Abstract
Lessons Learned
Hyperfractionation of lutetium‐177 (177Lu)‐J591 for patients with metastatic castration‐resistant prostate cancer did not appear to have any additional advantage over the single dose 177Lu‐J591 or fractionated two‐dose 177Lu‐J591 therapy.
Definite conclusions were challenging because of the small sample size of this study, and so further studies are needed to evaluate the viability of the hypothesis.
On the basis of available data, a registration study of 177Lu‐J591 (also known as TLX591) is planned and will use the two‐dose fractionation schedule (Telix Pharma Q3 2019 update https://telixpharma.com/news-media/).
Background
Phase I and II single‐dose studies of lutetium‐177 (177Lu)‐J591, a radio‐labeled antibody binding prostate‐specific membrane antigen (PSMA), demonstrated safety and efficacy with dose response. Modest dose fractionation of 177Lu‐J591 (2 doses) has less myelosuppression per similar cumulative dose, allowing higher doses to be administered safely. We hypothesized that additional dose fractionation would allow a higher cumulative dose, potentially with less toxicity and more efficacy.
Methods
Men with progressive metastatic castration‐resistant prostate cancer and adequate organ function were enrolled. 177Lu‐J591 was administered at 25 mCi/m2 every 2 weeks until the emergence of related grade 2 toxicity. 177Lu‐J591 imaging was performed and circulating tumor cell (CTC) counts were measured before and after treatment along with standard monitoring.
Results
Six subjects in a single cohort, with a median age of 68.6 years, were enrolled. Patients received three to six doses (cumulative 75−150 mCi/m2). Two (33%) patients had >30% prostate‐specific antigen (PSA) decline and three (50%) had CTC count decline. Two (33%) experienced grade (Gr) 4 neutropenia (without fever), three (50%) had Gr 4 thrombocytopenia (without hemorrhage), and two (33%) required platelet transfusions. Following hematological improvement, two patients developed worsening cytopenia during prostate cancer progression; bone marrow biopsies revealed infiltrative tumor replacing normal marrow elements without myelodysplasia. Targeting of known disease sites was seen on planar imaging in all.
Conclusion
Hyperfractionation of 177Lu‐J591 is feasible but does not seem to have significant advantages over the two‐dose fractionation regimen.
Discussion
Prostate cancer is radiosensitive and generally has high expression of PSMA 1 J591 is a deimmunized monoclonal antibody that binds with high specificity, sensitivity, and affinity to PSMA, followed by rapid internalization 2. Phase I and phase II studies have established that 177Lu‐J591 can lead to significant, measurable disease and PSA response when administered as a single dose (recommended phase II dose 70 mCi/m2) in patients with metastatic castration‐resistant prostate cancer (mCRPC) 3, 4. A dose‐response relationship was observed, with further dose escalation limited by reversible myelosuppression. Subsequently, a phase I/II study provided evidence that administering treatment in two divided doses allowed higher cumulative radiation activity with less or comparable treatment‐related toxicity compared with single‐dose administration 5. It stood to reason that further dose fractionation should be explored for maximizing tumor response and minimizing toxicity in a dose‐dependent manner. This pilot study examined the feasibility of hyperfractionated 177Lu‐J591 therapy in six patients with mCRPC. A total of 83% of these patients were previously treated with abiraterone, 50% with enzalutamide, 50% with docetaxel, 33% with cabazitaxel, 33% with sipuleucel‐T, and 17% with investigational agents. A total of 83% had unfavorable CTC count (≥5), most (67%) were in a high CALGB (Halabi) prognostic risk category, and two patients had visceral metastasis.
