Abstract

A hydrazone ligand, (E)-6-(2-((2-hydroxynaphthalen-1-yl)methylene)hydrazinyl)nicotinohydrazide (H2L), was synthesized and characterized by spectroscopic methods. The reaction of H2L with CuCl2·2H2O in methanol gave Cu(II) coordination compound, [Cu(HL′)(Cl)]·CH3OH (1), which was characterized by elemental analysis and spectroscopic methods (Fourier transform infrared (FT-IR) and UV–vis). The structure of 1 was also determined by single-crystal X-ray analysis. Structural studies confirmed the formation of esteric group during the synthesis of 1. Compound 1 was immobilized on 3-aminopropyltriethoxysilane (APTS)-functionalized silica gel through the amidification reaction and the obtained heterogeneous coordination compound was utilized as a catalyst for the three-component azide–epoxide–alkyne cycloaddition reaction in water as a green solvent. The structural properties of the heterogeneous catalyst were characterized by a combination of FT-IR, UV–vis, thermogravimetric analysis (TGA), scanning electron microscopy (SEM) and energy-dispersive spectrometry (EDS) analyses. The effect of the amount of catalyst and temperature on the cycloaddition reaction was studied, and the obtained 1,2,3-triazoles were characterized by spectroscopic studies and single-crystal X-ray analysis. The catalytic investigations revealed that this catalytic system has high activity in the synthesis of β-hydroxy-1,2,3-triazoles. It was also found that the aromatic and aliphatic substituents on the alkyne and epoxide together with the reaction temperature have considerable effects on the activity and regioselectivity of this catalytic system.
Introduction
The green synthesis of nitrogen-based heterocyclic compounds has attracted great attention in pharmaceutical and medicinal chemistry during the recent years. In this line, 1,2,3-triazoles,1 by revealing a wide range of biological and pharmacological activities (such as antimicrobial,2 antiviral,3 anticonvulsants,4 anti-human immunodeficiency virus (anti-HIV)5 and anti-allergic6 activities), play a prominent role in medicinal chemistry. Additionally, this type of heterocycles, due to their unique chemical and structural properties, has received much attention in materials sciences and they have also some industrial applications such as dyes, sensors, agrochemicals, corrosion inhibitors, and photostabilizers.7 Therefore, the development of efficient, simple, novel, and green methods for the synthesis of 1,2,3-triazole derivatives has practical importance and are attractive in synthetic organic chemistry. The copper-catalyzed azide–alkyne cycloaddition (CuAAC) reaction is the most popular method for the construction of 1,2,3-triazoles, which is independently discovered by Sharpless and Meldal in 2002, and is well known as “click reaction”.8 Due to the explosive property of the low-molecular-weight (MW) organic azides and the difficulties in their handling, in situ generation of organic azides by the reaction of NaN3 with organic reagents like benzyl halides,9 epoxides,10 and aryl boronic acids11 has been introduced to create a wide range of 1,2,3-triazole derivatives. Among them, using epoxides for the production of β-hydroxy-1,2,3-triazoles have attracted the highest interest because of their importance in drugs and pharmaceuticals.12 β-Hydroxy-1,2,3-triazoles are also found in peptide surrogates of HIV-1 protease inhibitors.13
The investigations in catalytic systems show that heterogeneous catalysts are often preferred over homogeneous catalysts due to their reusability and also their faster and simpler isolation process from the reaction products.14 Therefore, some heterogeneous catalysts such as Cu(I)-modified zeolite,15 CuFe2O4 magnetic nanoparticles,16 copper nanoparticles on activated carbon,17 Cu(I) supported on alumina (Cu/Al2O3),18 and Cu(II)-hydrotalcite19 have been attained for the Huisgen 1,3-dipolar cycloaddition reaction.
The development of nontoxic and clean methods for carrying out organic reactions is one of the main goals of green chemistry.20 The immobilization of metal-based catalysts on a safe heterogeneous surface does meet both economical and green chemistry requirements. Silica gel by having several advantages, such as high chemical and thermal stability, low price, good accessibilities, adaptability with different catalytic systems, and also surface modification ability with various organic groups, has generated the most interest in preparing heterogeneous catalysts.21 Physical adsorption and chemical grafting of the functional groups are two usual methods for the surface modification of silica.22 In the chemical modification, the organic and inorganic components are linked together through strong covalent or coordinative bonds on the surface of silica. The chemical immobilization of metal coordination compounds on the silica surface is more attractive than physical adsorption because it offers unique advantages such as high catalytic efficiency, higher stability, and better recyclability.23 Nevertheless, using strong synthetic strategies and suitable materials that can connect to the surface of silica is the primary and important requirement of this method. Due to this, the design and synthesis of materials containing suitable functional groups to attach the catalysts on the surface of silica are the challenges in preparing silica-supported heterogeneous catalysts.
In most of the previously reported copper-based catalysts for the synthesis of 1,2,3-triazoles, the Cu(I) compounds or a combination of Cu(II) salts with a reducing agent (such as sodium ascorbate)24 have been employed. These methods by considering the difficulty in handling Cu(I) compounds and also using other reagents are not ideal for green chemistry. Thus, in recent years, the development of a new class of catalysts based on Cu(II) coordination compounds has attracted considerable attention.25 Based on the facts above, the development of high-performance heterogeneous systems containing Cu(II) ions with high stability, ecofriendly, and economically viable materials for the catalytic production of 1,2,3-triazoles is still highly desirable. Thus, in this paper, we report the design and catalytic activity of a new Cu(II)-based silica-supported heterogeneous catalyst for this reaction. By considering the high stability and activity of hydrazone-based coordination compounds in various catalytic reactions,26 we selected this type of ligand for preparing Cu(II) coordination compounds. The obtained compound containing ester functionality was characterized by spectroscopic methods and single-crystal X-ray analysis. In the next step, the heterogeneous silica-supported copper(II) catalyst was obtained by the immobilization of the obtained coordination compound on the silica surface. The catalytic investigations indicated that this catalytic system is an active and selective catalyst for the green synthesis of β-hydroxy-1,2,3-triazoles in water.
Results and Discussion
Synthesis and Spectroscopy
The hydrazone Schiff base ligand, (E)-6-(2-((2-hydroxynaphthalen-1-yl)methylene)hydrazinyl)nicotinohydrazide (H2L), was synthesized by the refluxing of equimolar amounts of 6-hydrazinonicotinic hydrazide hydrate with 2-hydroxy-1-naphthaldehyde in methanol (Scheme 1). The formation of H2L was confirmed by elemental analyses and Fourier transform infrared (FT-IR) and NMR spectroscopic studies. In the 1H NMR spectrum of H2L (Figure S1), the singlet peak at δ 9.07 ppm is due to azomethine (−CH=N) hydrogen. The hydrogen of phenolic O–H, amidic (=N–NH–C=O), and hydrazine (Py–NH–N=) appears at δ 11.70, 11.34, and 9.65 ppm, respectively.27 The broad peak at δ 4.46 ppm is due to the hydrogen atoms of the −NH2 moiety. By the addition of D2O to the NMR tube, these peaks were eliminated (see Figure S2). The aromatic hydrogen atoms are observed in δ 8.81–6.97 ppm. Seventeen peaks were observed in the 13C NMR spectrum of H2L (Figure S3), which is in good agreement with the proposed structure for H2L in Scheme 1. In the FT-IR spectrum of H2L (Figure S4), the bands at 1664, 1625, and 1523 cm–1 are due to amidic C=O, C=N (azomethine), and aromatic C=C functionalities of the ligand, respectively.28 The bands at 3272 and 3204 cm–1 are due to the NH groups, and the broad band at 3335 cm–1 is due to the phenolic OH group.29 These bands are relatively broad, which indicates that they participate in the formation of strong intermolecular hydrogen bond interactions. The reaction of copper(II) chloride dihydrate and H2L with 1:1 molar ratios in methanol leads to compound [Cu(HL′)Cl]·CH3OH (1). In the FT-IR spectrum of compound 1 (Figure S5), the absorption band at 1633 cm–1 can be assigned to the imine (C=N) stretching frequency of the coordinated ligand. Furthermore, in the FT-IR spectra of 1, one strong band at 1717 cm–1 is observed, which can be assigned to the esteric (C=O) stretching vibration.30 The formation of an esteric functionality was confirmed by other analyses like single-crystal X-ray diffraction studies. The elimination of −NH2 peaks (observed at 3272 cm–1 in the FT-IR spectrum of ligand) in the FT-IR spectrum of compound 1 confirms the elimination of hydrazide moiety (−NH–NH2) during the formation of a coordination compound. The weak peak at 3180 cm–1 in the FT-IR spectrum of compound 1 is due to the hydrazine (R–NH–N=) vibration. This peak is overlapped with the strong and broad peak of the OH group at 3419 cm–1, which is observed due to the presence of uncoordinated methanol molecule in the structure of compound 1.31 Further, the peaks at 669 and 447 cm–1 were observed, which might be attributed to Cu–O and Cu–N stretching vibrations, respectively.32
Scheme 1. Synthesis of (a) Ligand (H2L) and (b) Compound [Cu(HL′)(Cl)]·CH3OH (1).
The electronic absorption spectra of H2L and compound 1 in methanol are shown in Figure 1. The Schiff base ligand displays strong bands at 212, 240, 266, 334, 374, and 390 nm. These bands are assigned to intraligand π → π* (characteristic of π-bonds of the aromatic ring and C=N and C=O groups) and n → π* (characteristic of nonbonded electrons available on C=N and C=O groups) transitions. The UV–vis spectrum of compound 1 in the region of 200–350 nm is relatively similar to the UV–vis spectrum of the Schiff base ligand, and the shifts of the bands indicate the coordination of the ligand to Cu(II) ions. In the UV–vis spectrum of compound 1, the elimination of the n → π* band of free ligand (at 374 and 390 nm) together with the observation of a new broad band at about 450 nm can be attributed to the coordination of C=N and C=O groups to the metal ion. The band at 450 nm in the electronic spectrum of compound 1 is due to the ligand to metal charge transfer (LMCT) transitions. The band for the d–d transitions appears as a broad weak peak at around 600 nm. The thermal stability of compound 1 was examined by thermogravimetric analysis (TGA) in the range of 25–800 °C under a nitrogen atmosphere. The TGA curve of 1 (Figure S6) shows that the uncoordinated methanol molecule is removed between 100 and 130 °C. This curve also indicates that 1 is stable up to 150 °C, where the 3% of weight, probably coordinated Cl ion, is removed. Then, at 260 °C, the organic ligand is removed during three steps (260–300, 300–500, and 500–700 °C) with different rates of weight loss. The overall mass loss of up to 800 °C is equal to −79.34% of starting mass and indicates that the final product is CuO (calcd −82.37%).
Figure 1.
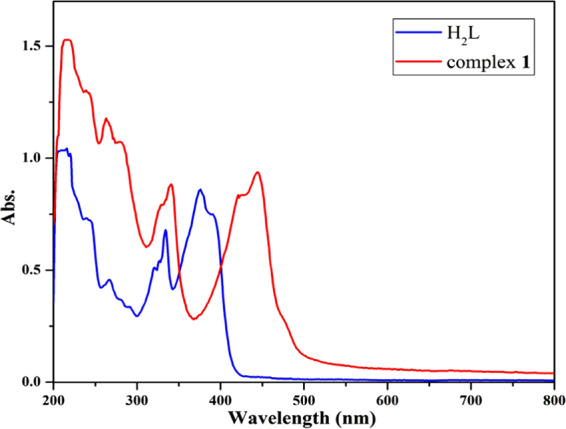
UV–vis spectra of H2L and compound 1 in methanol.
