Abstract

In the present study, we have successfully synthesized nitrogen-rich graphitic carbon nitride (g-C3N4) nanosheets by a simple direct thermal polymerization approach. The synthesized g-C3N4 nanosheets were exfoliated using HCl to make their surface a few nanometers thick. The ultrathin surface was achieved by simply mixing g-C3N4 in 3 M HCl. After that, palladium nanoparticles were uniformly immobilized on the surface of g-C3N4. The synthesized materials were characterized by various physiochemical techniques such as X-ray diffraction, energy-dispersive X-ray spectroscopy, and Fourier transform infrared spectroscopy. Information about morphology and size was obtained through transmission electron microscopy and scanning electron microscopy. The Brunauer–Emmett–Teller surface area, pore volume, and pore diameter were determined using nitrogen adsorption–desorption measurements. The prepared material (Pd/g-C3N4) was utilized as an efficient catalyst for the reduction of hazardous nitroarenes and degradation of organic dyes. The catalyst could be easily recovered through centrifugation and then could be reused multiple times for the further catalytic cycles with a little loss in its catalytic activity. The work presented here illustrates the sustainable anchoring of metal nanoparticles over the surface of nitrogen-rich g-C3N4 nanosheets and could be utilized for different types of catalytic reactions.
1. Introduction
Global environmental pollution is becoming a core issue for the modern society, which is directly threatening the terrestrial and aquatic life. Water pollution is considered as one of the foremost challenges.1 Colored pigments including organic dyes and aromatic nitro compounds are commonly used in various industries including clothing, paper, fiber, pharmaceutical, food, printing, and leather.2−4 These industries release a massive amount of effluent with some amount of hazardous compounds such as organic dyes and aromatic nitro compounds.5 These effluents with hazardous compounds contaminate water bodies such as river and ponds and so forth because of inadequate treatments before disposal from the industries.6 Many of these aromatic nitro compounds and organic dyes have been reported to be carcinogenic, used as skin sensitizers, and are capable of causing methemoglobinemia.7 The hazardous effluent from various industries could cause a serious problem for aquatic as well as for terrestrial life, and their proper treatment before their disposal is the need of hour.8
The scientific community for environmental remediation is constantly developing new protocols. Various industrial effluent management strategies have been utilized, including bacterial treatment, coagulation, chemical oxidation, adsorption, photocatalytic degradation, and many more.9−11 Several procedures are being employed to reduce the environmental pollution, and a wide range of strategies could be applied to accomplish this purpose.12 In the recent years, nanotechnology has attracted significant interest because of its applications in different areas of scientific research having a direct impact on improving human life.
Nanostructured inorganic solid support materials are considered as highly efficient materials for various applications such as catalysis, sensing, energy production, and so forth.13,14 Because of properties such as high surface area, high thermal stability, and possibility to functionalize with active groups, inorganic solid support materials have been widely used in different fields of scientific research, including biomedical applications, chemical sensing, adsorption, and heterogeneous catalysis. An inorganic solid support material when employed in catalysis has the advantage of further functionalization with a variety of different catalytic moieties and could also be recycled and reused in multiple catalytic cycles.15 Heterogeneous materials furnish large surface area for the adsorption of reactant molecules on their surface that could result in an increased reaction rate. Heterogeneous catalysis based on the use of inorganic solid support materials has drawn massive interest as an enticing method for environmental remediation. Recently, several reports have been published, involving the use of heterogeneous nanocatalysts being extensively employed for the reduction and degradation of various organic pollutants and for the generation of green energy.16−19
Because of diverse applications, nanocarbons such as graphene, carbon nitride, and boron carbon nitride have aroused enormous attention.20−22 These materials have a wide range of applications in numerous research fields including energy storage, environmental remediation, biomedicine, and heterogeneous catalysis.23−26 Carbon nitride is a family of polymeric structures mainly composed of carbon and nitrogen.27 Because of its simplistic preparation approach, the minimal cost of production, and controllable electronic properties, graphitic carbon nitride (g-C3N4) has drawn enormous attention in recent years.9,28 In addition, its remarkable thermal stability (up to 600 °C in air)29 and stability in neutral, basic, and acidic medium make it a potent material for various applications including solid support materials for heterogeneous catalysis.30 These g-C3N4 materials can be easily prepared from carbon-containing sources, replacing some carbon atoms with nitrogen atoms. g-C3N4 is not only the strong carbon nitride allotrope in the natural medium, but it also has diverse surface features that are appealing for catalytic applications. Because of the presence of hydrogen and nitrogen sites on its surface, g-C3N4 could be easily functionalized with different catalytic active groups including metal and bimetallic nanoparticles.31 g-C3N4 has been reported as an efficient catalyst for a variety of reactions including oxygen reduction,32−34 hydrogen evolution,35−37 dye degradation,38,39 and various other chemical transformations.40,41
In the present study, nitrogen-rich g-C3N4 was synthesized by a simple direct thermal polymerization procedure, followed by the immobilization of Pd nanoparticles on its surface. Here, g-C3N4 has been utilized as a support material to synthesize a heterogeneous catalyst. The catalytic efficiency of palladium-supported g-C3N4 was determined for the degradation of organic dyes and the reduction of aromatic nitro compounds. The synthesized catalyst exhibited excellent catalytic efficiency and could be reused for multiple catalytic cycles without any appreciable loss in its activity.