Patients enrolled in this single cohort study received a “hyperfractionated” dosing schedule (25 mCi/m2 biweekly until greater than grade 2 toxicity) and received a higher cumulative radiation dose than can be administered as a single dose, ranging from 75 to 150 mCi/m2. In previous single‐dose studies, 40% of patients receiving 70 mCi/m2 required a platelet transfusion, whereas in the fractionated‐dose study with cumulative doses of 80 mCi/m2 and 90 mCi/m2 administered, prophylactic platelet transfusion rates of 25% and 65% were seen, respectively 4, 5. In the current hyperfractionated regimen, doses were administered until a greater than grade 2 myelosuppression occurred. As expected, myelosuppression was the most common adverse event, with two patients receiving two platelet transfusions. Two patients had partial blood count recovery, followed by worsening cytopenia coinciding with prostate cancer progression. Bone marrow biopsies revealed infiltrative tumor replacing normal marrow elements without myelodysplasia as previously observed 6. Overall rates of toxicity in patients receiving hyperfractionated therapy (displayed in Table 1) were not significantly different from single‐dose or fractionated two‐dose therapy regimens.
A single 70 mCi/m2 dose of 177Lu‐J591 resulted in ≥30% PSA decline in 47% of patients 4. When administered in two divided doses, 90 mCi/m2 resulted in >30% PSA decline in 59% of patients 5. With hyperfractionated therapy, no significant improvement was seen in overall response rates compared with those seen in patients receiving single‐dose or fractionated two‐dose therapy 4, 5. A small study sample size and multiple lines of treatment prior to enrollment could explain these findings. No selection for PSMA expression was employed, although as previously seen, accurate targeting of 177Lu‐J591 at known sites of disease was seen in all patients as seen in Figure 1. Given the lack of improvement in efficacy or toxicity profile in this pilot study, we recommend single doses of 70 mg/m2 or two fractionated doses of 80–90 mg/m2 (cumulative) for 177Lu‐J591.
Figure 1.
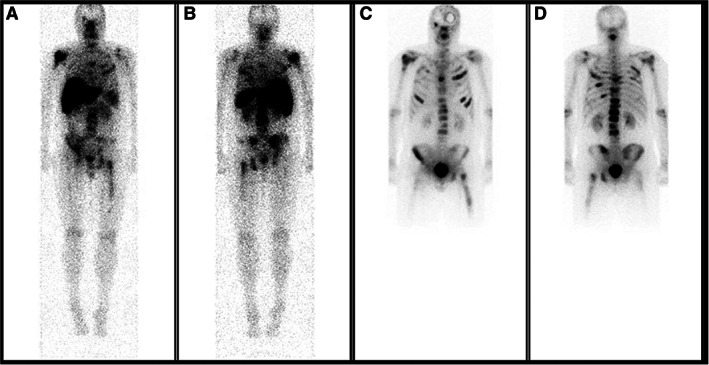
Whole body planar scan on day 7 after 177Lu‐J591 (A, B) of a 50‐year‐old man with metastatic castration‐resistant prostate cancer showing the accurate targeting of know disease sites, as seen on the pretreatment bone scan (C, D).
Trial Information
| Disease | Prostate cancer |
| Stage of Disease/Treatment | Metastatic/advanced |
| Prior Therapy | No designated number of regimens |
| Type of study – 1 | Phase I |
| Type of study – 2 | Pilot Study |
| Primary Endpoint | Maximum Tolerated Dose |
| Secondary Endpoint | Safety |
| Secondary Endpoint | Efficacy |
| Additional Details of Endpoints or Study Design | |
| Adult patients with progressive mCRPC were eligible for this proof of concept study. Two to six eligible subjects were planned to be enrolled. Should one of the initial three subjects or more than two subjects within the initial six subjects enrolled experience a dose‐limiting toxicity, further enrollment would be halted. The total planned enrollment for this pilot study was therefore two to six subjects without plans for formal hypothesis testing. If the hyperfractionated regimen appeared to be safe with a potential advantage over the two‐dose regimen, an additional prospective trial would be planned. Any type or number of lines of previous therapies were allowed except systemic beta‐emitting bone therapy. Additional entry criteria included ECOG performance status 0–2, absolute neutrophil count >2,000/mm3, platelet count ≥150,000/mm3, serum bilirubin <1.5× upper limit of normal (ULN), aspartate aminotransferase <2× ULN, and serum creatinine <2.5 mg/dL. This registered study (NCT00538668) was approved by the Weill Cornell Medicine Institutional Review Board. All subjects provided written informed consent. | |