X-ray Structure of [Cu(HL′)(Cl)]·CH3OH (1)
The crystal structure of [Cu(HL′)(Cl)]·CH3OH (1) is shown in Figure 2, and the selected bond lengths and angles are collected in Table 1. The dark brown block crystals of compound 1 crystallize in the monoclinic crystal system (P21/n space group). X-ray analysis showed that 1 is a mononuclear coordination compound of Cu(II), which is obtained by the coordination of a Schiff base ligand and one chloride anion to the Cu(II) ion. There is an uncoordinated methanol molecule in the structure of 1, which is located beside the molecules of the coordination compound and stabilized in the crystal structure by hydrogen bond interactions. The Schiff base ligand acts as a tridentate NNO-donor ligand by coordination of naphtholic oxygen and the nitrogen atoms of pyridine and azomethine groups. The coordination geometry of Cu(II) ion can be described as a distorted square planar with a CuN2OCl environment. Diffraction studies indicate that the structure of the primary Schiff base ligand, H2L, is changed during the formation of a coordination compound in methanol solvent. The hydrazide group of H2L, −C(=O)–NH–NH2, is changed to an esteric functionality, −C(=O)–OCH3, by in situ esterification by methanol during the coordination of ligand to the metal ion. Such in situ esterification reactions during the formation of coordination compounds have been previously reported in the literature.33 Therefore, the H2L is converted to a new ligand, H2L′, in the structure of the final Cu(II) coordination compound. By considering the elimination of the hydrogen atom of the naphtholic −OH group, the ligand acts as a mononegative ligand, (HL′)−, in compound 1.
Figure 2.
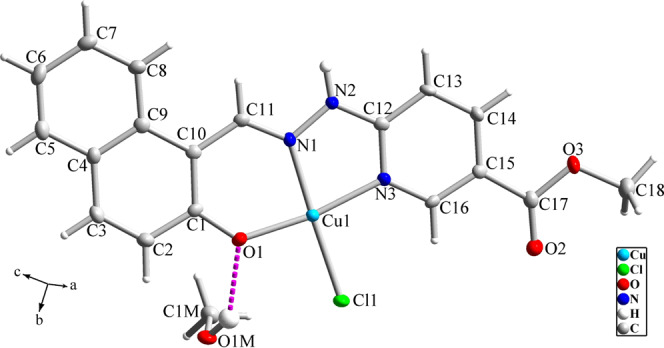
Molecular structure of compound 1 with the atom numbering scheme. The pink dashed line shows hydrogen bond interaction.
Table 1. Selected Bond Lengths (Å) and Angles (deg) in the Crystal Structure of Compound 1.
| bond | lengths (Å) | bond | angles (deg) |
|---|---|---|---|
| Cu1–O1 | 1.913(3) | O1–Cu1–N1 | 90.10(13) |
| Cu1–N1 | 1.944(3) | O1–Cu1–N3 | 171.41(13) |
| Cu1–N3 | 1.991(3) | N1–Cu1–N3 | 81.31(14) |
| Cu1–Cl1 | 2.2196(11) | O1–Cu1–Cl1 | 92.06(9) |
| N1–Cu1–Cl1 | 177.58(11) | ||
| N3–Cu1–Cl1 | 96.53(10) |
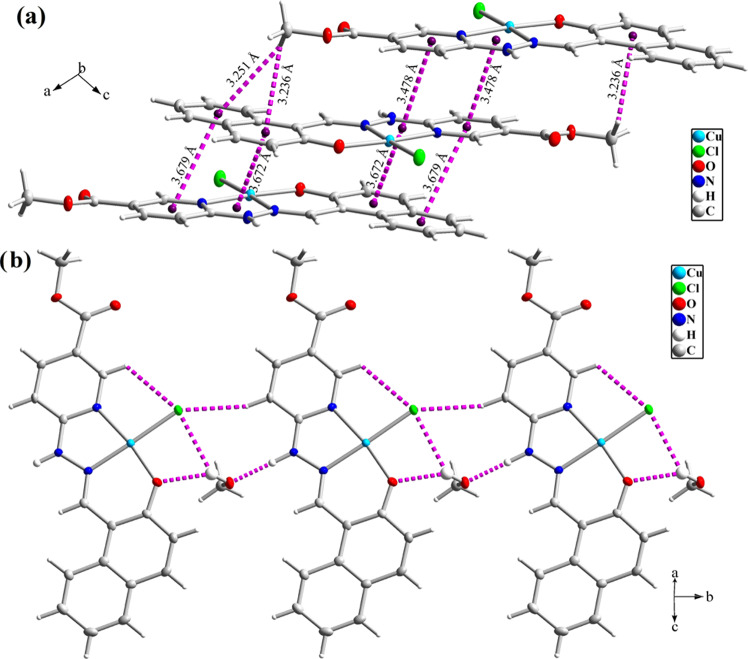
The crystal structure of compound 1 displays some directed π···π and C–H···π interactions (Figure 3a). Moreover, the O–H group of the uncoordinated methanol involves intermolecular O1M–H1M···O1 hydrogen bonds (see Table 2). The crystal packing of compound 1 is further stabilized by some other N2–H2···O1M and C1M–H1M3···O2ii interactions, where a one-dimensional (1D) polymeric network is generated by these intermolecular interactions (Figure 3b).
Figure 3.
(a) Intermolecular hydrogen bond interactions and (b) 1D polymeric chain in the crystal structure of 1.
Table 2. Hydrogen Bond Interactions in the Crystal Structure of Compounds 1, T2, and T3a.
| D–H···A | D–H | H···A | D···A | D–H···A |
|---|---|---|---|---|
| Compound 1 | ||||
| N2–H2N···O1Mi | 0.88 | 1.85 | 2.732(5) | 176 |
| O1M–H1M···Cl1 | 0.84 | 2.80 | 3.481(3) | 140 |
| O1M–H1M···O1 | 0.84 | 2.21 | 2.893(4) | 139 |
| C1M–H1M3···O2ii | 0.98 | 2.44 | 3.372(6) | 160 |
| C16–H16···Cl1 | 0.95 | 2.76 | 3.313 (4) | 118 |
| T2 | ||||
| O9–H9A···N10 | 0.82 | 2.49 | 2.900(5) | 112.2 |
| O9–H9A···O18iii | 0.82 | 2.14 | 2.879(4) | 149.2 |
| O18–H18A···O9iv | 0.82 | 2.04 | 2.833(4) | 161.2 |
| T3 | ||||
| C11–H11A···N9v | 0.93 | 2.64 | 3.326(5) | 130.8 |
| C12–H12A···O15v | 0.97 | 2.61 | 3.514(5) | 154.9 |
| O15–H15A···N8vi | 0.82 | 2.09 | 2.889(4) | 164.4 |
Symmetry codes: (i) x, y – 1, z; (ii) −x + 1, −y + 1, −z + 1; (iii) x, −y + 1/2, z + 1/2; (iv) = x, y, z – 1; (v) −x – 1/2, y, z – 1/2; and (vi) x + 1/2, −y – 2, z.
Preparing Heterogeneous Catalyst by Supporting Compound 1 on the Surface of Silica Gel
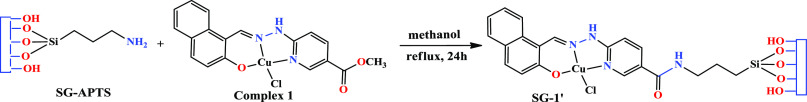
By considering the applications of 1,2,3-triazoles as important molecules in medicinal chemistry and pharmaceutical drugs, we are interested to employ compound 1 as a catalyst in the three-component (azide, epoxide, and terminal alkyne) 1,3-Huisgen cycloaddition reaction, known as “click reaction”, to produce β-hydroxy-1,2,3-triazoles. Considering the advantages of heterogeneous catalysts over their homogeneous counterparts, in this study, we decided to support compound 1 on silica gel by the simple reaction shown in Scheme 2 and convert it into a silica-supported heterogeneous catalyst. The reaction of compound 1 with 3-aminopropyltriethoxysilane-functionalized silica gel (SG-APTS) in refluxing methanol gave silica-supported Cu(II)-hydrazone coordination compound (SG-1′). In this reaction, the coordination compound is supported on the silica gel by the amidification reaction. The −NH2 group of the functionalized silica gel attacks the ester functionality of compound 1. Therefore, the coordination compound is attached to the surface of silica gel by strong amide functionality obtained by this reaction. The supporting of the coordination compound on the surface of silica gel was confirmed by a mixture of spectroscopic studies (FT-IR and UV–vis), scanning electron microscopy (SEM), TGA, and energy-dispersive spectrometry (EDS) analyses.
Scheme 2. Preparation of Silica Gel-Supported Copper(II) Catalyst (SG-1′).
Figure 4 shows the FT-IR spectra of SG-APTS, SG-1′, and compound 1. The infrared spectrum of SG-APTS displays bands at 1046 and 798 cm–1, which are the characteristic antisymmetric and symmetric stretching modes of [SiO4] units (Si–O–Si), respectively.34 The peak at 473 cm–1 is attributed to the bending vibrations of Si–O–Si. Also, the bands at 2924 and 2873 cm–1 are characteristics of the asymmetric and symmetric CH2 bands, confirming the existence of organic groups on the surface of the silica gel.35 After the reaction of SG-APTS with compound 1, an absorption band was observed at 1654 cm–1 in the FT-IR spectrum of SG-1′, which can be attributed to the amidic C=O vibration. The shift of C=O peak from 1717 cm–1 (in compound 1) to 1654 cm–1 (in SG-1′) confirms the formation of amide linkage between compound 1 and SG-APTS. The absorption band at 1618 cm–1 can be assigned to the imine (C=N) stretching frequency. The strong bands at 1098 and 450 cm–1 are due to the influence of the strong absorption background of the silica gel. The appearance of characteristic bands of the silica support in the FT-IR spectra of SG-APTS and SG-1′ indicates that the fundamental structure of the parent silica support is not disturbed even after the formation of the catalyst. Solid-state UV–vis spectra of compound 1 and SG-1′ are shown in Figure S7. These spectra are very close to each other, which confirms the supporting of compound 1 on the surface of silica gel. The small differences and some shifts in the spectrum of SG-1′ can be attributed to the changes in the intermolecular interactions, converting the esteric group to an amidic functionality, and also the effects of silica gel on the electronic transitions of the supported coordination compound.
Figure 4.
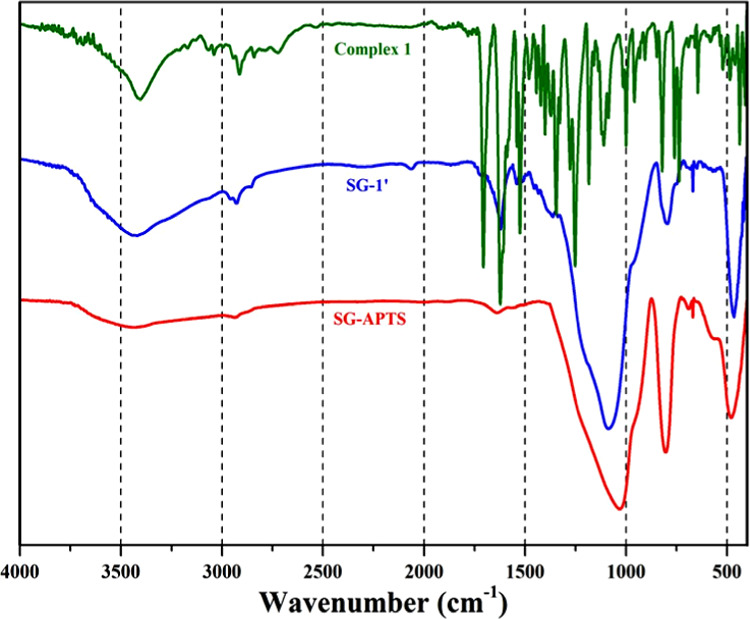
FT-IR spectra of compound 1, SG-APTS, and SG-1′.
