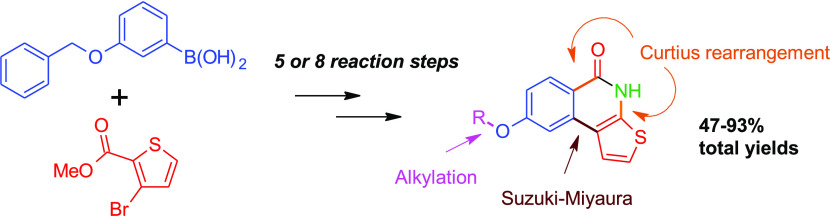
Abstract
Thieno[2,3-c]isoquinolin-5(4H)-one is known for its potential as an anti-ischemic agent through the inhibition of poly(ADP-ribose) polymerase 1 (PARP1). However, the compound also inhibits many other enzymes of the PARP family, potentially limiting its usability. The broad inhibition profile, on the other hand, indicates that this molecule backbone could be potentially used as a scaffold for the development of specific inhibitors for certain PARP enzymes. These efforts call for novel synthetic strategies for substituted thieno[2,3-c]isoquinolin-5(4H)-one that could provide the needed selectivity. In this article, an efficient synthetic strategy for 8-alkoxythieno[2,3-c]isoquinolin-5(4H)-ones through eight steps is presented and other tested synthetic pathways are discussed in detail. Synthesis of 7-methoxythieno[2,3-c]isoquinolin-5(4H)-one is also demonstrated to show that the strategy can be applied widely in the syntheses of substituted alkoxythieno[2,3-c]isoquinolin-5(4H)-ones.
Introduction
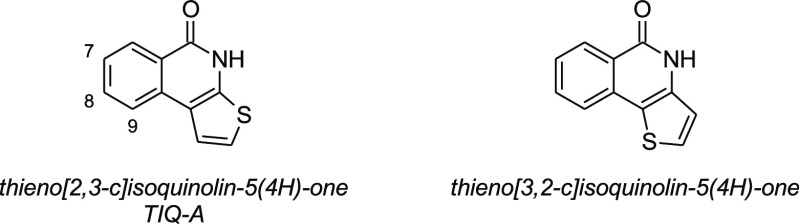
The reports on thieno[2,3-c]isoquinolin-5(4H)-one (TIQ-A) (Figure 1) and also its isomeric form thieno[3,2-c]isoquinolin-5(4H)-one are relatively rare. Originally, TIQ-A was studied as a potential anti-ischemic agent targeting poly(ADP-ribose) polymerase 1 (PARP1).1−5 The efficacy of TIQ-A has been demonstrated in many in vivo models where the compound has been used as a chemical probe to investigate various consequences of PARP1 inhibition.1,6,7 However, TIQ-A has poor selectivity, as the compound equally binds and inhibits many other enzymes of the PARP family.8 The broad inhibition profile, on the other hand, indicates that TIQ-A could be potentially used as a scaffold for the development of specific inhibitors for certain PARP enzymes. In contrast to TIQ-A, an analogous thieno[3,2-c]isoquinolin-5(4H)-one has been utilized as an acceptor unit of the donor–acceptor-type semiconducting material in organic solar cells where the devices based on this material showed excellent power conversion efficiencies.9
Figure 1.
Chemical structures of TIQ-A and its structural isomer.
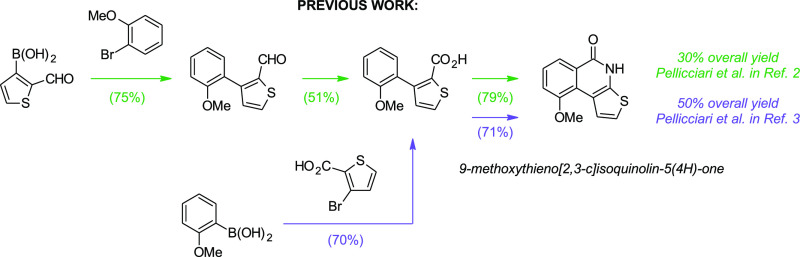
Syntheses of TIQ-A have been performed by Pellicciari et al. using Suzuki–Miyaura cross-coupling followed by multistep reaction sequence giving TIQ-A in 14–33% overall yields.2,3 Using continuous flow synthesis, the overall yield of TIQ-A was further improved to 50%.10 The only known alkoxy-substituted derivatives are 8-methoxythieno[2,3-c]isoquinolin-5(4H)-one, which has been presented in a patent,11 and 9-methoxythieno[2,3-c]isoquinolin-5(4H)-one (Scheme 1), which has been synthesized in 30–50% overall yields using two different Suzuki–Miyaura cross-coupling strategies followed by Curtius rearrangement.2,3
Scheme 1. Previous Syntheses of 9-Methoxythieno[2,3-c]isoquinolin-5(4H)-one.
In this article, a method for the synthesis of 8-alkoxy-substituted thieno[2,3-c]isoquinolin-5(4H)-ones is presented. The developed method or its slight variation can be potentially used for the syntheses of other alkoxy-substituted thieno[2,3-c]isoquinolin-5(4H)-ones.
Results and Discussion
Synthesis
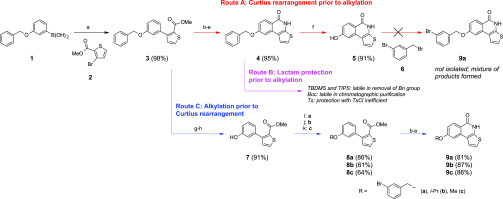
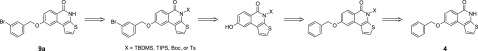
Our strategies for the synthesis of 8-alkoxythieno[2,3-c]isoquinolin-5(4H)-ones are presented in Scheme 2. In this plan, 3-(benzyloxy)phenylboronic acid (1) is utilized as a starting material, which offers benzyl ether-protected phenol functionality for later alkylations. In this way, the use of different alkoxy-substituted arylboronic acids, which can be expensive or whose commercial availability may be limited, can be avoided. Suzuki–Miyaura cross-coupling between 1 and methyl 3-bromothiophene-2-carboxylate (2) afforded product 3 in excellent yield, catalyzed by Pd(PPh3)4 efficiently in the presence of K3PO4 base in dioxane–water.
