Short abstract
The FDA has ordered the withdrawal of all ranitidine products from the marketplace based on recent findings of increased and unacceptable levels of N‐nitrosodimethylamine (NDMA). This commentary reviews the sources and properties of NDMA, assesses the dangers it poses, and suggests measures to mitigate contamination.
The causes of cancer are manifold. About one quarter to one third of cancers, depending on the specific tumor and population, are caused by infectious agents, while a smaller fraction can be attributed to genetic predisposition. A larger number (perhaps 50% or more) arise from environmental and behavioral causes, such as smoking, alcohol, dietary factors, obesity, and pollution. In modern society, where innovation through chemistry leads to exposure to a broad range of new chemicals and drugs, chemical carcinogenesis is a concern. Recent announcements of withdrawal of the commonly used medications, ranitidine and valsartan, from the market due to contamination with the carcinogen N‐nitrosodimethylamine (NDMA) have raised questions about the safety of these pharmaceuticals. This commentary will review the sources and properties of NDMA, assess the dangers it poses as a contaminant in foods and medicines, and suggest measures to mitigate contamination by such products.
Nitrosoamines as Carcinogens
The alert about NDMA contamination arose from the discovery of this carcinogen in several members of the sartan class of antihypertensives and similar findings of NDMA in ranitidine and related acid pump inhibitors. NDMA and other nitrosoamines are found ubiquitously in outdoor air, water, and soil in minor amounts. They are formed by the chemical interaction of a substituted (secondary or tertiary) amine and an oxidizing agent, usually a nitrite. Their chemical structure and the relevant reaction sequence are shown in Figure 1. In foods, the nitrosating agent responsible for forming NDMA is usually nitrous anhydride, which arises from a nitrite in acidic aqueous solution, as in the stomach 1. Beer, cured meats such as bacon or sausage, and even water contain nitrosoamines in small amounts. Tobacco (either smoke or smokeless) contains nitrosoamines 2, 3. Many different nitrosoamines have been evaluated for carcinogenic activity, with positive findings in many animal species, as they induce tumors in the liver, kidney, and respiratory tract 4, 5. Three nitrosoamines cause hepatocellular carcinoma (HCC) and other solid tumors in non‐human primates. NDMA, the specific contaminant discovered in the medications, produced cancer in a number of experimental animal species and caused cirrhosis and hyperplastic nodules in monkeys, but not hepatocellular cancer 5, 6, 7. On the basis of this evidence, nitrosoamines, including NDMA, have been classified as probable carcinogens in humans 8.
Figure 1.
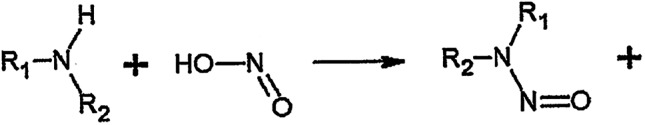
The formation of nitrosoamines. For NDMA R1 and R2 are methyl groups.
The mechanism of nitrosoamine carcinogenicity appears to be through its metabolic activation and covalent interaction with DNA, causing promutagenic DNA adducts. Structural and functional integrity can be restored to damaged DNA by various DNA repair processes, but if these fail or are overwhelmed by high exposures and adducts persist through a cycle of DNA replication, point mutations at critical sites in DNA may result.
The Presence of NDMA in Medications
Estimates suggest that the average intake of the volatile nitrosoamines (including NDMA) from food sources is about 1 microgram per day. The Food and Drug Administration has identified 96 nanograms per day as the upper limit of safe daily ingestion from medicines.
Recent discoveries of NDMA in sartans and ranitidine have raised concerns of a potential risk for people taking these common medications. In 2018, the European Medicines Agency (EMA) called attention to valsartan contaminated by NDMA, as manufactured by Zhejiang Huahai Pharmaceuticals in China, leading to a recall of this medication in European Union (EU) countries 9. In 2018, the FDA announced a voluntary recall of several valsartan products, manufactured by Zhejiang Huahai Pharmaceutical Co. Ltd. in China, and the same product made by Mylan Pharmaceuticals in India 10, 11. Other sartans (candesartan, irbesartan, losartan, and olmesartan 12) were found to contain, or were likely to contain, nitrosoamines, as the sartans all possess a tetrazole ring formed chemically through a nitrite reaction with amines. While manufacturing processes and products are evaluated by the FDA before the final products are accepted for marketing, it is noteworthy that approximately 40% of finished medications in the U.S. are manufactured in overseas facilities, and approximately 80% of ingredients in medications finished in the U.S. come from abroad, principally from China and India, where FDA oversight of quality controls is challenging. The nitrosoamine contaminants in sartans likely arose as a result of a change in the manufacturing process in recent years 12, although formation of the toxic product could also result from contamination during any stage of drug production or use.