2. Results and Discussion
Nitrogen-rich g-C3N4 was synthesized by a simple approach. The synthesized g-C3N4 nanosheets were exfoliated using HCl to make their surface a few nanometers thick. The ultrathin surface was achieved by simply mixing g-C3N4 in 3 M HCl.42 Palladium nanoparticles were immobilized on the surface of as-synthesized g-C3N4 by using NaBH4 as a reducing agent. The immobilization of metal nanoparticles could be confirmed by various physio-chemical techniques. The prepared nanocatalyst was employed for the reduction of aromatic nitro compounds to their corresponding amine derivatives. Organic dyes were also efficiently degraded by employing the prepared nanocatalyst.
2.1. Characterization
X-ray diffraction (XRD) technique was used to examine the structure of g-C3N4 and Pd/g-C3N4 (Figure 1a). As depicted in Figure 1a, a small peak (100) is observed at 13.1°, which can be attributed to the in-plane structural packing motif between nitride planes.43−45 One strong peak (002) observed at 27.4° for g-C3N4 is attributed to the interlayer stacking peak of the aromatic system.46−48 After immobilization of the Pd nanoparticle, the peak at 13.1° (100) disappeared because of the disturbance caused by the Pd nanoparticle in a g-C3N4 structure.43 Another peak at 39.5 and 46.1° appeared, which can be attributed to the (111) and (200) planes of the Pd nanoparticle, respectively. From the above analysis of these two peaks, it can be concluded that loading of the Pd nanoparticle on the g-C3N4 nanosheet is successful.
Figure 1.
(a) Powder XRD pattern of Pd/g-C3N4 and g-C3N4; (b) TEM image of the synthesized g-C3N4 showing a sheet-like architecture; (c) SEM images of g-C3N4; and (d) N2 adsorption–desorption isotherm of Pd/g-C3N4. (Inset) Pore size distribution of Pd/g-C3N4; (e) TEM image of Pd/g-C3N4 showing Pd nanoparticles on the g-C3N4 nanosheet and (f) SEM image showing Pd/g-C3N4.
In material science, transmission electron microscopy (TEM) is a vital tool for investigating the shape and size of the particle at the nanoscale. Scanning electron microscopy (SEM) and TEM instruments were used in order to examine the morphology of as-synthesized g-C3N4 and Pd-supported g-C3N4 nanomaterials. The TEM images were captured at various magnifications to investigate the morphology and size. The TEM images (Figure 1b) clearly show that g-C3N4 has a sheet-like structure forming several layers. g-C3N4 sheets can be analyzed by merely looking at the images. The Pd-supported g-C3N4 TEM image (Figures 1e and S4) indicates that the average size of Pd particles is approximately 3–3.5 nm. Upon immobilization of Pd nanoparticles, there was zero effect on the morphology of g-C3N4 nanosheets. Pd particles can be seen uniformly distributed on the g-C3N4 surface, although only a few Pd particles are aggregated on the g-C3N4 surface. SEM images have also shown a layered structure of g-C3N4 (Figure 1c) and Pd/g-C3N4 (Figure 1f) materials. Both techniques come up with a conclusion that the synthesized nanomaterials have a sheet-like structure and that Pd nanoparticles are perfectly distributed on the graphitic surface. The energy-dispersive X-ray spectroscopy (EDS) analysis (integrated with SEM) of Pd/g-C3N4 (Figure S3) and g-C3N4 (Figure S2) was performed in order to find the chemical composition of the synthesized materials. In the EDS mapping of g-C3N4 (Figure S2), the characterization peak of carbon and nitrogen appeared in the spectra, confirming the presence of carbon and nitrogen in as-synthesized g-C3N4. The EDS mapping obtained from the SEM image of Pd/g-C3N4 (Figure S3) shows elemental distribution of palladium, carbon, and nitrogen. The presence of a palladium peak in the EDS spectra confirmed the immobilization of palladium on g-C3N4. Carbon, hydrogen, and nitrogen (CHN) analysis (Table S1) was also performed in order to investigate the composition of total carbon and nitrogen in a synthesized material. EDS and CHN analyses confirmed that the synthesized material is nitrogen-rich and that the stoichiometry of the material is C3N4.8 (Table S1). The exact amount of the metal content in Pd/g-C3N4 was determined by inductively coupled plasma-atomic emission spectroscopy (ICP-AES) analysis. ICP-AES results reveal that the amount of Pd in Pd/g-C3N4 is 3.98 wt %.
The Brunauer–Emmett–Teller (BET) surface area, pore volume, and pore diameter were recorded by nitrogen adsorption–desorption measurement. For Pd/g-C3N4, the BET surface area of 27.14 m2/g was observed. The Pd/g-C3N4 material has a porous structure, which was observed through the Barrett–Joyner–Halenda adsorption pore size distribution curve. A pore size diameter of 3.824 nm and a pore volume of 0.15 cm3/g were observed. The observation indicates that the synthesized Pd/g-C3N4 is highly porous.