Scheme 2. Synthetic Routes to 8-Alkoxythieno[2,3-c]isoquinolin-5(4H)-ones.
Reagents and conditions: (a) Pd(PPh3)4, K3PO4, dioxane, H2O, 90 °C, and 24 h; (b) NaOH, EtOH, H2O, reflux, and 1 h; (c) SOCl2, toluene, dimethylformamide (DMF), 80 °C, and 105 min; (d) NaN3, tetrahydrofuran (THF), H2O, ice bath, and 20 min; (e) 1,2-dichlorobenzene, 210 °C, and 20 h; (f) BBr3, CH2Cl2, rt, and 2 h; (g) BBr3, CH2Cl2, rt, and overnight; (h) MeOH, conc. H2SO4, 85 °C, and 19 h; (i) 3-bromobenzyl bromide, K2CO3, DMF, rt, and 19 h; (j) 2-bromopropane, NaH, DMF, 60 °C, and 19 h; and (k) MeOTf, NaH, DMF, 60 °C, and 19 h.
The synthesis of compound 4 was started with the base catalyzed hydrolysis of the ester group of 3 (Route A in Scheme 2). The resulting acid was converted to its acid chloride with thionyl chloride and a catalytic amount of dry DMF following a slightly modified method presented by Pellicciari et al.2 Treatment with NaN3 gave the corresponding carbonyl azide, which readily underwent Curtius rearrangement at an elevated temperature in 1,2-dichlorobenzene, affording 4 in very high yield. The structure of 4 was verified with 1H, 13C, HSQC, and HMBC NMR measurements and high-resolution mass spectrometry (HRMS). The NMR spectra and detailed assignments are presented in the Supporting Information.
Initial attempts to remove benzyl protection from 4 by hydrogenolysis were unsuccessful, probably owing to the poisoning of the Pd/C catalyst by S(II). However, BBr3 in dichloromethane efficiently removed the benzyl group within 2 h. Compound 5 was isolated in high yield (91%) after a short work-up procedure without an extra purification step.
Alkylation of the phenol of 5 with 3-bromobenzyl bromide (6) (1.2 equiv) using K2CO3 in DMF proved to be problematic. On thin-layer chromatography (TLC), three intensive product spots were observed with no starting material 6. 1H NMR analysis of the reaction mixture revealed that the reaction mainly gave a disubstituted product, arising from the alkylation of phenolic OH and at the lactam nitrogen. All attempts to form and isolate 9a in good yield by varying solvents (acetone, 1,4-dioxane) and the base (NaH and without base) failed. We have previously shown that the alkylation of isoquinolin-1-ones needs careful optimization of reaction conditions.12−14 Alkylation can be directed at the lactam N under mildly basic conditions and at lactam O under Mitsunobu conditions affording the target compounds in moderate yields. In comparison with our previous studies of 4-hydroxy-8-tosyloxyquinoline, the N-alkylation of the quinoline derivative was a problem only with methylation affording both N-methylated and the desired O-methylated products.15 Other alkylations solely gave O-alkylated products.
The encountered problems with the trials to carry out alkylation after lactam formation forced us to reassess our synthetic strategy. In the second approach (Route B in Schemes 2 and 3), the protection of the lactam nitrogen of 4 prior to alkylation with 3-bromobenzyl bromide was attempted. After evaluation of the previous literature reports,16 four different protecting groups were selected to be tested. Based on TLC analyses, both tert-butyldimethylsilyl ether (TBDMS) and triisopropylsilyl (TIPS) protecting groups were successfully attached on compound 4 with imidazole as a base. However, these silyl protecting groups did not survive either Pd/C-catalyzed hydrogenolysis or the presence of BBr3. Protection with Boc also failed, as the NBoc group cleaved during the chromatographic purification with both silica and neutral Al2O3. Finally, tosyl protection of 4 was tried with no success. Based on TLC, only a minor amount of N-tosylated compound was formed in the reaction between compound 4 and TsCl.
Scheme 3. Route B: Potential Strategy for the Synthesis of 9a from Compound 4 via Lactam Protection.
In the light of these problems, the third synthetic plan (Route C, Scheme 2) was sketched. The solution was to remove the benzyl group and carry out the desired alkylation of phenol prior to the formation of lactam. Deprotection of 3 with BBr3 simultaneously removed the benzyl group and converted the methyl ester to the acid. Acid-catalyzed esterification restored the methyl thiophenecarboxylate functionality and afforded compound 7 in high (91%) overall yield over the two reaction steps. Previously, the exposure of BBr3 has been supposed to leave methyl thiophenecarboxylate functionalities intact.17 The studies of Finn et al. showed that methyl esters18 can be highly tolerant against BBr3 and it is evident that BBr3 is not capable to efficiently remove ethyl ester groups.19,2017,18 On the other hand, methyl esters can be readily converted to acids using excess BBr3.21,22 Similarly, our results show that excess BBr3 readily removes methyl thiophenecarboxylate functionality giving the carboxylic acid group.
Alkylations of the released phenolic hydroxyl group of 7 with 3-bromobenzyl bromide, 2-bromopropane, and methyl trifluoromethanesulfonate gave compounds 8a–c, respectively, in moderate to good yields (61–86%) without optimization. This shows that compound 7 can be used as a precursor for different alkylation products. Finally, the desired 8-alkoxy-substituted thieno[2,3-c]isoquinolin-5(4H)-ones 9a–c were synthesized from compounds 8a–c and isolated in good (81–87%) yields following the synthetic procedure developed for compound 4. The developed synthetic method (Route C, Scheme 2) afforded compounds 9a–c in 47–62% overall yields over eight reaction steps.
