Abstract
The outbreak of coronavirus disease 2019 (COVID‐19) has rapidly spread globally since being identified as a public health emergency of major international concern and has now been declared a pandemic by the World Health Organization (WHO). In December 2019, an outbreak of atypical pneumonia, known as COVID‐19, was identified in Wuhan, China. The newly identified zoonotic coronavirus, severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2), is characterized by rapid human‐to‐human transmission. Many cancer patients frequently visit the hospital for treatment and disease surveillance. They may be immunocompromised due to the underlying malignancy or anticancer therapy and are at higher risk of developing infections. Several factors increase the risk of infection, and cancer patients commonly have multiple risk factors. Cancer patients appear to have an estimated twofold increased risk of contracting SARS‐CoV‐2 than the general population. With the WHO declaring the novel coronavirus outbreak a pandemic, there is an urgent need to address the impact of such a pandemic on cancer patients. This include changes to resource allocation, clinical care, and the consent process during a pandemic. Currently and due to limited data, there are no international guidelines to address the management of cancer patients in any infectious pandemic. In this review, the potential challenges associated with managing cancer patients during the COVID‐19 infection pandemic will be addressed, with suggestions of some practical approaches.
Implications for Practice
The main management strategies for treating cancer patients during the COVID‐19 epidemic include clear communication and education about hand hygiene, infection control measures, high‐risk exposure, and the signs and symptoms of COVID‐19. Consideration of risk and benefit for active intervention in the cancer population must be individualized. Postponing elective surgery or adjuvant chemotherapy for cancer patients with low risk of progression should be considered on a case‐by‐case basis. Minimizing outpatient visits can help to mitigate exposure and possible further transmission. Telemedicine may be used to support patients to minimize number of visits and risk of exposure. More research is needed to better understand SARS‐CoV‐2 virology and epidemiology.
Keywords: Coronavirus, COVID‐19, Influenza, Neoplasm, Pandemic, SARS‐CoV‐2
Short abstract
Cancer patients have an increased risk of contracting COVID‐19. This article addresses the challenges associated with managing cancer patients during the COVID‐19 infection pandemic and suggests some practical approaches.
Introduction
The emergence of coronavirus disease 2019 (COVID‐19) has caused a global public health emergency. In December 2019, an outbreak of respiratory disease caused by a novel coronavirus was first detected in China and has now spread to more than 150 countries 1. The virus was named severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) and has a phylogenetic similarity to SARS‐CoV‐1 that caused the SARS pandemic in 2002 2. This new type of respiratory illness is characterized by rapid human‐to‐human transmission, having achieved pandemic spread 3. There are currently no therapeutics or vaccines available and, presumably, no pre‐existing immunity in the population.
With the World Health Organization (WHO) declaring the novel coronavirus outbreak a pandemic 4, 5, 6, focus is needed on the impact of this rapidly spreading viral infection on cancer patients. Patients with cancer are more susceptible to infection than individuals without cancer, because malignancy and anticancer therapy result in an immunosuppressive state 7. In a retrospective study during the 2009 influenza A (H1N1) virus pandemic, the cancer patient population was at higher incidence of pneumonia (66%) and 30‐day mortality (18.5%) compared with the general population 8. A recent small case series study that evaluated SARS‐CoV‐2 in cancer patients found that patients with cancer had worse outcomes from SARS‐CoV‐2 than other individuals without cancer 7.
Limited studies and research regarding preparedness plans for the care of cancer patients during an infectious pandemic exist 9, 10. In this review, we aim specifically to address challenges associated with cancer care during the COVID‐19 pandemic. Recent literature 1, 9, 10, 11, 12, 13, 14 has already extensively addressed the virology, diagnosis, and treatment of COVID‐19 and is beyond the scope of this paper 2, 11, 12, 13, 14, 15, 16.
Cancer Patients Are a High‐Risk Population
Accumulating evidence suggests that cancer patients are at higher risk of COVID‐19 infection and more likely to have higher morbidity and mortality than the general population. In a study with a total of 1,524 patients with cancer, cancer patients had a twofold increased risk of COVID‐19 infection when compared with the general population 3. In another series from a single institution in the Wuhan region, the infection rate of SARS‐CoV‐2 in patients with cancer was 0.79% (95% CI = 0.3–1.2), which was higher than the cumulative incidence of all diagnosed COVID‐19 cases that was reported over the same time period (0.37%, 41,152/11,081,000 cases, data cutoff on February 17, 2020) 3. The Chinese Center for Disease Control and Prevention described the epidemiological characteristics of 72,314 COVID‐19 cases in mainland China as of February 11, 2020. They reported that 107 patients (0.5%) had cancer, and 6 of them died. The case fatality was 5.6%, which is higher than the overall reported case fatality (2.3%) from COVID‐19 17. Similarly, the WHO—China Joint Mission on COVID‐19 identified significantly higher case fatality amongst patients with pre‐existing malignancy (7.6%) compared with patients without comorbid conditions (1.4%) 18. In the series by Liang et al. 7, cancer was associated with higher risk of severe events (i.e., admission to the intensive care unit, invasive ventilation, or death seen in 7 of 18 patients [39%] with cancer vs. 124 of 1,572 patients [8%] without cancer; p = .0003) 7. These findings have been corroborated internationally, as an Italian study assessing the case fatality of COVID‐19 found that amongst 355 patients who died and underwent detailed chart review, 72 (20.3%) had active cancer 19. While these analyses are preliminary and require validation from larger international cohorts, several factors could account for an elevated risk for acquiring COVID‐19 and consequent complications amongst cancer patients, including frequent hospital visits and admissions 3, immunocompromised state, advanced age, and poor functional status.
Resource Allocation During Pandemic
A primary challenge when planning for a pandemic is human resource management. Strong leadership with clear chains of command within oncology and hematology teams nationally and locally will be needed to ensure timely and proportionate implementation of contingency plans that balance risks and protect patients and health care workers (HCWs) as infections rise 20.
In preparation for increased care and resource utilization during the COVID‐19 pandemic, strategies should be implemented to minimize interruption of cancer treatment, particularly in patients being treated with curative intent 21. However, to promote physical distancing and in anticipation of increased workload from a pandemic infection, it is recommended that elective surgeries and nonurgent outpatient clinics be deferred 21, 22, 23.