Fourier transform infrared spectroscopy (FT-IR) spectra for g-C3N4 (Figure S1) were recorded, and three different major IR regions were observed. The sharp peak of around 820 cm–1 often belongs to the C–N heterocyclic framework. It stems from a heptane ring system.49 The peaks in between 1200 and 1700 cm–1 can be attributed to the stretching vibrations of C=N, C–N heptazine-derived repeating units.50 The broad peak of 3000–3600 cm–1 is attributed to the N–H stretching of an amino functional group. The bunch of peaks between 850 and 1800 cm–1 corresponds to the stretching modes of s-triazine derivatives. The peaks around 1400, 1300, and 1250 cm–1 belong to the C–N bond stretching modes. The FTIR spectra of Pd/g-C3N4 (Figure S1) indicate that there is no notable peak with respect to g-C3N4 spectra, suggesting no covalent bonding between g-C3N4 and metal nanoparticles.43
2.2. Catalytic Activity
To investigate the activity of the synthesized nanocatalyst (Pd/g-C3N4), it was utilized as a heterogeneous catalyst for the reduction of toxic aromatic nitro compounds to their corresponding amine derivatives. The activity of the prepared nanocatalyst was also checked for the degradation of hazardous organic dyes.
2.2.1. Catalytic Reduction of Nitro Compounds
We chose the catalytic reduction of 4-nitrophenol (4-NP) to the corresponding aromatic amine, that is, 4-aminophenol (4-AP) as a preliminary model reaction by using NaBH4 as a hydrogen source to assess the catalytic performance of the prepared nanocatalyst (Pd/g-C3N4). The reduction reaction was easily monitored through UV–visible absorption spectroscopy. The aqueous solution of 4-NP was light yellow in color and showed an absorption peak at 400 nm; addition of NaBH4 to the aqueous solution of 4-NP converts it into a 4-nitrophenolate ion with an absorption peak at 400 nm in UV–vis spectra. Light yellow color changes into a deep yellow color because of the change in alkalinity (pH) of the mixture, thus resulting in the formation of a 4-nitrophenolate ion. After the addition of 100 μL of the synthesized nanocatalyst (Pd/g-C3N4), the peak intensity at 400 nm decreases rapidly over a while, and a new peak of the corresponding amine emerged at 300 nm (Figure 2A). After 60 s, the peak at 400 nm completely vanished, indicating the complete reduction of 4-NP. Upon visual observation of the cuvette, the color of the reaction mixture changed from bright yellow to colorless. Another set of reaction was monitored without the addition of a catalyst, and it was observed that the absorption peak of 4-nitrophenolate ions at 400 nm remained consistent for 30 min without any change, indicating that the reaction proceeds in the presence of a catalyst, that is, Pd/g-C3N4 (Figure S4). g-C3N4 sheets without the immobilization of palladium nanoparticles were also utilized for the reduction of 4-NP, and it was observed that the reaction did not proceed even in 460 s (Figure S5), indicating that the metal nanoparticles are necessary for the reaction to proceed. The catalytic activity of the prepared catalyst, that is, Pd, was compared with some of the reported catalyst for the same reaction, and the results are presented in the Supporting Information (Table S3). We observed that the catalytic activity of the prepared catalyst is much superior to that of the reported catalyst for the same reaction.
Figure 2.
UV–vis absorption spectra for the reduction of 0.2 mM (a) 4-NP; (b) 4-NA; and (c) 2-NA in the presence of 0.2 mg of Pd/g-C3N4 as the nanocatalyst. (Inset) Absorbance vs time plot and (d) plot of ln(Ct/C0) vs time representing first-order kinetics for the reduction of nitroarenes.
A similar procedure was applied to investigate the activity of the prepared nanocatalyst for the reduction of 2-NA and 4-NA. In the case of reduction of 4-NA, the initial absorption peak observed at 405 nm in UV–vis spectra gradually decreased after the addition of 100 μL of the synthesized nanocatalyst. After 60 s, the absorption peak at 405 nm completely disappeared, and a new peak emerged at 300 nm, indicating the formation of the corresponding aromatic amine product (Figure 2B). The yellow colored reaction mixture of 4-NA also changes to the colorless solution. Similarly, 2-NA was reduced to the corresponding amine product by utilizing 100 μL of Pd/g-C3N4 (0.2 mg/mL) as the nanocatalyst in the presence of NaBH4. The initial absorption peak appeared at 408 nm in UV–vis spectra completely vanished after 80 s upon the addition of a nanocatalyst (Figure 2C).
The reactions are supposed to follow first-order kinetics because the concentration of NaBH4 is much higher than that of aromatic nitro compounds, and it remains almost constant during the course of reaction. Therefore, first-order kinetics is applied to calculate the efficiency of the Pd/g-C3N4 nanocatalyst. The value of (Ct/C0) can be determined easily by measuring the relative absorbance intensity (At/A0), where At and A0 denote the absorbance values at t = t and t = 0, respectively. The value of rate constant k could be calculated simply by plotting linear plots of ln(Ct/C0) versus time, and the slope of the linear plot gives value of the rate constant (Figure 2D). The rate constant was normalized and reformed in terms of activity parameter. The values of rate constant, activity parameter, and reduction time have been presented in Table1. The activity parameter K was determined by utilizing the rate constant as stated in eq 1.
| 1 |
k = rate constant, m = amount of the catalyst.
Table 1. Summary of the Correlation Coefficient of ln(Ct/C0) vs Time Plot, Reduction Time, Rate Constant, and Activity Parameter for the Reduction of Nitroarenes Using Pd/g-C3N4 as a Heterogeneous Nanocatalyst.
| sample | R2 | reduction time (s) | k (× 10–3 s–1) | activity parameter (K = s–1 g–1) |
|---|---|---|---|---|
| 4-nitro phenol (4-NP) | 0.9989 | 60 | 4.21 | 21.05 |
| 2-nitro aniline (4-NA) | 0.9644 | 80 | 2.15 | 10.75 |
| 4-nitro aniline (2-NA) | 0.9637 | 60 | 2.49 | 12.45 |
2.2.2. Catalytic Degradation of Organic Dyes
Dyes such as methylene blue (MB), methyl red (MR), and rhodamine B (RhB) are the main coloring substances used in textiles, paper, leather, and other industries. These dyes have various biological and industrial applications. Because of their harmful effects, these dyes need to be degraded before their discharge into aquatic bodies. Here, we have utilized the synthesized Pd/g-C3N4 nanocatalyst for the degradation of few organic dyes such as RhB, MB, and MR.
