Abstract
On March 28, 2019, the Committee for Medicinal Products for Human Use adopted a positive opinion recommending the marketing authorization for the medicinal product plerixafor. The marketing authorization holder for this medicinal product is Genzyme Europe B.Th. The adoption was for an extension of the existing adult indication in combination with granulocyte colony‐stimulating factor (G‐CSF) to pediatric patients (aged 1 year to <18 years) to enhance mobilization of hematopoietic stem cells to the peripheral blood for collection and subsequent autologous transplantation in children with lymphoma or solid malignant tumors. This treatment is indicated either preemptively, when circulating stem cell count on the predicted day of collection after adequate mobilization with G‐CSF (with or without chemotherapy) is expected to be insufficient with regard to desired hematopoietic stem cells yield, or in children who previously failed to collect sufficient hematopoietic stem cells.
The efficacy and safety of plerixafor were evaluated in an open label, multicenter, phase I/II, dose‐ranging, and randomized controlled study (DFI12860) in pediatric patients with solid tumors, including neuroblastoma, sarcoma, Ewing sarcoma, or lymphoma, who were eligible for autologous hematopoietic stem cell transplantation. Forty‐five patients (aged 1 year to <18 years) were randomized, 2:1, using 0.24 mg/kg of plerixafor plus standard mobilization (G‐CSF with or without chemotherapy) versus control (standard mobilization alone). The primary analysis showed that 80% of patients in the plerixafor arm experienced at least a doubling of the peripheral blood (PB) CD34+ count, observed from the morning of the day preceding the first planned apheresis to the morning prior to apheresis, versus 28.6% of patients in the control arm (p = .0019). The median increase in PB CD34+ cell counts from baseline to the day of apheresis was 3.2‐fold in the plerixafor arm versus by 1.4‐fold in the control arm.
The observed safety profile in the pediatric population was consistent with that in adults, with adverse events mainly related to injection site reactions, hypokalemia, and increased blood bicarbonate. Importantly, plerixafor exposure did not seem to negatively affect transplant efficiency. This article summarizes the scientific review of the application leading to regulatory approval in the European Union.
Implications for Practice
This review of the marketing authorization of plerixafor will raise awareness of pediatric indication granted for this medicinal product.
Keywords: Plerixafor (Mozobil), High‐dose chemotherapy, Granulocyte‐colony stimulating factor, Hematopoietic stem cell transplantation, Hematopoietic stem cells
Short abstract
This article addresses development and authorization of novel agents for the treatment of cancer in the pediatric population, summarizing the scientific review of the application leading to regulatory approval of plerixafor in the European Union.
Introduction
In children, single or tandem myeloablative chemotherapy with autologous stem cell support is used during the treatment of refractory lymphoma and solid tumors, such as (but not limited to) medulloblastoma, neuroblastoma, Ewing's sarcoma, and germ cell tumors. Pediatric patients with these relatively chemotherapy‐resistant tumors receive high‐dose chemotherapy (HDC), causing severe and potentially fatal myeloablation that requires stem cell rescue. Autologous hematopoietic stem cell transplantation using hematopoietic stem cells (HSCs) mobilized from the bone marrow and collected by apheresis is a common strategy for repopulation of the bone marrow. Successful hematopoietic stem cell transplant requires the infusion of a sufficient number of hematopoietic stem and progenitor cells (HSPCs) that are capable of homing to the bone marrow cavity and regenerating durable trilineage hematopoiesis in a timely manner.
The majority of HSPCs reside in the bone marrow in a highly organized microenvironment, consisting of marrow stromal cells, osteoblasts, osteoclasts, and other extracellular matrix proteins (e.g., collagens, fibronectins, proteoglycans) 1, 2, 3, 4, 5. HSPCs express a number of cell surface molecules, such as stromal cell–derived factor 1 (SDF‐1)/CXCL12‐CXCR4, implicated in chemotaxis 6, 7, 8, 9, homing 10, 11, 12, and survival and antiapoptosis 13, 14.
Granulocyte‐colony stimulating factor (G‐CSF) is the most frequently used agent for stem cell mobilization. However, the use of G‐CSF alone results in suboptimal stem cell yields in a significant proportion of patients 15.