The obtained heterogeneous catalyst was also characterized by EDS and EDS mapping analyses, which are shown in Figure 5a,b, respectively. The EDS analysis of SG-1′ showed that the catalyst contains Si (≈28.10%), O (≈45.62%), C (≈15.52%), N (≈4.56%), Cl (≈2.07%), and Cu (≈4.13%). The amount of Cu indicates the presence of approximately 0.667 mmol of copper(II) ion per 1 g of catalyst (calculated 4.24%, experimental 4.13%), which is in agreement with the obtained results from the synthetic procedure. Relatively higher amounts of C (calculated 13.81%, experimental 15.52%) and N atoms (calculated 2.80%, experimental 4.56%) with respect to the expected amount for the supported coordination compound are due to the presence of the 3-aminopropyl linker on the surface of silica gel, which increases their percentage on the surface of the catalyst.
Figure 5.
(a) EDS and (b) EDS mapping analysis of the heterogeneous catalyst obtained by the reaction of SG-APTS with compound 1.
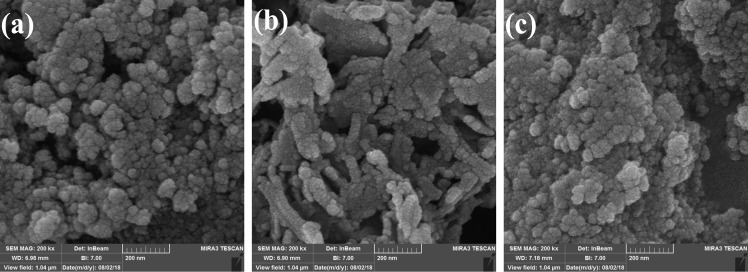
The SEM images of SG-APTS and SG-1′ and recovered SG-APTS and SG-1′ are shown in Figure 6a–c, respectively. The changes in the shape of the surface of SG-APTS in SG-1′ confirm the changes on the surface of SG-APTS, which can be attributed to the supporting of the coordination compound on SG-APTS. The thermal stability of the catalyst was studied by TGA analysis (see Figure S8). TGA analysis of SG-1′ indicated the total weight loss of 25% up to 800 °C, which is due to the decomposition of the supported coordination compound and the loss of the organic ligand and also solvent molecules from the structure of the catalyst.
Figure 6.
Field-emission SEM (FESEM) image of (a) SG-ATPS, (b) SG-1′, and (c) recovered SG-1′ after the catalytic reaction.
General Procedure for Catalytic Production of β-Hydroxy-1,2,3-triazoles in the Presence of Heterogeneous Catalyst (SG-1′)
The catalytic cycloaddition reactions in the presence of the obtained heterogeneous catalyst were carried out in water as a green solvent, which is also used by nature in biological systems and has other advantages like being a cheap, safe, and easily accessible solvent. To optimize the reaction conditions, initially, the three-component reaction of styrene oxide, sodium azide, and phenylacetylene in the presence of SG-1′ were studied in different conditions. The results of these experiments are shown in Table 3. In a preliminary step, the reaction was performed at room temperature (r.t.) and the progress of the reaction was monitored by thin-layer chromatography (TLC) until one of the primary materials (phenylacetylene or styrene oxide) was consumed. After this observation, the obtained product was extracted by ethyl acetate and purified by chromatography and crystallization. It was found that the amount of the catalyst has a significant effect on the time of reaction. Depending on the amount of the catalyst, the reactions were completed after 2.5–8 h (see Table 3). In the absence of the catalyst, the reaction did not take place and there was not any product. By increasing the amount of the catalyst, the reaction was completed in a shorter time. The best-isolated yield in this stage was 94% after 3.5 h when 20 mg of catalyst was employed. When the amount of the catalyst was reduced from 20 to 15 and 10 mg, almost the same yield of triazole was obtained, but in these cases, the reaction was completed a little later. It was found that a further decrease in the catalyst to 7.5 or 5 mg led to lower yield or longer reaction time (entries 3 and 4). Therefore, 10 mg was considered as the optimal amount of catalyst for this cycloaddition reaction.
Table 3. SG-1′-Catalyzed Cycloaddition Reaction: Optimization of the Catalytic Conditions for the Epoxystyrene–Azide–Phenylacetylene Cycloaddition Reactiona.
| entry | catalyst (mg) | temp. | time (h:min) | yield (%)b | selectivity (%) |
|---|---|---|---|---|---|
| 1 | 0 | r.t. | 8:00 | 0 | |
| 2 | 3 | r.t. | 8:00 | 30 | 100 |
| 3 | 5 | r.t. | 6:00 | 60 | 100 |
| 4 | 7.5 | r.t. | 5:00 | 90 | 100 |
| 5 | 10 | r.t. | 4:00 | 94 | 100 |
| 6 | 15 | r.t. | 3:45 | 94 | 100 |
| 7 | 20 | r.t. | 3:30 | 92 | 100 |
| 8 | 10 | 50 | 3:00 | 93 | 100 |
| 9 | 10 | 60 | 3:00 | 93 | >95 |
| 10 | 10 | 70 | 2:45 | 92 | 82 |
| 11 | 10 | 80 | 2:30 | 94 | 60 |
| 12 | 10 | 100 | 2:30 | 93 | 50 |
Reaction conditions: epoxystyrene (1 mmol), phenylacetylene (1 mmol), NaN3 (1 mmol), SG-1′, and water (2 mL).
Isolated yield.
In the next step, the effect of temperature on the reaction was investigated. The reactions were done at 40, 50, 60, 80, and 100 °C, and the results showed the high influence of temperature on the activity and selectivity of this catalytic system. Increasing the reaction temperature from 25 to 50 °C increased the catalytic activity of the catalyst since the reaction was completed after almost 3 h. By increasing the temperature to 60, 70, 80, and 100 °C, the reaction time was slightly decreased, but in these temperatures, one other product was also obtained. This observation is similar to our previous report in preparing 1,2,3-triazoles by similar reagents.10b It is obvious that the new product is generated by the epoxide ring-opening reaction from the attack of azide to the less substituted carbon atom of the epoxide ring. Therefore, increasing the temperature increases the catalytic activity, but the selectivity of the reaction decreases at temperatures higher than 50 °C.
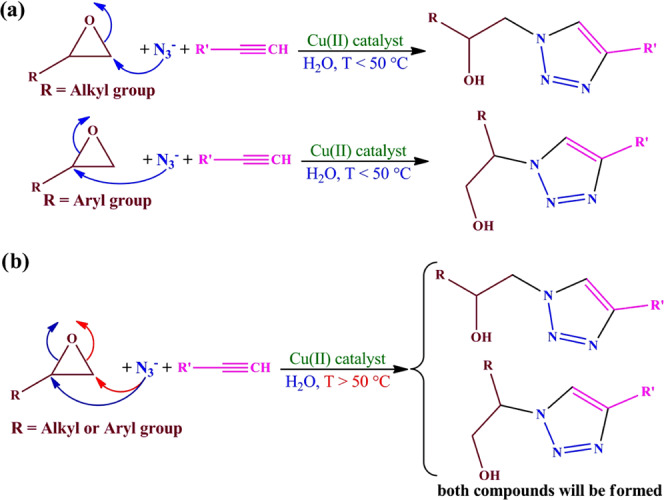
After determining the optimized conditions (the amount of the catalyst and temperature), the synthetic scope of 1,2,3-triazole derivatives via the three-component ring-opening cycloaddition reaction was investigated in the presence of SG-1′. For this purpose, a series of aliphatic or aromatic epoxides and alkynes were employed, of which the results are collected in Table 4. The structure of products was characterized by elemental analysis and spectroscopic methods (FT-IR, 1H NMR, and 13C NMR; their information is available in the Supporting Information). As shown in Table 4, the activity of aliphatic alkynes was lower than phenylacetylene. This matter indicated that the electronic properties (electron-withdrawing (EW) or electron-releasing (ER)) of the substituent connected to the C≡C group have considerable influence on the activity of this catalytic system. On the other hand, in the case of aliphatic-substituted epoxides, the ring-opening reaction mainly occurs through the attack of azide to the less substituted carbon atom of the epoxide ring. A primary alcoholic functionality can be obtained by the attack to the more substituted position of the epoxide ring, while a secondary alcoholic functionality can be generated by an attack to the less substituted position. Therefore, we investigated the effect of temperature on the regioselectivity of the epoxide ring-opening reaction. The results showed that in aliphatic-substituted epoxides the azide anion only attacks to the less substituted carbon atom up to 50 °C, but at higher temperatures, it can also attack the more substituted carbon atom of the epoxide ring. At 60 °C, the byproduct was trace and hardly detectable by TLC, but at 70 °C, its amount was relatively considerable. However, even at higher temperatures, the amount of the product obtained by the attack to the more substituted position is low, which indicates that the less substituted carbon is the most active position in the case of aliphatic-substituted epoxides. Previous studies on the epoxide ring-opening reactions indicate that, depending on the reaction conditions and on the nature of the epoxide, this reaction can proceed by nucleophilic attack to the less or more substituted carbon atom in the case of asymmetric epoxide rings.17,36 Undoubtedly, besides the steric considerations, this matter is mainly related to the electron-withdrawing (EW) or electron-releasing (ER) properties of the substituents connected to the epoxide ring. Due to this, the regioselectivity of the ring-opening reaction in preparing β-hydroxyl-1,2,3-triazoles from the epoxide–azide–alkyne cycloaddition reaction was one of the major subjects for discussion in previous reports. Finding effective factors for obtaining better regioselectivity in the production of β-hydroxyl-1,2,3-triazoles still is one of the attractive subjects in this field. Most of the reports confirm the nucleophilic attack of azide to the more substituted carbon atom in the case of aromatic-substituted epoxides and attack to the less substituted carbon atom in the case of aliphatic-substituted epoxides.17,36 Nevertheless, we found that the regioselectivity of the epoxide ring-opening reaction, in the production of β-hydroxyl-1,2,3-triazoles, considerably depends on the reaction temperature (see Scheme 3). At low temperatures (like room temperature), the mentioned observation in previous reports is correct, but at higher temperatures, both reactions (attack to less and more substituted carbon of the epoxide ring) can take place in both of the aromatic- and aliphatic-substituted epoxides. It should be mentioned that our studies indicated that in the aromatic-substituted epoxides the ratio of two products is almost the same at about 100 °C, but in the aliphatic-substituted epoxides, the attack to the less substituted position still is higher. This matter can be visually followed by a simple TLC method and also by NMR spectroscopic studies.
Table 4. Synthesis of β-Hydroxy-1,2,3-triazoles by Catalytic Reaction of Azide, Epoxide, and Alkyne in the Presence of a Heterogeneous Catalyst (SG-1′)a.
Reaction conditions: epoxide (1 mmol), alkyne (1 mmol), NaN3 (1 mmol), SG-1′ (10 mg), and water (2 mL).
Scheme 3. Structures of β-Hydroxy-1,2,3-triazoles Obtained Using Aliphathic- or Aromatic-Substituted Epoxides at (a) Low and (b) High Temperatures.
