Abstract
Breast cancer is the most common malignancy in young women worldwide, accounting for an estimated 30% of new cancer diagnoses and 25% of cancer deaths. Approximately two thirds of young women with breast cancer have hormone receptor–positive (HR+)/human epidermal growth receptor 2–negative (HER2−) tumors. Numerous studies, primarily in early‐stage breast cancer, have demonstrated that young age is an independent risk factor for more aggressive disease and worse outcomes. Although more limited data are available regarding outcomes in young patients with advanced disease, these age‐related disparities suggest that breast cancer in premenopausal women has distinct clinicopathologic and molecular features that can impact treatment outcomes. Until recently, limited data were available on the intrinsic molecular subtypes and genetics of young patients with HR+/HER2− metastatic breast cancer (mBC). In this review, we explore insights into the clinical and pathologic features of HR+/HER2− mBC in younger women derived from recent clinical trials of the cyclin‐dependent kinase 4/6 inhibitors palbociclib (PALOMA‐3), ribociclib (MONALEESA‐7), and abemaciclib (MONARCH 2) and the implications of these findings for clinical practice, guideline development, and future research.
Implications for Practice
This review provides clinicians with an overview of emerging data on the unique clinicopathologic and molecular features of hormone receptor–positive/human epidermal growth receptor 2–negative metastatic breast cancer (mBC) in premenopausal women, summarizes findings from the most recent clinical trials of endocrine‐based treatment in this patient population, and explores the implications of these findings for clinical practice, guideline development, and future research. Improved understanding of the key factors influencing disease course and treatment response in premenopausal patients with mBC may lead to more timely incorporation of evidence‐based treatment approaches, thereby improving patient care and outcomes.
Keywords: Metastatic breast cancer, Hormone receptor positive, Human epidermal growth factor negative, Premenopausal, Intrinsic subtype
Short abstract
Age‐related disparities in breast cancer care suggest that breast cancer in premenopausal women has distinct features that can affect treatment outcomes. On the basis of results of recent related clinical trials, this review explores insights into the clinical and pathologic features of HR+/HER2− breast cancer in younger women.
Introduction
Breast cancer is the most common malignancy in young women (aged 20–39 years) worldwide, accounting for an estimated 30% of new cancer diagnoses and 25% of cancer deaths 1. Population‐based studies in the U.S. and other countries have consistently shown that young women are more likely than postmenopausal patients to present with aggressive disease and de novo stage IV breast cancer; those who present with less advanced disease frequently progress to metastatic breast cancer (mBC) 2, 3, 4, 5. In the U.S., women under the age of 40 diagnosed with breast cancer between 1988 and 2003 were 39% more likely to die of the disease than those 40 or older 6.
Between 1976 and 2009, the incidence of mBC in U.S. women 25–39 years of age increased by 2.1% annually—from 1.53 to 2.90 per 100,000—and this trend has continued in more recent years 7, 8. Although some of the rise is a result of more accurate staging, after controlling for this variable the increase in de novo mBC among young black women remained statistically significant 8. Of note, the number of young women with hormone receptor–positive (HR+) disease is also on the rise, with an 8.15% annual increase from 1992 to 2009 7.
Age‐related disparities in disease presentation and course suggest that breast cancer in premenopausal women has distinct clinicopathologic and molecular features that can affect treatment outcomes and should be considered when developing treatment plans. To date, most research on age‐related issues has focused on early‐stage disease. However, recent research has begun to shed light on the unique characteristics of mBC in younger patients.
Unique Features of Metastatic Breast Cancer in Young Women
The incidence, morbidity, and mortality of breast cancer among young women vary by region and ethnicity. In the U.S., for example, black women have the highest proportion of grade 3/4 and advanced (stage III/IV) breast tumors 9. On a global scale, the mortality burden of breast cancer in young women is highest in Africa and other low‐income regions 1, 10, 11, whereas incidence is highest in middle‐ to high‐income areas such as North America, Europe, Australia, and Japan 1, 10, 12.
Socioeconomic and environmental factors such as access to care, funding for prevention and screening programs, and exposure to carcinogens account for some, but not all, of this variability 10. It has been suggested that the high rate of breast cancer among young women in high‐income regions such as the U.S., Australia, and the European Union may be due in part to a higher incidence of BRCA1/2 in people of European ancestry 1. Similarly, the high proportion of premenopausal breast cancer observed in Asian countries has been linked to a range of hereditary factors, including a higher prevalence of oncogenic alterations, differences in HR expression, and differences in the tumor immune microenvironment as compared with Western populations 13, 14.
Young age is itself an independent risk factor for more aggressive disease and worse outcomes 6. A 2009 population‐based study of more than 22,000 patients with breast cancer in Sweden (including 2% with metastatic disease at diagnoses) found that 5‐year relative survival was lowest in women <35 years of age (74.8%; 95% confidence interval [CI]: 70.1–78.9) 15. More recently, a study of more than 25,000 patients in Japan (2% of whom had metastatic disease at onset) found that age <35 years was an independent negative prognostic factor for both overall survival (OS; hazard ratio: 1.58; 95% CI: 1.16–2.15; p = .004) and disease‐free survival (hazard ratio: 1.73; 95% CI: 1.42–2.10; p < .001) 4. Age‐based comparisons of the clinicopathologic features of early breast cancer have consistently found that younger women are more likely to be diagnosed with estrogen receptor–negative (ER−) tumors, to have higher‐grade and larger tumors, and to have lymph node involvement 16, 17, 18.
Intrinsic Molecular Subtypes
Intrinsic molecular subtypes of breast cancer have been identified that are associated with specific single‐gene mutations that affect response to systemic therapies and survival outcomes 19, 20. Luminal A—which is characterized by expression of estrogen and progesterone hormone receptors, an absence of human epidermal growth receptor 2 (HER2) expression, and less expression of proliferation genes than luminal B—is the most common subtype, accounting for up to 60% of all breast cancers and approximately two thirds of cancers arising in premenopausal patients 21, 22.
Recently, researchers analyzed age‐related differences in somatic mutations in 780 patients with early‐stage breast cancer from The Cancer Genome Atlas (TCGA) data set 23. Gene expression profiling revealed a similar distribution of subtypes across age groups (≤45, 46–69, and ≥70 years); however, younger patients were somewhat more likely to have triple‐negative (basal) tumors (Fig. 1). A similar analysis of samples from 1,319 participants in the Life After Cancer Epidemiology and Pathways studies found that the odds ratio (OR) for having luminal B versus the luminal A breast cancer was 2.48 (95% CI: 0.98–6.29) for women aged <40 years and 1.27 (95% CI: 0.72–2.27) for those 40–49 years of age, with a trend toward a more aggressive variant in women with otherwise low‐risk HR+ disease 24. In addition, gene expression profiling indicates that luminal B tumors in younger (≤40 years) patients are more aggressive compared with tumors in older patients 25.
