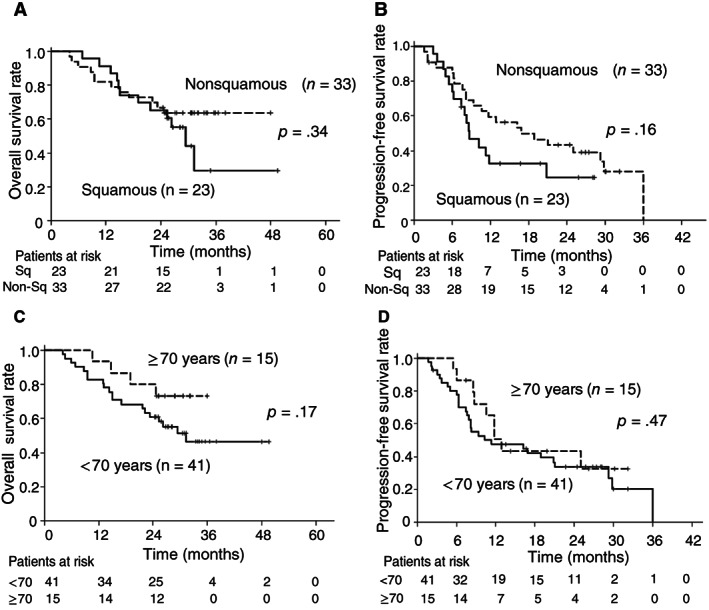
Kaplan‐Meier curves for overall survival and progression‐free survival according to tumor histology or patient age.
Adverse Events
| All Cycles | |||||||
|---|---|---|---|---|---|---|---|
| Name | NC/NA | 1 | 2 | 3 | 4 | 5 | All grades |
| Pneumonitis | 93% | 5% | 2% | 0% | 0% | 0% | 7% |
| Esophagitis | 100% | 0% | 0% | 0% | 0% | 0% | 0% |
| Peripheral sensory neuropathy | 100% | 0% | 0% | 0% | 0% | 0% | 0% |
| Pneumothorax | 98% | 0% | 0% | 2% | 0% | 0% | 2% |
| Pleural effusion | 96% | 2% | 2% | 0% | 0% | 0% | 4% |
Late toxicities in patients who received treatment at the recommended dose (RD).
All 56 patients who received treatment at the RD were eligible for safety analysis. Main toxicities at the RD during treatment were described in our previous report 7. The principal late toxicities of the 56 patients that occurred more than 1 month after the completion of study treatment are listed. There were also no treatment‐related deaths during the study period.
Abbreviation: NC/NA, no change from baseline/no adverse event.
Assessment, Analysis, and Discussion
| Completion | Study completed |
| Investigator's Assessment | Active and should be pursued further |
Lung cancer remains the leading cause of cancer‐related death worldwide 8, with locally advanced (stage III) non‐small cell lung cancer (NSCLC) accounting for ∼25% of all lung cancer cases 9. For individuals with unresectable locally advanced NSCLC and a good performance status, the standard of care is platinum‐based doublet chemotherapy with concurrent radiotherapy (CRT) followed by durvalumab, an antibody to programmed cell death–ligand 1 1, 2, 10. However, cytotoxic agents still play an important role in chemoradiation, and the optimal concurrent chemotherapy regimen has not been determined. Commonly administered regimens for locally advanced NSCLC include weekly solvent‐based paclitaxel (sb‐paclitaxel) plus carboplatin (sb‐P/C) as a result of its favorable toxicity profile and efficacy 3, 4, 5.The updated analysis of our study has revealed that weekly carboplatin plus nab‐paclitaxel (nab‐P/C) with CRT yielded an OS rate at 2 years of 66.1% (95% CI, 52.1%–76.8%) and a PFS rate at 2 years of 35.9% (95% CI, 23.1%–48.9 %) in patients with locally advanced NSCLC. Our updated survival data underscore the activity of weekly nab‐P/C with CRT compared with weekly sb‐P/C with CRT 3, 4, 5, although the two regimens are not directly comparable.