Of additional concern, in 2019, the Valisure pharmacy reported that ranitidine (the over‐the‐counter brand Zantac) and a related product, nizatidine, both used to counter gastric hyperacidity and reflux, contained unacceptable amounts of NDMA 13, 14. Their report suggested that spontaneous breakdown of the ranitidine molecule could yield dimethylamine and nitrites, leading to NDMA formation. The amounts of NDMA in ranitidine, as tested by the FDA, if ingested as prescribed on a daily basis, would exceed the 96 nanogram daily limit by as much as ninefold, 860 nanograms 15.
The Risk of NDMA Carcinogenesis
It is difficult to calculate a specific cancer risk related to taking valsartan at the levels of contamination found. The generally acceptable risk for potential carcinogens in pharmaceuticals is one case of cancer per 100,000 subjects. The estimated risk calculated for valsartan ranges from 12 to almost 30 cases per 100,000 subjects, based on the European Medicines Agency assessment for an individual taking 320 mg valsartan, containing 24.1 micrograms NDMA and 3.7 micrograms NDEA per day for 4 years 12. These estimates depend on an accurate accounting of the level of contamination in the available medication over time and the duration of exposure. The risk from NDMA in ranitidine and in its over‐the‐counter version, Zantac, is more problematic and may be greater. In use since 1981, it is the 50th most prescribed medication (>15 million prescriptions annually, plus over‐the‐counter use). The amount of NDMA found in ranitidine by the FDA, while lower than that found by the Valisure pharmacy, still exceeds the allowable daily limit (96 nanograms) by ninefold 15. The actual amount of NDMA ingested by subjects taking ranitidine is still in question, although NMDA excreted in a 24‐hour urine collection test of volunteers taking ranitidine increased 400‐fold compared with baseline measurements 16. An additional factor is the amount of nitrosoamine generated during storage of drug or nitrosoamine formed in gastric fluid, once the drug is internalized and contacts nitrite‐containing foods, e.g., processed meats or nitrate‐containing vegetables such as lettuce, spinach, celery, or beets. Once ingested, nitrates can be converted to nitrites in the mouth or stomach. Thus, the total exposure of people taking ranitidine or nizatidine is not known at this time and may be subject to multiple factors, such as diet and gastric acidity, as well as impurities in the manufactured product and its storage.
Clinical Evidence of Carcinogenesis Due to Contamination of Medications
There is only limited clinical evidence at present suggesting that NDMA actually causes cancer in subjects taking sartans or ranitidine. A survey of 24,000 patients at Memorial Sloan Kettering Cancer Center compared subjects who reported ranitidine use at the time of diagnosis versus those who used other H‐2 blockers or proton pump blockers. Ranitidine use was associated with a significant increase in the odds of presenting with breast, testicular, thyroid, and kidney cancer 17. A negative association was reported for the incidence of colorectal cancer. Of interest is the absence of mention of an association with HCC, the primary tumor type that was found in preclinical carcinogenicity in multiple species. Although the specific organ targeted by a carcinogen may not be congruent across species, including human populations, HCC is an important potential target based on the frequency of this cancer in preclinical NDMA experiments. Definitive epidemiological studies of the association of these medicines with specific cancers in human clearly need to be performed.
Aside from their role as complete carcinogens, the nitrosoamines are likely be co‐factors or promoters in patients with underlying hepatic damage due to alcoholism, hepatitis, or hepatic steatosis. It is notable that the incidence of HCC had been steadily rising in the U.S. in the years from 2000 to 2013, although it has more recently plateaued and then declined with the introduction of antiviral therapy for hepatitis C virus 18.
Are there potential preventative agents or antidotes to nitrosoamine formation or induced DNA damage? Reducing agents such as sodium ascorbate (vitamin C) or sodium erythorbate might prevent or diminish damage in patients taking the drugs in question. Current formulations of ranitidine, including the ranitidine syrup taken by children, do not contain a reducing agent 19, 20.
In conclusion, NDMA contamination poses a potential carcinogenic risk of undetermined effect at present for those taking ranitidine, valsartan, or related medications on a regular basis. It is thus incumbent upon industry and the FDA to take steps to identify and eliminate the sources of contamination of medications with this class of carcinogen. At the same time, pharmaco‐epidemiology studies should be performed to establish if there is excess risk in patients taking these medications.
Disclosures
Bruce Chabner: PharmaMar, EMD Serono, Cyteir (C/A, H), Biomarin, Seattle Genetics, PharmaMar, Loxo, Blueprint, Immunomedics, Constellation (OI), Eli Lilly & Co., Genentech (ET). Richard H. Adamson indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Editor's Note.