Initially, the degradation of RhB was studied by utilizing Pd/g-C3N4 as a heterogeneous nanocatalyst in the presence of NaBH4. The absorption peak for 2 mL of RhB (0.02 mM) appeared at 554 nm, and after the addition of the aqueous solution of 100 μL of the Pd/g-C3N4 (0.2 mg/mL) nanocatalyst, this peak at 554 nm gradually started decreasing and completely disappeared after 60 s, indicating the complete degradation of RhB (Figure 3A). A similar procedure was applied for the degradation of MR and MB. The absorption peak for the aqueous solution of MR appeared at 425 nm in the presence of NaBH4 and started decreasing after the addition of the aqueous solution of Pd/g-C3N4 and vanished after 120 s (Figure 3B). Similarly, the absorption peak of the aqueous solution of MB in the presence of NaBH4 at 663 completely disappeared after 80 s upon the addition of the prepared nanocatalyst. The characteristic peak appeared at 663 nm; after the addition of the catalyst, the peak started decreasing and disappeared after 80 s (Figure 3C). All the three dyes became colorless upon complete degradation. The BH4– anions get absorbed on the g-C3N4 nanosheet, and the electrons contributed by NaBH4 (BH4– ions) to palladium nanoparticles through the g-C3N4 nanosheet are taken up by organic dyes, which ultimately lead to the degradation of dyes.51 The graphical representation of the proposed mechanism for the degradation of dyes is depicted in Figure 3.
Figure 3.
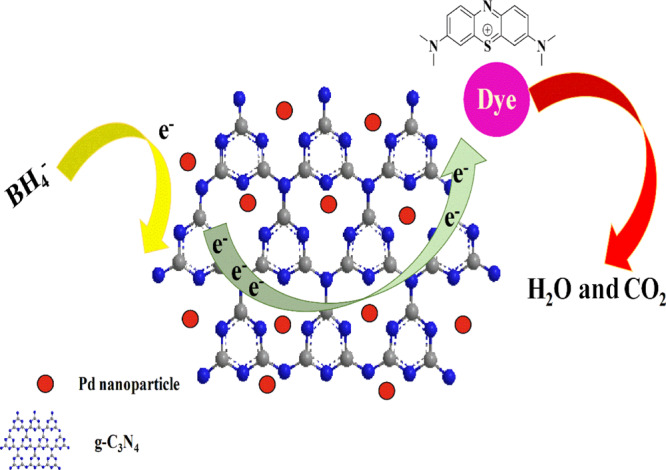
Graphical representation of the mechanism for dye degradation.
The rate constant “k” and activity parameter were calculated in a similar way as previously calculated for nitro compounds, and the results are reported in Table 2.
Table 2. Correlation Coefficient of ln(Ct/C0) vs Time Plot, Reduction Time, Rate Constant, and Activity Parameter for the Reduction of Dyes Using Pd/g-C3N4 as a Heterogeneous Nanocatalyst.
| sample | R2 | reduction time (s) | k (× 10–3 s–1) | activity parameter (K = s–1 g–1) |
|---|---|---|---|---|
| MR | 0.99462 | 120 | 3.78 | 12.6 |
| MB | 0.95577 | 80 | 2.04 | 6.8 |
| RhB | 1 | 60 | 2.11 | 7.03 |
2.2.3. Effect of Catalyst Loading on the Catalytic Efficiency
The effect of catalyst loading was also studied by choosing the reduction of 4-NP and degradation of MR as model reactions. Figure 4A,B shows the absorbance spectra of 4-NP for different catalytic loadings while keeping other parameters constant. It was observed that when the concentration of the catalyst, that is, aqueous solution of Pd/g-C3N4, was reduced from 100 to 50 μL and 25 μL, the time for the complete reduction of 4-NP was increased to 140 and 240 s, respectively (Figure 4A,B). Similarly, the degradation of MR was also studied with 50 and 25 μL of aqueous solution of Pd/g-C3N4, and we observed that the degradation was completed in 240 and 340 s, respectively (Figure 4C,D). This study suggests that 100 μL is the ideal amount of the catalyst. The rate constant k could be calculated simply by plotting linear plots of ln(Ct/C0) versus time, and the slope of the linear plot gives the value of the rate constant (Figure S6). The values of the rate constant, reduction time, and activity parameter for the reduction of 4-NP and degradation of MR with different loadings of the catalyst are given in Table S2.
Figure 4.
UV–vis absorption spectra of degradation of 0.02 mM (a) RhB; (b) MB; and (c) MR by NaBH4 (0.4 M) in the presence of 0.2 mg of Pd/g-C3N4 as the nanocatalyst. (Inset) Absorbance vs time plot and (d) plot of ln(Ct/C0) vs reaction time representing first-order kinetics for the degradation of organic dyes.