When additional mobilization fails, patients may need bone marrow harvest or become ineligible for an autologous transplant procedure. Alternatively, patients may have to undergo allogeneic transplantation, which is a more complex procedure with higher morbidity and mortality. Although plerixafor (Mozobil, Genzyme Europe B.Th., Amsterdam, The Netherlands) can be used to enhance mobilization in adults whose HSPCs mobilize poorly, this was not indicated for children, hence the need to extend the indication to the pediatric population. This would potentially allow more pediatric patients to undergo HDC with autologous stem cell support.
Plerixafor is a small‐molecule bicyclam derivative that reversibly antagonizes the CXCR4 chemokine receptor and blocks binding of its cognate ligand, SDF‐1α, also known as CXCL12. The SDF‐1/CXCL12‐CXCR4 interaction has been suggested to be involved in retention of HSCs and hematopoietic progenitor cells (HPCs) within the marrow (Fig. 1) 7, 11. This suggests that antagonizing interactions of marrow produced SDF‐1/CXCL12 with CXCR4 expressed on HSCs and HPCs or that changing the SDF‐1/CXCL12 gradient between marrow and blood might be useful as an HSC/HPC mobilizing strategy 16, 17, 18, 19.
Figure 1.
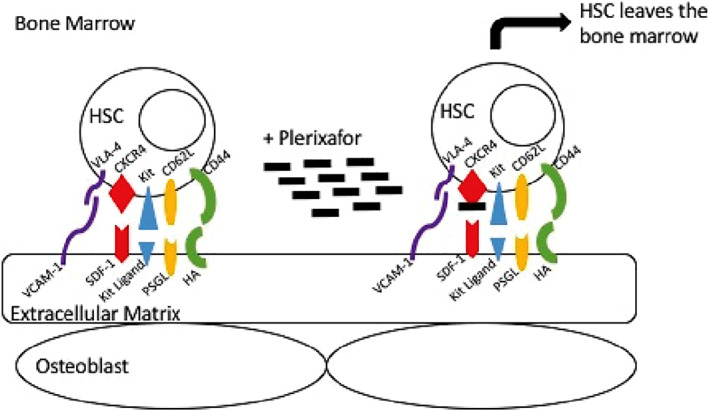
Trafficking of HSCs out of the bone marrow after Plerixafor administration. Plerixafor blocks the interaction between CXCR4 on the HSC and SDF‐1 in the bone marrow extracellular matrix. Source: Trajman, 2016 20. Abbreviations: HA, hyaluronan; HSC, hematopoietic stem cell; PSGL, P‐selectin glycoprotein ligand; SDF‐1, stromal cell–derived factor 1; VCAM‐1, vascular cell adhesion protein 1; VLA‐4, very late antigen 4.
Clinical Efficacy
Although the mechanism of action of plerixafor and the expression of CXCR4 and SDF‐1 is similar between children and adults, direct extrapolation of adult data is not possible as there may be differences in pharmacokinetics and pharmacodynamics between adults and children. Furthermore, the underlying malignancies requiring HDC are different between the adult and pediatric populations, and also the chemotherapy regimen is likely to be different between adults and children. In principle these differences might affect the response to mobilization regimen as well as the marrow recovery and ability for HSC engraftment. To address these issues, the applicant designed a two‐phase study (DFI12860) containing a dose‐ranging part (phase I) followed by a small randomized comparative study (phase II). The design of this study was agreed to by the Pediatric Committee in March 2009. It was an open‐label, comparative study to support the extension of the indication to pediatric patients (aged 1 year to less than 18 years), to be used in combination with G‐CSF to enhance mobilization of hematopoietic stem cells to the peripheral blood for collection and subsequent autologous transplantation in children with lymphoma or solid malignant tumors and who either had low circulating stem cell count on the predicted day of collection after mobilization with G‐CSF (with or without chemotherapy) or who previously failed to collect sufficient HSPCs. After the phase I dose‐ranging part of the clinical study, the company decided to proceed with a dose of 240 μg/kg for phase II. However, upon closer examination it was found that the exposure (area under the curve and maximum serum concentration) depended on both age and weight (increased clearance in younger age group), roughly leading to a 2‐fold lower exposure in the group aged <6 years compared with the group aged 12 to 18 years and a more than 2‐fold lower exposure in the <15 kg weight cohort compared with the >40 kg cohort.
In phase II, patients started on a standard mobilization (G‐CSF ± chemotherapy as per site standard practice). The dose of once daily G‐CSF was to be 10 μg/kg (which could be increased up to a maximum of 15 μg/kg in poor mobilizers). When the trigger point minimum of seven CD34+ cells per microliter in peripheral blood (measured locally) was achieved, patients were randomized two‐to‐one to receive either plerixafor at a dose of 240 μg/kg daily starting on the same day in the evening plus standard mobilization or standard mobilization alone (Fig. 2).