Figure 1.
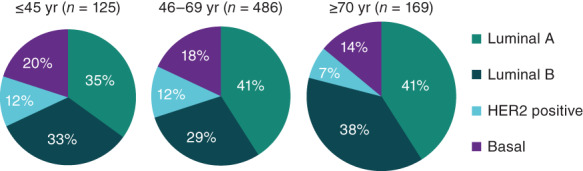
Breast cancer intrinsic subtypes by age, The Cancer Genome Atlas 23.Abbreviations: HER2, human epidermal growth receptor 2; yr, years.
Limited data are available on the molecular subtypes and genetics of young patients with HR+/HER2− mBC. Recognizing the need for a broader evidence base to inform treatment decisions in this group, opinion leaders and professional organizations have repeatedly called for the inclusion of premenopausal women in clinical trials 26, 27, 28. As a result, data are now emerging from a new generation of trials that have included premenopausal patients. The first large data sets to provide insights into the clinical and pathologic features of HR+/HER2− mBC in younger women have come from phase III clinical trials of the cyclin‐dependent kinase (CDK) 4/6 inhibitors palbociclib (PALOMA‐3), ribociclib (MONALEESA‐7), and abemaciclib (MONARCH 2; Table 1).
Table 1.
Clinical trials of cyclin‐dependent kinase 4/6 inhibitors in premenopausal patients with hormone receptor–positive/human epidermal growth receptor 2–negative mBC 30, 43, 44, 45, 46, 48, 49
| Trial | |||
|---|---|---|---|
| Characteristic | PALOMA‐3NCT01942135 | MONALEESA‐7NCT02278120 | MONARCH 2NCT02107703 |
| Inclusion criteria |
|
|
|
| Treatment arms |
|
|
|
| Pre/perimenopausal women enrolled, n (% of total population) | 108 (21) | 672 (100) | 114 (17) |
| Prior chemotherapy for ABC/mBC, %a | 33 | 14 | 0 |
| Patients with visceral disease, %a | 59 | 58 | 55 |
| Median PFS, mo, experimental vs. control | 11.2 vs. 4.6 | 23.8 vs. 13.0 | 16.4 vs. 9.3 |
| Median OS, mo, experimental vs. control (hazard ratio: 95% CI) | 34.9 vs. 28.0 (0.81; 0.64–1.03; p = .09) | NR vs. 40.9 (0.71; 0.54–0.95; p = .01) | 46.7 vs. 37.3 (0.76; 0.61–0.94; p = .01) |
Experimental group only.
Abbreviations: ABC, advanced breast cancer; CDK, cyclin‐dependent kinase; GnRH, gonadotropin‐releasing hormone; HER2−, human epidermal growth receptor 2 negative; HR+, hormone receptor–positive; mBC, metastatic breast cancer; NR, not reached; OS, overall survival; PFS, progression‐free survival.
Both PALOMA‐3 and MONARCH 2 documented differing rates of endocrine resistance in premenopausal patients compared with postmenopausal patients. In PALOMA‐3, endocrine resistance was defined as the absence of a response (complete, partial, or stable) in the first 24 weeks of prior endocrine therapy for mBC or recurrence in the first 2 years after receiving adjuvant endocrine therapy. At baseline, 30% of premenopausal patients were endocrine resistant versus 21% of the overall population and 19% of postmenopausal women 29. Using a similar definition of endocrine resistance, 38% of premenopausal women in MONARCH 2 were found to have primary endocrine resistance versus 25% of the overall population 12, 30.
Genetics
Despite a relatively large volume of research on the relationship between age and genetics in early‐stage breast cancer and on the genomic profile of primary versus metastatic disease, the evidence base on the genomic landscape of mBC in premenopausal women is limited.
In the aforementioned analysis of age‐related differences in somatic mutations among patients from the TCGA data set, only one mutation—GATA3—was independently associated with breast cancer arising in young women, although others were associated with older age at diagnosis and with the luminal A subtype that is common in young patients with HR+/HER2− mBC (Table 2). The study authors noted that “age is associated with unique biological features at the DNA level” and that these features are independent of molecular subtype, tumor histology, or tumor stage 23.
Table 2.
Prevalent somatic mutations (%) by age and intrinsic subtype, The Cancer Genome Atlas data set 20, 23
| Age group, yr | Intrinsic subtype | ||||
|---|---|---|---|---|---|
| Gene | ≤45 | 46–69 | ≥70 | Luminal A | Luminal B |
| GATA3 | 15.2 | 8.2 | 9.0 | 14.0 | — |
| TP53 | 27.9 | 33.4 | 23.2 | 12.0 | 32.0 |
| PIK3CA | 28.8 | 32.7 | 41.9 | 49.0 | 32.0 |
| TTN | 13.5 | 15.1 | 29.0 | — | — |
| MAP3K1 | — | — | — | 14.0 | 5.0 |
Abbreviation: —, no data.
More recently, gene sequencing of 387 patients with HR+/HER2− breast cancer identified 11 genes that were more frequently mutated in mBC than early‐stage disease: TP53 (29%), KMT2C (13%), NCOR1 (8%), NF1 (7%), RB1 (4%), C16orf3 (2%), FRG1 (6%), ESR1 (21%), RIC8A (4%), AKT1 (7%), and PLSCR5 (2%). Patients with HR+/HER2− mBC were significantly more likely than those with early disease to present with an actionable mutation (73% and 55%, respectively; p < .01), with a higher prevalence of alterations in the MAPK/ERK (37% vs. 22%) and homologous recombination deficiency (22% vs. 10%) pathways 31.
Recent efforts from the MSK‐IMPACT and mBC Project data sets have provided detailed, publicly available information (via cBioPortal) regarding the genomic landscape of mBC 32, 33, 34. However, clinical annotation with age is only available in a subset of patients from the mBC Project (58 patients age ≤50 years had a total of 68 sequenced tumors from a metastatic site) 35, 36. Among these patients, the five most frequently mutated genes were TP53 (37%), PIK3CA (25%), CDH1 (12%), PTEN (12%), and GATA3 (9%) 35, 36.