| 177Lu‐J591 was administered at 25 mCi/m2 every 2 weeks until the emergence of grade >2 myelosuppression (CTCAE v4). No patient selection based on PSMA expression was performed, with planar imaging of 177Lu‐J591 at 6−8 days following the first (and optionally after fourth) dose. Radiolabeled J591 images were compared with baseline bone scintigraphy and cross‐sectional imaging; semiquantitative PSMA expression analysis was performed with a 5‐point visual score relative to background and liver uptake as previously published 4, 5. Complete blood counts were monitored at least weekly for 8 weeks. Chemistry and PSA were monitored at least every 4 weeks with computed tomography and bone scan repeated every 12 weeks. Circulating tumor cell (CTC) count (CellSearch) was assessed at baseline and at 4–6 weeks. | |
| Investigator's Analysis | Drug tolerable, efficacy indeterminant |
Drug Information
| Drug 1 | |
| Generic/Working Name | 177Lu‐J591 |
| Trade Name | None |
| Company Name | Weill Cornell Medical College |
| Drug Type | Antibody |
| Drug Class | Immune therapy |
| Dose | 25 mCi m2 |
| Route | IV |
| Schedule of Administration | 25 mCi/m2 every 2 weeks until the emergence of attributable at least grade 2 toxicity |
Patient Characteristics
| Number of Patients, Male | 6 |
| Age | Median: 68.6 years |
| Number of prior systemic therapies | Median (range): 2.5 (1–6) |
| Performance Status: ECOG |
0 — 0 1 — 4 2 — 2 3 — 0 Unknown — 0 |
| Cancer Types or Histologic Subtypes | Adenocarcinoma of prostate |
Adverse Events
| All Cycles | |||||||
|---|---|---|---|---|---|---|---|
| Name | NC/NA | 1 | 2 | 3 | 4 | 5 | All Grades |
| Anorexia | 33% | 50% | 17% | 0% | 0% | 0% | 67% |
| Arthralgia | 83% | 17% | 0% | 0% | 0% | 0% | 17% |
| Aspartate aminotransferase increased | 83% | 17% | 0% | 0% | 0% | 0% | 17% |
| Bruising | 83% | 17% | 0% | 0% | 0% | 0% | 17% |
| Creatinine increased | 83% | 17% | 0% | 0% | 0% | 0% | 17% |
| Cough | 83% | 17% | 0% | 0% | 0% | 0% | 17% |
| Generalized muscle weakness | 83% | 17% | 0% | 0% | 0% | 0% | 17% |
| Hyperkalemia | 83% | 0% | 17% | 0% | 0% | 0% | 17% |
| Depression | 83% | 17% | 0% | 0% | 0% | 0% | 17% |
| Anemia | 16% | 67% | 0% | 17% | 0% | 0% | 84% |
| Diarrhea | 83% | 17% | 0% | 0% | 0% | 0% | 17% |
| Dyspnea | 67% | 33% | 0% | 0% | 0% | 0% | 33% |
| Nausea | 67% | 33% | 0% | 0% | 0% | 0% | 33% |
| Edema limbs | 67% | 33% | 0% | 0% | 0% | 0% | 33% |
| White blood cell decreased | 0% | 33% | 0% | 17% | 50% | 0% | 100% |
| Dysgeusia | 83% | 17% | 0% | 0% | 0% | 0% | 17% |
| Fracture | 83% | 17% | 0% | 0% | 0% | 0% | 17% |
| Weight loss | 50% | 50% | 0% | 0% | 0% | 0% | 50% |
| Rash maculopapular | 83% | 17% | 0% | 0% | 0% | 0% | 17% |
| Anxiety | 83% | 17% | 0% | 0% | 0% | 0% | 17% |
| Hypocalcemia | 83% | 0% | 17% | 0% | 0% | 0% | 17% |
| Lymphocyte count decreased | 16% | 0% | 0% | 67% | 17% | 0% | 84% |
| Fatigue | 50% | 50% | 0% | 0% | 0% | 0% | 50% |
| Platelet count decreased | 0% | 17% | 0% | 33% | 50% | 0% | 100% |
| Neutrophil count decreased | 50% | 0% | 0% | 17% | 33% | 0% | 50% |
| Back pain | 66% | 17% | 17% | 0% | 0% | 0% | 34% |
| Bone pain | 67% | 33% | 0% | 0% | 0% | 0% | 33% |
| Myalgia | 83% | 17% | 0% | 0% | 0% | 0% | 17% |
| Dry mouth | 83% | 17% | 0% | 0% | 0% | 0% | 17% |
| Chest wall pain | 83% | 17% | 0% | 0% | 0% | 0% | 17% |
Abbreviation: NC/NA, no change from baseline/no adverse event.