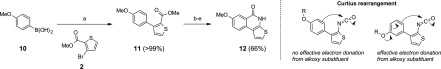
In addition, we wished to see if a similar protocol could be used in the synthesis of regioisomeric 7-alkoxythieno[2,3-c]isoquinolin-5(4H)-ones. To demonstrate this, Suzuki–Miyaura cross-coupling between 4-(methoxy)phenylboronic acid (10) and methyl 3-bromothiophene-2-carboxylate (2) was carried out to afford the coupling product 11 (Scheme 4). Previously, compound 11 has been synthesized in 77% yield using the same starting materials and reagents in anhydrous 1,4-dioxane.23 Using aq dioxane, we were able to collect 11 in quantitative yield. Using 11 as a starting material, the same four-step synthesis developed for compounds 4 and 9a–c was applied. Full conversion of the carbonyl azide to lactam was observed after 12 h and a longer reaction time led to formation of unidentified byproducts. The synthesis afforded 7-methoxythieno[2,3-c]isoquinolin-5(4H)-one (12) in 66% yield. These results show that both 7- and 8-alkoxythieno[2,3-c]isoquinolin-5(4H)-ones can be synthesized in a similar fashion. However, 8-alkoxy substitution, i.e., the para-position in relation to the new Ph–CO bond, is more favorable compared to 7-alkoxy substitution (i.e., the alkoxy substituent is at the meta-position in relation to the new Ph–CO bond) considering the Curtius rearrangement step. Also, the previous literature reports2,3 show that 9-methoxythieno[2,3-c]isoquinolin-5(4H)-one is produced in lower yields (71–79%) compared to 8-alkoxy-substituted thieno[2,3-c]isoquinolin-5(4H)-ones (9a–c) here. 9-Alkoxy substitution corresponds to a similar meta-type positioning of the alkoxy substituent in relation to the new Ph–CO bond as 7-alkoxy substitution, which does not efficiently support the high electron density required for an intramolecular nucleophilic attack of the phenyl core on the carbonyl group in Curtius rearrangement compared to the 8-alkoxy substitution (Scheme 4).
Scheme 4. Synthesis of 7-Methoxythieno[2,3-c]isoquinolin-5(4H)-one (12).
Reagents and conditions: (a) Pd(PPh3)4, K3PO4, dioxane, H2O, 90 °C, and 24 h; (b) NaOH, EtOH, H2O, reflux, and 1 1/2 h; (c) SOCl2, toluene, DMF, 80 °C, and 105 min; (d) NaN3, THF, H2O, ice bath, and 25 min; and (e) 1,2-dichlorobenzene, 210 °C, and 12 h.
Conclusions
An efficient synthetic sequence toward 8-alkoxythieno[2,3-c]isoquinolin-5(4H)-ones has been developed. A successful strategy was to carry out the alkylation prior to the formation of the lactam structure by Curtius rearrangement. In this way, the problematic competition between the alkylation of phenol and lactam positions could be avoided. By this strategy, the target 8-alkoxythieno[2,3-c]isoquinolin-5(4H)-ones 9a–c were obtained in 47–62% total yields after eight reaction steps. The results showed that the developed method or slight variations can be potentially used for the syntheses of other alkoxythieno[2,3-c]isoquinolin-5(4H)-ones. As a demonstration, the synthesis of 7-methoxythieno[2,3-c]isoquinolin-5(4H)-one has been presented. TIQ-A is a potent but nonselective PARP inhibitor that could provide a starting point for the development of specific inhibitors toward individual enzymes of the PARP family. Many PARP enzymes have been linked to cellular pathways often misregulated in diseases such as cancer and specific small molecule inhibitors could validate their potential as drug targets. However, such specificity using the TIQ-A scaffold would require modifications and additional substituents probing and interacting with nonconserved regions of the NAD+ substrate-binding site of the PARP isoforms. The efficient synthetic method presented in this article enables both the development of such compounds and optimization campaigns targeting specific PARP enzymes.
Experimental Section
All commercial starting materials and reagents were used without purification. The solvents were dried with appropriate molecular sieves when needed. The reaction progress was monitored with silica gel-coated aluminum TLC sheets. The chemical structures of all synthesized new compounds were characterized using 1H NMR, 13C NMR, and HRMS techniques. In addition, compound 4 was characterized using HSQC and HMBC measurements. 1H NMR assignments are presented in the Supporting Information.
Methyl 3-[3-(Benzyloxy)phenyl]thiophene-2-carboxylate (3)
A mixture of dioxane (9.0 mL) and H2O (1.5 mL) was bubbled with argon for 10 min. 3-Benzyloxybenzeneboronic acid (1) (344.4 mg, 1.51 mmol), methyl 3-bromothiophene-2-carboxylate (2) (309.3 mg, 1.40 mmol), K3PO4 (587.4 mg, 2.77 mmol), and Pd(PPh3)4 (79.9 mg, 69.1 μmol) were added. The sealed reaction tube was evacuated and backfilled with argon five times. The mixture was stirred and heated in an oil bath (90 °C) for 24 h. The cooled mixture was filtered through a thin pad of silica, which was rinsed with toluene. Evaporation and flash chromatography using the mixture of toluene and n-hexane (6:1) as the eluent gave 3 as a viscous oil (443.3 mg) in 98% yield. 1H NMR (400 MHz, CDCl3) δ ppm 3.78 (s, 3H), 5.10 (s, 2H), 7.01 (ddd, J = 8.3, 2.5, 0.8 Hz, 1H), 7.05–7.12 (m, 3H), 7.31–7.37 (m, 2H), 7.38–7.43 (m, 2H), 7.44–7.49 (m, 2H), 7.51 (d, J = 5.0 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ ppm 51.9, 70.1, 114.4, 115.8, 122.0, 127.1, 127.5, 128.0, 128.6, 128.8, 130.2, 131.5, 137.0, 148.3, 158.3, 162.4. HRMS (ESI) m/z: [M + H]+ calcd for C19H17O3S 325.0893; found 325.0888.