Resource allocation can be challenging, especially if a medical supply shortage occurs 9. Medication shortage, mainly chemotherapy and narcotics, can cause significant negative impact in cancer care delivery. For cancers in which certain regimens are clinically preferred or no other therapeutic alternatives exist, drug shortages may be life‐threatening 24. It is anticipated that the COVID‐19 pandemic will affect the medical product supply chains. On February 27, 2020, prior to the designation of pandemic, the U.S. Food and Drug Administration (FDA) issued a statement noting that one drug (drug was not named) is now in short supply because of COVID‐19 25. As of March 21, 2020, the FDA Drug Shortages list contained 26 oncology medications 26. While drug shortages might not manifest immediately, as many companies stockpile ingredients or supplies to protect against unexpected stoppages, identification and anticipation of such shortage can be particularly challenging during a pandemic 27. Clear communication and transparency between stakeholders, suppliers, and health organizations is a must for successful implementation of strategies for managing drug shortages. The American Society of Health‐System Pharmacists suggested the following steps to overcome drug shortage: establishment of contact with other sites or health systems, as large health systems can often survive drug shortages by shifting drug inventory among sites; identification of alternative substitute therapies; and development of criteria for patient prioritization during drug shortages, with a multidisciplinary team involving pharmacy, medical, and nursing staff 28.
Challenges of Cancer Diagnosis During a Pandemic
The diagnosis and timely treatment of cancer patients should not be compromised during an infectious disease pandemic; however, the management of such patients should be tailored to the best available resources. The necessity of any interventional procedure must be balanced against the increased risk during a pandemic and should be evaluated on a case‐by‐case basis to address the urgency of the procedure and the effect on the patient's outcome if the procedure is to be deferred. Endoscopy closure or restriction can create a great challenge in the diagnosis and interventions of malignant tumors of the digestive tract, especially in clinical situations with obstruction that require stenting or urgent biliary drainage. Alternative planning should be in place to have the procedure done in a negative‐pressure room, as necessary 29.
Cancer Patients in the Outpatient Setting
There are limited data to examine the effect of pandemics in outpatient cancer settings. Chen et al. examined a prospective case series of 79 patients with non‐small cell lung cancer (NSCLC) enrolled in chemotherapy trials in a Taiwanese hospital during the SARS epidemic. While five patients were under investigation, none were confirmed to have SARS. Results of a questionnaire showed that nearly two thirds of patients were afraid of entering a hospital for fear of acquiring SARS, and three patients ceased all further chemotherapy due to this concern. Chemotherapy delays occurred in 2.7% of administrations 30.
A more recent incident highlights the risk of exposure to cancer patients in clinical settings during a pandemic. A radiation oncologist in a regional cancer center in Ontario, Canada, was confirmed to be positive for SARS‐CoV‐2 after attending 14 patients in an outpatient clinic 31.
While outpatient visits for cancer patients should be reduced to the safest level without jeopardizing patient care, several measures may help reduce transmissions in outpatient settings. Clear communication and education about hand hygiene, infection control measures, the signs and symptoms of the COVID‐19, high‐risk travel or exposure, and the importance of reporting new symptoms to their HCWs should be reinforced. Clinic attendance should be limited to the patient and one visitor (or no visitors). Entry/exit points should be reduced, with signage and personnel at these ports to facilitate communication and policy adherence.
Ambulatory care clinics, including chemotherapy infusion units, must have strict screening and be prepared to identify and transfer potential cases safely without risking disease transmission 21. Suggested steps to reduce the risk of exposure include a call to the patient the day before their scheduled appointment to screen for any recent travel and contact history, as well as symptoms associated with COVID‐19. On the basis of this assessment, the patient may be directed to an acute care health care facility or dedicated COVID‐19 assessment center. Universal precautions remain essential, as 17.9% to 33.3% of patients may have asymptomatic COVID‐19 infection 32, 33.
Clinics should create a simple screening algorithm or questionnaire for early detection of potentially infectious persons. Travel advisory posters can be displayed to facilitate the screening process by prompting patients to be proactive by self‐reporting travel history 34. Individuals who meet criteria for highly communicable diseases requiring isolation, such as the novel COVID‐19 or other emerging respiratory infections, should be provided a mask and placed in a private exam room as soon as possible as per the infectious control guidance found on the WHO and Centers for Disease Control and Prevention (CDC) websites 35, 36, 37.
The chemotherapy infusion unit should function at usual capacity to avoid cancer treatment delays. Patients on active outpatient anticancer therapy can be categorized into oral or intravenous therapy, and consideration for switching intravenous chemotherapy to acceptable alternative oral anticancer drugs may be considered on a case‐by‐case basis. For example, fluorouracil can be substituted for capecitabine in concurrent neoadjuvant chemoradiation for rectal cancer without compromising oncological outcome 38, 39. This strategy can reduce the patient's visit time and, hence, risk of exposure to SARS‐CoV‐2. Another potential suggestion is to consider home infusion of chemotherapy if medically and logistically feasible 40.
The use of hematopoietic colony‐stimulating factors to prevent chemotherapy‐induced neutropenia is not routine with most chemotherapy protocols. There is no evidence to support a greater use of such factors to prevent neutropenia during a pandemic, especially considering that it also would increase the cost of treatment at a time when health care budgets will be severely strained 9.
For oral anticancer therapy in an outpatient setting, follow‐up visits may be minimized to the safest minimum number 21. Consideration for a chemotherapy break or holiday can be considered on a case‐by‐case basis. To reduce potential viral exposure at pharmacy departments, consideration should be given to the use of a drive‐through medicine collection facility, where patients are alerted by telephone when their medicine is available. Some hospitals may alternatively choose a courier service 20.
If blood work is needed to monitor side effects of therapy and response to treatment, a home‐drawn blood service can be considered, if feasible. Because of the paucity of literature available on the timing of baseline blood work before initiation of anticancer agents, the choice of accepted intervals between blood work and chemotherapy is ill defined 41. The HCWs can consider drawing blood work at expanded safe intervals to minimize the patient's visits to medical facilities.