Data from recent clinical trials, in particular PALOMA‐3 and MONALEESA‐7, have provided insights into the molecular profile of HR+ mBC in premenopausal women. The PALOMA‐3 trial analyzed circulating free DNA (cfDNA) at baseline in a subset of 79 premenopausal patients 29. The frequency of PIK3CA mutations in cfDNA was 39% in premenopausal women and 31% in postmenopausal women. In contrast, ESR1 mutations were less common in premenopausal women (19% vs. 29%), likely reflecting lack of exposure to aromatase inhibitors 29. Overall, the mutational burden in cfDNA at baseline was not significantly different in samples from premenopausal versus postmenopausal women in PALOMA‐3 29.
In the MONALEESA‐7 trial, NanoString nCounter (NanoString Technologies, Seattle, WA) analysis of baseline archival tumor samples from 360 pre/perimenopausal women with HR+/HER2− mBC (185 ribociclib‐treated, 175 from the placebo group) showed generally consistent progression‐free survival (PFS) benefit across gene expression subgroups; however, the magnitude of benefit varied in some subsets 37. A trend toward greater PFS benefit was observed in patients with high versus low expression of CCND1 (hazard ratio: 0.38 vs. 0.67), ERBB3 (hazard ratio: 0.33 vs. 0.76), and IGF1R (hazard ratio: 0.33 vs. 0.77) 37. Conversely, greater benefit was seen with low versus high expression of CCNE1 (hazard ratio: 0.38 vs. 0.65) and MYC (hazard ratio: 0.37 vs. 0.69). Of note, no difference in PFS benefit with ribociclib was observed based on expression of FGFR1, ESR1, or tumor proliferation genes such as MKI67 37. Similarly, immunohistochemistry analysis of of Ki67, Rb, and p16 protein expression showed consistent PFS benefit in high‐ and low‐expression subgroups 38.
The Evolving Treatment Landscape
The goal of therapy in mBC is to both prolong the patient's life and protect the quality of that life. Current clinical guidelines for HR+/HER2− mBC recommend the use of treatments associated with minimal toxicity, with cytotoxic chemotherapy reserved for symptomatic visceral metastases or disease that is refractory to endocrine agents 28, 39.
Endocrine therapy is the standard of care for HR+/HER2− mBC. In premenopausal women, treatment is based on ovarian function suppression/ablation (OFS) coupled with one or more antiendocrine agents: selective ER modulators (tamoxifen or toremifene); nonsteroidal aromatase inhibitors (NSAIs; anastrozole or letrozole), the selective ER downregulator fulvestrant, and the steroidal aromatase inactivator exemestane 39.
In recent years, the treatment armamentarium for HR+/HER2− mBC has expanded with the introduction of small‐molecule inhibitors that can be added to traditional endocrine backbones. Currently approved targeted agents include the CDK4/6 inhibitors palbociclib, ribociclib, and abemaciclib; the mammalian target of rapamycin inhibitor everolimus; the PI3K inhibitor alpelisib (for patients with PIK3CA mutations); and the poly adenosine diphosphate‐ribose polymerase (PARP) inhibitors olaparib and talazoparib (for patients with germline BRCA1/2 mutations) 21, 40, 41. These regimens have improved clinical outcomes in premenopausal patients with mBC 42 but have also made the process of selecting the appropriate therapy more complex (Fig. 2).
Figure 2.
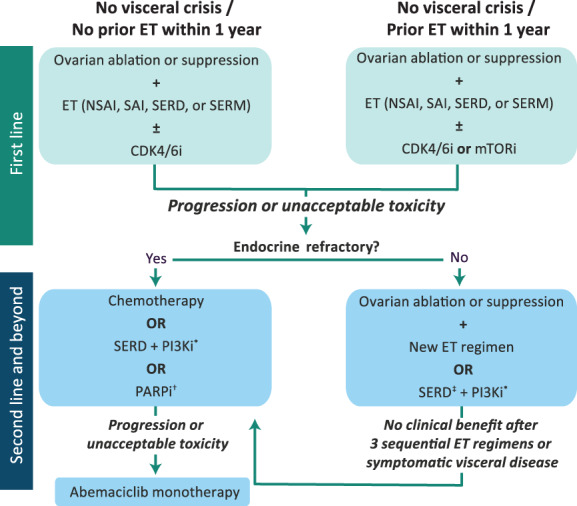
Treatment algorithm for premenopausal women with hormone receptor–positive/human epidermal growth receptor 2–negative mBC 21, 39, 40. *In patients with PIK3CA mutations as detected by a U.S. Food and Drug Administration–approved test. †In patients with germline BRCA1/2 mutations. ‡If not received in first line.Abbreviations: CDK4/6i, cyclin‐dependent kinase 4/6 inhibitor (palbociclib, ribociclib, abemaciclib); ET, endocrine therapy; mTORi, mammalian target of rapamycin inhibitor (everolimus); NSAI, nonsteroidal reversible aromatase inhibitor (anastrozole, letrozole); PARPi, poly ADP ribose polymerase inhibitor (olaparib, talazoparib); PI3Ki, phosphatidylinositol‐3‐kinase inhibitor (alpelisib); SAI, steroidal irreversible aromatase inactivator (exemestane); SERD, selective estrogen receptor degrader (fulvestrant); SERM, selective estrogen receptor modulator (tamoxifen, toremifene).
CDK4/6 Inhibitors
To date, three phase III clinical trials of CDK4/6 inhibitors have specifically included premenopausal and perimenopausal women: PALOMA‐3, MONARCH 2, and MONALEESA‐7. In all three trials, OFS was required for all peri/premenopausal patients (Table 1) 30, 43, 44, 45, 46.
Palbociclib
PALOMA‐3 compared palbociclib plus fulvestrant with placebo plus fulvestrant in pretreated patients with HR+/HER2− mBC and was the basis of the U.S. Food and Drug Administration (FDA) approval of palbociclib with fulvestrant for the treatment of advanced HR+/HER2− mBC following progression on prior endocrine therapy. Some of the study population had been heavily pretreated: one third had received prior chemotherapy for metastatic disease, and more than half had at least two prior lines of endocrine therapy 43. Pre/perimenopausal women made up 21% (n = 108) of patients. Eight percent (n = 42) of patients were ≤40 years of age, and 31% (n = 163) were ≤50 years 29.
The objective response rate among pre/perimenopausal women was 25% in the palbociclib arm compared with 11% in the placebo arm (OR: 3.06; 95% CI: 0.82–13.38), with a clinical benefit rate of 69.4% compared with 44.4% (OR: 2.89; 95% CI: 1.15–7.34) 29. Median PFS was 9.5 versus 5.6 months (hazard ratio: 0.50; 95% CI: 0.29–0.87). Median time to chemotherapy was 120 versus 75 days 29. Median OS was unchanged for peri/premenopausal women receiving palbociclib compared with placebo (38.0 vs. 38.0 months; hazard ratio: 1.07; 95% CI: 0.61–1.86) 45.