8-(Benzyloxy)thieno[2,3-c]isoquinolin-5(4H)-one (4)
Compound 3 (495.4 mg, 1.53 mmol) was added to a 100 mL round-bottom flask. EtOH (5.0 mL), H2O (5.0 mL), and ground NaOH (1.239 g, 30.98 mmol) were added and the reaction mixture was refluxed for 1 h. CH2Cl2 (15 mL) was added to the cooled mixture. Aq HCl (37%, ca. 2 mL) was added dropwise until the pH value reached 2. The aqueous phase was extracted with CH2Cl2 (2 × 15 mL). The combined organic phase was dried (Na2SO4) and filtered. The solvent was evaporated under vacuum to give the intermediate carboxylic acid as a white powder (475.5 mg; >99%). A portion (302.1 mg, 973 μmol) was transferred into a 50 mL two-neck round-bottom flask, and toluene (6.5 mL) was added. The mixture was stirred at 80 °C under argon. Thionyl chloride (0.12 mL, 1.65 mmol) and dry DMF (few drops) were added. After 105 min, the solvent and excess reagent were evaporated. THF (4.0 mL) was added under argon and the mixture was stirred at 0 °C. NaN3 (94.2 mg, 1.45 mmol) in H2O (1.4 mL) was added slowly to the mixture. After 20 min, ice water (15 mL) and CH2Cl2 (10 mL) were added. The phases were separated, and the aqueous layer was extracted with CH2Cl2 (2 × 10 mL). The combined organic phase was dried (Na2SO4) and filtered. The evaporation residue was dissolved in 1,2-dichlorobenzene (6.0 mL) and added to hot (210 °C) 1,2-dichlorobenzene (14 mL) in a 50 mL two-neck round-bottom flask equipped with a reflux condenser and an argon balloon. The reaction mixture was stirred at 210 °C for 20 h. The evaporation residue was refluxed in the mixture of methanol (5.0 mL) and ethanol (2.5 mL) for 10 min. The solid was collected by filtration and washed with a mixture of MeOH (7.5 mL) and EtOH (2.5 mL) and dried to afford 4 as a gray powder (282.9 mg) in 95% overall yield. 1H NMR (400 MHz, (CD3)2SO) δ ppm 5.30 (s, 2H), 7.15 (br dd, J = 8.9, 2.1 Hz, 1H), 7.21 (br d, J = 5.6 Hz, 1H), 7.36 (br d, J = 7.2 Hz, 1H), 7.42 (br t, J = 7.3 Hz, 2H), 7.52 (br d, J = 7.1 Hz, 2H), 7.68 (br d, J = 2.1 Hz, 1H), 7.73 (br d, J = 5.5 Hz, 1H), 8.16 (d, J = 8.8 Hz, 1H), 12.21 (br s, 1H). 13C NMR (100 MHz, (CD3)2SO) δ ppm 69.8 (Bn CH2), 106.2 (isoq. CH), 115.3 (isoq. CH), 116.4 (thiop. CH), 117.2 (isoq. C), 117.7 (thiop. C), 121.5 (thiop. CH), 128.1 (Bn CH), 128.6 (Bn CH), 130.1 (isoq. CH), 135.6 (isoq. C), 136.6 (Bn C), 141.4 (thiop. C), 161.0 (C=O), 162.0 (isoq. C). HRMS (ESI) m/z: [M + H]+ calcd for C18H14NO2S 308.0740; found 308.0737.
8-Hydroxythieno[2,3-c]isoquinolin-5(4H)-one (5)
BBr3 (0.81 mL, 1 M in CH2Cl2, 810 μmol) was added dropwise to vigorously stirred 4 (49.7 mg, 0.16 mmol) in CH2Cl2 (5.0 mL) under argon in an ice bath. The reaction was allowed to proceed at room temperature for 2 h. The reaction tube was cooled in an ice bath and sat. aq NaHCO3 (5.0 mL) was slowly added. The aqueous phase was washed with CH2Cl2 (4 × 8 mL). Aq HCl (2 M, ca. 2 mL) was added dropwise to the aqueous phase until the pH value reached 2. The precipitate from the aqueous phase was filtered and washed with water. Drying (desiccator) gave 5 as a gray powder (32.0 mg) in 91% yield. 1H NMR (400 MHz, (CD3)2SO) δ ppm 6.95 (dd, J = 8.7, 2.3 Hz, 1H), 7.18 (d, J = 5.6 Hz, 1H), 7.30 (d, J = 2.2 Hz, 1H), 7.54 (d, J = 5.6 Hz, 1H), 8.08 (d, J = 8.7 Hz, 1H), 10.41 (s, 1H) 12.06 (s, 1H). 13C NMR (100 MHz, (CD3)2SO) δ ppm 107.0, 115.5, 115.9, 116.3, 117.4, 121.1, 130.3, 135.7, 141.1, 161.1, 161.6. HRMS (ESI) m/z: [M + H]+ calcd for C11H8NO2S 218.0270; found 218.0271.