Telemedicine can be considered to support patients remotely to reduce in‐person hospital visits during an infectious pandemic 42. This may include providing a hospital hotline and expanded telehealth capabilities 21. Telemedicine has been demonstrated to improve access to care and decrease health care costs 43. Examples of successful telemedicine in oncology include remote chemotherapy supervision, symptom management, survivorship care, palliative care, and clinical trials 43. Patients who are not currently receiving active therapy may be especially well‐suited for telemedicine follow‐up. Major limitations to telemedicine include the jurisdictional boundaries of the physicians’ practice, the need for training on telemedicine tools that may be limited in a pandemic setting, limitation of physical exam, and telemedicine reimbursement‐related issues 43.
Radiation therapy during a pandemic has unique challenges; unlike medical or surgical therapies, radiotherapy patients need to attend daily and the nature of the treatment is such that an interruption of therapy is clinically unacceptable. Even if no new cases are started, the patients currently on treatment must continue 22. Patients who are at risk or who are a known contact should be seen and treated in a separate room. This is possible in the outpatient clinic setting or even in operating suites; however, radiotherapy treatment requires facilities that are unique and limited. There may not be excess capacity to have a linac, simulator, or mould room specifically set aside for at‐risk patients. Concerns that SARS‐CoV‐2 particles might remain viable for up to 72 hours have implications for subsequent patient and staff activity in that room 22, 44. The American Society for Radiation Oncology (ASTRO) recently published brief guidelines for COVID‐19 recommendations to radiation oncology practices 45.
Limited data are available regarding cancer survivors and the risk of SARS‐CoV‐2 infection. Considering the limited data, we continue to suggest that cancer survivors follow the recommendations found on the WHO and CDC websites for the general population 35. Deferring or reducing surveillance visits may be considered to minimize exposure. Rescheduling these visits must be tracked to avoid patients being lost to follow‐up. Furthermore, routine screening appointments and genetics oncology clinics may be deferred until the pandemic wave is over; however, patients with abnormal screening results before or at the beginning of the pandemic should be assessed on a case‐by‐case basis.
Hospitalized Patients with Cancer
Patients with cancer frequently need hospitalization, generally an unavoidable step in the cancer treatment trajectory 46. A cohort study described hospital admission and the course of 454 cancer patients. Most admissions were attributed to medical emergencies (74.1%) and caused by uncontrolled symptoms (79.6%), highlighting the challenges in avoiding admissions in this population during an infectious disease pandemic 46.
Limited descriptions exist from recent epidemics regarding specific infection prevention and control procedures for patients with cancer. While the application of designated units with cohorting of COVID‐19 patients would be advisable, the significantly larger scale of this pandemic may preclude dedicated nursing for all infected patients. However, where possible, it would be advisable to dedicate health care practitioners to the care of COVID‐19 patients to reduce opportunities for transmission to uninfected patients.
There should be strict and safe triaging procedures to assess any COVID‐19 symptoms, as well as the urgency and necessity of hospitalization, at entry points, especially in emergency rooms. Restrictions on hospital‐based ambulatory care, nonurgent hospital utilization, and hospital transfers may be a safe public health strategy to control an infectious epidemic and provide hospital surge capacity for up to several months during an epidemic 47.
Patients with suspected COVID‐19 infection should be admitted first to an isolation room, as per standard infection control protocol, as a precaution in case they test positive for SARS‐CoV‐2 infection. Grouped areas with minimization of staff overlap with other patient care areas will ideally be available for confirmed COVID‐19 cases. If COVID‐19 infection is excluded, consideration may be given to transfer to the appropriate service as medically indicated; however, patients should be evaluated for the need for quarantine if the patient has been exposed to a close contact of a confirmed case or has other epidemiologic risk. If the patient is positive for SARS‐CoV‐2, then COVID‐19 isolation procedures should be followed as per the medical facility's guidelines (Fig. 1).
Figure 1.
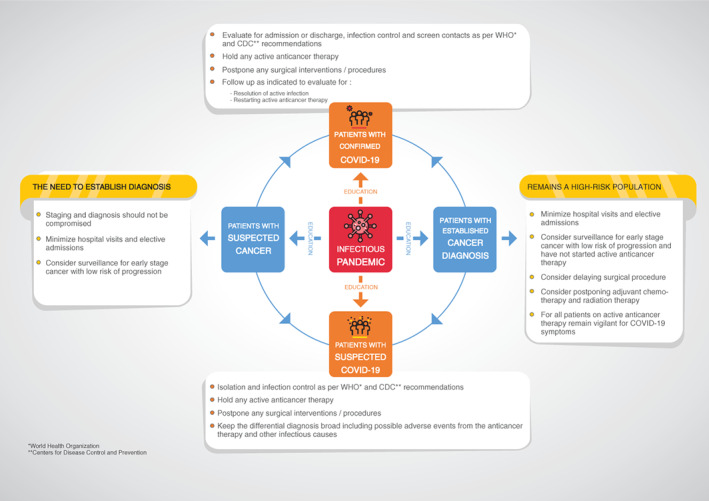
To minimize occupational exposure to the virus and reduce the risk of nosocomial transmission, provision of adequate personal protective equipment (PPE) for health care workers and rigorous application of infection prevention and control measures in health care facilities is mandatory 48, 49. While PPE kits should be made available, conservation efforts for all critical PPE should be implemented if resource constraints demand.
Active Anticancer Therapy for Cancer Patients with Infection or at risk of Infection
During a pandemic, the potential for benefit with chemotherapy would be unchanged, but the risk of harm would be increased to a degree that cannot be readily quantified. Patients who underwent chemotherapy or surgery in the month before diagnosis with COVID‐19 had a numerically higher risk (three of four patients) of clinically severe events than did those not receiving chemotherapy or surgery (six of 14 patients) 7.
During a pandemic, the consent process may change because the risk and benefit ratio may alter. For example, cancer patients need to know that anticancer therapy could carry greater risk during a pandemic. In addition, access to hospital beds will be limited both by increased demand and by potential staff shortage. In this circumstance, patients may well make an informed choice for a potentially less efficacious but less myelosuppressive treatment 9. Additionally, the risk of surgery may carry the risk of nosocomial infection with the pandemic pathogen.