No clinically relevant drug interactions were observed between palbociclib and goserelin, and the frequency of all‐grade and serious adverse events (AEs) were similar with palbociclib in both pre‐ and postmenopausal women. In pre‐ and postmenopausal women in the palbociclib arm, grade 3 or 4 AEs occurred in 83% versus 71%, dose interruptions occurred in 90% versus 82%, and dose reductions in 42% versus 32% of patients, respectively 29.
Recently reported results from the ongoing Young‐PEARL study (NCT02592746) provide additional evidence of the activity of palbociclib‐based combination therapy in premenopausal women with HR+/HER2− mBC. This prospective, open‐label phase II trial assessed the antitumor activity and safety of palbociclib plus exemestane and leuprolide (n = 92) versus capecitabine (n = 86) in premenopausal women with inoperable locally advanced or metastatic HR+/HER2− breast cancer. After a median follow‐up of 17 months, investigator‐assessed median PFS was 14.4 months in the capecitabine group (95% CI: 12.1–17.0) versus 20.1 in the palbociclib plus exemestane and leuprolide group (95% CI: 14.2–21.8). Palbociclib‐treated patients showed higher rates of hematologic toxicities (e.g., neutropenia and leukopenia) and lower rates of nausea, diarrhea, and hand‐foot syndrome 47.
Abemaciclib
The MONARCH 2 trial evaluated abemaciclib and fulvestrant versus fulvestrant alone in patients with advanced breast cancer whose disease had progressed on endocrine therapy 30. Women of any menopausal status were included, and 17% (n = 114) of patients were pre/perimenopausal. A greater proportion of Asian patients were pre/perimenopausal, with Asian patients making up two thirds of pre/perimenopausal women but just under one third of the whole study population 12, 30. As described earlier, a higher proportion of pre/perimenopausal women had primary endocrine resistance 12, 30. Forty percent of patients had received endocrine therapy for metastatic disease.
Median PFS for pre/perimenopausal women receiving abemaciclib and fulvestrant was not reached versus 10.5 months in pre/perimenopausal women receiving fulvestrant alone (hazard ratio: 0.45; 95% CI: 0.26–0.75; p = .002) 12. This compared with a hazard ratio of 0.55 (95% CI: 0.45–0.68; p < .0000001) in the entire intent‐to‐treat MONARCH‐2 patient cohort 12. Among pre/perimenopausal women receiving abemaciclib and fulvestrant, the response rate was 43% versus 19% in women receiving fulvestrant alone; clinical benefit rates were 78% and 69%, respectively 12. Median time to chemotherapy was not reached in the pre/perimenopausal abemaciclib and fulvestrant group versus 19.2 months (hazard ratio: 0.61; 95% CI: 0.32–1.15) in those taking fulvestrant alone 12. Dose reductions were needed in 39% of pre/perimenopausal women receiving abemaciclib plus fulvestrant versus 2% of those receiving fulvestrant alone; serious AEs occurred in 11% versus 5% of patients, respectively 12. The findings of this trial led to the FDA approval of abemaciclib with fulvestrant in women with HR+/HER2− advanced breast cancer and progression on prior endocrine therapy regardless of menopausal status.
Interim OS analysis after a median follow‐up of 47.4 months found a median OS of 46.7 months in the abemaciclib group versus 37.3 months in those treated with placebo (hazard ratio: 0.76; 95% CI: 0.61–0.95; p = .01). OS was similar in premenopausal/perimenopausal patients (hazard ratio: 0.69; 95% CI: 0.38–1.25) and those who were postmenopausal (hazard ratio: 0.77; 95% CI: 0.61–0.98) 48.
Ribociclib
The MONALEESA‐7 trial evaluated ribociclib with endocrine therapy (goserelin with tamoxifen, letrozole, or anastrozole) compared with endocrine therapy alone in patients with mBC 46. The study included 672 premenopausal patients, with 28% (n = 186) aged <40 years and 72% (n = 486) ≥40 years. Approximately 40% had de novo mBC, and 14% of patients received first‐line chemotherapy for metastatic disease before trial enrollment. Twenty‐six percent received tamoxifen, and the remainder received an NSAI. Median PFS was 23.8 months in the ribociclib group versus 13.0 months in the placebo group (hazard ratio: 0.55; 95% CI: 0.44–0.69; p < .0001), with similar hazard ratios for ribociclib with tamoxifen or an NSAI combination partner versus placebo 46. The response rate was 41% in the ribociclib group versus 30% in the placebo group; clinical benefit rates were 79% and 70%, respectively 46.
Estimated OS at 42 months was 70.2% (95% CI: 63.5–76.0) in the ribociclib group and 46.0% (95% CI: 32.0–58.9) in the placebo group, with a 29% lower risk of death in the ribociclib group (hazard ratio for death: 0.71; 95% CI: 0.54–0.95) 49. Subgroup analyses indicate that OS benefit was generally consistent regardless of age (<40 and ≥40 years) or endocrine therapy regimen; however, a greater benefit was observed in Asian (hazard ratio for death: 0.40; 95% CI: 0.22–0.72) versus non‐Asian patients (hazard ratio for death: 0.91; 95% CI: 0.64‐1.30) 49.
Deterioration in quality of life (QoL) was delayed in patients who received ribociclib, with a median time to deterioration of QoL of 24.0 months in women who received ribociclib with an NSAI compared with 19.4 months in the placebo group (hazard ratio: 0.76; 95% CI: 0.56–1.03). Patients receiving ribociclib also had improvement in their QoL scores 50.
Serious AEs occurred in 18% versus 12% of patients receiving endocrine therapy with and without ribociclib, respectively 46. Neutropenia and leukopenia were the most frequent grade 3 or 4 AEs 46. There was an increase in QTcF >60 milliseconds from baseline in 16% of patients with ribociclib and tamoxifen, 7% with ribociclib and NSAI, 7% with tamoxifen alone, and no patients with NSAI alone 46. These findings supported the approval of ribociclib by the FDA for use with an aromatase inhibitor in women of any menopausal status as initial endocrine therapy for HR+/HER2− advanced breast cancer. It is currently not indicated for use with tamoxifen, primarily because of concerns regarding QTc prolongation.