The pattern of first failure differed between nab‐P/C with CRT and sb‐P/C with CRT 5. The incidence of local recurrence for the nab‐P/C regimen was lower than that for the sb‐P/C regimen (42.9% vs. 61.3%) 5. Nab‐paclitaxel was found to deliver a higher drug concentration to tumors compared with sb‐paclitaxel in preclinical models 11, suggesting that the radiosensitizing effect of nab‐paclitaxel is higher than that of sb‐paclitaxel, and as a result, local recurrence was well controlled in our study. Distant failure was observed in 61.9% of patients in our study, with brain metastasis being the most common site of such failure. The frequency of distant relapse with the nab‐P/C regimen was also lower than that with the sb‐P/C regimen (80.4%) 5 and was similar to that for other platinum‐doublet chemotherapy with CRT (45.8%–65.7%) 12, 13, 14. However, a high risk of distant relapse remains a major problem for nab‐P/C and CRT. A recent phase III trial showed that administration of durvalumab as consolidative immunotherapy after chemoradiation conferred a significant increase in OS and PFS and had a favorable effect on the frequency of new metastases including brain metastases compared with a placebo control in patients with locally advanced NSCLC 1. Nab‐P/C combined with CRT followed by consolidative immunotherapy with durvalumab may thus have therapeutic potential for locally advanced NSCLC.
Common toxicities of grade 3 or 4 included leukopenia (60.7%) and neutropenia (28.6%), and one patient experienced pneumonitis of grade 3, during the concurrent chemoradiation phase of our study 7. The long‐term follow‐up of toxicities is necessary for evaluation of potential late effects of radiation. No new safety signals were identified at the median follow‐up time of 26.0 months in our study. A previous study found that the incidence of treatment‐related death in patients with lung cancer who received concurrent chemoradiation was 4.9% 15. In our study, there were no such deaths throughout the study period. The safety profile and treatment delivery for nab‐P/C with CRT in patients aged 70–74 years were similar to those in patients aged <70 years (data not shown). Our subset analysis revealed promising efficacy for the study treatment in patients aged 70–74 years as well as in those younger than 70. Its acceptable feasibility and efficacy thus support the use of nab‐P/C combined with CRT as a treatment option for older patients with locally advanced NSCLC.
Six epidermal growth factor receptor (EGFR) mutation–positive patients and one anaplastic lymphoma kinase (ALK) fusion–positive patient were enrolled in our study. All seven of these patients experienced relapse and were treated with EGFR or ALK tyrosine kinase inhibitors after nab‐P/C with CRT. However, the role of molecularly targeted therapy in locally advanced NSCLC remains unclear. The efficacy of molecularly targeted therapy in combination with radiotherapy in this patient population warrants further investigation 16.
In conclusion, our updated data provide further support for the activity and safety of concurrent chemoradiation with nab‐P/C in patients with locally advanced NSCLC.
Disclosures
Hiroyuki Yamaguchi: AstraZeneca, Bristol‐Myers Squibb, Chugai Pharmaceutical, Boehringer Ingelheim, Eli Lilly & Co. (H), Boehringer Ingelheim, Novartis Pharma K.K., Eli Lilly & Co. (RF); Katsuya Hirano: Taiho Pharmaceutical (H); Atsushi Horiike: Chugai Pharmaceutical, AstraZeneca, Taiho Pharmaceutical, Eli Lilly & Co., Pfizer (H), Chugai Pharmaceutical, MSD Oncology, Abbvie, AstraZeneca, Loxo (RF); Miyako Satouchi: Chugai Pharmaceutical, Taiho Pharmaceutical, Eli Lilly & Co., Pfizer, AstraZeneca, Bristol‐Myers Squibb, Ono Pharmaceutical, Merck Sharp & Dohme, Novartis, Boehringer Ingelheim (H), Chugai Pharmaceutical, Eli Lilly & Co., Pfizer, AstraZeneca, Bristol‐Myers Squibb, Ono Pharmaceutical, Merck Sharp & Dohme, Novartis, Takeda, Abbvie, Boehringr Ingelheim (RF, institutional); Kosuke Takahashi: AstraZeneca, Boehringer Ingelheim, Chugai Pharmaceutical, Eli Lilly & Co., Merck Sharp & Dohme, Taiho Pharmaceutical (H); Isamu Okamoto: Chugai Pharmaceutical, AstraZeneca, Taiho Pharmaceutical, Boehringer Ingelheim, Ono Pharmaceutical, MSD Oncology, Eli Lilly & Co., Bristol‐Myers Squibb, Pfizer (H), Chugai Pharmaceutical, AstraZeneca, Taiho Pharmaceutical, Boehringer Ingelheim, Ono Pharmaceutical, MSD Oncology, Eli Lilly & Co., Bristol‐Myers Squibb, Astellas Pharma, Novartis, AbbVie (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Acknowledgments
We thank all patients and investigators who participated in this study. This study was supported by Taiho Pharmaceutical Co. Ltd., Japan, under a study contract (no grant number applies).