To have better data about the structure of products and the type of epoxide ring-opening reaction, the structures of two products (T2 and T3) were determined by single-crystal X-ray analysis. The molecular structures of T2 and T3 are shown in Figures 7a and 8a, respectively, and the selected bond lengths and angles are available in Table S1. Diffraction studies confirmed the proposed structures for the obtained products from the cycloaddition reactions in the presence of SG-1′. In the case of T2, where an aromatic epoxide is used in the cycloaddition reaction, a primary alcoholic functionality is obtained by nucleophilic attack of the azide anion to the more substituted carbon atom of the epoxide ring. In T3, with the aliphatic group connected to the epoxide ring, a secondary alcoholic functionality is obtained by nucleophilic attack of azide to the less substituted position of the epoxide ring. The bond lengths and angles in the obtained 1,2,3-triazole rings are close to the previously reported 1,2,3-triazoles.37 The crystal structures of both T2 and T3 are stabilized by intermolecular O–H···O hydrogen bond interactions (see Figures 7b and 8b, respectively), and a 1D polymeric chain is generated by these interactions. Moreover, there are some other C–H···O, C–H···N, and π···π interactions in the crystal structures of T2 and T3, which convert 1D polymeric chains to 2D polymeric networks (see Table 2 and Figures 7b and 8b).
Figure 7.

(a) Molecular structure of product T2. (b) 1D polymeric chain obtained by intermolecular hydrogen-bonding interactions in the crystal structure of T2.
Figure 8.

(a) Molecular structure of product T3. (b) 2D polymeric chain obtained by intermolecular hydrogen-bonding interactions in the crystal structure of T3.
Recycling and the Stability of the Catalyst
The recycling test of the heterogeneous catalyst was investigated under the optimized reaction conditions. It is worth mentioning that despite a small amount of catalyst was utilized, it could be easily recovered by filtration (after treating the reaction mixture with ethyl acetate) and reused. The results of this reusability study are illustrated in Figure 9. The results indicate that the heterogeneous catalyst can be reused at least four times with only a small reduction in the rate of the reaction under similar experimental conditions. Generally, the catalyst lost below 10% of its original catalytic activity in the fourth catalytic run, and the selectivity of the products was also comparable in each catalytic run. It proves the good stability of the obtained heterogeneous catalyst covalently immobilized on silica gel. To study the changes in the structure of the catalyst after catalytic reactions, the recovered catalyst was analyzed by FT-IR spectroscopy, SEM, EDS, and TGA analyses. The FT-IR spectrum of the recovered catalyst (see Figure S9) was very similar to the FT-IR spectrum of the fresh catalyst, which confirms the stability of the catalyst. However, some minor changes were also observed, which can be attributed to the interactions of the reagents with the catalyst. The most important change is the observation of an azide peak at 2078 cm–1, which indicated that the azide is inserted to the structure of the catalyst. This matter can be attributed to the replacement of chloride anion with azide anion and the coordination of azide to the Cu(II) ion, which is also confirmed by EDS analysis. Figure 10 shows the EDS and EDS mapping analysis of the recovered catalyst. As it is seen, the chloride anion is absent in the structure of the recovered catalyst, which confirms its replacement by the azide anion. The presence of copper, nitrogen, and carbon atoms in the EDS analysis of the recovered catalyst confirms the stability and reusability of the catalyst. Finally, in the TGA analysis of the recovered catalyst (see Figure S10), the loss of approximately 30% of the weight is due to the decomposition of the supported coordination compound. This matter indicates that the coordination compound still remains in the structure of the recovered catalyst and is another evidence for the stability of the heterogeneous catalyst. The higher weight loss in the case of recovered catalyst (≈30%) with respect to the fresh catalyst (≈25%) can be attributed to the replacement of chloride anion with azide and also the absorption of reagents onto the surface of the catalyst.
Figure 9.
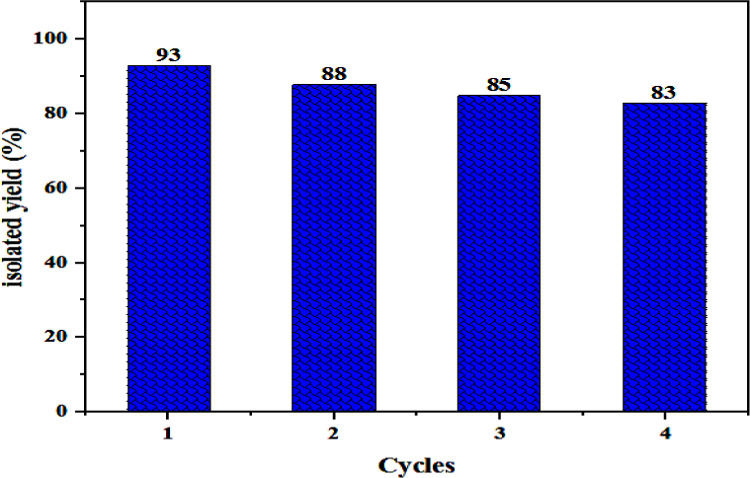
Isolated yield of β-hydroxy-1,2,3-triazole using the recovered SG-1′ catalyst.
Figure 10.
EDS and EDS mapping analysis of the recovered heterogeneous catalyst after the catalytic production of β-hydroxy-1,2,3-triazoles.
The details of the mechanism of copper-catalyzed azide–alkyne cycloaddition reactions have been theoretically and experimentally studied in the literature.38 Although mechanistic studies were not done in this project, by considering the characterization results of the recovered catalyst it is obvious that the reaction is proceeded by the coordination of azide to the copper core of the supported coordination compound and elimination of the chloride ligand. The copper core of the catalyst can also facilitate the in situ generation of alkyl–azide by the interaction with the oxygen atom of the epoxide ring.10b It is predictable that the mechanism of this reaction is similar to the previously reported mechanisms,10b,38 and the reaction is proceeded by the interaction of alkyne with the copper core of catalyst. Finally, the reaction is completed by the 3 + 2 cycloaddition reaction of the in-situ-generated alkyl-azide and alkyne and the copper core of the catalyst facilitates this process by interaction with the substrates.
Conclusions
In summary, a new copper(II) coordination compound was synthesized and characterized using spectroscopic methods and single-crystal X-ray analysis. Then, the obtained coordination compound was successfully supported on silica gel by the amidification reaction and a novel silica-supported Cu(II) catalyst was synthesized. The obtained heterogeneous catalyst was characterized by FT-IR, SEM, TGA, and EDS analyses, and it was employed in the catalytic production of β-hydroxy-1,2,3-triazoles from three-component azide–epoxide–alkyne cycloaddition reactions. Catalytic reactions were performed in water as a green solvent, and the effects the reaction temperature and the amounts of catalyst were studied. The investigations showed that the reported covalently supported Cu(II) coordination compound on the silica gel was an easily producible, stable, inexpensive, and highly active catalyst for cycloaddition reactions. The obtained β-hydroxy-1,2,3-triazoles were characterized by spectroscopic methods, and the structure of two products was determined by single-crystal X-ray analysis. The catalytic studies indicated that the aromatic and aliphatic substituents on the epoxide ring have considerable effects on the regioselectivity of this catalytic system. The results indicated that this catalytic system is regioselective at low temperatures, but at temperatures higher than 50 °C, two products can be obtained due to two kinds of epoxide ring-opening reactions. Moreover, the substituents on the alkyne group have an impressive effect on the activity of the obtained heterogeneous catalyst in cycloaddition reactions.
Experimental Section
Materials and Instrumentations
6-Hydrazinonicotinic hydrazide hydrate was purchased from Sigma-Aldrich. All of the other chemical reagents were prepared from Merck and used as received. Solvents of the highest-grade commercially available (Merck) were used without further purification. The surface of silica gel was functionalized by 3-aminopropyltriethoxysilane (APTS), according to the procedure reported in the literature.39 FT-IR spectra were recorded using a Bruker FT-IR spectrophotometer as KBr disks. UV–vis spectra of solutions were recorded using a thermospectronic Helios Alpha spectrophotometer. 1H and 13C NMR spectra in dimethyl sulfoxide (DMSO)-d6 solution were measured using a Bruker 250 MHz spectrometer, and the chemical shifts were indicated in parts per million (ppm) relative to tetramethylsilane (TMS). The elemental analyses (carbon, hydrogen, and nitrogen) of the compounds were recorded using a Carlo ERBA model EA 1108 analyzer. The copper content was determined by atomic absorption analysis using a Varian Spectra AA-220 equipment. Thermal gravimetric analyses (TGA) curves were recorded using a PerkinElmer Pyris 1 instrument in the range of 25–1000 °C. The particle size and morphology of the sample surfaces were studied using a scanning electron microscope (MIRA3, TE-SCAN) equipped with an energy-dispersive X-ray spectrometer (EDS). EDS and EDS mapping were performed to further confirm the composition of the prepared samples.
Synthesis of Ligand (E)-6-(2-((2-Hydroxynaphthalen-1-yl)methylene)hydrazinyl)nicotinohydrazide (H2L)
A methanol (10 mL) solution of 6-hydrazinonicotinic hydrazide hydrate (0.501 g, 3.0 mmol) was added dropwise to a methanol solution (10 mL) of 2-hydroxy-1-naphthaldehyde (0.516 g, 3.0 mmol), and the mixture was refluxed for 4 h. The solution was evaporated on a steam bath to 5 mL and cooled to room temperature. The resulting light yellow precipitate was separated and filtered off, washed with 5 mL of cooled methanol, and dried in air. Yield: 90% (0.867 g). m.p. 299–302 °C. Anal. Calcd for C17H15N5O2 (MW = 321.33): C, 63.54; H, 4.71; N, 21.79%. Found: C, 63.59; H, 4.67; N, 21.85%. FT-IR (KBr, cm–1): 3335 (w), 3272 (w, br), 3204 (m), 3050 (w), 2923 (w), 2859 (w), 1664 (m), 1625 (vs), 1589 (vs), 1523 (s), 1531 (m), 1468 (s), 1420 (w), 1403 (w), 1386 (w), 1327 (s), 1281 (m), 1240 (w), 1188 (m), 1158 (s), 1141 (m), 1014 (w), 956 (w), 930 (w), 913 (w), 885 (w), 855 (w), 812 (s), 776 (m), 743 (m), 718 (w), 685 (w), 666 (m), 656 (m), 543 (w), 526 (w), 509 (w), 499 (w), 477 (w), 442 (w), 422 (w). 1H NMR (250.13 MHz, DMSO-d6, 25 °C, TMS): δ = 11.70 (s, 1H, OH), 11.34 (s, 1H, NHamide), 9.65 (s, 1H, NHhydrazone), 9.07 (s, 1H, CH=N), 8.81 (s, 1H, CH=Npyridine), 8.44–6.97 (m, 8H, aromatic), 4.46 (s, 2H, NH2). 13C NMR (62.90 MHz, DMSO-d6): δ = 165.18, 157.56, 156.77, 148.38, 141.14, 137.48, 131.90, 131.60, 129.27, 128.48, 127.99, 123.83, 122.21, 120.59, 118.90, 110.36, 105.50 ppm. UV–vis (in CH3OH, c = 2.5 × 10–5 M, λmax [nm] with ε [M–1 cm–1]): 212 (57 600), 240 (29 300), 266 (18 700), 334 (27 400), 374 (34 600), 390 nm (30 000).