PARP Inhibitors
PARP inhibitors are relatively new additions to the mBC treatment armamentarium that target DNA repair deficiencies linked to BRCA1/2. The OlympiAD (olaparib) and EMBRACA (talazoparib) trials evaluated PARP inhibitors in women with HER2− mBC. As both trials included only patients with mutated BRCA1/2—which are highly prevalent in young women—they de facto selected for a younger patient population 51, 52, 53.
In the OlympiAD trial, 205 patients were randomized to olaparib and 97 to the single‐agent chemotherapy of the physician's choice (TPC) 54. Median age in the olaparib and TPC arms were 44 and 45 years, respectively, with only 5% of patients over the age of 65 51. Median OS was 19.3 months in the olaparib arm versus 17.1 months with TPC (hazard ratio: 0.90; 95% CI: 0.66–1.23; p = .513). Overall survival benefit with olaparib was consistent in patients <44 years of age (hazard ratio: 0.92; 95% CI: 0.60–1.46) and ≥44 years of age (hazard ratio: 0.87; 95% CI: 0.58–1.34) 54. The most frequently reported AEs with olaparib were nausea (58.0%), anemia (40.0%), vomiting (32.2%), fatigue (29.8%), and neutropenia (27.3%); most were grade 1 or 2 in severity 54. The overall rate of AE‐related discontinuations was 4.9% in the olaparib group versus 7.7% in the TPC arm 54.
The EMBRACA trial enrolled 431 patients who were randomly assigned in a 2:1 ratio to talazoparib (n = 287) or standard chemotherapy (n = 144) 52. Median age was 45 years in the talazoparib arm and 50 years in the standard therapy group, with 58% of the overall population under 50 years of age 52. After a median duration of follow‐up of 11.2 months, median PFS in the overall talazoparib group was 8.6 months (95% CI: 7.9–9.3) versus 5.6 months (95% CI: 4.2–6.7) in patients receiving standard therapy, with a hazard ratio for progression or death of 0.54 (95% CI: 0.41–0.71; p < .001) 52. In patients younger than 50 years of age, the hazard ratio for PFS was 0.51 (95% CI: 0.35–0.75), with an objective response rate of 62% (95% CI: 53.45–69.98) in the talazoparib arm versus 22.4% in the standard therapy group (OR: 5.77; 95% CI: 2.54–13.67; p < .0001) 55. The most frequently reported talazoparib‐related AEs were similar to those seen with olaparib: anemia (52.8%), fatigue (50.3%), nausea (48.6%), neutropenia (34.6%), headache (32.5%), and thrombocytopenia (26.9%), although a higher proportion of hematologic (55%) and nonhematologic (32%) AEs were grade ≥3 in severity. Treatment‐related AEs resulting in discontinuation of therapy were reported in 5.9% of talazoparib‐treatment patients and 8.7 of those on standard therapy 52.
Questions remain about the relative benefits of PARP inhibitors in patients with HR+ mBC. A recent meta‐analysis of data from the OlympiAD and EMBRACA trials found that single‐agent PARP inhibitors yielded a statistically significant improvement in PFS only in HR− patients (hazard ratio: 0.51; 95% CI: 0.37–0.71; p < .001) 56. These agents currently are recommended as second‐line therapy in HR+ patients with endocrine therapy–refractory disease (Fig. 2) 57.
Optimizing QoL in Premenopausal Patients with mBC
Young women with breast cancer typically report a significantly greater symptom burden and poorer QoL than age‐matched controls 58. Premenopausal women receiving endocrine therapy for early‐stage HR+/HER2− breast cancer frequently experience vasomotor, gynecologic, sexual, musculoskeletal, constitutional, and psychological symptoms that adversely affect QoL, with similar effects reported by patients aged <35 years and ≥35 years 59.
In addition to considering the distinct biology of the disease, treatment plans for younger women with HR+/HER2− mBC need to address other age‐related issues that can impact patients’ QoL and adherence to therapy 60. Young women frequently face unique social and economic challenges—including job instability, inadequate health benefits, and work and family obligations—that interfere with their ability to adhere to care plans 60. “Financial toxicity” can be a treatment‐limiting factor for younger patients with limited resources and conflicting priorities and should be considered when designing a treatment plan and counseling patients 61. For example, dose modification is central to reducing AEs and ensuring that treatment is completed; however, the costs associated with drug wastage and prescription overlap can place a significant financial burden on younger patients and actually increase the risk of nonadherence 62. Conversely, therapies that delay time to chemotherapy, preserve QoL, and reduce cancer‐related pain can help allay patients’ fears and promote treatment adherence. Recognizing these issues and working with the patient to address them is an essential component of an effective multidisciplinary care plan.
Discussion
Although HR+/HER2− mBC occurs less frequently in younger than older women, it is more common globally than previously understood. Until recently, there have been limited data to define unique disease features or identify optimal therapy in this group.
Consensus guidelines issued by the European School of Oncology and the European Society for Medical Oncology have consistently stressed that the treatment of younger women should be guided by the biological characteristics of the tumor and the patient's comorbidities and preferences, noting that young age is not in itself a reason to prescribe more aggressive therapies (e.g., combination chemotherapy) 26, 27. However, current treatment recommendations for HR+/HER2− mBC in premenopausal patients are largely extrapolated from data gathered on postmenopausal patients and do not address the unique characteristics of mBC in this population 27, 63.
Retrospective studies have documented persistent gaps in guideline‐concordant care in patients treated in real‐world settings in the U.S. and other countries. Up to 40% of premenopausal women with HR+/HER2− mBC are still receiving first‐line treatment with cytotoxic chemotherapy rather than endocrine therapy 64, 65, 66. A recent retrospective chart review of 652 women treated with palbociclib following FDA approval found that although the median age of patients receiving the drug was similar to that seen in clinical trials, only about 13% were premenopausal 67. A larger retrospective cohort study of more than 4,500 women with HR+/HER2− mBC found that 30% of women ≤50 years of age received palbociclib during first‐line endocrine therapy 68. Another retrospective chart review of first‐line therapy in 201 premenopausal women diagnosed with HR+/HER2− mBC between January 2015 and January 2017 revealed more extensive incorporation of CDK4/6 inhibitors—52.7% of women received a CDK4/6 inhibitor–based regimen—but also found that 20.9% received a chemotherapy regimen. Among CDK4/6 inhibitor patients, median time on treatment was 26.8 months. More than half of all premenopausal patients studied also received an ovarian suppressant during first‐line treatment 69.