Figure 2.
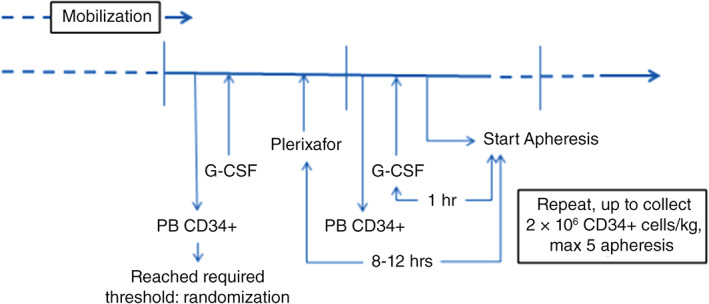
Schematic time schedule for administration of G‐CSF, plerixafor, and apheresis, and respective time for measurement of CD34+ cells in peripheral blood. Abbreviations: G‐CSF, granulocyte‐colony stimulating factor; PB, peripheral blood.
Plerixafor was to be administered as a subcutaneous injection at a separate anatomical site from the patient's standard mobilization treatment. In exceptional circumstances (e.g., significant thrombocytopenia), plerixafor may have been administered via the intravenous route, but only with prior authorization from the sponsor. The patients were to begin apheresis the next day (approximately 1 hour after administration of G‐CSF, 8 to 12 hours after administration of plerixafor). If necessary, treatment with plerixafor and G‐CSF was to be continued according to the same schedule until a yield of at least 2 × 106 CD34+ cells per kilogram was reached, or for a maximum of five aphereses. Apheresis was to occur if the PB CD34+ count on the scheduled day of apheresis was ≥20 cells per microliter.
Primary and Secondary Efficacy Endpoints
The primary efficacy endpoint was successful mobilization defined as doubling of the PB CD34+ count from the morning before the apheresis day to the morning of the apheresis day. The proportion of patients with successful mobilization was significantly greater in the plerixafor plus standard mobilization arm (80%, 24 of 30 patients) compared with the standard mobilization only arm (28.6%, 4 of 14 patients; p = .0019; Table 1).
Table 1.
Summary of efficacy for phase II of trial DFI12860
| Primary analysis of full analysis set (FAS) | Results | |
|---|---|---|
| Standard mobilization alone | Plerixafor + standard mobilization | |
| Descriptive statistics and estimate variability | ||
| Treatment group | ||
| Patients, n | 15 | 30 |
| Successful mobilization, % | 28.6 (8.4–58.1) | 80.0 (61.4–92.3) |
| Difference, % | 51.4 (18.5–84.3), p = .0019 | |
| Effect estimate per comparison | ||
| Secondary endpoint: Days of apheresis required to reach >2 × 106 CD34+ cells/kg | ||
| 1 | 1 | |
| Secondary endpoint: Patients reaching the threshold, % | 92.9 | 89.7 |
| Secondary endpoint: Total CD34+ yield, median, cells/kg | 10.15 × 106 | 9.13 × 106 |
| Secondary endpoint: Patients proceeding to transplant, % | 66.7 | 76.7 |
| Secondary endpoint: Patients successfully engrafting, % | 100 | 100 |
| Secondary endpoint: Patients with durable engraftment at 3, 6, 12, and 24 months after transplant, % | ||
| 3 months | 100 | 91.3 |
| 6 months | 90 | 87.0 |
| 12 months | 80.0 | 87.0 |
| 24 months | 80.0 | 82.6 |
Furthermore, no difference between the arms was noted in the number of patients reaching the threshold of collecting ≥2 × 106 CD34+ cells per kilogram at first apheresis (26 of 29 [89.7%] evaluated patients in the plerixafor arm; 13 of 14 [92.9%] evaluated patients in the control arm). The median number of apheresis days required to collect ≥2 × 106 CD34+ cells per kilogram was identical (1 day) in both treatment arms. One patient in the standard mobilization alone group and three patients in the plerixafor plus standard mobilization group failed to reach 2 × 106 CD34+ cells per kilogram by central laboratory assessment. The percentage of patients proceeding to transplant was numerically higher in the plerixafor arm (23 of 30, 76.7%) than in the control arm (10 of 15, 66.7%), but patients’ numbers were low and there was no apparent difference in reasons for not proceeding to transplant. All patients in each treatment arm who were transplanted (23 in the plerixafor arm and 10 in the standard mobilization arm) successfully engrafted. The summary of durable engraftment at the 3‐, 6‐, 12‐, and 24‐month assessments showed no consistent differences between treatment arms.