Synthesis of [Cu(HL′)(Cl)]·CH3OH (1)
Compound 1 was synthesized by the reaction of H2L (0.321 g, 1.00 mmol) and CuCl2·2H2O (0.170 g, 1.00 mmol) in methanol using the thermal gradient method in a branched tube. The above-mentioned amounts of the materials were placed in the main arm of a branched tube. The tube was carefully filled with methanol and sealed. The reagent-containing arm was immersed in an oil bath at 65 °C, while the other arm was kept at ambient temperature. After a 1 week, dark brown crystals were deposited in the cooler arm. Yield 75% (0.33 g). Anal. Calcd for C19H18ClCuN3O4 (MW = 451.35): C, 50.56; H, 4.02; N, 9.31; Cu, 14.08%. Found: C, 50.48; H, 3.98; N, 9.39; Cu, 14.17%. FT-IR (KBr, cm–1): 3419 (br, m), 3180 (w), 3050 (w), 2960 (w), 2923 (w), 2851 (w), 1717 (vs), 1633 (vs), 1598 (m), 1551 (m), 1536 (s), 1491 (w), 1455 (w), 1433 (w), 1412 (m), 1383 (w), 1356 (s), 1339 (w), 1278 (m), 1263 (s), 1194 (m), 1169 (s), 1130 (m), 1119 (m), 1096 (w), 1004 (w), 1009 (m), 968 (m), 924 (w), 916 (w), 857 (w), 831 (m), 770 (m), 747 (m), 669 (w), 654 (w), 531 (w), 496 (w), 471 (w), 447 (m), 420 (m). UV–vis (in CH3OH, c = 2.5 × 10–5 M, λmax [nm] with ε [M–1 cm–1]): 216 (61 500), 241 (52 100), 263 (47 200), 339 (35 500), 422 (33 700), 444 nm (51 100).
Synthesis of Heterogeneous Catalyst from Compound 1 (SG-1′)
The silica gel-supported heterogeneous catalyst was prepared by reaction of compound 1 (1.354 g, 3.00 mmol) with 3.00 g of functionalized silica gel and APTS (SG-APTS) in 25 mL of methanol. The mixture was stirred under reflux conditions for 24 h. Then, the product was filtered off, washed with cold methanol, and dried under vacuum at 50 °C for 24 h to obtain dry heterogeneous catalyst (SG-1′). By considering the weight of the final product (4.05 g), the loading of compound 1 on the surface of silica gel was calculated to be approximately ≈0.90 mmol per 1 g of silica gel, which is equal to ≈0.667 mmol of Cu(II) coordination compound per 1 g of final heterogeneous catalyst. FT-IR (KBr, cm–1): 3426 (m, br), 2923 (w), 2853 (w), 1617 (m), 1532 (m), 1486 (w), 1409 (w), 1356 (w), 1338 (w), 1193 (m), 1093 (vs), 968 (w), 919 (w), 825 (m), 805 (m), 749 (w), 668 (w), 450 (vs).
X-ray Crystallography
The brown crystals of 1 crystallize in P21/n (monoclinic) space group, but colorless crystals of T2 and T3 crystallize in P21/c (monoclinic) and Pca21 (orthorhombic) space groups, respectively. A summary of the crystal data and refinement details for compound 1, T2, and T3 is given in Table 5. Single-crystal data collection for 1 was performed using an Xcalibur diffractometer with a charge-coupled device (CCD) ruby detector equipped with an Oxford Cryosystem open-flow nitrogen cryostat using ω scan and a graphite-monochromated Mo Kα (λ = 0.71073 Å) radiation at 100 K. The X-ray data for T2 and T3 were collected at 293(2) K by an Oxford Sapphire CCD diffractometer using Mo Kα radiation. The structures were solved by direct methods and refined with full-matrix least-squares techniques on F2 with SHELXL-2014.40 H atoms were found in the difference Fourier maps or were included using geometrical considerations. The molecular structure plot was prepared using Diamond.41 The structural data have been deposited at the Cambridge Crystallographic Data Centre (CCDC no. 1921721 for T2, 1935505 for T3, and 1980597 for 1).
Table 5. Crystal Data and Structure Refinement Parameters for Compounds 1, T2, and T3.
| identification code | compound 1 | T2 | T3 |
|---|---|---|---|
| net formula | C19H18ClCuN3O4 | C13H17N3O2 | C11H13N3O |
| formula weight (g mol–1) | 451.35 | 247.29 | 203.24 |
| T (K) | 100 | 293 | 293 |
| crystal size (mm3) | 0.06 × 0.08 × 0.18 | 0.22 × 0.08 × 0.07 | 0.61 × 0.12 × 0.09 |
| crystal shape, color | needle, dark brown | block, colorless | needle, colorless |
| crystal system | monoclinic | monoclinic | orthorhombic |
| space group | P21/n | P21/c | Pca21 |
| a (Å) | 10.822(5) | 5.7635(13) | 9.873(2) |
| b (Å) | 8.919(3) | 24.996(6) | 12.958(3) |
| c (Å) | 19.088(8) | 8.917(2) | 8.353(2) |
| β (deg) | 105.07(5) | 97.92(2) | 90 |
| volume (Å3) | 1779.0(12) | 1272.4(5) | 1068.6(4) |
| Z | 4 | 4 | 4 |
| density (calcd) (g cm–3) | 1.685 | 1.291 | 1.263 |
| absorption coefficient (mm–1) | 1.41 | 0.09 | 0.09 |
| F(000) | 924 | 528 | 432 |
| θ range (deg) | 3.0–27.6 | 2.5–28.3 | 2.6–28.6 |
| measured reflections | 19 300 | 8569 | 6748 |
| independent reflections | 4101 | 2914 | 2431 |
| reflections with I > 2σ(I) | 2926 | 1079 | 1295 |
| index ranges hkl | –12 → 14, –11 → 11, –24 → 24 | –7 → 7, –32 → 31, –11 → 9 | –12 → 13, –17 → 15, –10 → 10 |
| restraints/parameters | 1/257 | 0/163 | 1/137 |
| goodness of fit on F2 | 1.07 | 0.998 | 0.956 |
| Rint | 0.130 | 0.160 | 0.057 |
| R[F2 > 2σ(F2)] | 0.060 | 0.094 | 0.048 |
| wR(F2) | 0.157 | 0.226 | 0.087 |
| max/min electron density (e Å–3) | 0.82/–0.68 | 0.30/–0.35 | 0.14/–0.12 |
General Procedure for the Catalytic Production of β-Hydroxy-1,2,3-triazoles from the One-Pot Tricomponent (Epoxide, Azide, Alkyne) Ring-Opening/Huisgen Cycloaddition Reaction
The catalytic click syntheses of β-hydroxy-1,2,3-triazoles were carried out by the method described in our previous report.10b Briefly, sodium azide (0.065 g, 1.0 mmol), epoxide (1 mmol), and terminal alkyne (1 mmol) were added to a suspension of SG-1′ (0.002 g) in water (2 mL). The reaction mixture was stirred at the desired temperature, and the progress of the reaction was monitored by TLC until the total conversion of the starting materials. In all cases, white precipitates (or oily organic layer) were formed after the formation of the product. After completion of the reaction, the organic product was extracted by the addition of chloroform (3 × 10 mL) to the mixture. The silica-supported heterogeneous catalyst was separated from the remaining solution by filtration. The pure β-hydroxy-1,2,3-triazoles were isolated through silica gel column chromatography. The products of catalytic reactions were characterized by spectroscopic methods and elemental analysis, and their detailed information is presented in the Supporting Information. The structures of the two products were further characterized by single-crystal X-ray analysis. The products of the reaction among phenylacetylene, sodium azide, and epoxystyrene, 2-phenyl-2-(4-phenyl-1H-1,2,3-triazol-1-yl)ethanol (T1a) and 1-phenyl-2-(4-phenyl-1H-1,2,3-triazol-1-yl)ethanol (T1b), were similar to our previous report;10b their information is available in the Supporting Information.
Acknowledgments
The authors are thankful to the University of Zanjan, Imam Khomeini International University, and Nicolaus Copernicus University for supporting this study.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c01491.
Analysis of the conformers of FT-IR, 1H NMR and 13C{1H} NMR information of all compounds, TGA analysis of compound 1, SG-1′ and recovered SG-1′, and selected geometric parameters (Å, deg) for T2 and T3 (PDF)
Crystallographic data file for compound 1 (CIF)
Crystallographic data file for compound T2 (CIF)
Crystallographic data file for compound T3 (CIF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Wang Y.-C.; Xie Y.-Y.; Qu H.-E.; Wang H.-S.; Pan Y.-M.; Huang F.-P. Ce(OTf)3-Catalyzed [3 + 2] Cycloaddition of Azides with Nitroolefins: Regioselective Synthesis of 1,5-Disubstituted 1,2,3-Triazoles. J. Org. Chem. 2014, 79, 4463–4469. 10.1021/jo5004339. [DOI] [PubMed] [Google Scholar]; b Zhu A.; Xing X.; Wang S.; Yuan D.; Zhu G.; Geng M.; Guo Y.; Zhang G.; Li L. Multi-component syntheses of diverse 5-fluoroalkyl-1,2,3-triazoles facilitated by air oxidation and copper catalysis. Green Chem. 2019, 21, 3407–3412. 10.1039/C9GC00647H. [DOI] [Google Scholar]; c Huang K.; Sheng G.; Lu P.; Wang Y. From 1-Sulfonyl-4-aryl-1,2,3-triazoles to 1-Allenyl-5-aryl-1,2,3-triazoles. J. Org. Chem. 2017, 82, 5294–5300. 10.1021/acs.joc.7b00627. [DOI] [PubMed] [Google Scholar]
- Kumar S. V.; Scottwell S. Ø.; Waugh E.; McAdam J.; Hanton L. R.; Brooks H. J. L.; Crowley J. D. Antimicrobial Properties of Tris(homoleptic) Ruthenium(II) 2-Pyridyl-1,2,3-triazole “Click” Complexes against Pathogenic Bacteria, Including Methicillin-Resistant Staphylococcus aureus (MRSA). Inorg. Chem. 2016, 55, 9767–9777. 10.1021/acs.inorgchem.6b01574. [DOI] [PubMed] [Google Scholar]
- a Elkanzi N. A. A.; El-Sofany W. I.; Gaballah S. T.; Mohamed A. M.; Kutkat O.; El-Saye W. A. Synthesis, Molecular Modeling, and Antiviral Activity of Novel Triazole Nucleosides and Their Analogs. Russ. J. Gen. Chem. 2019, 89, 1896–1904. 10.1134/S1070363219090263. [DOI] [Google Scholar]; b Bangalore P. K.; Vagolu S. K.; Bollikanda R. K.; Veeragoni D. K.; Choudante P. C.; Misra S.; Sriram D.; Sridhar B.; Kantevari S. Usnic Acid Enaminone-Coupled 1,2,3-Triazoles as Antibacterial and Antitubercular Agents. J. Nat. Prod. 2020, 83, 26–35. 10.1021/acs.jnatprod.9b00475. [DOI] [PubMed] [Google Scholar]
- Ekhlass M. N.; Fathy M. A.; Rezk R. A.; Ahmed F. E.-F. Synthesis and Some Reactions of 1-aryl-4-acetyl-5-methyl-1,2,3-triazole Derivatives with Anticonvulsant Activity. Mini-Rev. Med. Chem. 2016, 16, 926–936. 10.2174/1389557516666160118105505. [DOI] [PubMed] [Google Scholar]