2.2.4. Reusability Test
In order to check the reusability of the catalyst, that is, Pd/g-C3N4, the reduction of 4-NP and degradation of RhB were chosen as model reactions. After the completion of the reaction, the catalyst was separated from the reaction mixture and reused for five consecutive catalytic cycles. We observed a negligible loss in its activity. In the fifth cycle, the reduction of 4-NP and degradation of RhB were completed in 95 and 100 s, respectively. In order to check the stability of the synthesized catalyst, CHN and EDS analyses of the reused catalyst were studied (Table S1 and Figure S8), and the results demonstrate that no appreciable loss in the elemental composition of the reused catalyst could be observed (Figures 5 and 6).
Figure 5.
UV–vis absorption spectra of reduction of (a) 4-NA (50 μL of Pd/g-C3N4); (b) 4-NA (25 μL of Pd/g-C3N4); (c) MR (50 μL of Pd/g-C3N4); and (d) MR (25 μL of Pd/g-C3N4) using Pd/g-C3N4 as a nanocatalyst.
Figure 6.

Recyclability test of Pd/g-C3N4 for the reduction of 4-NP and degradation of RhB.
3. Conclusions
In summary, we have synthesized N-rich g-C3N4 nanosheets by a thermal polymerization approach. In the next step of the synthesis procedure, palladium nanoparticles were uniformly anchored over the surface of g-C3N4 using NaBH4 as a reducing agent. The characterization of the prepared materials was carried out by various techniques such as TEM, SEM, XRD, BET, CHN, EDS, and FTIR. Subsequent to the characterization, the prepared material, that is, Pd/g-C3N4, was checked for its catalytic activity for the reduction of aromatic nitro compounds and the degradation of organic dyes. The prepared catalyst showed excellent activity and could be reused in multiple catalytic cycles with a very little loss in its activity.
4. Experimental Section
4.1. Synthesis of g-C3N4 Sheets
The synthesis of a carbon nitride nanosheet has been achieved by a direct thermal polymerization procedure. In the typical synthesis procedure, melamine (3 g) and ammonium carbonate (3 g) were mixed and placed in the aluminum crucible. The mixture was heated (2 °C/min) in air at 550 °C and kept for 5 h for polymerization.42 As a result, a yellow colored material was obtained, and it was crushed into powder. Furthermore, the obtained powder was transferred into a 250 mL conical flask, 30 mL of 3 M HCl was poured into it, and the mixture was stirred for 15 h. The mixture was centrifuged, and the solid product was washed multiple times with water. Finally, the solid product was collected and dried for 10 h at 60 °C in a vacuum oven.
4.2. Synthesis of Pd/g-C3N4
The heterogeneous nanocatalyst was synthesized by an ultrasonic deposition method. In the synthesis process, the as-prepared g-C3N4 (1 g) was added into 60 mL of water under ultrasonication for 3 h, followed by the addition of PdCl2 (25 mg), and the mixture was ultrasonicated for another 40 min. Then, 5 mL of NaBH4 (0.5 M) solution was added dropwise, and the mixture was stirred for 1 h. Finally, the solid product was collected by centrifugation and washed multiple times with water to remove impurities. The product was dried in a vacuum oven at 60 °C for 5 h.
4.3. Procedure for the Catalytic Reduction of Nitro Compounds
Typically, 0.2 mL of aqueous solution of the aromatic nitro compound (0.2 mM) was taken in a standard cuvette containing a 3 mL volume and a 1 cm path length, followed by the addition of freshly prepared 0.5 mL of NaBH4 (0.4 M). After that, 100 μL of the synthesized nanocatalyst, that is, Pd/g-C3N4 (0.2 mg/mL), was added in the same cuvette. The progress of the reaction was monitored through UV–vis absorption spectroscopy, which was recorded immediately after the addition of a nanocatalyst at room temperature.
4.4. Procedure for the Catalytic Degradation of Dyes
Typically, 0.2 mL of aqueous solution of the organic dye (0.02 mM) was taken into a standard cuvette containing a 3 mL volume and a 1 cm path length, followed by the addition of freshly prepared 0.5 mL of NaBH4 (0.4 M). After that, 100 μL of the synthesized nanocatalyst, that is, Pd/g-C3N4 (0.2 mg/mL), was added in the same cuvette. The progress of the reaction was monitored through UV–vis absorption spectroscopy, which was recorded immediately after the addition of a nanocatalyst at room temperature.
Acknowledgments
Y.K. thanks SAIF (Sophisticated Analytical Instrumentation Facility), AIIMS, New Delhi, for providing the advanced characterization facilities. The authors thank School of Sciences, IGNOU, for providing the laboratory facilities.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c01280.