Delaying curative adjuvant chemotherapy can be considered within the accepted duration for each disease site 7 (e.g., adjuvant chemotherapy for stage III colorectal cancer can be safely delayed up to 8 weeks, but more than 12 weeks of delay is not recommended) 50. Recommendations for prioritization, treatment, and triage of patients with breast cancer during the COVID‐19 pandemic has been recently published 51.
During a pandemic, cancer patients will be classified as either confirmed infection or at high risk for COVID‐19. Patients who have confirmed COVID‐19 infection should be assessed for holding anticancer therapy until they are deemed medically clear as per the clinical guidance found on the WHO and CDC websites 35, 52. Cancer patients who are on active anticancer therapy continue to be high risk and should remain vigilant for COVID‐19 symptoms. (Fig. 1).
Surgical Consideration for Cancer Patients at Risk of Infection
As mentioned earlier, patients who receive surgery in the month before contracting SARS‐CoV‐2 are likely to be at higher risk of clinically severe events than those who did not have surgery 7. During an infectious disease pandemic, patients may face difficulties, including access to hospitalization and deferred surgeries. Currently, there are no published international guidelines for decisions regarding special considerations for surgical intervention in cancer patients during an infectious disease pandemic.
Delaying elective surgical intervention may be more appropriate for select patients (e.g., early asymptomatic small breast cancer tumors detected on routine screening mammograms). These can be followed until the infectious disease pandemic is more controlled or over. Sixty‐day delays in surgical intervention in clinical stage I or stage II breast cancer were not associated with worse oncological outcomes 53. One of the challenges of delaying the elective surgery is the uncertainty regarding the ending of the infectious pandemic. The concept of delaying elective surgery is complex when considered across different disease sites, as each site has a unique oncological multidisciplinary approach. The suggested approach to solid tumor diagnosis and treatment decisions during an infectious disease pandemic, including oncological surgical consideration, is shown in Figure 2. The American College of Surgeons recently published guidance for triage of nonemergent surgical procedures during the COVID‐19 pandemic, based on the Elective Surgery Acuity Scale (ESAS) 54.
Figure 2.
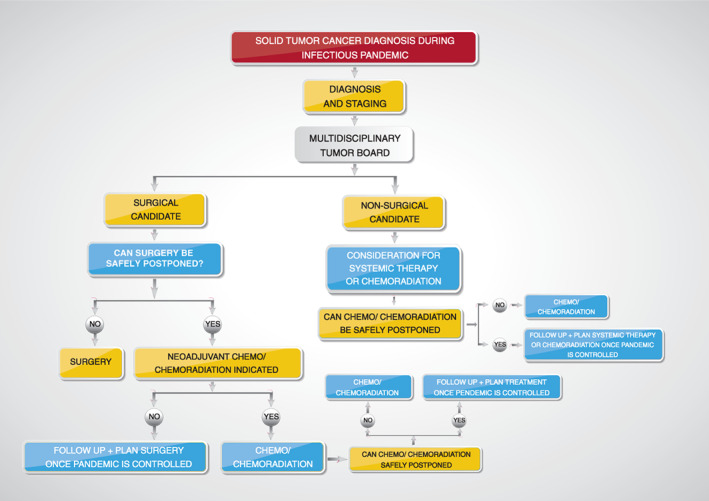
Special Consideration: Patients with Lung Cancer
Patients with lung cancer usually have compromised lung function with associated dyspnea, cough, and polypnea. They might be at higher risk of severe forms of COVID‐19 infection due to decreased pulmonary function. In the aforementioned studies that evaluated COVID‐19 in a small cohort of patients with cancer, the investigators found that lung cancer was the most frequent type of malignancy in this cohort of COVID‐19–infected patients (five [28%] of 18 patients) 7. Whether this reflects a true increase in the susceptibly of the lung cancer population to SARS‐CoV‐2 infection or is simply due to the fact that lung cancer is the most cancer in China is yet to be determined 55.
There are no published data regarding the risk of susceptibility to SARS‐CoV‐2 based on the type of the anticancer therapy used (e,g., immunotherapy, chemotherapy, or anti‐EGFR [epidermal growth factor receptor] therapies).
The Chinese expert groups (Lung Cancer Study Group, Chinese Thoracic Society, Chinese Medical Association, and Chinese Respiratory Oncology Collaboration) recommend, whenever possible, to treat patients with advanced NSCLC as outpatients at the nearest medical center during the COVID‐19 pandemic. Patients who need to be hospitalized should be screened for COVID‐19. Clinicians should remain vigilant not to miss alternative or co‐infecting respiratory infections 56. In addition, pulmonary adverse reactions caused by anticancer treatment (e.g., drug‐ or radiation‐induced pneumonitis) should also be sought in the right clinical setting 56. Lastly, other potential causes for the respiratory deterioration of NSCLC patients, which could mimic COVID‐19 symptoms, must be considered; these may include obstructive pneumonia, pleural or pericardial effusion, pulmonary embolism, and heart failure; therefore, rapid access to SARS‐CoV‐2 assays is of utmost importance.
For NSCLC patients at risk of acquiring SARS‐CoV‐2, health care workers should stress the importance of using PPE and handwashing. Postponing anticancer treatment should be considered according to the patient's risk of infection, performance, and clinical status. Currently, there are no clinical trials to examine the safety and efficacy of antiviral prophylaxis for SARS‐CoV‐2 in cancer patients.
Special Consideration: Hematopoietic Cell Transplant Recipients
Hematopoietic cell transplant (HCT) recipients are at increased risk of various infections, including viral infections due to the underlying disease and immunosuppression. Respiratory viral infections (RVI) are prevalent in both the pre‐ and the post‐engraftment periods. The incidence of RVI is 7.8% among allogeneic and 2.3% among autologous transplant recipients 57. Using molecular testing, up to 15% of patients developed RVI in the setting of allo‐HCT 58. Most patients develop upper respiratory tract infection, and up to half progress to complicated lower respiratory tract infection (LRTI), with an average mortality of 32% 59, 60, 61. In addition to other common factors, prolonged neutropenia and lymphopenia, as well as graft‐versus‐host disease, make HCT recipients and patients with hematological malignancies at increased risk of viral infections. HCT recipients present with prolonged viral shedding and have a higher rate of progression to LRTI 61. Since about half of these patients develop nosocomial RVI 62, the CDC recommends implementing strict adherence to infection control measures, including contact isolation with mask 36.