- a Tian Y.; Liu Z.; Liu J.; Huang B.; Kang D.; Zhang H.; Clercq E. D.; mans D. D.; Pannecouque C.; Lee K. H.; Chen C. H.; Zhan P.; Liu X. Targeting the entrance channel of NNIBP: Discovery of diarylnicotinamide 1,4-disubstituted 1,2,3-triazoles as novel HIV-1 NNRTIs with high potency against wild-type and E138K mutant virus. Eur. J. Med. Chem. 2018, 151, 339–350. 10.1016/j.ejmech.2018.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Mohammed I.; Kummetha I. R.; Singh G.; Sharova N.; Lichinchi G.; Dang J.; Stevenson M.; Rana T. M. 1,2,3-Triazoles as Amide Bioisosteres: Discovery of a New Class of Potent HIV-1 Vif Antagonists. J. Med. Chem. 2016, 59, 7677–7682. 10.1021/acs.jmedchem.6b00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckle D. R.; Outred D. J.; Rockell C. J. M.; Smith H.; Spicer B. A. Studies on v-triazoles. 7. Antiallergic 9-oxo-1H, 9H-benzopyrano [2, 3-d]-v-triazoles. J. Med. Chem. 1983, 26, 251–254. 10.1021/jm00356a025. [DOI] [PubMed] [Google Scholar]
- a Duan T.; Fan K.; Fu Y.; Zhong C.; Chen X.; Peng T.; Qin J. Triphenylamine-based organic dyes containing a 1,2,3-triazole bridge for dye-sensitized solar cells via a ‘Click’ reaction. Dyes Pigm. 2012, 94, 28–33. 10.1016/j.dyepig.2011.11.008. [DOI] [Google Scholar]; b Debia N. P.; Saraiva M. T.; Martins B. S.; Beal R.; Gonçalves P. F. B.; Rodembusch F. S.; Alves D.; Lüdtke D. S. Synthesis of Amino Acid-Derived 1,2,3-Triazoles: Development of a Nontrivial Fluorescent Sensor in Solution for the Enantioselective Sensing of a Carbohydrate and Bovine Serum Albumin Interaction. J. Org. Chem. 2018, 83, 1348–1357. 10.1021/acs.joc.7b02852. [DOI] [PubMed] [Google Scholar]; c Ghosh D.; Rhodes S.; Hawkins K.; Winder D.; Atkinson A.; Ming W.; Padgett C.; Orvis J.; Aiken K.; Landge S. A simple and effective 1,2,3-triazole based “turn-on” fluorescence sensor for the detection of anions. New J. Chem. 2015, 39, 295–303. 10.1039/C4NJ01411A. [DOI] [Google Scholar]; d Meisner Q. J.; Accardo J. V.; Hu G.; Clark R. J.; Jiang D.-e.; Zhu L. Fluorescence of Hydroxyphenyl-Substituted “Click” Triazoles. J. Phys. Chem. A 2018, 122, 2956–2973. 10.1021/acs.jpca.8b00577. [DOI] [PubMed] [Google Scholar]; e Sinopoli A.; Black F. A.; Wood C. J.; Gibson E. A.; Elliott P. I. P. Investigation of a new bis(carboxylate) triazole-based anchoring ligand for dye solar cell chromophore complexes. Dalton Trans. 2017, 46, 1520–1530. 10.1039/C6DT02905A. [DOI] [PubMed] [Google Scholar]
- a Rostovtsev V. V.; Green L. G.; Fokin V. V.; Sharpless K. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem., Int. Ed. 2002, 41, 2596–2599. . [DOI] [PubMed] [Google Scholar]; b Tornøe C. W.; Christensen C.; Meldal M. Peptidotriazoles on solid phase:[1,2,3]-triazoles by regiospecific copper (I)-catalyzed 1, 3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002, 67, 3057–3064. 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]; c Liang L.; Astruc D. The copper(I)-catalyzed alkyne-azide cycloaddition (CuAAC) “click” reaction and its applications. An overview. Coord. Chem. Rev. 2011, 255, 2933–2945. 10.1016/j.ccr.2011.06.028. [DOI] [Google Scholar]; d Wei F.; Wang W.; Ma Y.; Tunga C.-H.; Xu Z. Regioselective synthesis of multisubstituted 1,2,3-triazoles: moving beyond the copper-catalyzed azide–alkyne cycloaddition. Chem. Commun. 2016, 52, 14188–14199. 10.1039/C6CC06194J. [DOI] [PubMed] [Google Scholar]; e Alexander J. R.; Ott A. A.; Liu E.-C.; Topczewski J. J. Kinetic Resolution of Cyclic Secondary Azides, Using an Enantioselective Copper-Catalyzed Azide–Alkyne Cycloaddition. Org. Lett. 2019, 21, 4355–4358. 10.1021/acs.orglett.9b01556. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Michinobu T.; Diederich F. The [2+2] Cycloaddition-Retroelectrocyclization (CA-RE) Click Reaction: Facile Access to Molecular and Polymeric Push-Pull Chromophores. Angew. Chem., Int. Ed. 2018, 57, 3552–3577. 10.1002/anie.201711605. [DOI] [PubMed] [Google Scholar]; g Lal S.; Díez-González S. [CuBr(PPh3)3] for Azide–Alkyne Cycloaddition Reactions under Strict Click Conditions. J. Org. Chem. 2011, 76, 2367–2373. 10.1021/jo200085j. [DOI] [PubMed] [Google Scholar]
- a Hassan S.; Muller T. J. J. Multicomponent Syntheses Based upon Copper Catalyzed Alkyne-Azide Cycloaddition. Adv. Synth. Catal. 2015, 357, 617–666. 10.1002/adsc.201400904. [DOI] [Google Scholar]; b Guo S.; Zhou Y.; Dai B.; Huo C.; Liu C.; Zhao Y. CuI/Et2NH-catalyzed one-pot highly efficient synthesis of 1,4-disubstituted 1,2,3-triazoles in green solvent glycerol. Synthesis 2018, 50, 2191–2199. 10.1055/s-0036-1591557. [DOI] [Google Scholar]; c Deng X.; Liang J.; Allison B. B.; Dvorak C.; McAllister H.; Savall B. M.; Mani N. S. Allyl-Assisted, Cu(I)-Catalyzed Azide–Alkyne Cycloaddition/Allylation Reaction: Assembly of the [1,2,3]Triazolo-4,5,6,7-tetrahydropyridine Core Structure. J. Org. Chem. 2015, 80, 11003–11012. 10.1021/acs.joc.5b02174. [DOI] [PubMed] [Google Scholar]
- a Eisavi R.; Karimi A. CoFe2O4/Cu(OH)2 magnetic nanocomposite: an efficient and reusable heterogeneous catalyst for one-pot synthesis of β-hydroxy-1,4-disubstituted-1,2,3-triazoles from epoxides. RSC Adv. 2019, 9, 29873–29887. 10.1039/C9RA06038C. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Noshiranzadeh N.; Emami M.; Bikas R.; Kozakiewicz A. Green click synthesis of β-hydroxy-1,2,3-triazoles in water in the presence of a Cu (II)–azide catalyst: a new function for Cu(II)–azide complexes. New J. Chem. 2017, 41, 2658–2667. 10.1039/C6NJ03865D. [DOI] [Google Scholar]; c Kumaraswamy G.; Ankamma K.; Pitchaiah A. Tandem Epoxide or Aziridine Ring Opening by Azide/Copper Catalyzed [3+2] Cycloaddition: Efficient Synthesis of 1,2,3-Triazolo β-Hydroxy or β-Tosylamino Functionality Motif. J. Org. Chem. 2007, 72, 9822–9825. 10.1021/jo701724f. [DOI] [PubMed] [Google Scholar]; d Mishra K. B.; Tiwari V. K. Click Chemistry Inspired Synthesis of Morpholine-Fused Triazoles. J. Org. Chem. 2014, 79, 5752–5762. 10.1021/jo500890w. [DOI] [PubMed] [Google Scholar]
- a Yu X.; Xu J.; Zhou Y.; Song Q. A facile synthesis of diverse 5-arylated triazoles via a Cu-catalyzed oxidative interrupted click reaction with arylboronic acids in air. Org. Chem. Front. 2018, 5, 2463–2467. 10.1039/C8QO00590G. [DOI] [Google Scholar]; b Zhang Q.; Su H.; Luo J.; Wei Y. “Click” magnetic nanoparticle-supported palladium catalyst: a phosphine-free, highly efficient and magnetically recoverable catalyst for Suzuki–Miyaura coupling reactions. Catal. Sci. Technol. 2013, 3, 235–243. 10.1039/C2CY20532G. [DOI] [Google Scholar]; c Garg A.; Ali A. A.; Damarla K.; Kumar A.; Sarma D. Aqueous bile salt accelerated cascade synthesis of 1,2,3-triazoles from arylboronic acids. Tetrahedron Lett. 2018, 59, 4031–4035. 10.1016/j.tetlet.2018.09.064. [DOI] [Google Scholar]
- a Rani G. S.; Vijay M.; Devi B. L. A. P. SO3Cu-Carbon: A Novel Heterogeneous Catalyst for the Synthesis of β-Hydroxy 1,2,3-Triazoles by One Pot Cycloaddition Reaction. ChemistrySelect 2019, 4, 10133–10142. 10.1002/slct.201902289. [DOI] [Google Scholar]; b Genin M. J.; Allwine D. A.; Anderson D. J.; Barbachyn M. R.; Emmert D. E.; Garmon S. A.; Graber D. R.; Grega K. C.; Hester J. B.; Hutchinson D. K.; Morris J.; Reischer R. J.; Ford C. W.; Zurenko G.; Hamel J. C. E.; Schaadt R. D.; Stapert D.; Yagi B. H. Substituent Effects on the Antibacterial Activity of Nitrogen–Carbon-Linked (Azolylphenyl)oxazolidinones with Expanded Activity Against the Fastidious Gram-Negative Organisms Haemophilus influenzae and Moraxella catarrhalis. J. Med. Chem. 2000, 43, 953–970. 10.1021/jm990373e. [DOI] [PubMed] [Google Scholar]; c Buckle D. R.; Outred D. J.; Rockell C. J. M.; Smith H.; Spicer B. A. Studies on v-triazoles. 7. Antiallergic 9-oxo-1H, 9H-benzopyrano [2, 3-d]-v-triazoles. J. Med. Chem. 1983, 26, 251–254. 10.1021/jm00356a025. [DOI] [PubMed] [Google Scholar]
- a Giffin M. J.; Heaslet H.; Brik A.; Lin Y.-C.; Cauvi G.; Wong C.-H.; McRee D. E.; Elder J. H.; Stout C. D.; Torbett B. E. A copper (I)-catalyzed 1,2,3-triazole azide–alkyne click compound is a potent inhibitor of a multidrug-resistant HIV-1 protease variant. J. Med. Chem. 2008, 51, 6263–6270. 10.1021/jm800149m. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Brik A.; Alexandratos J.; Lin Y.-C.; Elder J. H.; Olson A. J.; Wlodawer A.; Goodsell D. S.; Wong C.-H. 1,2,3-Triazole as a peptide surrogate in the rapid synthesis of HIV-1 protease inhibitors. ChemBioChem 2005, 6, 1167–1169. 10.1002/cbic.200500101. [DOI] [PubMed] [Google Scholar]
- a Liang J.; Liang Z.; Zou R.; Zhao Y. Heterogeneous catalysis in zeolites, mesoporous silica, and metal–organic frameworks. Adv. Mater. 2017, 29, 1701139 10.1002/adma.201701139. [DOI] [PubMed] [Google Scholar]; b Matsumoto K.; Tachikawa S.; Hashimoto N.; Nakano R.; Yoshida M.; Shindo M. Aerobic C–H Oxidation of Arenes Using a Recyclable, Heterogeneous Rhodium Catalyst. J. Org. Chem. 2017, 82, 4305–4316. 10.1021/acs.joc.7b00300. [DOI] [PubMed] [Google Scholar]; c Xie W.; Hu Li.; Yang X. Basic ionic liquid supported on mesoporous SBA-15 silica as an efficient heterogeneous catalyst for biodiesel production. Ind. Eng. Chem. Res. 2015, 54, 1505–1512. 10.1021/ie5045007. [DOI] [Google Scholar]