Assessment, Analysis, and Discussion
| Completion | Study completed |
| Investigator's Assessment | Drug tolerable, efficacy indeterminant |
Prostate cancer is the most common noncutaneous solid tumor in men. To date, prostate‐specific membrane antigen (PSMA) is the most widely recognized target for targeted radionuclide therapy for prostate cancer; it is overexpressed on approximately 90% of metastatic castration‐resistant prostate cancer (mCRPC) tumors and internalizes after binding of the therapeutic agent 1, 7, 8, 9. J591 is a deimmunized monoclonal antibody, which specifically binds with high affinity to the extracellular domain of PSMA, followed by rapid internalization of the complex 8, 9.
After phase I and II single‐dose lutetium‐177 (177Lu)‐J591 studies demonstrated safety and efficacy 3, 4 a phase I/II study of fractionated two‐dose 177Lu‐J591 demonstrated that higher cumulative doses can be administered with an improved dose‐response and less toxicity compared with similar single‐dose therapy 5. Based upon these data, the idea of further dose fractionation of 177Lu‐J591 was proposed. Adult patients with progressive mCRPC were eligible for this proof of concept study. Any type or number of lines of previous therapies were allowed except systemic beta‐emitting bone therapy. Lu‐J591 was administered at 25 mCi/m2 every 2 weeks until the emergence of grade >2 myelosuppression (CTCAE v4). No patient selection based on PSMA expression was performed.
Six men with progressive metastatic CRPC were treated between November 2014 and October 2015. In total, four patients received three doses each, one patient five doses, and one patient received six doses, with a cumulative dose of radioactivity ranging from 75 to 150 mCi/m2. As expected based upon the study design plus the known myelotoxic effects of radioimmunotherapy in general, myelosuppression was the most commonly observed adverse event in this study: three patients (50%) experienced grade (Gr) 3 or 4 neutropenia (without fever) and five patients (83%) had Gr 3 or 4 thrombocytopenia (without hemorrhage), two (33%) of whom received two prophylactic platelet transfusions each. Two patients developed worsening cytopenia following partial bone marrow function recovery. In both patients, bone marrow biopsies revealed disease progression with prostate cancer replacing normal marrow elements but no bone marrow dysplasia as observed in prior studies 6. There were no grade 3–5 nonhematologic toxicities, and overall toxicity profile in these patients was similar to the recommended phase II dose of single‐dose or fractionated two‐dose therapy 4, 5.
Two patients experienced decline in prostate‐specific antigen (PSA) from baseline, one with 74% and the other with 24% decline in PSA, whereas the others had an increase in PSA level. With small numbers, there was no apparent difference in response based upon prognostic category nor by PSMA visual scores. Overall with hyperfractionated therapy, no significant improvement was seen in response rates compared with those seen in patients receiving single‐dose or fractionated two‐dose therapy 4, 5, although accurate targeting of 177Lu‐J591 at known sites of disease was seen in all patients in this unselected patient population. Aside from the methodology of dosing until toxicity occurred, a possible reason for why hyperfractionated low dose was not safer might have been the phenomenon of exposing recovering bone marrow (i.e., stem cells) to radioactivity during recovery from earlier doses. Responses were not observed to be cumulative dose related, in contrast to previous studies, although this analysis is clearly limited by small sample size 4, 5. All six patients had detectable baseline CTC count, with 5 of 6 having unfavorable CTC count (>5). Of the five patients with baseline unfavorable CTC count, one converted to favorable, one decreased, two went up, and one could not be assessed because of insufficient blood sample. Of the four patients with measurable disease at baseline, one (25%) had stable disease and three (26.1%) had progressive disease according to RECIST 1.1.