Results from trials of CDK4/6 inhibitors that include premenopausal women indicate that combination regimens with CDK4/6 inhibitors, endocrine therapy, and gonadotropin‐releasing hormone (GnRH) agonists are generally well tolerated in younger patients, with similar toxicities and effects on QoL as seen in the overall mBC population 12, 29, 46. It is worth noting that both the MONALEESA‐7 and MONARCH‐2 trials enrolled patients who had received fewer lines of treatment for advanced/metastatic disease (Table 1). In addition, in MONALEESA‐7, the GnRH agonist and study treatment could be initiated simultaneously versus the 28‐day delay required in PALOMA‐2 and MONARCH‐2 41. These data suggest that early initiation of combination therapy with CDK4/6 inhibitors may be of benefit in premenopausal patients. Current and ongoing clinical trials of new and established combination therapies (Table 3) promise to yield further insights and new treatment options for premenopausal patients with HR+/HER2− disease.
Table 3.
Ongoing clinical trials in premenopausal patients with hormone receptor–positive/human epidermal growth receptor 2–negative mBC
| Identifier (name) | Phase | Target enrollment | Treatment arm(s) | Outcome measures |
|---|---|---|---|---|
| NCT03096847 | 3b | 504 | Ribociclib + letrozole + goserelin |
Primary: CBR Secondary: PFS, OS, QoL |
| NCT03839823 (RIGHT Choice) | 2 | 222 |
Experimental: ribociclib + letrozole/anastrozole + goserelin Control: combination chemotherapy |
Primary: PFS Secondary: TTF, ORR, CBR, OS, AEs, QoL |
| NCT02384239 | 2 | 70 |
Palbociclib (100 or 125 mg) + fulvestrant or tamoxifen |
Primary: Tumor progression (RECIST v1.1) Secondary: PFS, CBR, biomarkers |
| NCT02917005 (FATIMA) | 2 | 160 |
Experimental: palbociclib + exemestane + goserelin Control: exemestane + goserelin |
Primary: PFS Secondary: ORR, CBR, OS, TRAEs |
| NCT02592746 (KCSG BR 15‐10; Young‐PEARL) | 2 | 182 |
Experimental: palbociclib + exemestane + goserelin Control: capecitabine |
Primary: PFS |
| NCT03481998 | 1/2 | 146 |
SHR6390 + letrozole, anastrozole, or fulvestrant |
Primary: AEs Secondary: PK/PD, ORR, PFS, DCR |
| NCT02990845 (PEER) | 1/2 | 25 |
Pembrolizumab + exemestane + leuprolide |
Primary: PFS Secondary: TRAEs, ORR, CBR, DOR |
Abbreviations: AE, adverse event; CBR, clinical benefit rate; DCR, disease control rate; DOR, duration of response; mBC, metastatic breast cancer; ORR, overall response rate; OS, overall survival; PD, pharmacodynamics; PFS, progression‐free survival; PK, pharmacokinetics; QoL, quality of life; TRAE, treatment‐related adverse event; TTF, time to treatment failure.
Conclusion
The totality of clinical evidence points to the superiority of endocrine therapy given in combination with targeted therapies for young/premenopausal patients with metastatic HR+ breast cancer. The addition of a CDK4/6 inhibitor in the first‐ or second‐line setting is a significant advance in the management of young women with mBC, but endocrine resistance is still observed and the optimal timing and sequencing of these agents have yet to be determined. Accordingly, future research focused on better understanding disease biology and the most effective therapy in premenopausal women with HR+ mBC is needed.
Author Contributions
Conception/design: Ami N. Shah, Otto Metzger, Cynthia Huang Bartlett, Yuan Liu, Xin Huang, Massimo Cristofanilli
Provision of study material or patients: Ami N. Shah, Cynthia Huang Bartlett, Yuan Liu, Xin Huang
Collection and/or assembly of data: Ami N. Shah, Cynthia Huang Bartlett, Yuan Liu, Xin Huang
Data analysis and interpretation: Ami N. Shah, Otto Metzger, Cynthia Huang Bartlett, Yuan Liu, Xin Huang, Massimo Cristofanilli
Manuscript writing: Ami N. Shah, Otto Metzger, Cynthia Huang Bartlett, Yuan Liu, Xin Huang, Massimo Cristofanilli
Final approval of manuscript: Ami N. Shah, Otto Metzger, Cynthia Huang Bartlett, Yuan Liu, Xin Huang, Massimo Cristofanilli
Disclosures
Otto Metzger: Pfizer (RF); Cynthia Huang Bartlett: Pfizer (E); Yuan Liu: Pfizer (E), Pfizer, Novartis (OI); Massimo Cristofanilli: CytoDyn, Novartis, Merus, Genentech (C/A), Pfizer, Eli Lilly and Company (H).
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Acknowledgments
Editorial and medical writing support was provided by Catherine Grillo of Complete Healthcare Communications, LLC (North Wales, PA), a CHC Group company, and was funded by Pfizer.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Fidler MM, Gupta S, Soerjomataram I et al. Cancer incidence and mortality among young adults aged 20‐39 years worldwide in 2012: A population‐based study. Lancet Oncol 2017;18:1579–1589. [DOI] [PubMed] [Google Scholar]
- 2. Johnson RH, Anders CK, Litton JK et al. Breast cancer in adolescents and young adults. Pediatr Blood Cancer 2018;65:e27397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fredholm H, Magnusson K, Lindstrom LS et al. Long‐term outcome in young women with breast cancer: A population‐based study. Breast Cancer Res Treat 2016;160:131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kataoka A, Iwamoto T, Tokunaga E et al. Young adult breast cancer patients have a poor prognosis independent of prognostic clinicopathological factors: A study from the Japanese Breast Cancer Registry. Breast Cancer Res Treat 2016;160:163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Loibl S, Jackisch C, Lederer B et al. Outcome after neoadjuvant chemotherapy in young breast cancer patients: A pooled analysis of individual patient data from eight prospectively randomized controlled trials. Breast Cancer Res Treat 2015;152:377–387. [DOI] [PubMed] [Google Scholar]
- 6. Anders CK, Johnson R, Litton J et al. Breast cancer before age 40 years. Semin Oncol 2009;36:237–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Johnson RH, Chien FL, Bleyer A. Incidence of breast cancer with distant involvement among women in the United States, 1976 to 2009. JAMA 2013;309:800–805. [DOI] [PubMed] [Google Scholar]
- 8. DeSantis CE, Ma J, Jemal A. Trends in stage at diagnosis for young breast cancer patients in the United States. Breast Cancer Res Treat 2019;173:743–747. [DOI] [PubMed] [Google Scholar]
- 9. Shoemaker ML, White MC, Wu M et al. Differences in breast cancer incidence among young women aged 20‐49 years by stage and tumor characteristics, age, race, and ethnicity, 2004‐2013. Breast Cancer Res Treat 2018;169:595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bellanger M, Zeinomar N, Tehranifar P et al. Are global breast cancer incidence and mortality patterns related to country‐specific economic development and prevention strategies? J Glob Oncol 2018;4:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Villarreal‐Garza C, Aguila C, Magallanes‐Hoyos MC et al. Breast cancer in young women in Latin America: An unmet, growing burden. The Oncologist 2013;18:1298–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Neven P, Rugo H, Tolaney S et al. Abemaciclib for pre/perimenopausal women with HR+, HER2– advanced breast cancer [abstract 1002]. Presented at: 2018 ASCO Annual Meeting; June 3, 2018; Chicago, IL.