Clinical Safety
During phase I, treatment‐emergent adverse events (TEAEs) were reported in 59% of patients, with TEAEs assessed by the investigator as related to study treatment reported in only one patient. The only grade 3/4 TEAEs reported in two or more patients were febrile neutropenia (two patients with grade 3 and one patient with grade 4 TEAEs) and pancytopenia (one patient with grade 3 and one patient with grade 4 TEAEs).
During phase II, TEAEs were reported in 77% of patients in the plerixafor plus standard mobilization arm and in 67% patients in the standard mobilization only arm. TEAEs assessed by the investigator as related to study treatment were reported in four (13.3%) patients in the plerixafor plus standard mobilization arm and none in the standard mobilization alone arm. The events reported were mild (grade 1 in severity) included injection site reactions (two patients, 6.7%), and hypokalemia and increased blood bicarbonate (one patient each, 3.3%). These were considered consistent with the known safety profile of plerixafor.
The observed events were consistent with the known safety profile of plerixafor in adults. Data on survival, relapse rate, hospitalization, and tumor cell mobilization also did not point toward any unexpected safety concerns, and no obvious differences were noted between the two treatment arms in phase II of the study. The observed effect on hematology parameters (i.e., increased neutrophils, decreased platelets) were in line with the intended effects of CD34+ mobilization, with again no major differences between the study arms.
Benefit‐Risk Assessment
As the mechanism of action of plerixafor and the expression of CXCR4 and SDF‐1 is similar between children and adults, a similar response in children as in adults is to be expected. However, direct extrapolation of adult efficacy data based on pharmacokinetics only is not possible as there may be differences between adults and children. This issue was addressed in phase I of the study. Furthermore, as the underlying malignancies requiring HDC are different between adult and pediatric populations, the chemotherapy regimen is also likely to be different. This concerns both the chemotherapy administered before HSC collection and the HDC regimes for which HCS rescue is needed. In principle, these differences might affect the response of the patient to the mobilization regimen as well as marrow recovery and ability for HSC engraftment. This issue was addressed in phase II of the study.
In phase II of the study, doubling of the PB CD34+ count was seen in a larger proportion of the population in the plerixafor arm than in the control arm, and the primary endpoint of the study was met. The percentage of patients who proceeded to transplant was higher than that seen in the control (76.7% vs. 66.7%, respectively), and all were successfully engrafted. Thus, plerixafor exposure did not seem to negatively affect transplant efficiency. Although statistically significant difference is seen in favor of the study arm in term of the proportion of patients with successful mobilization, the number of patients reaching the threshold of collecting ≥2 × 106 CD34+ cells per kilogram was similar in both arms. For both treatment groups the median number of apheresis days needed to collect the threshold number of cells was 1 day.
The total amount of CD34+ cell yield was slightly lower for the plerixafor arm than for the control arm (9.13 × 106 cells/kg vs. 10.15 × 106 cells/kg, respectively), which may, in part, be explained by the slightly lower blood volume which was processed in the plerixafor plus standard mobilization compared with the control arm. Also, the small differences in the percentage of patients who received chemotherapy as part of the standard mobilization regimen (23.3% vs. 33.3%, plerixafor vs. control) and the median total G‐CSF dose (89.59 μg/kg vs. 97.60 μg/kg, plerixafor vs. control) between the study arms may have contributed to this imbalance.
Importantly, the median amount of CD34+ cells at baseline was significantly lower (15 × 106 cells/mL vs. 35 × 106 cells/mL) in the plerixafor plus standard mobilization arm. Thus the absolute increase in PB CD34+ count (also needed to reach the relatively similar CD34+ yield) was higher in the plerixafor plus standard mobilization arm. Also, the relative increase in PB CD34+ counts was higher in the plerixafor plus standard mobilization arm (+220% vs. +39%).
The results of the efficacy endpoints demonstrate that the pharmacodynamic effect of plerixafor added to a standard mobilization regime in the pediatric population showed an additional increase (absolute and relative) in the amount of circulating CD34+ cells at the day of apheresis when compared with a standard mobilization regime (G‐CSF ± chemotherapy) alone, taking the day before apheresis as baseline. This pharmacodynamic effect was the first step needed for proof of clinical benefit.