On April 1, 2020, the U.S. Food and Drug Administration ordered the withdrawal of all ranitidine (Zantac) products from the commercial market and advised consumers to dispose of any of the product in their possession. This action was based on the finding of increased and unacceptable levels of NDMA in ranitidine stores at high temperature.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Contributor Information
Richard H. Adamson, Email: radamson.tpn@gmail.com.
Bruce A. Chabner, Email: bruce.chabner@theoncologist.com.
References
- 1. Scanlan RA. Formation and occurrence of nitrosamines in food. Cancer Res 1983;43(suppl 5):2435s–2440s. [PubMed] [Google Scholar]
- 2. Agency for Toxic Substance & Disease Registry . Public Health Statement for n‐Nitrosodimethylamine. Available at https://www.atsdr.cdc.gov/PHS/PHS.asp?id=882&tid=173. Accessed January 21, 2015.
- 3. Park JE, Seo JE, Lee JY et al. Distribution of seven N‐nitrosamines in food. Toxicol Res 2015;31:279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Magee PN, Barnes JM. The production of malignant primary hepatic tumors in the rat by feeding dimethylnitrosamine. Br J Cancer 1956;10:114–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Health Organization, International Agency for Research on Cancer . Some N‐nitroso‐compounds In: IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans, Vol. 17 Lyon, France, 1978. [Google Scholar]
- 6. Adamson RH, Sieber SM. Chemical carcinogenesis in non‐human primates In: Longenbach R, Nesnow S, Rice JM, eds. Organ and Species Specificity in Chemical Carcinogenesis. New York and London: Plenum Publishing Corp, 1983:129–156. [Google Scholar]
- 7. Thorgeirsson UP, Dalgard DW, Reeves J et al. Tumor incidence in a chemical carcinogenesis study of nonhuman primates. Regul Toxicol Pharmacol 1994;19:130–151. [DOI] [PubMed] [Google Scholar]
- 8.Agents Classified by the IARC Monographs, Volumes 1–123. Available at https://monographs.iarc.fr/wp‐content/uploads/2018/09/ClassificationsCASOrder.pdf.
- 9. European Medicines Agency . EMA reviewing medicines containing valsartan from Zhejiang Huahai following detection of an impurity: Some valsartan medicines being recalled across the EU. Available at https://www.ema.europa.eu/en/news/ema‐reviewing‐medicines‐containing‐valsartan‐zhejiang‐huahai‐following‐detection‐impurity‐some. Accessed December 10, 2019.
- 10. U.S. Food and Drug Administration . FDA announces voluntary recall of several medicines containing valsartan following detection of an impurity. Available at https://www.fda.gov/news‐events/press‐announcements/fda‐announces‐voluntary‐recall‐several‐medicines‐containing‐valsartan‐following‐detection‐impurity. Accessed Decembr 14, 2019.
- 11. Palmer E. Pfizer Japan drawn into valsartan recall after finding API from Mylan is tainted. Available at https://www.fiercepharma.com/manufacturing/pfizer-japan-finds-impurities-its-valsartan-drugs-made-by-mylan. Accessed December 12, 2019.
- 12. European Medicines Agency . Assessment report EMA/217823/2019. Referral under Article 31 of Directive 2001/83/EC. Angiotensin‐II‐receptor antagonists (sartans) containing a tetrazole group. 2019;1–41.
- 13. Johnson C. A tiny pharmacy raises big doubts about drugs. The Washington Post. November 17, 2019; Section G1 and G4.
- 14. Division of Dockets Management . Valisure citizen petition on ranitidine. September 9, 2019.
- 15. U.S. Food and Drug Administration . Laboratory tests–Ranitidine. Available at https://www.fda.gov/drugs/drug‐safety‐and‐availability/laboratory‐tests‐ranitidine. Accessed January 4, 2020.
- 16. Zeng T, Mich WA. Oral intake of ranitidine increases urinary excretion of N‐nitrosodimethylamine. Carcinogenesis 2016;37:625–634. [DOI] [PubMed] [Google Scholar]
- 17. Braunstein LZ, Kantor ED, Mitch WA et al. Ranitidine use, N‐nitrosodimethylamine (NDMA) production and variations in cancer diagnosis. Under Review.
- 18. Shiels MS, O'Brien TR. Recent decline in hepatocellular carcinoma rates in the United States. Gastroenterology 2020:158:1503–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zantac – FDA prescribing information, side effects and uses. Available at https://www.drugs.com/pro/zantac.html. Accessed December 21, 2019.
- 20. Precision Dose Inc . Ranitidine syrup (ranitidine oral solution, USP). Available at https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=2cd2a198‐36e4‐43d7‐a7b2‐dc40620ad514&type=display. Accessed January 12, 2020.