Materials, characterization techniques, FTIR/EDS/CHN/TEM of different samples, particle size distribution histogram, UV–vis absorption spectra, ln(Ct/C0) versus reduction time plot, rate constant, activity parameter, and activity comparison (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Keiser D. A.; Shapiro J. S. Consequences of the Clean Water Act and the demand for water quality. Q. J. Econ. 2019, 134, 349–396. 10.1093/qje/qjy019. [DOI] [Google Scholar]
- Jayalakshmi R.; Jeyanthi J. Simultaneous removal of binary dye from textile effluent using cobalt ferrite-alginate nanocomposite: Performance and mechanism. Microchem. J. 2019, 145, 791–800. 10.1016/j.microc.2018.11.047. [DOI] [Google Scholar]
- Zazouli M. A.; Ghanbari F.; Yousefi M.; Madihi-Bidgoli S. Photocatalytic degradation of food dye by Fe3O4–TiO2 nanoparticles in presence of peroxymonosulfate: The effect of UV sources. J. Environ. Chem. Eng. 2017, 5, 2459–2468. 10.1016/j.jece.2017.04.037. [DOI] [Google Scholar]
- Pérez-Ibarbia L.; Majdanski T.; Schubert S.; Windhab N.; Schubert U. S. Safety and regulatory review of dyes commonly used as excipients in pharmaceutical and nutraceutical applications. Eur. J. Pharm. Sci. 2016, 93, 264–273. 10.1016/j.ejps.2016.08.026. [DOI] [PubMed] [Google Scholar]
- Rai H. S.; Bhattacharyya M. S.; Singh J.; Bansal T. K.; Vats P.; Banerjee U. C. Removal of dyes from the effluent of textile and dyestuff manufacturing industry: a review of emerging techniques with reference to biological treatment. Crit. Rev. Environ. Sci. Technol. 2005, 35, 219–238. 10.1080/10643380590917932. [DOI] [Google Scholar]
- Sahoo A.; Patra S. A Combined Process for the Degradation of Azo-Dyes and Efficient Removal of Aromatic Amines Using Porous Silicon Supported Porous Ruthenium Nanocatalyst. ACS Appl. Nano Mater. 2018, 1, 5169–5178. 10.1021/acsanm.8b01152. [DOI] [Google Scholar]
- Woo Y.-T.; Lai D. Y.. Aromatic amino and nitro–amino compounds and their halogenated derivatives. Patty’s Toxicology; American Cancer Society, 2001; pp 1–96. [Google Scholar]
- Ahmad A.; Mohd-Setapar S. H.; Chuong C. S.; Khatoon A.; Wani W. A.; Kumar R.; Rafatullah M. Recent advances in new generation dye removal technologies: novel search for approaches to reprocess wastewater. RSC Adv. 2015, 5, 30801–30818. 10.1039/c4ra16959j. [DOI] [Google Scholar]
- Hu F.; Luo W.; Hu Y.; Dai H.; Peng X. Insight into the kinetics and mechanism of visible-light photocatalytic degradation of dyes onto the P doped mesoporous graphitic carbon nitride. J. Alloys Compd. 2019, 794, 594–605. 10.1016/j.jallcom.2019.04.235. [DOI] [Google Scholar]
- Liu X.; Jin A.; Jia Y.; Xia T.; Deng C.; Zhu M.; Chen C.; Chen X. Synergy of adsorption and visible-light photocatalytic degradation of methylene blue by a bifunctional Z-scheme heterojunction of WO3/g-C3N4. Appl. Surf. Sci. 2017, 405, 359–371. 10.1016/j.apsusc.2017.02.025. [DOI] [Google Scholar]
- Liang B.; Zhang P.; Wang J.; Qu J.; Wang L.; Wang X.; Guan C.; Pan K. Membranes with selective laminar nanochannels of modified reduced graphene oxide for water purification. Carbon 2016, 103, 94–100. 10.1016/j.carbon.2016.03.001. [DOI] [Google Scholar]
- Guerra F.; Attia M.; Whitehead D.; Alexis F. Nanotechnology for environmental remediation: materials and applications. Molecules 2018, 23, 1760. 10.3390/molecules23071760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R.; Duay J.; Lee S. B. Heterogeneous nanostructured electrode materials for electrochemical energy storage. Chem. Commun. 2011, 47, 1384–1404. 10.1039/c0cc03158e. [DOI] [PubMed] [Google Scholar]
- Zhao H.; Dong Y.; Wang G.; Jiang P.; Zhang J.; Wu L.; Li K. Novel magnetically separable nanomaterials for heterogeneous catalytic ozonation of phenol pollutant: NiFe2O4 and their performances. Chem. Eng. J. 2013, 219, 295–302. 10.1016/j.cej.2013.01.019. [DOI] [Google Scholar]
- Xie K.; He Y.; Zhao Q.; Shang J.; Gu Q.; Qiao G. G.; Webley P. A. Pd(0) loaded Zn2(azoBDC)2(dabco) as a heterogeneous catalyst. CrystEngComm 2017, 19, 4182–4186. 10.1039/c6ce02447e. [DOI] [Google Scholar]
- Truppi A.; Petronella F.; Placido T.; Striccoli M.; Agostiano A.; Curri M. L.; Comparelli R. Visible-light-active TiO2-based hybrid nanocatalysts for environmental applications. Catalysts 2017, 7, 100. 10.3390/catal7040100. [DOI] [Google Scholar]