- Boningari T.; Olmos A.; Reddy B. M.; Sommer J.; Pale P. Zeo, Click Chemistry: Copper (I)–Zeolite Catalyzed Cascade Reaction; One-Pot Epoxide Ring-Opening and Cycloaddition. Eur. J. Org. Chem. 2010, 6338–6347. 10.1002/ejoc.201000802. [DOI] [Google Scholar]
- Kumar B. S. P. A.; Reddy K. H. V.; Satish G.; Kumar R. U.; Nageswar Y. V. D. Synthesis of β-hydroxy-1,4-disubstituted-1,2,3-triazoles catalyzed by copper ferrite nanoparticles in tap water using click chemistry. RSC Adv. 2014, 4, 60652–60656. 10.1039/C4RA12061B. [DOI] [Google Scholar]
- Alonso F.; Moglie Y.; Radivoy G.; Yus M. Multicomponent click synthesis of 1,2,3-triazoles from epoxides in water catalyzed by copper nanoparticles on activated carbon. J. Org. Chem. 2011, 76, 8394–8405. 10.1021/jo2016339. [DOI] [PubMed] [Google Scholar]
- Mukherjee N.; Ahammed S.; Bhadra S.; Ranu B. C. Solvent-free one-pot synthesis of 1,2,3-triazole derivatives by the ‘Click’ reaction of alkyl halides or aryl boronic acids, sodium azide and terminal alkynes over a Cu/Al2O3 surface under ball-milling. Green Chem. 2013, 15, 389–397. 10.1039/C2GC36521A. [DOI] [Google Scholar]
- Prasad A. N.; Thirupathi B.; Raju G.; Srinivas R.; Reddy B. M. Green Chemical Synthesis and Click Reactions. Catal. Sci. Technol. 2012, 2, 1264–1268. 10.1039/c2cy20052j. [DOI] [Google Scholar]
- a Erythropel H. C.; Zimmerman J. B.; de Winter T. M.; Petitjean L.; Melnikov F.; Lam C. H.; Lounsbury A. W.; Mellor K. E.; Janković N. Z.; Tu Q.; Pincus L. N.; Falinski M. M.; Shi W.; Coish P.; Plata D. L.; Anastas P. T. The Green ChemisTREE: 20 years after taking root with the 12 principles. Green Chem. 2018, 20, 1929–1961. 10.1039/C8GC00482J. [DOI] [Google Scholar]; b Frontana-Uribe B. A.; Little R. D.; Ibanez J. G.; Palma A.; Vasquez-Medrano R. Organic electrosynthesis: a promising green methodology in organic chemistry. Green Chem. 2010, 12, 2099–2119. 10.1039/c0gc00382d. [DOI] [Google Scholar]
- a Ferré M.; Pleixats R.; Man M. W. C.; Cattoën X. Recyclable organocatalysts based on hybrid silicas. Green Chem. 2016, 18, 881–922. 10.1039/C5GC02579F. [DOI] [Google Scholar]; b Gao K.; Huang C.; Yang Y.; Li H.; Wu J.; Hou H. Cu(I)-Based Metal–Organic Frameworks as Efficient and Recyclable Heterogeneous Catalysts for Aqueous-Medium C–H Oxidation. Cryst. Growth Des. 2019, 19, 976–982. 10.1021/acs.cgd.8b01527. [DOI] [Google Scholar]; c Lo H. K.; Thiel I.; Copéret C. Efficient CO2 Hydrogenation to Formate with Immobilized Ir-Catalysts Based on Mesoporous Silica Beads. Chem. – Eur. J. 2019, 25, 9443–9446. 10.1002/chem.201901663. [DOI] [PubMed] [Google Scholar]
- a Gu T.; Chen J.; Qiu H. A novel green approach for the chemical modification of silica particles based on deep eutectic solvents. Chem. Commun. 2015, 51, 9825–9828. 10.1039/C5CC02553B. [DOI] [PubMed] [Google Scholar]; b Alipour K.; Nasirpouri F. Effect of Morphology and Surface Modification of Silica Nanoparticles on the Electrodeposition and Corrosion Behavior of Zinc-Based Nanocomposite Coatings. J. Electrochem. Soc. 2019, 166, D1–D9. 10.1149/2.0191902jes. [DOI] [Google Scholar]; c Ahn B.; Kim D.; Kim K.; Kim I.; Kim H. J.; Kang C. H.; Lee J.-Y.; Kim W. Effect of the functional group of silanes on the modification of silica surface and the physical properties of solution styrene-butadiene rubber/silica composites. Compos. Interface 2019, 26, 585–596. 10.1080/09276440.2018.1514145. [DOI] [Google Scholar]
- a Hauser P. M.; Hunger M.; Buchmeiser M. R. Silica-Supported Molybdenum Alkylidyne N-Heterocyclic Carbene Catalysts: Relevance of Site Isolation to Catalytic Performance. ChemCatChem 2018, 10, 1829–1834. 10.1002/cctc.201701654. [DOI] [Google Scholar]; b Rather R. A.; Siddiqui Z. N. Synthesis, characterization and application of Nd-Salen schiff base complex Immobilized Mesoporous Silica in solvent free synthesis of pyranopyrazoles. J. Organomet. Chem. 2018, 868, 164–174. 10.1016/j.jorganchem.2018.05.008. [DOI] [Google Scholar]
- a Rostovtsev V. V.; Green L. G.; Fokin V. V.; Sharpless K. B. A Stepwise Huisgen Cycloaddition Process: Copper(I) Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem., Int. Ed. 2002, 41, 2596–2605. . [DOI] [PubMed] [Google Scholar]; b Shao C.; Wang X.; Xu J.; Zhao J.; Zhang Q.; Hu Y. Carboxylic Acid-Promoted Copper (I)-Catalyzed Azide–Alkyne Cycloaddition. J. Org. Chem. 2010, 75, 7002–7005. 10.1021/jo101495k. [DOI] [PubMed] [Google Scholar]; c Deraedt C.; Pinaud N.; Astruc D. Recyclable Catalytic Dendrimer Nanoreactor for Part-Per-Million CuI Catalysis of “Click” Chemistry in Water. J. Am. Chem. Soc. 2014, 136, 12092–12098. 10.1021/ja5061388. [DOI] [PubMed] [Google Scholar]; d Jiang Y.; Kong D.; Zhao J.; Qi Q.; Li W.; Xu G. Cu(OAc)2·H2O/NH2NH2·H2O: an efficient catalyst system that in situ generates Cu2O nanoparticles and HOAc for Huisgen click reactions. RSC Adv. 2014, 4, 1010–1019. 10.1039/C3RA45437A. [DOI] [Google Scholar]
- a Guru M. M.; Punniyamurthy T. Copper(II)-Catalyzed Aerobic Oxidative Synthesis of Substituted 1,2,3- and 1,2,4-Triazoles from Bisarylhydrazones via C–H Functionalization/C–C/N–N/C–N Bonds Formation. J. Org. Chem. 2012, 77, 5063–5073. 10.1021/jo300592t. [DOI] [PubMed] [Google Scholar]; b Li P.; Liu Y.; Wang L.; Xiao J.; Tao M. Copper(II) Schiff Base Complex-Functionalized Polyacrylonitrile Fiber as a Green Efficient Heterogeneous Catalyst for One-Pot Multicomponent Syntheses of 1,2,3-Triazoles and Propargylamines. Adv. Synth. Catal. 2018, 360, 1673–1684. 10.1002/adsc.201701475. [DOI] [Google Scholar]; c Tavassoli M.; Landarani-Isfahani A.; Moghadam M.; Tangestaninejad S.; Mirkhani V.; Mohammadpoor-Baltork I. Copper Dithiol Complex Supported on Silica Nanoparticles: A Sustainable, Efficient, and Eco-friendly Catalyst for Multicomponent Click Reaction. ACS Sustainable Chem. Eng. 2016, 4, 1454–1462. 10.1021/acssuschemeng.5b01432. [DOI] [Google Scholar]; d Brassard C. J.; Zhang X.; Brewer C. R.; Liu P.; Clark R. J.; Zhu L. Cu(II)-Catalyzed Oxidative Formation of 5,5′-Bistriazoles. J. Org. Chem. 2016, 81, 12091–12105. 10.1021/acs.joc.6b01907. [DOI] [PubMed] [Google Scholar]; e Chen Y.; Nie G.; Zhang Q.; Ma S.; Li H.; Hu Q. Copper-Catalyzed [3 + 2] Cycloaddition/Oxidation Reactions between Nitro-olefins and Organic Azides: Highly Regioselective Synthesis of NO2-Substituted 1,2,3-Triazoles. Org. Lett. 2015, 17, 1118–1121. 10.1021/ol503687w. [DOI] [PubMed] [Google Scholar]
- a Bikas R.; Lippolis V.; Noshiranzadeh N.; Farzaneh-Bonab H.; Blake A. J.; Siczek M.; Hosseini-Monfared H.; Lis T. Electronic Effects of Aromatic Rings on the Catalytic Activity of Dioxidomolybdenum(VI)–Hydrazone Complexes. Eur. J. Inorg. Chem. 2017, 999–1006. 10.1002/ejic.201601359. [DOI] [Google Scholar]; b Vignesh A.; Bhuvanesh N. S. P.; Dharmaraj N. Conversion of Arylboronic Acids to Tetrazoles Catalyzed by ONO Pincer-Type Palladium Complex. J. Org. Chem. 2017, 82, 887–892. 10.1021/acs.joc.6b02277. [DOI] [PubMed] [Google Scholar]; c Watanabe K.; Mino T.; Abe T.; Kogure T.; Sakamoto M. Hydrazone–Palladium-Catalyzed Allylic Arylation of Cinnamyloxyphenylboronic Acid Pinacol Esters. J. Org. Chem. 2014, 79, 6695–6702. 10.1021/jo501235w. [DOI] [PubMed] [Google Scholar]
- a Deng J.; Yu P.; Zhang Z.; Zhang J.; Zhewen S.; Cai M.; Yuan H.; Liang H.; Yang F. Novel Pt (II) complexes with modified aroyl-hydrazone Schiff-base ligands: synthesis, cytotoxicity and action mechanism. Metallomics 2019, 11, 1847–1863. 10.1039/C9MT00193J. [DOI] [PubMed] [Google Scholar]; b Bikas R.; Farzaneh-Bonab H.; Noshiranzade N.; Aygun M.; Emami M.; Lis T. Coumarin-naphthohydrazone ligand with a rare coordination mode to form Mn(II) and Co(II) 1-D coordination polymers: synthesis, characterization, and crystal structure. J. Coord. Chem. 2018, 71, 1127–1146. 10.1080/00958972.2018.1446083. [DOI] [Google Scholar]; c Ghorbanloo M.; Jafari S.; Bikas R.; Krawczyk M. S.; Lis T. Dioxidovanadium (V) complexes containing thiazol-hydrazone NNN-donor ligands and their catalytic activity in the oxidation of olefins. Inorg. Chim. Acta 2017, 455, 15–24. 10.1016/j.ica.2016.10.005. [DOI] [Google Scholar]