Regarding the unfavorable effects, it is important to note that the observed safety profile in the pediatric population is consistent with that in adults and that no new safety signals have been observed. Moreover, plerixafor exposure did not seem to negatively affect transplant efficiency.
Data on the effect of plerixafor in the group aged <2 years are limited to one patient. A high increase in PB CD34+ cells after treatment was noted, suggesting that plerixafor was active in this small infant (aged 13 months, 8.9 kg). Thus the available (but very limited) data do not suggest that the potential lower exposure in this age group is clinically relevant. However, because of the lack of data, the final indication is defined in children aged at least 1 year.
The similarity in the relationship between the pharmacodynamic response (fold increase) and preapheresis PB CD34+ count between adult and pediatric patients and the established efficacy in the adult population was supportive of this extrapolation approach. The higher frequency of potentially poorly mobilizing patients with a strong increase (>4‐fold) in CD34+ counts in plerixafor‐treated patients in the pediatric study further supported the claim of efficacy.
Conclusion
Together, the data from the pediatric study with an extrapolation approach, building on the established clinical benefit in poorly mobilizing adults by comparing the effects seen in adults with those in the pediatric population, are sufficient to support a pediatric indication of the addition of plerixafor to the standard mobilization for children who (are expected to) mobilize poorly.
This led to approval of the use of plerixafor in pediatric patients (aged 1 year to less than 18 years) in combination with G‐CSF to enhance mobilization of hematopoietic stem cells to the peripheral blood for collection and subsequent autologous transplantation in children with a lymphoma or a solid malignant tumor, either preemptively, when circulating stem cell count on the predicted day of collection after adequate mobilization with G‐CSF (with or without chemotherapy) is expected to be insufficient with regard to desired hematopoietic stem cells yield, or for patients who previously failed to collect sufficient hematopoietic stem cells.
Author Contributions
Conception/design: Sahra Ali, Paula B. van Hennik, Sabine Straus, Filip Josephson, Geanne Thole, Pieter J. Glerum, Carla Herberts, Negar Babae, Ralf Herold, Irene Papadouli, Francesco Pignatti
Provision of study material or patients: Sahra Ali, Paula B. van Hennik, Sabine Straus, Filip Josephson, Geanne Thole, Pieter J. Glerum, Carla Herberts, Negar Babae, Ralf Herold, Irene Papadouli, Francesco Pignatti
Collection and/or assembly of data: Sahra Ali, Paula B. van Hennik, Sabine Straus, Filip Josephson, Geanne Thole, Pieter J. Glerum, Carla Herberts, Negar Babae, Ralf Herold, Irene Papadouli, Francesco Pignatti
Data analysis and interpretation: Sahra Ali, Paula B. van Hennik, Sabine Straus, Filip Josephson, Geanne Thole, Pieter J. Glerum, Carla Herberts, Negar Babae, Ralf Herold, Irene Papadouli, Francesco Pignatti
Manuscript writing: Sahra Ali, Paula B. van Hennik, Sabine Straus, Filip Josephson, Geanne Thole, Pieter J. Glerum, Carla Herberts, Negar Babae, Ralf Herold, Irene Papadouli, Francesco Pignatti
Final approval of manuscript: Dominik Karres, Sahra Ali, Paula B. van Hennik, Sabine Straus, Filip Josephson, Geanne Thole, Pieter J. Glerum, Carla Herberts, Negar Babae, Ralf Herold, Irene Papadouli, Francesco Pignatti
Disclosures
The authors indicated no financial relationships.