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Footnotes
- ClinicalTrials.gov Identifier: UMIN000012719
- Sponsor: Taiho Pharmaceutical Co. Ltd., Japan
- Principal Investigator: Isamu Okamoto
- IRB Approved: Yes
References
- 1. Antonia SJ, Villegas A, Daniel D et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 2018;379:2342–2350. [DOI] [PubMed] [Google Scholar]
- 2. Antonia SJ, Villegas A, Daniel D et al. Durvalumab after chemoradiotherapy in stage III non‐small‐cell lung cancer. N Engl J Med 2017;377:1919–1929. [DOI] [PubMed] [Google Scholar]
- 3. Yamamoto N, Nakagawa K, Nishimura Y et al. Phase III study comparing second‐ and third‐generation regimens with concurrent thoracic radiotherapy in patients with unresectable stage III non‐small‐cell lung cancer: West Japan Thoracic Oncology Group WJTOG0105. J Clin Oncol 2010;28:3739–3745. [DOI] [PubMed] [Google Scholar]
- 4. Belani CP, Choy H, Bonomi P et al. Combined chemoradiotherapy regimens of paclitaxel and carboplatin for locally advanced non‐small‐cell lung cancer: A randomized phase II locally advanced multi‐modality protocol. J Clin Oncol 2005;23:5883–5891. [DOI] [PubMed] [Google Scholar]
- 5. Vokes EE, Herndon JE 2nd, Kelley MJ et al. Induction chemotherapy followed by chemoradiotherapy compared with chemoradiotherapy alone for regionally advanced unresectable stage III non‐small‐cell lung cancer: Cancer and Leukemia Group B. J Clin Oncol 2007;25:1698–1704. [DOI] [PubMed] [Google Scholar]
- 6. Socinski MA, Bondarenko I, Karaseva NA et al. Weekly nab‐paclitaxel in combination with carboplatin versus solvent‐based paclitaxel plus carboplatin as first‐line therapy in patients with advanced non‐small‐cell lung cancer: Final results of a phase III trial. J Clin Oncol 2012;30:2055–2062. [DOI] [PubMed] [Google Scholar]
- 7. Kawano Y, Sasaki T, Yamaguchi H et al. Phase I/II study of carboplatin plus nab‐paclitaxel and concurrent radiotherapy for patients with locally advanced non‐small cell lung cancer. Lung Cancer 2018;125:136–141. [DOI] [PubMed] [Google Scholar]
- 8. Cheema PK, Rothenstein J, Melosky B et al. Perspectives on treatment advances for stage III locally advanced unresectable non‐small‐cell lung cancer. Curr Oncol 2019;26:37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tsim S, O'Dowd CA, Milroy R et al. Staging of non‐small cell lung cancer (NSCLC): A review. Respir Med 2010;104:1767–1774. [DOI] [PubMed] [Google Scholar]
- 10. Yoon SM, Shaikh T, Hallman M. Therapeutic management options for stage III non‐small cell lung cancer. World J Clin Oncol 2017;8:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Desai N, Trieu V, Yao Z et al. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor‐free, albumin‐bound paclitaxel, ABI‐007, compared with cremophor‐based paclitaxel. Clin Cancer Res 2006;12:1317–1324. [DOI] [PubMed] [Google Scholar]
- 12. Sasaki T, Seto T, Yamanaka T et al. A randomised phase II trial of S‐1 plus cisplatin versus vinorelbine plus cisplatin with concurrent thoracic radiotherapy for unresectable, locally advanced non‐small cell lung cancer: WJOG5008L. Br J Cancer 2018;119:675–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Senan S, Brade A, Wang LH et al. PROCLAIM: Randomized phase III trial of pemetrexed‐cisplatin or etoposide‐cisplatin plus thoracic radiation therapy followed by consolidation chemotherapy in locally advanced nonsquamous non‐small‐cell lung cancer. J Clin Oncol 2016;34:953–962. [DOI] [PubMed] [Google Scholar]
- 14. Segawa Y, Kiura K, Takigawa N et al. Phase III trial comparing docetaxel and cisplatin combination chemotherapy with mitomycin, vindesine, and cisplatin combination chemotherapy with concurrent thoracic radiotherapy in locally advanced non‐small‐cell lung cancer: OLCSG 0007. J Clin Oncol 2010;28:3299–3306. [DOI] [PubMed] [Google Scholar]
- 15. Minami‐Shimmyo Y, Ohe Y, Yamamoto S et al. Risk factors for treatment‐related death associated with chemotherapy and thoracic radiotherapy for lung cancer. J Thorac Oncol 2012;7:177‐182. [DOI] [PubMed] [Google Scholar]
- 16. Iwama E, Nakanishi Y, Okamoto I. Combined therapy with epidermal growth factor receptor tyrosine kinase inhibitors for non‐small cell lung cancer. Expert Rev Anticancer Ther 2018;18:267–276. [DOI] [PubMed] [Google Scholar]