- a Conley R. T.The Near-Infrared Region. Infrared Spectroscopy; Allyn & Bacon: Boston, MA, 1966. [Google Scholar]; b Mishra A. K.; Chattopadhyay D. K.; Sreedhar B.; Raju K. V. S. N. FT-IR and XPS studies of polyurethane-urea-imide coatings. Prog. Org. Coat. 2006, 55, 231–243. 10.1016/j.porgcoat.2005.11.007. [DOI] [Google Scholar]; c Noshiranzadeh N.; Heidari A.; Haghi F.; Bikas R.; Lis T. Chiral lactic hydrazone derivatives as potential bioactive antibacterial agents: Synthesis, spectroscopic, structural and molecular docking studies. J. Mol. Struct. 2017, 1128, 391–399. 10.1016/j.molstruc.2016.09.006. [DOI] [Google Scholar]; d Aligholivand M.; Shaghaghi Z.; Bikas R.; Kozakiewicz A. Electrocatalytic water oxidation by a Ni(II) salophen-type complex. RSC Adv. 2019, 9, 40424–40436. 10.1039/C9RA08585H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Nakamoto K.Infrared and Raman Spectra of Inorganic and Coordination Compounds, 6th ed.; John Wiley & Sons: Hoboken, 2009. [Google Scholar]; b Kamyabi M. A.; Soleymani-Bonoti F.; Alirezaei F.; Bikas R.; Noshiranzadeh N.; Emami M.; Krawczyk M. S.; Lis T. Electrocatalytic properties of a dinuclear cobalt (III) coordination compound in molecular oxygen reduction reaction. Appl. Organomet. Chem. 2019, e5214 10.1002/aoc.5214. [DOI] [Google Scholar]; c Kamyabi M. A.; Soleymani-Bonoti F.; Bikas R.; Hosseini-Monfared H.; Arshadi N.; Siczek M.; Lis T. Molecular oxygen reduction catalyzed by a highly oxidative resistant complex of cobalt–hydrazone at the liquid/liquid interface. Phys. Chem. Chem. Phys. 2015, 17, 32161–32172. 10.1039/C5CP04695E. [DOI] [PubMed] [Google Scholar]
- a Edington S. C.; Flanagan J. C.; Baiz C. R. An Empirical IR Frequency Map for Ester C-O Stretching Vibrations. J. Phys. Chem. A 2016, 120, 3888–3896. 10.1021/acs.jpca.6b02887. [DOI] [PubMed] [Google Scholar]; b Reisenauer H. P.; Wagner J. P.; Schreiner P. R. Gas-Phase Preparation of Carbonic Acid and Its Monomethyl Ester. Angew. Chem. 2014, 126, 11960–11965. 10.1002/ange.201406969. [DOI] [PubMed] [Google Scholar]
- a Bikas R.; Ajormal F.; Emami M.; Noshiranzadeh N.; Kozakiewicz A. Catalytic oxidation of benzyl alcohols by new Cu(II) complexes of 1,3-oxazolidine based ligand obtained from a solvent free reaction. Inorg. Chim. Acta 2018, 478, 77–87. 10.1016/j.ica.2018.03.038. [DOI] [Google Scholar]; b Bikas R.; Shahmoradi E.; Reinoso S.; Emami M.; Lezama L.; Sanchiz J.; Noshiranzadeh N. The effect of the orientation of the Jahn–Teller distortion on the magnetic interactions of trinuclear mixed-valence Mn(II)/Mn(III) complexes. Dalton Trans. 2019, 48, 13799–13812. 10.1039/C9DT01652J. [DOI] [PubMed] [Google Scholar]
- a Emami M.; Noshiranzadeh N.; Bikas R.; Gutierrez A.; Kozakiewicz A. Synthesis, crystal structure and magnetic studies of linear and cubane-type tetranuclear Cu(II) complexes obtained by stoichiometric control of the reagents. Polyhedron 2017, 122, 137–146. 10.1016/j.poly.2016.11.010. [DOI] [Google Scholar]; b Roy S.; Mondal P.; Sengupta P. S.; Dhak D.; Santra R. C.; Das S.; Guin P. S. Spectroscopic, computational and electrochemical studies on the formation of the copper complex of 1-amino-4-hydroxy-9,10-anthraquinone and effect of it on superoxide formation by NADH dehydrogenase. Dalton Trans. 2015, 44, 5428–5440. 10.1039/C4DT03635B. [DOI] [PubMed] [Google Scholar]
- a Chesman A. S. R.; Turner D. R.; Price D. J.; Moubaraki B.; Murray K. S.; Deacon G. B.; Batten S. R. Solvothermal vs. bench-top reactions: Control over the formation of discrete complexes and coordination polymers. Chem. Commun. 2007, 3541–3543. 10.1039/b707709b. [DOI] [PubMed] [Google Scholar]; b Martin N. P.; Volkringer C.; Henry N.; Trivelli X.; Stoclet G.; Ikeda-Ohno A.; Loiseau T. Formation of a new type of uranium (IV) poly-oxo cluster {U 38} based on a controlled release of water via esterification reaction. Chem. Sci. 2018, 9, 5021–5032. 10.1039/C8SC00752G. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Wang J.-J.; Cao Z.; Wang X.; Tang L.; Hou X.-Y.; Ju P.; Ren Y.-X.; Chen X.-L.; Zhang Y.-Q. A novel 3D Cd (II) coordination polymer generated via in situ ligand synthesis involving C–O ester bond formation. RSC Adv. 2019, 9, 307–312. 10.1039/C8RA06112B. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Squitieri R. A.; Shearn-Nance G. P.; Hein J. E.; Shaw J. T. Synthesis of esters by in situ formation and trapping of diazoalkanes. J. Org. Chem. 2016, 81, 5278–5284. 10.1021/acs.joc.6b00408. [DOI] [PubMed] [Google Scholar]
- a Tian Y.; Yin P.; Qu R. J.; Wang C. H.; Zheng H. G.; Yu Z. X. Removal of transition metal ions from aqueous solutions by adsorption using a novel hybrid material silica gel chemically modified by triethylenetetraminomethylenephosphonic acid. Chem. Eng. J. 2010, 162, 573–579. 10.1016/j.cej.2010.05.065. [DOI] [Google Scholar]; b Chen J.; Qu R.; Zhang Y.; Sun C.; Wang C.; Ji C.; Yin P.; Chen H.; Niu Y. Preparation of silica gel supported amidoxime adsorbents for selective adsorption of Hg (II) from aqueous solution. Chem. Eng. J. 2012, 209, 235–244. 10.1016/j.cej.2012.08.030. [DOI] [Google Scholar]; c Naz A.; Arun S.; Narvi S. S.; Alam M. S.; Singha A.; Bhartiya P.; Dutta P. K. Cu(II)-carboxymethyl chitosan-silane schiff base complex grafted on nano silica: Structural evolution, antibacterial performance and dye degradation ability. Int. J. Biol. Macromol. 2018, 110, 215–226. 10.1016/j.ijbiomac.2017.11.112. [DOI] [PubMed] [Google Scholar]
- a Li Y.; Ren M.; Lv P.; Liu Y.; Shao H.; Wang C.; Tang C.; Zhou Y.; Shuai M. A robust and flexible bulk superhydrophobic material from silicone rubber/silica gel prepared by thiol–ene photopolymerization. J. Mater. Chem. A 2019, 7, 7242–7255. 10.1039/C8TA11111A. [DOI] [Google Scholar]; b Qu R.; Zhang Y.; Qu W.; Sun C.; Chen J.; Ping Y.; Chen H.; Niu Y. Mercury adsorption by sulfur-and amidoxime-containing bifunctional silica gel based hybrid materials. Chem. Eng. J. 2013, 219, 51–61. 10.1016/j.cej.2012.12.070. [DOI] [Google Scholar]
- a Lee M.; Lamb R.; Sanford M. J.; Pointe A. M. L.; Coates G. W. Nucleophilic ring opening of trans-2, 3-disubstituted epoxides to β-amino alcohols with catalyst-controlled regioselectivity. Chem. Commun. 2018, 54, 12998–13001. 10.1039/C8CC07200K. [DOI] [PubMed] [Google Scholar]; b Shi X.-L.; Sun B.; Hu Q.; Chen Y.; Duan P. Fiber-supported Fe (III) complex catalyst in spinning basket reactor for cleaner ring-opening of epoxides with alcohols. Green Chem. 2019, 21, 3573–3582. 10.1039/C9GC00987F. [DOI] [Google Scholar]; c Lu J.; Ma E.-Q.; Liu Y.-H.; Li Y.-M.; Mo L.-P.; Zhang Z.-H. One-pot three-component synthesis of 1,2,3-triazoles using magnetic NiFe2O4–glutamate–Cu as an efficient heterogeneous catalyst in water. RSC Adv. 2015, 5, 59167–59185. 10.1039/C5RA09517D. [DOI] [Google Scholar]; d Naeimi H.; Nejadshafiee V. Efficient one-pot click synthesis of β-hydroxy-1,2,3-triazoles catalyzed by copper (i)@ phosphorated SiO2 via multicomponent reaction in aqueous media. New J. Chem. 2014, 38, 5429–5435. 10.1039/C4NJ00909F. [DOI] [Google Scholar]
- a Sahay I. I.; Ghalsasi P. S. Water-Assisted Self-Aggregation of Benzimidazole and Triazole Adducts. ACS Omega 2019, 4, 437–443. 10.1021/acsomega.8b02688. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Gupta A.; Jamatia R.; Patil R. A.; Ma Y.-R.; Pal A. K. Copper Oxide/Reduced Graphene Oxide Nanocomposite-Catalyzed Synthesis of Flavanones and Flavanones with Triazole Hybrid Molecules in One Pot: A Green and Sustainable Approach. ACS Omega 2018, 3, 7288–7299. 10.1021/acsomega.8b00334. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Aneja B.; Azam M.; Alam S.; Perwez A.; Maguire R.; Yadava U.; Kavanagh K.; Daniliuc C. G.; Rizvi M. M.; Haq Q. M. R.; Abid M. Natural Product-Based 1,2,3-Triazole/Sulfonate Analogues as Potential Chemotherapeutic Agents for Bacterial Infections. ACS Omega 2018, 3, 6912–6930. 10.1021/acsomega.8b00582. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Singh K.; Gangrade A.; Jana A.; Mandal B. B.; Das N. Design, Synthesis, Characterization, and Antiproliferative Activity of Organoplatinum Compounds Bearing a 1,2,3-Triazole Ring. ACS Omega 2019, 4, 835–841. 10.1021/acsomega.8b02849. [DOI] [Google Scholar]
- a Ben El Ayouchia H.; Bahsis L.; Anane H.; Domingo L. R.; Stiriba S.-E. Understanding the mechanism and regioselectivity of the copper(I) catalyzed [3 + 2] cycloaddition reaction between azide and alkyne: a systematic DFT study. RSC Adv. 2018, 8, 7670–7678. 10.1039/C7RA10653J. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Himo F.; Lovell T.; Hilgraf R.; Rostovtsev V. V.; Noodleman L.; Sharpless K. B.; Fokin V. V. Copper(I)-Catalyzed Synthesis of Azoles. DFT Study Predicts Unprecedented Reactivity and Intermediates. J. Am. Chem. Soc. 2005, 127, 210–216. 10.1021/ja0471525. [DOI] [PubMed] [Google Scholar]; c Ye W.; Xiao X.; Wang L.; Hou S.; Hu C. Synthesis of Mono- and Binuclear Cu(II) Complexes Bearing Unsymmetrical Bipyridine–Pyrazole–Amine Ligand and Their Applications in Azide–Alkyne Cycloaddition. Organometallics 2017, 36, 2116–2125. 10.1021/acs.organomet.7b00154. [DOI] [Google Scholar]; d Wang Y.; Liu J.; Xia C. Insights into Supported Copper(II)-Catalyzed Azide-Alkyne Cycloaddition in Water. Adv. Synth. Catal. 2011, 353, 1534–1542. 10.1002/adsc.201000868. [DOI] [Google Scholar]
- Khdary N. H.; Ghanem M. A. Highly dispersed platinum nanoparticles supported on silica as catalyst for hydrogen production. RSC Adv. 2014, 4, 50114–50122. 10.1039/C4RA09341K. [DOI] [Google Scholar]
- Sheldrick G. M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr., Sect. A: Found. Adv. 2015, 71, 3–8. 10.1107/S2053273314026370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandenburg K.Diamond, Crystal, Molecular Structure Visualization, version 3.2d; Crystal Impact, Dr. H. Putz & K.Brandenburg GbR: Bonn, Germany, 2009.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.