General recommendations for treatment are limited because of the lack of effective antiviral agents and powered clinical studies. Initial evaluation of patients with underlying hematological malignancies and/or undergoing HCT includes full clinical history and examination (Table 1) along with chest x‐ray and computerized tomography (CT) scan of the chest 60. Laboratory evaluation for respiratory viruses is essential to document active infection (i.e., nasopharyngeal aspirate, nasal and/or throat swabs, saline throat gargles or sputum samples by polymerase chain reaction) 63, 64.
Table 1.
Definitions of community‐acquired respiratory virus respiratory tract infectious disease
| ECIL‐4 Definitions |
|---|
Case Classification
|
Clinical criteria
|
Epidemiological criteria
|
Abbreviations: ECIL‐4, Fourth European Conference on Infections in Leukemia; RTID, respiratory tract infectious disease.
Reproduced from 60 with permission from Oxford University Press under Open Access article distributed under the terms of the Creative Commons Attribution License.
Infection control plays an integral part in the management of COVID‐19 in pandemic. Good practice measures as per WHO/CDC guidelines should be applied. Suspected or confirmed cases should be placed in single room with neutral air pressure and antechamber. Patients with hematological malignancies or post‐HCT should have immunosuppression to minimum, where possible and screening for other co‐pathogens is warranted 60. In Italy during the 2020 COVID‐19 pandemic, HCWs attending malignant hematology patients were screened weekly for SARS‐CoV‐2 21 as part of the infection control plan.
Pre‐HCT recipients should be screened for RVI and treated appropriately until resolution of the RVI, since the presence of RVI prior to HCT has been linked with poorer outcomes post‐HCT 65. European bone marrow transplant panel experts’ recommendations (released on March 1, 2020) for recipients and donors of hematopoietic cells before the beginning of any of the transplant procedures (mobilization, apheresis, marrow harvest, and conditioning) are summarized in Table 2 66.
Table 2.
European Society for Blood and Marrow Transplantation recommendations on SARS‐CoV‐2 diagnosis
| EBMT recommendations |
|---|
Recipient:
|
As defined by health authorities.
Adapted from 19 with permission from The European Society for Blood and Marrow Transplantation.
Another critical aspect related to blood products supply in pandemics is that the shortage of these products is more likely to arise 67. Blood donor centers are likely to experience loss of donors, workers, and reliable transport of specimens to national testing laboratories and degradation of response times from national testing labs. The WHO published a detailed transfusion services guideline in maintaining a safe and adequate blood supply during pandemic influenza 68.
Plans for rationing medical care need to take the vulnerability of the blood transfusion system into account. Some of the measures that may be used to support transfusion services during a pandemic include conducting blood drives with acceptable donors one at a time and encouraging HCWs to donate in their workplace. On March 19, 2020, the FDA issued a statement encouraging the community to donate blood, as the number of blood donations was dramatically reduced 67.
Psychological Aspect of Patients with Cancer During a Pandemic
Social distancing measures, quarantine, and visitor limitations will limit opportunities for family support and advocacy, affecting an important sense of connection and source of strength and well‐being for cancer patients. It is likely that many cancer patients and their families will understandably be concerned about how a pandemic might affect their care and treatment. Patients will be concerned about contracting the virus and the subsequent impact on their treatment and how they will continue to access the services of oncology during the pandemic 69. New patients may be worried about whether their treatments will be delayed and what the implications might be on their outcome. It is important to recognize the increased level of distress that cancer patients and their families might face during this time, over and above the distress already experienced in relation to their diagnosis and treatment and the pandemic itself. As a result, it is important that supports are in place in each cancer program and hospital to assess the level of distress and intervene appropriately to the best of the available resources. This may mean that psychosocial staff will be more utilized to assess distress and available to address the ongoing needs of patients and families during this pandemic.
Impact of the Pandemic on Cancer Research
Clinical and basic cancer research is likely to be severely affected by a pandemic. There will most likely be a decrease in trial initiations and accruals, and the pace of progress will slowed 70. However, the impact will be magnified, perhaps exponentially, by protocol deviations and violations for missed and delayed visits, leading to countless queries and estimated dates of confinement. The burden on research teams will increase markedly, and precious time and resources will be devoted to these tasks rather than accelerating clinical investigation to make up for lost time. During the pandemic, there is a need to carefully reconsider the clinical cancer research processes and procedures that contribute to data integrity and patient safety versus tasks that might ultimately detract from cancer research goals. Further research is needed in this area to address these issues 71. On March 18, 2020, the FDA published guidance for industry, investigators, and institutional review boards on conduct of clinical trials of medical products during the COVID‐19 pandemic 72.
Health Care Workers in Oncology During the Pandemic
The spread of COVID‐19 disease can be rapid and may overwhelm primary and acute care facilities. This may be compounded by COVID‐19 infection of medical personnel, quarantine requirements, and school closures, all of which may affect staffing levels and increase stress of the HCWs 73. Clinicians and other support staff may need to work flexibly to facilitate safe service provision in alternative settings. Social distancing and separation of clinic work spaces are important steps to reduce the risk of infection. If staff are required to self‐isolate due to contact with a confirmed case of coronavirus, consider ways they can continue to provide care and/or support multidisciplinary tumor boards 74 (e.g., virtual attendance at MDT meetings; telephone or video consultations, especially follow‐ups; identifying vulnerable patients and making contact to discuss changes to care and treatment; identifying patients suitable for remote monitoring/follow‐up; and data entry (where remote access enabled).
Conclusion
In this review, we addressed some of the current challenges associated with managing cancer patients during the COVID‐19 pandemic and provided some guidance and recommendations. This approach is likely applicable to various infectious pandemics. Health care authorities in cancer care should immediately start planning for cancer care delivery during a pandemic. The limited but accumulating evidence suggests that patients with cancer are at higher risk of COVID‐19 infection than individuals without cancer. The main management strategies for patients with cancer in this COVID‐19 pandemic include clear communication and education about hand hygiene, infection control measures, high‐risk exposure, and the signs and symptoms of the COVID‐19. Consideration of risk and benefit for active intervention in the cancer population during an infectious disease pandemic must be individualized. Consideration for postponing elective surgery or chemotherapy for cancer patients with low risk of progression should be considered on a case‐by‐case basis. Minimizing outpatients’ visits and elective admissions can help in mitigating exposure and possible further transmission. Telemedicine may be used to support patients during an infectious pandemic to minimize visits and risk of exposure. More research is needed to further understand SARS‐CoV‐2 virology and epidemiology in the cancer population.