- Meng X.; Wang T.; Liu L.; Ouyang S.; Li P.; Hu H.; Kako T.; Iwai H.; Tanaka A.; Ye J. Photothermal conversion of CO2 into CH4 with H2 over Group VIII nanocatalysts: an alternative approach for solar fuel production. Angew. Chem., Int. Ed. 2014, 53, 11478–11482. 10.1002/anie.201404953. [DOI] [PubMed] [Google Scholar]
- Xie H.; Wang T.; Liang J.; Li Q.; Sun S. Cu-based nanocatalysts for electrochemical reduction of CO2. Nano Today 2018, 21, 41–54. 10.1016/j.nantod.2018.05.001. [DOI] [Google Scholar]
- Sakla R.; Kaushik R.; Kumar V.; Jose D. A.; Ghosh A.; Mariappan C. R. Light-induced water oxidation by polymorphs of the Zn–Co–Ni oxide spinel catalyst: a comparative study. Sustainable Energy Fuels 2019, 3, 786–792. 10.1039/c8se00536b. [DOI] [Google Scholar]
- Yan S. C.; Li Z. S.; Zou Z. G. Photodegradation of rhodamine B and methyl orange over boron-doped g-C3N4 under visible light irradiation. Langmuir 2010, 26, 3894–3901. 10.1021/la904023j. [DOI] [PubMed] [Google Scholar]
- Wang X.; Blechert S.; Antonietti M. Polymeric graphitic carbon nitride for heterogeneous photocatalysis. ACS Catal. 2012, 2, 1596–1606. 10.1021/cs300240x. [DOI] [Google Scholar]
- Thomas A.; Fischer A.; Goettmann F.; Antonietti M.; Müller J.-O.; Schlögl R.; Carlsson J. M. Graphitic carbon nitride materials: variation of structure and morphology and their use as metal-free catalysts. J. Mater. Chem. 2008, 18, 4893–4908. 10.1039/b800274f. [DOI] [Google Scholar]
- Gogotsi Y. Not just graphene: The wonderful world of carbon and related nanomaterials. MRS Bull. 2015, 40, 1110–1121. 10.1557/mrs.2015.272. [DOI] [Google Scholar]
- Brennan L. J.; Byrne M. T.; Bari M.; Gun’ko Y. K. Carbon nanomaterials for dye-sensitized solar cell applications: a bright future. Adv. Energy Mater. 2011, 1, 472–485. 10.1002/aenm.201100136. [DOI] [Google Scholar]
- Pyun J. Graphene oxide as catalyst: application of carbon materials beyond nanotechnology. Angew. Chem., Int. Ed. 2011, 50, 46–48. 10.1002/anie.201003897. [DOI] [PubMed] [Google Scholar]
- Luo B.; Liu S.; Zhi L. Chemical approaches toward graphene-based nanomaterials and their applications in energy-related areas. Small 2012, 8, 630–646. 10.1002/smll.201101396. [DOI] [PubMed] [Google Scholar]
- Liu A. Y.; Cohen M. L. Prediction of new low compressibility solids. Science 1989, 245, 841–842. 10.1126/science.245.4920.841. [DOI] [PubMed] [Google Scholar]
- Yu H.; Shi R.; Zhao Y.; Bian T.; Zhao Y.; Zhou C.; Waterhouse G. I. N.; Wu L.-Z.; Tung C.-H.; Zhang T. Alkali-assisted synthesis of nitrogen deficient graphitic carbon nitride with tunable band structures for efficient visible-light-driven hydrogen evolution. Adv. Mater. 2017, 29, 1605148. 10.1002/adma.201605148. [DOI] [PubMed] [Google Scholar]
- Zhu J.; Wei Y.; Chen W.; Zhao Z.; Thomas A. Graphitic carbon nitride as a metal-free catalyst for NO decomposition. Chem. Commun. 2010, 46, 6965–6967. 10.1039/c0cc01432j. [DOI] [PubMed] [Google Scholar]
- Zhu J.; Xiao P.; Li H.; Carabineiro S. A. C. Graphitic Carbon Nitride: Synthesis, Properties, and Applications in Catalysis. ACS Appl. Mater. Interfaces 2014, 6, 16449–16465. 10.1021/am502925j. [DOI] [PubMed] [Google Scholar]
- Su F.; Antonietti M.; Wang X. mpg-C3N4 as a solid base catalyst for Knoevenagel condensations and transesterification reactions. Catal. Sci. Technol. 2012, 2, 1005–1009. 10.1039/c2cy00012a. [DOI] [Google Scholar]
- Fu X.; Hu X.; Yan Z.; Lei K.; Li F.; Cheng F.; Chen J. Template-free synthesis of porous graphitic carbon nitride/carbon composite spheres for electrocatalytic oxygen reduction reaction. Chem. Commun. 2016, 52, 1725–1728. 10.1039/c5cc08897f. [DOI] [PubMed] [Google Scholar]
- He F.; Li K.; Yin C.; Wang Y.; Tang H.; Wu Z. Single Pd atoms supported by graphitic carbon nitride, a potential oxygen reduction reaction catalyst from theoretical perspective. Carbon 2017, 114, 619–627. 10.1016/j.carbon.2016.12.061. [DOI] [Google Scholar]
- Feng J.-J.; Chen L.-X.; Song P.; Wu X.-l.; Wang A.-J.; Yuan J. Bimetallic AuPd nanoclusters supported on graphitic carbon nitride: one-pot synthesis and enhanced electrocatalysis for oxygen reduction and hydrogen evolution. Int. J. Hydrogen Energy 2016, 41, 8839–8846. 10.1016/j.ijhydene.2016.03.108. [DOI] [Google Scholar]
- Wang X.; Zhou C.; Shi R.; Liu Q.; Waterhouse G. I. N.; Wu L.; Tung C.-H.; Zhang T. Supramolecular precursor strategy for the synthesis of holey graphitic carbon nitride nanotubes with enhanced photocatalytic hydrogen evolution performance. Nano Res. 2019, 12, 2385–2389. 10.1007/s12274-019-2357-0. [DOI] [Google Scholar]