Methyl 3-(3-Hydroxyphenyl)thiophene-2-carboxylate (7)
BBr3 (3.50 mL, 1 M in CH2Cl2) was added dropwise to vigorously stirred 3 (434.3 mg, 1.34 mmol) in CH2Cl2 (12 mL) under argon in an ice bath. The reaction was allowed to proceed overnight at room temperature. The reaction tube was cooled in an ice bath and aq NaOH (1 M, 15 mL) was slowly added. The separated organic phase was extracted with aq NaOH (1 M, 4 × 15 mL). EtOAc (15 mL) was added to the combined aqueous phase and aq HCl (37%) was added dropwise until the pH value reached 2. The separated aqueous layer was extracted with EtOAc (4 × 15 mL). The combined EtOAc layers were dried (Na2SO4) and filtered. Evaporation gave the intermediate as a sticky solid (287.6 mg, 98%). A portion (148.5 mg, 674 μmol) was stirred with H2SO4 (1.0 mL) at 85 °C in MeOH (20 mL) for 19 h. The solvent was evaporated to a small volume and EtOAc (10 mL) was added at 0 °C, followed by sat. aq NaHCO3 (10 mL). The organic layer was separated and the aqueous phase was extracted with EtOAc (2 × 10 mL). The combined organic phases were dried (Na2SO4) and filtered. Evaporation gave 7 as a sticky solid (148.0 mg) in 91% overall yield. H NMR (400 MHz, CDCl3) δ ppm 3.79 (s, 3H), 6.83 (ddd, J = 8.2, 2.6, 0.9 Hz, 1H), 6.93–6.94 (m, 1H), 7.00 (dt, J = 7.7, 1.2 Hz, 1H), 7.07 (d, J = 5.0 Hz, 1H), 7.26 (t, J = 7.9 Hz, 1H), 7.50 (d, J = 5.0 Hz, 1H), 8.33 (br s, 1 H). 13C NMR (100 MHz, CDCl3) δ ppm 52.0, 115.0, 116.3, 121.7, 126.9, 129.1, 130.3, 131.5, 137.1, 148.3, 155.1, 162.6. HRMS (ESI) m/z: [M + H]+ calcd for C12H11O3S 235.0423; found 235.0427.
Methyl 3-{3-[(3-Bromophenyl)methoxy]phenyl}thiophene-2-carboxylate (8a)
Compound 7 (57.2 mg; 244 μmol) in DMF (5.0 mL) was added to 3-bromobenzyl bromide (6) (73.3 mg; 293 μmol) and K2CO3 (50.7 mg, 367 μmol), and the mixture was stirred at room temperature for 19 h. The mixture was filtered through a thin pad of silica, which was rinsed with toluene. Evaporation and flash chromatography using the mixture of toluene and n-hexane (6:1) as an eluent gave 8a as a sticky solid (84.3 mg) in 86% yield. 1H NMR (400 MHz, CDCl3) δ ppm 3.77 (s, 3H), 5.05 (s, 2H), 6.96–6.99 (m, 1H), 7.06–7.08 (m, 3H), 7.23–7.27 (m, 1H), 7.30–7.37 (m, 2H), 7.44–7.46 (m, 1H), 7.50 (d, J = 5.0 Hz, 1H), 7.61 (s, 1H). 13C NMR (100 MHz, CDCl3) δ ppm 51.9, 69.1, 114.4, 115.8, 122.3, 122.6, 125.9, 127.1, 128.9, 130.1, 130.2, 130.4, 131.0, 131.5, 137.1, 139.4, 148.2, 158.0, 162.3. HRMS (ESI) m/z: [M + H]+ calcd for C19H16O3BrS 402.9998; found 402.9993.
Methyl 3-{3-[(Propan-2-yl)oxy]phenyl}thiophene-2-carboxylate (8b)
NaH (60%) in mineral oil (45.0 mg, 1.88 mmol) was added to 7 (288.7 mg, 1.23 mmol) in DMF (10 mL). The sealed tube was evacuated and backfilled with argon five times. 2-Bromopropane (0.58 mL, 6.18 mmol) was added using a syringe. The reaction mixture was stirred at 60 °C for 19 h. The mixture was filtered through a thin pad of silica, which was rinsed with toluene. Evaporation and flash chromatography using toluene as an eluent gave 8b as a sticky solid (209.2 mg) in 61% yield. 1H NMR (400 MHz, CDCl3) δ ppm 1.36 (d, J = 6.1 Hz, 6H), 3.79 (s, 3H), 4.58 (spt, J = 6.0 Hz, 1H), 6.91 (ddd, J = 8.2, 2.5, 0.6 Hz, 1H), 6.98–6.99 (m, 1H), 7.01–7.03 (m, 1H), 7.09 (d, J = 5.1 Hz, 1H), 7.30 (t, J = 7.9 Hz, 1H), 7.50 (d, J = 5.0 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ ppm 22.1, 51.9, 69.9, 115.5, 116.8, 121.5, 127.0, 128.8, 130.1, 131.6, 136.9, 148.5, 157.4, 162.4. HRMS (ESI) m/z: [M + H]+ calcd for C15H17O3S 277.0893; found 277.0891.
Methyl 3-(3-Methoxyphenyl)thiophene-2-carboxylate (8c)
NaH in mineral oil (23.2 mg, 968 μmol) was added to 7 (150.3 mg, 642 μmol) in DMF (10 mL). The sealed reaction tube was evacuated and backfilled with argon five times. Methyl trifluoromethanesulfonate (0.11 mL, 972 μmol) was added using a syringe. The reaction mixture was stirred at 60 °C for 19 h. The mixture was filtered through a thin pad of silica, which was rinsed with toluene. Evaporation and flash chromatography using toluene as an eluent gave 8c as a sticky solid (101.4 mg) in 64% yield. 1H NMR (400 MHz, CDCl3) δ ppm 3.79 (s, 3H), 3.84 (s, 3H), 6.93 (ddd, J = 8.2, 2.6, 0.8 Hz, 1H), 7.01–7.02 (m, 1H), 7.04–7.06 (m, 1H), 7.10 (d, J = 5.0 Hz, 1H), 7.33 (t, J = 8.00 Hz, 1H), 7.51 (d, J = 5.1 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ ppm 51.9, 55.3, 113.5, 114.9, 121.7, 127.1, 128.8, 130.2, 131.5, 136.9, 148.4, 159.0, 162.4. HRMS (ESI) m/z: [M + H]+ calcd for C13H13O3S 249.0580; found 249.0582.