Author Contributions
Conception/Design: Humaid O. Al‐Shamsi
Collection and/or assembly of data: Humaid O. Al‐Shamsi, Waleed Alhazzani, Ahmad Alhuraiji
Manuscript writing: Humaid O. Al‐Shamsi, Waleed Alhazzani, Ahmad Alhuraiji, Eric A. Coomes, Roy F. Chemaly, Meshari Almuhanna, Robert Wolff, Ibrahim K. Nuhad, Melvin L.K. Chua, Sebastien J. Hotte, Brandon M. Meyers, Tarek Elfiki, Giuseppe Curigliano, Cathy Eng, Axel Grothey, Conghua Xie
Final approval of manuscript: Humaid O. Al‐Shamsi, Waleed Alhazzani, Ahmad Alhuraiji, Eric A. Coomes, Roy F. Chemaly, Meshari Almuhanna, Robert Wolff, Ibrahim K. Nuhad, Melvin L.K. Chua, Sebastien J. Hotte, Brandon M. Meyers, Tarek Elfiki, Giuseppe Curigliano, Cathy Eng, Axel Grothey, Conghua Xie
Disclosures
Melvin Chua Lee Kiang: Ferring, Varian (RF), Janssen, Astellas, Merck, Illumina, Varian (H); Giuseppe Curigliano: Roche, Lilly, Pfizer, Seattle Genetics, Daichii‐Sankyo, AstraZeneca, Merck (C/A), Unicancer, Ellipsis (SAB). The other authors indicated no financial relationships.
Acknowledgments
Funding for this study was provided by the Khalifa Foundation, Abu Dhabi, United Arab Emirates and by Roche Pharmaceuticals Middle East FZCO, Dubai, United Arab Emirates to Humaid O. Al‐Shamsi. Melvin L.K. Chua received funding from the National Medical Research Council Clinician‐Scientist Award (CSA/0027/2018). Conghua Xie received funding from the Health Commission of Hubei Province Scientific Research Project (WJ2019H002). We express special appreciation to Ms. Fatma Al Falasi for her help with the illustration of the figures in this article.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Worldometer . COVID‐19 Coronovirus Pandemic. Available at https://www.worldometers.info/coronavirus/#countries. Accessed March 16, 2020.
- 2. Guan WJ, Ni ZY, Hu Y et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yu J, Ouyang W, Chua MLK et al. SARS‐CoV‐2 transmission in cancer patients of a tertiary hospital in Wuhan. JAMA Oncol 2020 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 4. World Health Organization . WHO Director‐General's opening remarks at the media briefing on COVID‐19, March 11, 2020. Available at https://www.who.int/dg/speeches/detail/who‐director‐general‐s‐opening‐remarks‐at‐the‐media‐briefing‐on‐covid‐19—11‐march‐2020. Accessed March 11, 2020.
- 5. Anderson RM, Heesterbeek H, Klinkenberg D et al. How will country‐based mitigation measures influence the course of the COVID‐19 epidemic? Lancet 2020;395:931–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organization . Coronavirus disease (COVID‐19) Pandemic. Available at https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019. Accessed March 11, 2020.
- 7. Liang W, Guan W, Chen R et al. Cancer patients in SARS‐CoV‐2 infection: A nationwide analysis in China. Lancet Oncol 2020;21:335–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dignani MC, Costantini P, Salgueira C et al. Pandemic 2009 influenza A (H1N1) virus infection in cancer and hematopoietic stem cell transplant recipients; a multicenter observational study. F1000Res 2014;3:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Battershill PM. Influenza pandemic planning for cancer patients. Curr Oncol 2006;13:119–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ASCO COVID‐19 Clinical Oncology Frequently Asked Questions (FAQs). Last updated – March 12, 2020. Available at https://www.asco.org/sites/new‐www.asco.org/files/content‐files/blog‐release/pdf/COVID‐19‐Clinical%20Oncology‐FAQs‐3‐12‐2020.pdf. Accessed March 14, 2020.
- 11.World Health Organization. Disease outbreak news: Novel coronavirus – Republic of Korea (ex‐China). January 21, 2020. Available at https://www.Who.Int/csr/don/21‐january‐2020‐novel‐coronavirus‐republic‐of‐korea‐ex‐china/en/. Accessed January 21, 2020.
- 12.Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID‐19). Cases in the U.S. Available at https://www.cdc.gov/coronavirus/2019-ncov/cases-in-us.html. Accessed March 10, 2020).
- 13. Lu R, Zhao X, Li J et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020;395:565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Perlman S. Another decade, another coronavirus. N Engl J Med 2020;382:760–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li Q, Guan X, Wu P et al. Early transmission dynamics in Wuhan, China, of novel coronavirus‐infected pneumonia. N Engl J Med 2020;382:1199–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chan JF, Yuan S, Kok KH et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person‐to‐person transmission: A study of a family cluster. Lancet 2020;395:514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Novel Coronavirus Pneumonia Emergency Response Epidemiology Team . The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID‐19) in China [in Chinese]. Zhonghua Liu Xing Bing Xue Za Zhi 020;41:145–151.
- 18.Report of the WHO–China Joint Mission on Coronavirus Disease 2019 (COVID‐19). Available at https://www.Who.Int/docs/default‐source/coronaviruse/who‐china‐joint‐mission‐on‐covid‐19‐final‐report.pdf. Accessed March 12, 2020.
- 19. Onder G, Rezza G, Brusaferro S. Case‐fatality rate and characteristics of patients dying in relation to COVID‐19 in Italy. JAMA 2020. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 20. Willan J, King AJ, Hayes S et al. Care of haematology patients in a COVID‐19 epidemic. Br J Haematol 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. The Cancer Letter . What to expect: Oncology's response to coronavirus in Italy: “It's like being in a war”. Available at https://cancerletter.Com/articles/20200311_1/. Accessed March 13, 2020.