Acknowledgments
The scientific assessment summarized in this report is based on important contributions from the rapporteur and co‐rapporteur assessment teams, the Committee for Medicinal Products for Human Use (CHMP), Pharmacovigilance Risk Assessment Committee (PRAC) members, and additional experts after the application for a marketing authorization from the company. This publication is a summary of the European Public Assessment Report (EPAR), CHMP, and PRAC assessment. The EPAR is published on the European Medicines Agency Web site (www.ema.europa.eu). For the most current information on this marketing authorization, please refer to the European Medicines Agency Web site. The authors of this article remain solely responsible for the opinions expressed in this publication.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Xie T, Spradling AC. A niche maintaining germ line stem cells in the Drosophila ovary. Science 2000;290:328–330. [DOI] [PubMed] [Google Scholar]
- 2. Kiger AA, White‐Cooper H, Fuller MT. Somatic support cells restrict germline stem cell self‐renewal and promote differentiation. Nature 2000;407:750–754. [DOI] [PubMed] [Google Scholar]
- 3. Adams GB, Scadden DT. The hematopoietic stem cell in its place. Nat Immunol 2006;7:333–337. [DOI] [PubMed] [Google Scholar]
- 4. Wilson A, Trumpp A. Bone‐marrow haematopoietic‐stem‐cell niches. Nat Rev Immunol 2006;6:93–106. [DOI] [PubMed] [Google Scholar]
- 5. Kollet O, Dar A, Lapidot T. The multiple roles of osteoclasts in host defense: Bone remodeling and hematopoietic stem cell mobilization. Annu Rev Immunol 2007;25:51–69. [DOI] [PubMed] [Google Scholar]
- 6. Aiuti A, Webb IJ, Bleul C et al. The chemokine SDF‐1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J Exp Med 1997;185:111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim CH, Broxmeyer HE. In vitro behavior of hematopoietic progenitor cells under the influence of chemoattractants: Stromal cell‐derived factor‐1, steel factor and the bone marrow environment. Blood 1998;91:100–110. [PubMed] [Google Scholar]
- 8. Jo DY, Rafii S, Hamada T et al. Chemotaxis of primitive hematopoietic cells in response to stromal cell‐derived factor‐1. J Clin Invest 2000;105:101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wright DE, Bowman EP, Wagers AJ et al. Hematopoietic stem cells are uniquely selective in their migratory response to chemokines. J Exp Med 2002;195:1145–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Peled A, Petit I, Kollet O et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science 1999;283:845–848. [DOI] [PubMed] [Google Scholar]
- 11. Lapidot T, Kollet O. The essential roles of the chemokine SDF‐1 and its receptor CXCR4 in human stem cell homing and repopulation of transplanted immune‐deficient NOD/SCID and NOD/SCID/β2mnull mice. Leukemia 2002;16:1992–2203. [DOI] [PubMed] [Google Scholar]
- 12. Christopherson KW II, Hangoc G, Mantel C et al. Modulation of hematopoietic stem cell homing and engraftment by CD26. Science 2004;305:1000–1003. [DOI] [PubMed] [Google Scholar]
- 13. Broxmeyer HE, Cooper S, Kohli L et al. Transgenic expression of stromal cell derived factor‐1/CXCL12 enhances myeloid progenitor cell survival/anti‐apoptosis in vitro in response to growth factor withdrawal and enhances myelopoiesis in vivo. J Immunol 2003;170:421–429. [DOI] [PubMed] [Google Scholar]
- 14. Broxmeyer HE, Kohli L, Kim CH et al. Stromal cell derived factor‐1/CXCL12 enhances survival/anti‐apoptosis of hematopoietic stem and myeloid progenitor cells: Direct effects mediated through CXCR4 and Gαi proteins. J Leuk Biol 2003;73:630–638. [DOI] [PubMed] [Google Scholar]
- 15. Rettig MP, Ansstas G, DiPersio JF. Mobilization of hematopoietic stem and progenitor cells using inhibitors of CXCR4 and VLA‐4. Leukemia 2012;26:34–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shen H, Cheng T, Olszak I et al. CXCR‐4 desensitization is associated with tissue localization of hematopoietic progenitor cells. J Immunol 2001;166:5027–5033. [DOI] [PubMed] [Google Scholar]
- 17. Hattori K, Heissig B, Tashiro K et al. Plasma elevation of stromal cell‐derived factor‐1 induced mobilization of mature and immature hematopoietic progenitor and stem cells. Blood 2001;97:3354–3360. [DOI] [PubMed] [Google Scholar]
- 18. Sweeny EA, Lortat‐Jacob H, Priestley GV et al. Sulfated polysaccharides increase plasma levels of SDF‐1 in monkeys and mice: Involvement in mobilization of stem/progenitor cells. Blood 2002;99:44–51. [DOI] [PubMed] [Google Scholar]
- 19. Petit I, Szyper‐Kravitz M, Nagler A et al. G‐CSF induces stem cell mobilization by decreasing bone marrow SDF‐1 and up‐regulating CXCR4. Nat Immunol 2002;3:687–694. [DOI] [PubMed] [Google Scholar]
- 20. Trajman LC. Stem Cell Mobilization Protocols: Filgrastim vs. Mozobil. Pleasanton, CA: CepheusBio, September 2016. [Google Scholar]