- 13. Kan Z, Ding Y, Kim J et al. Multi‐omics profiling of younger Asian breast cancers reveals distinctive molecular signatures. Nat Commun 2018;9:1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang K, Ren Y, Li H et al. Comparison of clinicopathological features and treatments between young (</=40 years) and older (>40 years) female breast cancer patients in West China: A retrospective, epidemiological, multicenter, case only study. PLoS One 2016;11:e0152312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fredholm H, Eaker S, Frisell J et al. Breast cancer in young women: Poor survival despite intensive treatment. PLoS One 2009;4:e7695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Anders CK, Hsu DS, Broadwater G et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol 2008;26:3324–3330. [DOI] [PubMed] [Google Scholar]
- 17. Gnerlich JL, Deshpande AD, Jeffe DB et al. Elevated breast cancer mortality in women younger than age 40 years compared with older women is attributed to poorer survival in early‐stage disease. J Am Coll Surg 2009;208:341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kolečková M, Kolář Z, Ehrmann J et al. Age‐associated prognostic and predictive biomarkers in patients with breast cancer. Oncol Lett 2017;13:4201–4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Haque R, Ahmed SA, Inzhakova G et al. Impact of breast cancer subtypes and treatment on survival: An analysis spanning two decades. Cancer Epidemiol Biomarkers Prev 2012;21:1848–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cancer Genome Atlas Network . Comprehensive molecular portraits of human breast tumours. Nature 2012;490:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bardia A, Hurvitz S. Targeted therapy for premenopausal women with HR(+), HER2(‐) advanced breast cancer: Focus on special considerations and latest advances. Clin Cancer Res 2018;24:5206–5218. [DOI] [PubMed] [Google Scholar]
- 22. Eroles P, Bosch A, Perez‐Fidalgo JA et al. Molecular biology in breast cancer: Intrinsic subtypes and signaling pathways. Cancer Treat Rev 2012;38:698–707. [DOI] [PubMed] [Google Scholar]
- 23. Azim HA Jr, Nguyen B, Brohée S et al. Genomic aberrations in young and elderly breast cancer patients. BMC Med 2015;13:266–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sweeney C, Bernard PS, Factor RE et al. Intrinsic subtypes from PAM50 gene expression assay in a population‐based breast cancer cohort: Differences by age, race, and tumor characteristics. Cancer Epidemiol Biomarkers Prev 2014;23:714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jézéquel P, Sharif Z, Lasla H et al. Gene‐expression signature functional annotation of breast cancer tumours in function of age. BMC Med Genomics 2015;8:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Partridge AH, Rumble RB, Carey LA et al. Chemotherapy and targeted therapy for women with human epidermal growth factor receptor 2‐negative (or unknown) advanced breast cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2014;32:3307–3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Paluch‐Shimon S, Pagani O, Partridge AH et al. ESO‐ESMO 3rd International Consensus Guidelines for Breast Cancer in Young Women (BCY3). Breast 2017;35:203–217. [DOI] [PubMed] [Google Scholar]
- 28. Cardoso F, Senkus E, Costa A et al. 4th ESO–ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 4). Ann Oncol 2018;29:1634–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Loibl S, Turner NC, Ro J et al. Palbociclib combined with fulvestrant in premenopausal women with advanced breast cancer and prior progression on endocrine therapy: PALOMA‐3 results. The Oncologist 2017;22:1028‐1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sledge GW Jr, Toi M, Neven P et al. MONARCH 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2‐ advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol 2017;35:2875–2884. [DOI] [PubMed] [Google Scholar]
- 31. Andre F, Filleron T, Ng C et al. Abstract GS1‐08: Genomic characterisation of metastatic breast cancer. Cancer Res 2019;79:GS1–08. [Google Scholar]
- 32. Zehir A, Benayed R, Shah RH et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med 2017;23:703–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Razavi P, Chang MT, Xu G et al. The genomic landscape of endocrine‐resistant advanced breast cancers. Cancer Cell 2018;34:427–438 e426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Parry M. Introducing the Metastatic Breast Cancer Project: A novel patient‐partnered initiative to accelerate understanding of MBC. ESMO Open 2018;3:e000452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cerami E, Gao J, Dogrusoz U et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov 2012;2:401–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gao J, Aksoy BA, Dogrusoz U et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 2013;6:pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lu Y‐S, Hurvitz SA, Su F et al. In‐depth gene expression analysis of premenopausal patients with HR+/HER2− advanced breast cancer (ABC) treated with ribociclib‐containing therapy in the phase III MONALEESA‐7 trial. Presented at: American Society of Clinical Oncology (ASCO) Annual Meeting; May 31–June 4, 2019; Chicago, IL.
- 38. Bardia A, Colleoni M, Campos‐Gomez S et al. Abstract PD2‐08: Ribociclib with endocrine therapy for premenopausal patients with hormone receptor‐positive, HER2‐negative advanced breast cancer: Biomarker analyses from the phase III randomized MONALEESA‐7 trial. Cancer Res 2019;79:PD2–08. [Google Scholar]
- 39. National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Breast Cancer Version 1.2019. Plymouth Meeting, PA: National Comprehensive Cancer Network; 2019.
- 40. PIQRAY (alpelisib) . Prescribing Information. Novartis Pharmaceuticals Corporation; East Hanover, NJ, 2019.
- 41. National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Breast Cancer Version 2.2019. Plymouth Meeting, PA: National Comprehensive Cancer Network; July 2, 2019.