- 22. Mukherjee RK, Back MF, Lu JJ et al. Hiding in the bunker: Challenges for a radiation oncology department operating in the severe acute respiratory syndrome outbreak. Australas Radiol 2003;47:143–145. [DOI] [PubMed] [Google Scholar]
- 23.Hospitals are canceling elective surgeries to make space for a potential flood of coronavirus patients. Available at https://www.cnbc.com/2020/03/13/hospitals‐cancel‐elective‐surgery‐to‐make‐room‐for‐coronavirus‐influx.html. Accessed March 20, 2020.
- 24. Alpert A, Jacobson M. Impact of oncology drug shortages on chemotherapy treatment. Clin Pharmacol Ther 2019;106:415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. U.S. Food & Drug Administration . FDA statement: Coronavirus (COVID‐19) supply chain update. Available at https://www.fda.gov/news‐events/press‐announcements/coronavirus‐covid‐19‐supply‐chain‐update?utm_campaign=022720_statement_coronavirus%20%28covid‐19%29%20supply%20chain%20update&utm_medium=email&utm_source=eloqua. Accessed March 20, 2020.
- 26. U.S. Food & Drug Administration . FDA drug shortages. Available at https://www.accessdata.fda.gov/scripts/drugshortages/default.cfm. Accessed March 21, 2020.
- 27. Vox . Could the coronavirus spark drug shortages in the US? Available at https://www.vox.com/2020/3/9/21163356/coronavirus‐drug‐shortage‐potential‐fda‐china‐india. Accessed March 20, 2020.
- 28. Ventola CL. The drug shortage crisis in the United States: Causes, impact, and management strategies. P T 2011;36:740–757. [PMC free article] [PubMed] [Google Scholar]
- 29. Repici A, Maselli R, Colombo M et al. Coronavirus (COVID‐19) outbreak: What the department of endoscopy should know. Gastrointest Endosc 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 30. Chen YM, Perng RP, Chu H et al. Impact of severe acute respiratory syndrome on the status of lung cancer chemotherapy patients and a correlation of the signs and symptoms. Lung Cancer 2004;45:39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. CBC News . 14 cancer patients at Hamilton hospital saw doctor who tested positive for COVID‐19. Available at https://www.cbc.ca/news/canada/hamilton/covid19-first-case-doctor-hamilton-health-sciences-1.5493530. Accessed March 12, 2020.
- 32. Nishiura H, Kobayashi T, Suzuki A et al. Estimation of the asymptomatic ratio of novel coronavirus infections (COVID‐19). Int J Infect Dis 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mizumoto K, Kagaya K, Zarebski A et al. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID‐19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill 2020;25:pii=2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Steinkuller F, Harris K, Vigil KJ et al. Outpatient infection prevention: A practical primer. Open Forum Infect Dis 2018;5:ofy053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. World Health rganization . Coronavirus disease (2019‐COVID‐19) technical guidance: Patient management. Available at https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019/technical‐guidance/patient‐management. Accessed March 4, 2020.
- 36. Tablan OC, Anderson LJ, Besser R et al. Guidelines for preventing health‐care‐associated pneumonia, 2003: Recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee. MMWR Recomm Rep 2004;53:1–36. [PubMed] [Google Scholar]
- 37. Centers for Disease Contro and Prevention . Interim infection prevention and control recommendations for patients with suspected or confirmed coronavirus disease 2019 (COVID‐19) in healthcare settings. Available at https://www.cdc.gov/coronavirus/2019‐ncov/infection‐control/control‐recommendations.html?cdc_aa_refval=https%3a%2f%2fwww.cdc.gov%2fcoronavirus%2f2019‐ncov%2fhcp%2finfection‐control.html. Accessed March 6, 2020.
- 38. Hofheinz RD, Wenz F, Post S et al. Chemoradiotherapy with capecitabine versus fluorouracil for locally advanced rectal cancer: A randomised, multicentre, non‐inferiority, phase 3 trial. Lancet Oncol 2012;13:579–588. [DOI] [PubMed] [Google Scholar]
- 39. Zou XC, Wang QW, Zhang JM. Comparison of 5‐fu‐based and capecitabine‐based neoadjuvant chemoradiotherapy in patients with rectal cancer: A meta‐analysis. Clin Colorectal Cancer 2017;16:e123–e139. [DOI] [PubMed] [Google Scholar]
- 40. Shereen NG, Salman D. Delivering chemotherapy at home: How much do we know? Br nursing Community Nurs 2019;24:482–484. [DOI] [PubMed] [Google Scholar]
- 41. Warr J, Hird AE, DeAngelis C et al. Baseline blood work before initiation of chemotherapy: What is safe in the real world? J Oncol Pract 2013;9:e182–185. [DOI] [PubMed] [Google Scholar]
- 42. The New York Times . Doctors and patients turn to telemedicine in the coronavirus outbreak. Available at https://www.nytimes.com/2020/03/11/health/telemedicine‐coronavirus.html. Accessed March 12, 2020.
- 43. Sirintrapun SJ, Lopez AM. Telemedicine in cancer care. Am Soc Clin Oncol Educ Book 2018;38:540–545. [DOI] [PubMed] [Google Scholar]
- 44. van Doremalen N, Bushmaker T, Morris DH et al. Aerosol and surface stability of SARS‐CoV‐2 as compared with SARS‐CoV‐1. N Engl J Med 2020 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 45. ASTRO . COVID‐19 recommendations to radiation oncology practices. Available at https://www.astro.org/daily-practice/covid-19-recommendations-and-information. Accessed March 20, 2020.
- 46. Numico G, Cristofano A, Mozzicafreddo A et al. Hospital admission of cancer patients: Avoidable practice or necessary care? PLoS One 2015;10:e0120827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Stukel TA, Schull MJ, Guttmann A et al. Health impact of hospital restrictions on seriously ill hospitalized patients: Lessons from the Toronto SARS outbreak. Med Care 2008;46:991–997. [DOI] [PubMed] [Google Scholar]
- 48. European Centre for Disease Prevention and Control . Outbreak of novel coronavirus disease 2019 (COVID‐19): Increased transmission globally – fifth update. Available at https://www.ecdc.europa.eu/en/publications‐data/rapid‐risk‐assessment‐outbreak‐novel‐coronavirus‐disease‐2019‐covid‐19‐increased. Accessed March 6, 2020.