Given the small sample size of this study, it is difficult to make sweeping conclusions about the overall efficacy of hyperfractionated radioimmunotherapy. Furthermore, the studied patients are relatively heterogeneous in terms of their baseline pretreatment characteristics. Still, further studies to determine the optimal target population and dosing regimen required to maximize tumor response and minimize toxicity are warranted, and various approaches are being tried to achieve this 10. In addition to randomized trials utilizing repetitive doses of 177Lu‐labeled PSMA ligand 6 weeks apart (VISION, NCT03511664 and TheraP, NCT03392428), fractionated dosing of 177Lu‐PSMA‐617 is also being studied 10, 11.
Disclosures
David M. Nanus: DSMB Roche Genentech (other: phone consultations); Neil H. Bander: Weill Cornell Medicine (IP), BZL Biologics, LLC, Telix Pharmaceuticals, LTD (C/A, OI); Scott T. Tagawa: Astellas, Bayer, Tolmar, Medivation, Janssen, Amgen, Pfizer, Dendreon, Abbvie, Sanofi, Clovis, Genentech, Endocyte, Immunomedics, Karyopharm, QED (C/A), Janssen, Millennium, Merck, Clovis, Bayer, Abbvie, Medivation, BMS, AstraZeneca, Aveo, Exelixis, Newlink, Immunomedics, Dendreon, Genentech, Rexahn, Inovio, Endocyte, Sanofi, Karyopharm, Lilly, Astellas, Amgen, Novrtis, Boehinger Ingelheim (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Table
Table 1.
Adverse events
| CTCAE toxicity | Grade 1–2 | Grade 3 | Grade 4 | Total |
|---|---|---|---|---|
| Nonhematologic, n (%) | ||||
| Anorexia | 4 (66.67) | 4 (66.67) | ||
| Ankle fracture | 1 (16.67) | 1 (16.67) | ||
| Anxiety | 1 (16.67) | 1 (16.67) | ||
| Arthralgia | 1 (16.67) | 1 (16.67) | ||
| AST (SGOT) | 1 (16.67) | 1 (16.67) | ||
| Bruising | 1 (16.67) | 1 (16.67) | ||
| Chest wall pain | 1 (16.67) | 1 (16.67) | ||
| Depression | 1 (16.67) | 1 (16.67) | ||
| Diarrhea | 1 (16.67) | 1 (16.67) | ||
| Dry mouth | 1 (16.67) | 1 (16.67) | ||
| Dysgeusia | 1 (16.67) | 1 (16.67) | ||
| Dyspnea | 2 (33.3) | 2 (33.3) | ||
| Edema: limb | 2 (33.3) | 2 (33.3) | ||
| Fatigue | 3 (50) | 3 (50) | ||
| Fall | 1 (16.67) | 1 (16.67) | ||
| Generalized muscle weakness | 1 (16.67) | 1 (16.67) | ||
| Hyperkalemia | 1 (16.67) | 1 (16.67) | ||
| Hypocalcemia | 1 (16.67) | 1 (16.67) | ||
| Increased creatinine | 1 (16.67) | 1 (16.67) | ||
| Nausea | 2 (33.3) | 2 (33.3) | ||
| Productive cough | 1 (16.67) | 1 (16.67) | ||
| Pain: bone | 1 (16.67) | 1 (16.67) | ||
| Pain: joint | 1 (16.67) | 1 (16.67) | ||
| Pain: back | 2 (33.3) | 2 (33.3) | ||
| Rash maculopapular | 1 (16.67) | 1 (16.67) | ||
| Weight loss | 3 (50) | 3 (50) | ||
| Hematologic, n (%) | ||||
| Anemia | 4 (66.67) | 1 (33.33) | 5 (83.33) | |
| Decreased leukocytes | 2 (33.3) | 1 (16.67) | 3 (50) | 6 (100) |
| Decreased lymphocytes | 4 (66.67) | 1 (16.67) | 5 (83.33) | |
| Decreased neutrophils (ANC) | 1 (16.67) | 2 (33.3) | 3 (50) | |
| Decreased platelets | 1 (16.67) | 2 (33.3) | 3 (50) | 6 (100) |
Abbreviations: ANC, absolute neutrophil count; AST, aspartate aminotransferase; SGOT, serum glutamic‐oxaloacetic transaminase.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Footnotes
- ClinicalTrials.gov Identifier: NCT00538668
- Sponsors: Department of Defense (W81XWH‐04‐1‐0267), Prostate Cancer Foundation, National Institutes of Health (ULI RR024996, 1‐KL2‐RR024997‐01, PTBF5405), David H. Koch Foundation, Robert Dow Foundation, Lawrence and Carol Zicklin Charitable Trust
- Principal Investigator: Scott T. Tagawa
- IRB Approved: Yes
Contributor Information
Muhammad Junaid Niaz, Email: mjn3001@med.cornell.edu.