- 42. Nur Husna SM, Tan H‐TT, Mohamud R et al. Inhibitors targeting CDK4/6, PARP and PI3K in breast cancer: A review. Ther Adv Med Oncol 2018;10:1758835918808509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cristofanilli M, Turner NC, Bondarenko I et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone‐receptor‐positive, HER2‐negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA‐3): Final analysis of the multicentre, double‐blind, phase 3 randomised controlled trial. Lancet Oncol 2016;17:425–439. [DOI] [PubMed] [Google Scholar]
- 44. Turner NC, Ro J, Andre F et al. Palbociclib in hormone‐receptor‐positive advanced breast cancer. N Engl J Med 2015;373:209–219. [DOI] [PubMed] [Google Scholar]
- 45. Turner NC, Slamon DJ, Ro J et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med 2018;379:1926–1936. [DOI] [PubMed] [Google Scholar]
- 46. Tripathy D, Im SA, Colleoni M et al. Ribociclib plus endocrine therapy for premenopausal women with hormone‐receptor‐positive, advanced breast cancer (MONALEESA‐7): A randomised phase 3 trial. Lancet Oncol 2018;19:904–915. [DOI] [PubMed] [Google Scholar]
- 47. Park YH, Kim TY, Kim GM et al. A randomized phase II study of palbociclib plus exemestane with GNRH agonist versus capecitabine in premenopausal women with hormone receptor‐positive metastatic breast cancer (KCSG‐BR 15‐10, NCT02592746). Presented at: 2019 ASCO Annual Meeting; May 31–June 4, 2019; Chicago, IL.
- 48. Sledge GW Jr, Toi M, Neven P et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor‐positive, ERBB2‐negative breast cancer that progressed on endocrine therapy‐MONARCH 2: A randomized clinical trial. JAMA Oncol 2019;6:116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Im SA, Lu YS, Bardia A et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med 2019;381:307–316. [DOI] [PubMed] [Google Scholar]
- 50. Beck JT, Neven P, Esteva FJ et al. Abstract P6‐18‐14: Patient‐reported outcomes with ribociclib‐based therapy in hormone receptor‐positive, HER2‐negative advanced breast cancer: Results from the phase III MONALEESA‐2, ‐3, and ‐7 trials. Cancer Res 2019;79:P6‐18‐14. [Google Scholar]
- 51. Robson M, Im SA, Senkus E et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med 2017;377:523–533. [DOI] [PubMed] [Google Scholar]
- 52. Litton JK, Rugo HS, Ettl J et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med 2018;379:753–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Copson ER, Maishman TC, Tapper WJ et al. Germline BRCA mutation and outcome in young‐onset breast cancer (POSH): A prospective cohort study. Lancet Oncol 2018;19:169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Robson ME, Tung N, Conte P et al. OlympiAD final overall survival and tolerability results: Olaparib versus chemotherapy treatment of physician's choice in patients with a germline BRCA mutation and HER2‐negative metastatic breast cancer. Ann Oncol 2019;30:558–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rugo HS, Ettl J, Woodward NE et al. EMBRACA: Efficacy outcomes in clinically relevant subgroups comparing talazoparib (TALA), an oral poly ADP ribose polymerase (PARP) inhibitor, to physician's choice of therapy (PCT) in patients with advanced breast cancer and a germline BRCA mutation. J Clin Oncol 2018;36:1069–1069. [Google Scholar]
- 56. Poggio F, Bruzzone M, Ceppi M et al. Single‐agent PARP inhibitors for the treatment of patients with BRCA‐mutated HER2‐negative metastatic breast cancer: A systematic review and meta‐analysis. ESMO Open 2018;3:e000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Paradiso A, Andreopoulou E, Conte P et al. PARP inhibitors in breast cancer: Why, how, and when. Breast Care 2018;13:216–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Morrow PK, Broxson AC, Munsell MF et al. Effect of age and race on quality of life in young breast cancer survivors. Clin Breast Cancer 2014;14:e21–e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Saha P, Regan MM, Pagani O et al. Treatment efficacy, adherence, and quality of life among women younger than 35 years in the International Breast Cancer Study Group TEXT and SOFT Adjuvant Endocrine Therapy Trials. J Clin Oncol 2017;35:3113–3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Adolescent and Young Adult (AYA) Oncology, Version 2.2019. Plymouth Meeting, PA: 2019. Available at https://www.nccn.org/professionals/physician_gls/pdf/aya.pdf. Accessed July 9, 2019.
- 61. Rosenzweig M, West M, Matthews J et al. Financial toxicity among women with metastatic breast cancer. Oncol Nurs Forum 2019;46:83–91. [DOI] [PubMed] [Google Scholar]
- 62. Dalal AA, Gagnon‐Sanschagrin P, Burne R et al. Dosing patterns and economic burden of palbociclib drug wastage in HR+/HER2‐ metastatic breast cancer. Adv Ther 2018;35:768–778. [DOI] [PubMed] [Google Scholar]
- 63. Suter MB, Pagani O. Should age impact breast cancer management in young women? Fine tuning of treatment guidelines. Ther Adv Med Oncol 2018;10:1758835918776923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Dalal AA, Gauthier G, Gagnon‐Sanschagrin P et al. Treatment and monitoring patterns among premenopausal women with HR+/HER2‐ advanced breast cancer. Adv Ther 2018;35:1356–1367. [DOI] [PubMed] [Google Scholar]
- 65. Hartkopf AD, Huober J, Volz B et al. Treatment landscape of advanced breast cancer patients with hormone receptor positive HER2 negative tumors ‐ Data from the German PRAEGNANT breast cancer registry. Breast 2018;37:42–51. [DOI] [PubMed] [Google Scholar]
- 66. Lobbezoo DJ, van Kampen RJ, Voogd AC et al. In real life, one‐quarter of patients with hormone receptor positive metastatic breast cancer receive chemotherapy as initial palliative therapy. A study of the Southeast Netherlands Breast Cancer Consortium. Ann Oncol 2016;27:256–262. [DOI] [PubMed] [Google Scholar]
- 67. Taylor‐Stokes G, Mitra D, Waller J et al. Treatment patterns and clinical outcomes among patients receiving palbociclib in combination with an aromatase inhibitor or fulvestrant for HR+/HER2‐negative advanced/metastatic breast cancer in real‐world settings in the US: Results from the IRIS study. Breast 2019;43:22–27. [DOI] [PubMed] [Google Scholar]
- 68. Burstein HJ, Mayer EL, DeMichele A et al. Abstract P3‐11‐01: Treatment patterns for young women with HR+/HER2‐ metastatic breast cancer in the United States in the era of CDK 4/6 inhibitors. Cancer Res 2018;78:P3‐11‐01. [Google Scholar]
- 69. Dalal AA, Goldschmidt D, Romdhani H et al. Abstract P6‐18‐38: Treatment patterns and sequences among pre‐menopausal women with HR+/HER2‐ metastatic breast cancer: A chart review study. Cancer Res 2019;79:P6‐18‐38. [Google Scholar]