8-[(3-Bromophenyl)methoxy]thieno[2,3-c]isoquinolin-5(4H)-one (9a)
Compound 8a (108.9 mg, 270 μmol) was added to a round-bottom vessel. EtOH (5.0 mL), water (5.0 mL), and ground NaOH (216.9 mg, 5.42 mmol) were added and the reaction mixture was stirred and refluxed for 160 min. Water (10 mL) was added and the mixture was extracted with CH2Cl2 (3 × 10 mL). The combined organic phase was dried (Na2SO4) and filtered. Evaporation gave the intermediate acid (99.7 mg, 256 μmol), which was transferred in a 50 mL two-neck round-bottom flask with toluene (2.5 mL). The acid was subjected to the same reaction sequence as for the synthesis of 4 to give 9a as a gray powder (84.9 mg) in 81% overall yield. 1H NMR (400 MHz, (CD3)2SO) δ ppm 5.32 (s, 2H), 7.17 (dd, J = 8.9, 2.5 Hz, 1H), 7.22 (d, J = 5.6 Hz, 1H), 7.39 (t, J = 7.8 Hz, 1H), 7.53–7.57 (m, 2H), 7.69 (d, J = 2.5 Hz, 1H), 7.73–7.75 (m, 2H), 8.17 (d, J = 8.8 Hz, 1H), 12.21 (s, 1H). 13C NMR (100 MHz, (CD3)2SO) δ ppm 68.7, 106.3, 115.2, 116.4, 117.3, 117.6, 121.4, 121.8, 126.9, 130.1, 130.6, 130.7, 130.9, 135.6, 139.4, 141.5, 161.0, 161.7. HRMS (ESI) m/z: [M + H]+ calcd for C18H13NO2BrS 385.9845; found 385.9841.
8-[(Propan-2-yl)oxy]thieno[2,3-c]isoquinolin-5(4H)-one (9b)
Compound 9b was synthesized from 8b, following the synthetic procedure for 9a. Flash chromatography using EtOAc as an eluent gave a solid, which was washed with n-hexane (3 × 5.0 mL) and dried to afford 9b as a gray powder (171.4 mg) in 87% overall yield. 1H NMR (400 MHz, CDCl3) δ ppm 1.43 (d, J = 6.1 Hz, 6H), 4.78 (spt, J = 6.0 Hz, 1H), 6.99 (d, J = 5.6 Hz, 1H), 7.06 (dd, J = 8.9, 2.5 Hz, 1H), 7.26 (d, J = 2.5 Hz, 1H), 7.42 (d, J = 5.6 Hz, 1H), 8.45 (d, J = 8.9 Hz, 1H), 12.06 (br s, 1H). 13C NMR (100 MHz, CDCl3) δ ppm 22.0, 70.2, 106.7, 115.6, 115.9, 116.7, 118.9, 120.6, 130.9, 136.1, 140.8, 161.9, 163.5. HRMS (ESI) m/z: [M + H]+ calcd for C14H15NO2S 260.0740; found 260.0742.
8-Methoxythieno[2,3-c]isoquinolin-5(4H)-one (9c)
Compound 9c was synthesized from 8c, following the synthetic procedure for 9a. Flash chromatography using EtOAc as an eluent gave a solid, which was washed with n-hexane (3 × 2.5 mL) and dried to afford 9c as a gray powder (75.7 mg) in 86% overall yield. 1H NMR (400 MHz, CDCl3) δ ppm 3.98 (s, 3H), 7.00 (d, J = 5.6 Hz, 1H), 7.08 (dd, J = 8.9, 2.5 Hz, 1H), 7.26 (d, J = 1.7 Hz, 1H), 7.44 (d, J = 5.6 Hz, 1H), 8.44 (d, J = 8.9 Hz, 1H), 11.44 (br s, 1H). 13C NMR (100 MHz, CDCl3) δ ppm 55.6, 104.7, 114.9, 115.9, 117.1, 118.9, 120.7, 130.9, 136.0, 140.7, 163.2, 163.5. HRMS (ESI) m/z: [M + H]+ calcd for C12H10NO2S 232.0427; found 232.0423.
Methyl 3-(4-Methoxyphenyl)thiophene-2-carboxylate (11)
Synthesis was carried out following the method described for 3, using dioxane (9.0 mL), H2O (1.5 mL), 4-(methoxy)phenylboronic acid (10) (226.8 mg, 1.49 mmol), methyl 3-bromothiophene-2-carboxylate (2) (300.1 mg, 1.36 mmol), K3PO4 (576.8 mg, 2.72 mmol), and Pd(PPh3)4 (78.1 mg, 67.6 μmol). Flash chromatography using toluene as the eluent gave 11 as a light yellow solid (334.5 mg) in >99% yield. 1H NMR (400 MHz, CDCl3) δ ppm 3.80 (s, 3H), 3.86 (s, 3H), 6.94–6.97 (m, 2H), 7.08 (d, J = 5.0 Hz, 1H), 7.41–7.45 (m, 2H), 7.50 (d, J = 5.0 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ ppm 51.8, 55.2, 113.2, 126.0, 127.8, 130.1, 130.5, 131.5, 148.5, 159.4, 162.5.