- Ma L.; Fan H.; Fu K.; Lei S.; Hu Q.; Huang H.; He G. Protonation of graphitic carbon nitride (g-C3N4) for an electrostatically self-assembling carbon@ g-C3N4 core–shell nanostructure toward high hydrogen evolution. ACS Sustainable Chem. Eng. 2017, 5, 7093–7103. 10.1021/acssuschemeng.7b01312. [DOI] [Google Scholar]
- Yang S.; Gong Y.; Zhang J.; Zhan L.; Ma L.; Fang Z.; Vajtai R.; Wang X.; Ajayan P. M. Exfoliated graphitic carbon nitride nanosheets as efficient catalysts for hydrogen evolution under visible light. Adv. Mater. 2013, 25, 2452–2456. 10.1002/adma.201204453. [DOI] [PubMed] [Google Scholar]
- Ouedraogo S.; Chouchene B.; Desmarets C.; Gries T.; Balan L.; Fournet R.; Medjahdi G.; Bayo K.; Schneider R. Copper octacarboxyphthalocyanine as sensitizer of graphitic carbon nitride for efficient dye degradation under visible light irradiation. Appl. Catal., A 2018, 563, 127–136. 10.1016/j.apcata.2018.06.036. [DOI] [Google Scholar]
- Tiwari B.; Ram S. Biogenic Synthesis of Graphitic Carbon Nitride for Photocatalytic Degradation of Organic Dyes. ACS Omega 2019, 4, 10263–10272. 10.1021/acsomega.9b00989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J.; Fu Y.; He G.; Sun X.; Wang X. Green Suzuki–Miyaura coupling reaction catalyzed by palladium nanoparticles supported on graphitic carbon nitride. Appl. Catal., B 2015, 165, 661–667. 10.1016/j.apcatb.2014.10.072. [DOI] [Google Scholar]
- Xu J.; Shen K.; Xue B.; Li Y.-X. Microporous carbon nitride as an effective solid base catalyst for Knoevenagel condensation reactions. J. Mol. Catal. A: Chem. 2013, 372, 105–113. 10.1016/j.molcata.2013.02.019. [DOI] [Google Scholar]
- Antil B.; Kumar L.; Reddy K. P.; Gopinath C. S.; Deka S. Direct thermal polymerization approach to N-rich holey carbon nitride nanosheets and their promising photocatalytic H2 evolution and charge-storage activities. ACS Sustainable Chem. Eng. 2019, 7, 9428–9438. 10.1021/acssuschemeng.9b00626. [DOI] [Google Scholar]
- Zhao Y.; Tang R.; Huang R. Palladium supported on graphitic carbon nitride: an efficient and recyclable heterogeneous catalyst for reduction of nitroarenes and Suzuki coupling reaction. Catal. Lett. 2015, 145, 1961–1971. 10.1007/s10562-015-1600-x. [DOI] [Google Scholar]
- Martha S.; Nashim A.; Parida K. M. Facile synthesis of highly active gC3N4 for efficient hydrogen production under visible light. J. Mater. Chem. A 2013, 1, 7816–7824. 10.1039/c3ta10851a. [DOI] [Google Scholar]
- Zhang L.; He X.; Xu X.; Liu C.; Duan Y.; Hou L.; Zhou Q.; Ma C.; Yang X.; Liu R.; Yang F.; Cui L.; Xu C.; Li Y. Highly active TiO2/g-C3N4/G photocatalyst with extended spectral response towards selective reduction of nitrobenzene. Appl. Catal., B 2017, 203, 1–8. 10.1016/j.apcatb.2016.10.003. [DOI] [Google Scholar]
- Zhang J.; Chen X.; Takanabe K.; Maeda K.; Domen K.; Epping J. D.; Fu X.; Antonietti M.; Wang X. Synthesis of a carbon nitride structure for visible-light catalysis by copolymerization. Angew. Chem., Int. Ed. 2010, 49, 441–444. 10.1002/anie.200903886. [DOI] [PubMed] [Google Scholar]
- Yao F.; Li X.; Wan C.; Xu L.; An Y.; Ye M.; Lei Z. Highly efficient hydrogen release from formic acid using a graphitic carbon nitride-supported AgPd nanoparticle catalyst. Appl. Surf. Sci. 2017, 426, 605–611. 10.1016/j.apsusc.2017.07.193. [DOI] [Google Scholar]
- Liu G.; Tang R.; Wang Z. Metal-free allylic oxidation with molecular oxygen catalyzed by gC3N4 and N-hydroxyphthalimide. Catal. Lett. 2014, 144, 717–722. 10.1007/s10562-014-1200-1. [DOI] [Google Scholar]
- Fang L. J.; Li Y. H.; Liu P. F.; Wang D. P.; Zeng H. D.; Wang X. L.; Yang H. G. Facile fabrication of large-aspect-ratio g-C3N4 nanosheets for enhanced photocatalytic hydrogen evolution. ACS Sustainable Chem. Eng. 2017, 5, 2039–2043. 10.1021/acssuschemeng.6b02721. [DOI] [Google Scholar]
- Zou L.-R.; Huang G.-F.; Li D.-F.; Liu J.-H.; Pan A.-L.; Huang W.-Q. A facile and rapid route for synthesis of gC3N4 nanosheets with high adsorption capacity and photocatalytic activity. RSC Adv. 2016, 6, 86688–86694. 10.1039/c6ra20514c. [DOI] [Google Scholar]
- Mogha N. K.; Gosain S.; Masram D. T. Gold nanoworms immobilized graphene oxide polymer brush nanohybrid for catalytic degradation studies of organic dyes. Appl. Surf. Sci. 2017, 396, 1427–1434. 10.1016/j.apsusc.2016.11.182. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