- 49. Adams JG, Walls RM. Supporting the health care workforce during the COVID‐19 global epidemic. JAMA 2020. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 50. Bos AC, van Erning FN, van Gestel YR et al. Timing of adjuvant chemotherapy and its relation to survival among patients with stage iii colon cancer. Eur J Cancer 2015;51:2553–2561. [DOI] [PubMed] [Google Scholar]
- 51. American College of Surgeons . Recommendations for prioritization, treatment and triage of breast cancer patients during the COVID‐19 pandemic. Available at https://www.facs.org/quality-programs/cancer/executive-summary. Accessed March 26, 2020. [DOI] [PMC free article] [PubMed]
- 52. Centers for Disease Control And Prevention . Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID‐19). Available at https://www.cdc.gov/coronavirus/2019‐ncov/hcp/clinical‐guidance‐management‐patients.html. Accessed March 4, 2020.
- 53. Mansfield SA, Abdel‐Rasoul M, Terando AM et al. Timing of breast cancer surgery‐how much does it matter? Breast J 2017;23:444–451. [DOI] [PubMed] [Google Scholar]
- 54. American College of Surgeons . COVID‐19: Guidance for triage of non‐emergent surgical procedures. Available at https://www.facs.org/about‐acs/covid‐19/information‐for‐surgeons/triage. Accessed March 20, 2020.
- 55. Feng RM, Zong YN, Cao SM et al. Current cancer situation in china: Good or bad news from the 2018 global cancer statistics? Cancer Commun (Lond) 2019;39:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yang L, Xu HY, Wang Y. Diagnostic and therapeutic strategies of lung cancer patients during the outbreak of 2019 novel coronavirus disease (COVID‐19) [in Chinese]. Zhonghua Zhong Liu Za Zhi 2020;42:E006. [DOI] [PubMed] [Google Scholar]
- 57. Ohrmalm L, Wong M, Rotzen‐Ostlund M et al. Flocked nasal swab versus nasopharyngeal aspirate for detection of respiratory tract viruses in immunocompromised adults: A matched comparative study. BMC Infect Dis 2010;10:340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sim SA, Leung VKY, Ritchie D et al. Viral respiratory tract infections in allogeneic hematopoietic stem cell transplantation recipients in the era of molecular testing. Biol Blood Marrow Transplant 2018;24:1490–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lee I, Barton TD. Viral respiratory tract infections in transplant patients: Epidemiology, recognition and management. Drugs 2007;67:1411–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hirsch HH, Martino R, Ward KN et al. Fourth European Conference on Infections in Leukaemia (ECIL‐4): Guidelines for diagnosis and treatment of human respiratory syncytial virus, parainfluenza virus, metapneumovirus, rhinovirus, and coronavirus. Clin Infect Dis 2013;56:258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chemaly RF, Shah DP, Boeckh MJ. Management of respiratory viral infections in hematopoietic cell transplant recipients and patients with hematologic malignancies. Clin Infect Dis 2014;59 Suppl 5:S344–S351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Whimbey E, Champlin RE, Couch RB et al. Community respiratory virus infections among hospitalized adult bone marrow transplant recipients. Clin Infect Dis 1996;22:778–782. [DOI] [PubMed] [Google Scholar]
- 63. Dignan FL, Clark A, Aitken C et al. BCSH/BSBMT/UK clinical virology network guideline: Diagnosis and management of common respiratory viral infections in patients undergoing treatment for haematological malignancies or stem cell transplantation. Br J Haematol 2016;173:380–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Blaschke AJ, Allison MA, Meyers L et al. Non‐invasive sample collection for respiratory virus testing by multiplex PCR. J Clin Virol 2011;52:210–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Campbell AP, Guthrie KA, Englund JA et al. Clinical outcomes associated with respiratory virus detection before allogeneic hematopoietic stem cell transplant. Clin Infect Dis 2015;61:192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. EBMT . Coronavirus disease COVID‐19: EBMT recommendations. Available at https://www.ebmt.org/ebmt/news/ebmt‐recommendation‐coronavirus‐disease‐covid‐19. Accessed March 5, 2020.
- 67. U.S. Food & Drug Administration . Coronavirus (COVID‐19) update: Blood donations. Available at https://www.fda.gov/news‐events/press‐announcements/coronavirus‐covid‐19‐update‐blood‐donations. Accessed March 20, 2020.
- 68. World Health Organization . Maintaining a safe and adequate blood supply during pandemic influenza guidelines for blood transfusion services, July 2011. Available at https://www.who.int/bloodsafety/transfusion_services/WHO_Guidelines_on_Pandemic_Influenza_and_Blood_Supply.pdf?ua=1. Accessed March 16, 2020.
- 69. https://twitter.com/itsnot_pink/status/1238590023571832834. Accessed March 15, 2020.
- 70. https://twitter.com/mattgalsky/status/1238933344135544833. Accessed March 15, 2020.
- 71. Busta ER, Mancher M, Cuff PA et al. Integrating clinical research into epidemic response: The Ebola experience. National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Sciences Policy; Board on Global Health; Committee on Clinical Trials During the 2014–2015 Ebola Outbreak. Washington (DC): National Academies Press (US); 2017. June. [PubMed] [Google Scholar]
- 72.FDA guidance on conduct of clinical trials of medical products during COVID‐19 pandemic, guidance for industry, investigators, and institutional review boards. Available at https://www.fda.gov/media/136238/download. Accessed March 20, 2020.
- 73. The Medical Journal of Australia . Managing haematology and oncology patients during the COVID‐19 pandemic: Interim consensus guidance. Available at https://www.mja.com.au/journal/2020/212/10/managing‐haematology‐and‐oncology‐patients‐during‐covid‐19‐pandemic‐interim. Accessed March 26, 2020. [DOI] [PMC free article] [PubMed]
- 74.Clinical guide for the management of cancer patients during the coronavirus pandemic, 17 March 2020, version 1, Available at https://www.england.nhs.uk/coronavirus/wp‐content/uploads/sites/52/2020/03/specialty‐guide_cancer‐and‐coronavirus_17‐march.pdf. Accessed March 17, 2020.