7-Methoxythieno[2,3-c]isoquinolin-5(4H)-one (12)
The synthesis was carried out following the slightly modified method used for the synthesis of 4. Compound 11 (334.5 mg, 1.45 mmol) was added to a 100 mL round-bottom flask. EtOH (16 mL), H2O (16 mL), and ground NaOH (1.040 g, 26.00 mmol) were added, and the reaction mixture was refluxed for 90 min. CH2Cl2 (30 mL) was added to the cooled mixture. Aq HCl (37%, ca. 2 mL) was added dropwise until the pH value reached 2. The aqueous phase was extracted with CH2Cl2 (30 mL). The combined organic phase was dried (Na2SO4) and filtered. Evaporation gave the intermediate acid as a white powder (308.8 mg, 97.8%). A portion (163.0 mg, 696 μmol) was transferred into a 50 mL two-neck round-bottom flask with toluene (4.7 mL). The mixture was stirred at 85 °C under argon. Thionyl chloride (0.09 mL, 1.23 mmol) and a few droplets of dry DMF were added. After 105 min, the solvent and excess reagent were evaporated. THF (2.9 mL) was added under argon and the mixture was stirred in an ice bath. NaN3 (67.9 mg, 1.04 mmol) in H2O (1.0 mL) was slowly added in the reaction system. After 25 min, ice water (15 mL) and CH2Cl2 (10 mL) were added. The phases were separated, and the aqueous layer was extracted with CH2Cl2 (2 × 10 mL). The combined organic phase was dried (Na2SO4) and filtered. The evaporation residue was dissolved in 1,2-dichlorobenzene (5.0 mL) and the solution was added in hot (210 °C) 1,2-dichlorobenzene (6.5 mL) in a 50 ml two-neck round-bottom flask equipped with a reflux condenser and an argon balloon. The reaction mixture was stirred at 210 °C for 12 h. A spoonful of Celite 521 was added and the solvent was evaporated. Neutral Al2O3 was placed in a sintered glass funnel with EtOAc. The Celite–crude product mixture was placed on the top of the Al2O3 layer. The package was eluated with EtOAc until the brown impurity could not be observed. Next, the package was eluated with wet acetone (ca. 10 mL of water in 300 mL of acetone). The solvents were evaporated from the fractions containing the product and the powder was washed with n-hexane (3 × 5.0 mL) and dried to afford 12 as a gray powder (107.9 mg) in 66% yield. 1H NMR (400 MHz, DMSO-d6) δ ppm 3.88 (s, 3H), 7.22 (d, J = 5.6 Hz, 1H), 7.40 (dd, J = 8.8, 2.8 Hz, 1H), 7.66 (d, J = 5.6 Hz, 1H), 7.68 (d, J = 2.8 Hz, 1H), 8.07 (d, J = 8.8 Hz, 1H), 12.36 (br s, 1H). 13C NMR (100 MHz, CDCl3) δ ppm 55.4, 108.7, 116.6, 117.8, 121.0, 122.1, 124.7, 124.9, 127.6, 138.4, 157.6, 161.0. HRMS (ESI) m/z: [M + H]+ calcd for C12H10NO2S 232.0427; found 232.0429.
Acknowledgments
The authors would like to thank Dr. Ulrich Bergmann (Proteomics and protein analysis core facility of Biocenter Oulu, University of Oulu) for measuring the HRMS data. This work was funded by the Academy of Finland (grant nos. 287063 and 294085 for L.L.) and by the Emil Aaltonen Foundation (for M.M.M.).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c01879.
NMR spectra of synthesized compounds with 1H NMR assignments (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Chiarugi A.; Meli E.; Calvani M.; Picca R.; Baronti R.; Camaioni E.; Costantino G.; Marinozzi M.; Pellegrini-Giampietro D. E.; Pellicciari R.; Moroni F. Novel isoquinolinone-derived inhibitors of poly(ADP-ribose) polymerase-1: Pharmacological characterization and neuroprotective effects in an in vitro model of cerebral ischemia. J. Pharmacol. Exp. Ther. 2003, 305, 943–949. 10.1124/jpet.103.048934. [DOI] [PubMed] [Google Scholar]
- Pellicciari R.; Camaioni E.; Costantino G.; Marinozzi M.; Macchiarulo A.; Moroni F.; Natalini B. Towards new neuroprotective agents: design and synthesis of 4H-thieno[2,3-c] isoquinolin-5-one derivatives as potent PARP-1 inhibitors. Il Farmaco 2003, 58, 851–858. 10.1016/S0014-827X(03)00143-5. [DOI] [PubMed] [Google Scholar]
- Pellicciari R.; Camaioni E.; Gilbert A. M.; Macchiarulo A.; Bikker J. A.; Shah F.; Bard J.; Costantino G.; Gioiello A.; Robertson G. M.; Sabbatini P.; Venturoni F.; Liscio P.; Carotti A.; Bellocchi D.; Cozzi A.; Wood A.; Gonzales C.; Zaleska M. M.; Ellingboe J. W.; Moroni F. Discovery and characterization of novel potent PARP-1 inhibitors endowed with neuroprotective properties: From TIQ-A to HYDAMTIQ. Med. Chem. Commun. 2011, 2, 559–565. 10.1039/c1md00021g. [DOI] [Google Scholar]
- Moroni F.; Cozzi A.; Chiarugi A.; Formentini L.; Camaioni E.; Pellegrini-Giampietro D. E.; Chen Y.; Liang S.; Zaleska M. M.; Gonzales C.; Wood A.; Pellicciari R. Long-lasting neuroprotection and neurological improvement in stroke models with new, potent and brain permeable inhibitors of poly(ADP-ribose) polymerase. Br. J. Pharmacol. 2012, 165, 1487–1500. 10.1111/j.1476-5381.2011.01666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand J.-R.; Carotti A.; Passeri D.; Filipponi P.; Liscio P.; Camaioni E.; Pellicciari R.; Gioiello A.; Macchiarulo A. Investigating the allosteric reverse signalling of PARP inhibitors withmicrosecond molecular dynamic simulations andfluorescence anisotropy. Biochim. Biophys. Acta, Proteins Proteomics 2014, 1844, 1765–1772. 10.1016/j.bbapap.2014.07.012. [DOI] [PubMed] [Google Scholar]