Scott T. Tagawa, Email: stt2007@med.cornell.edu.
References
- 1. Bostwick DG, Pacelli A, Blute M et al. Prostate specific membrane antigen expression in prostatic intraepithelial neoplasia and adenocarcinoma: A study of 184 cases. Cancer 1998;82:2256–2261. [DOI] [PubMed] [Google Scholar]
- 2. Bander NH, Nanus DM, Milowsky MI et al. Targeted systemic therapy of prostate cancer with a monoclonal antibody to prostate‐specific membrane antigen. Semin Oncol 2003;30:667–676. [DOI] [PubMed] [Google Scholar]
- 3. Bander NH, Milowsky MI, Nanus DM et al. Phase I trial of 177lutetium‐labeled J591, a monoclonal antibody to prostate‐specific membrane antigen, in patients with androgen‐independent prostate cancer. J Clin Oncol 2005;23:4591–4601. [DOI] [PubMed] [Google Scholar]
- 4. Tagawa ST, Milowsky MI, Morris M et al. Phase II study of lutetium‐177–labeled anti‐prostate‐specific membrane antigen monoclonal antibody J591 for metastatic castration‐resistant prostate cancer. Clin Cancer Res 2013;19:5182–5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tagawa ST, Vallabhajosula S, Christos PJ et al. Phase 1/2 study of fractionated dose lutetium‐177‐labeled anti‐prostate‐specific membrane antigen monoclonal antibody J591 (177Lu‐J591) for metastatic castration‐resistant prostate cancer. Cancer 2019;125:2561–2569. [DOI] [PubMed] [Google Scholar]
- 6. Tagawa S, Akhtar N, Nikolopoulou A et al. Bone marrow recovery and subsequent chemotherapy following radiolabeled anti‐prostate‐specific membrane antigen monoclonal antibody J591 in men with metastatic castration‐resistant prostate cancer. Front Oncol 2013;3:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Silver DA, Pellicer I, Fair WR et al. Prostate‐specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res 1997;3:81–85. [PubMed] [Google Scholar]
- 8. Liu H, Moy P, Kim S et al. Monoclonal antibodies to the extracellular domain of prostate‐specific membrane antigen also react with tumor vascular endothelium. Cancer Res 1997;57:3629–3634. [PubMed] [Google Scholar]
- 9. Liu H, Rajasekaran AK, Moy P et al. Constitutive and antibody‐induced internalization of prostate‐specific membrane antigen. Cancer Res 1998;58:4055–4060. [PubMed] [Google Scholar]
- 10. Miyahira AK, Pienta KJ, Morris MJ et al. Meeting report from the Prostate Cancer Foundation PSMA‐directed radionuclide scientific working group. Prostate 2018;78:775–789. [DOI] [PubMed] [Google Scholar]
- 11. Tagawa ST, Osborne J, Hackett A et al. Preliminary results of a phase I/II dose‐escalation trial of fractionated dose 177Lu‐PSMA‐617 for progressive metastatic castration‐resistant prostate cancer (mCRPC). Ann Oncol 2019;30(suppl 5):849PD. [Google Scholar]