- Hans C. P.; Zerfaoui M.; Naura A. S.; Troxclair D.; Strong J. P.; Matrougui K.; Boulares A. H. Thieno[2,3-c]isoquinolin-5-one, a potent poly(ADP-ribose) polymerase inhibitor, promotes atherosclerotic plaque regression in high-fat diet-fed apolipoprotein E-deficient mice: Effects on inflammatory markers and lipid content. J. Pharmacol. Exp. Ther. 2009, 329, 150–158. 10.1124/jpet.108.145938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba H.; Tsukagoshi A.; Kida S. PARP-1 activity is required for the reconsolidation and extinction of contextual fear memory. Mol. Brain 2015, 8, 63 10.1186/s13041-015-0153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlberg E.; Karlberg T.; Kouznetsova E.; Markova N.; Macchiarulo A.; Thorsell A.-G.; Pol E.; Frostell Å.; Ekblad T.; Öncü D.; Kull B.; Robertson G. M.; Pellicciari R.; Schüler H.; Weigelt J. Family-wide chemical profiling and structural analysis of PARP and tankyrase inhibitors. Nat. Biotechnol. 2012, 30, 283–288. 10.1038/nbt.2121. [DOI] [PubMed] [Google Scholar]
- Li D.; Xiao Z.; Wang S.; Geng X.; Yang S.; Fang J.; Yang H.; Ding L. A thieno[3,2-c]isoquinolin-5(4H)-one building block for efficient thick-film solar cells. Adv. Energy Mater. 2018, 8, 1800397 10.1002/aenm.201800397. [DOI] [Google Scholar]
- Filipponi P.; Ostacolo C.; Novellino E.; Pellicciari R.; Gioiello A. Continuous flow synthesis of thieno[2,3-c]isoquinolin-5(4H)-one scaffold: A valuable source of PARP-1 inhibitors. Org. Process Res. Dev. 2014, 18, 1345–1353. 10.1021/op500074h. [DOI] [Google Scholar]
- Pellicciari R.; Moroni F.; Hansen E. C.; Gilbert A. M.; Larkin P. J.. Preparation of thienyl- and furanyl-isoquinolinones as therapeutic poly(ADP-ribose) polymerase modulators. U.S. Patent US8,633,215, Dec 23, 2014.
- Parveen I.; Naughton D. P.; Whish W. J. D.; Threadgill M. D. 2-Nitroimidazol-5-ylmethyl as a potential bioreductively activated prodrug system: reductively triggered release of the PARP inhibitor 5-bromoisoquinolinone. Bioorg. Med. Chem. Lett. 1999, 9, 2031–2036. 10.1016/S0960-894X(99)00306-6. [DOI] [PubMed] [Google Scholar]
- Ferrer S.; Naughton D. P.; Threadgill M. D. Studies on the reductively triggered release of heterocyclic and steroid drugs from 5-nitrothien-2-ylmethyl prodrugs. Tetrahedron 2003, 59, 3437–3444. 10.1016/S0040-4020(03)00481-2. [DOI] [Google Scholar]
- Ferrer S.; Naughton D. P.; Parveen I.; Threadgill M. D. O-Alkylation of isoquinolin-1-ones in the Mitsunobu reaction: Development of potential drug delivery systems. J. Chem. Soc., Perkin Trans. 2002, 1, 335–340. 10.1039/b109776h. [DOI] [Google Scholar]
- Heiskanen J. P.; Omar W. A. E.; Ylikunnari M. K.; Haavisto K. M.; Juan M. J.; Hormi O. E. O. Synthesis of 4-alkoxy-8-hydroxyquinolines. J. Org. Chem. 2007, 72, 920–922. 10.1021/jo062175i. [DOI] [PubMed] [Google Scholar]
- Wuts P. G. M.; Greene T. W.. Greene’s Protective Groups in Organic Synthesis, 4th ed.; John Wiley & Sons, Inc: Hoboken, New Jersey, 2007; pp 901–913. [Google Scholar]
- Iaroshenko V. O.; Ali S.; Mkrtchyan S.; Gevorgyan A.; Babar T. M.; Semeniuchenko V.; Hassan Z.; Villinger A.; Langer P. Design and synthesis of condensed thienocoumarins by Suzuki-Miyaura reaction/lactonization tandem protocol. Tetrahedron Lett. 2012, 53, 7135–7139. 10.1016/j.tetlet.2012.10.096. [DOI] [Google Scholar]
- Punna S.; Meunier S.; Finn M. G. A hierarchy of aryloxide deprotection by boron tribromide. Org. Lett. 2004, 6, 2777–2779. 10.1021/ol0489898. [DOI] [PubMed] [Google Scholar]
- Nicolaou K. C.; Montagnon T.; Vassilikogiannakis G.; Mathison C. J. N. The total synthesis of coleophomones B, C, and D. J. Am. Chem. Soc. 2005, 127, 8872–8888. 10.1021/ja0509984. [DOI] [PubMed] [Google Scholar]
- Huang X.; Shao N.; Palani A.; Aslanian R.; Buevich A. The total synthesis of psymberin. Org. Lett. 2007, 9, 2597–2600. 10.1021/ol071068n. [DOI] [PubMed] [Google Scholar]
- Lee T. S.; Das A.; Khosla C. Structure–activity relationships of semisynthetic mumbaistatin analogs. Bioorg. Med. Chem. 2007, 15, 5207–5218. 10.1016/j.bmc.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Miley G. P.; Rote J. C.; Silverman R. B.; Kelleher N. L.; Thomson R. J. Total synthesis of tambromycin enabled by indole C–H functionalization. Org. Lett. 2018, 20, 2369–2373. 10.1021/acs.orglett.8b00700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iaroshenko V. O.; Ali S.; Mkrtchyan S.; Gevorgyan A.; Babar T. M.; Semeniuchenko V.; Hassan Z.; Villinger A.; Langer P. Design and synthesis of condensed thienocoumarins by Suzuki–Miyaura reaction/lactonization tandem protocol. Tetrahedron Lett. 2012, 53, 7135–7139. 10.1016/j.tetlet.2012.10.096. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.