Abstract
A novel biostimulant, Paecilomyces variotii extracts (ZNC), with the ability to promote N absorption in the plant at a very low level has been proved in the lab experiment, but its chemical composition and practical effect in the field remain unclear. In this work, we determined the molecular composition of ZNC. Then, a three-year field experiment was conducted to investigate the synergistic effects of controlled-release urea (CRU) without ZNC or with ZNC at three doses (87.5, 175, and 262.5 mL ha–1) on the yield, nitrogen use efficiency (NUE), and net returns of rice. Results indicated that ZNC contained more carbohydrates, amino acids, alkyl structures, and less aromatic structures with a molecular weight between 140 and 2507 Da. Rice yield was 6.9–21.0% higher with CRU than with conventional urea. Combining CRU with ZNC at a dose of 87.5 mL ha–1 performed the best and significantly increased rice yields by 8.7–12.1%, NUE by 15.0–20.2%, and average net returns by 10.9–15.4% during three rice-growing seasons compared to the application of CRU only, which is attributed to the positively increasing panicles and N uptake of rice. With the increased dose of ZNC, the yield of rice showed a decreasing trend, but the yield was still higher/not significant than the CFF treatment without ZNC. Therefore, the planting patterns with the combination of CRU and biostimulant are an efficient way to increase the rice grain yield and net returns.
1. Introduction
Substantial increase in rice yield is largely attributed to the large inputs of synthetic nitrogen (N) fertilizers over the last 50 years.1,2 Urea is the major N fertilizer used in the world due to its high N content and high solubility.3 Nevertheless, mismanagement of urea, especially excessive application, has induced negative physiological impacts, such as increased pest/disease outbreak,4 reduced plant resistance and extended maturity achievement,5,6 and environmental pollution, including greenhouse gaseous emissions,7,8 surface water eutrophication,9 and soil degradation.10 So far, the average nitrogen use efficiency (NUE) in rice cropping systems of the world is only ranging from 25 to 45%, which means over half of the applied urea is not utilized by plants.11
Controlled-release urea (CRU), a new generation of N fertilizer, can enhance NUE and minimize N losses by gradually releasing N to match the demand of crops over the entire growing period.12,13 Numerous studies have demonstrated that the yield and NUE of rice are unarguably increased by the application of CRU.14,15 For example, rice yield with the application of CRU was 7.4% higher than that of applying urea at the same application N rate.16 A 1/3 reduction of the recommended application rate of N was also practicable with CRU to obtain comparably high yield with preserved soil fertility and saved labor compared with application of urea.14,17 Even when the N rate with CRU was reduced by 50% relative to urea, no prominent difference of rice gain yield was observed.17 However, the yield-increasing effect of CRU still has a threshold even though it performs more advantages than urea.18,19 Therefore, more agronomic measures are still needed to ensure a high rice yield as to feed the increasing population.
Biostimulants are defined as materials consisting of one or more substances and/or microorganisms with functionalities to promote plant’s nutrient uptake and utilization, enhance plant tolerance to abiotic/biotic stresses, and improve crop quality at very low doses.20,21 The mechanism of biostimulants in increasing crop yields is mainly by the regulation of physiological and biochemical responses of crops rather than the provision of nutrients for crops.20 Biostimulants have been widely used in the agricultural system with reports of increased nutrient absorption through regulating metabolic changes in tissues,21 promoting root growth by increased root hair length and density,22 enhanced activities of rhizosphere microorganisms, and increased photosynthesis of plants.23 However, whether biostimulants can further improve rice yield with application of CRU is not reported in the literature.
A novel biostimulant, Paecilomyces variotii extracts (ZNC), has the functionality to promote N absorption of a plant with the report that ZNC increased the number of lateral roots and promoted plant growth when applied at the concentrations as low as 1–10 ng/mL in the model plant Arabidopsis thaliana.(24) Subsequent transcriptome analysis further revealed that ZNC enhanced the expression of genes associated with N absorption and auxin biosynthesis genes. However, no experimental studies were conducted to test whether the promotion effects of ZNC in Arabidopsis thaliana can be achieved in other plants such as rice, and no researchers ever verify whether ZNC can promote the N absorption of crops in the field.
In the present study, we sprayed ZNC on the surface of CRU and simultaneously applied just once in the soil before rice planting to investigate their synergy effects on the yield, NUE, and net returns of rice. Before the application of ZNC, we employed transmission electron microscopy (TEM), nuclear magnetic resonance (NMR), and size exclusion chromatography (SEC) to determine the molecular composition of ZNC. The objectives of the current research were to characterize ZNC and CRU and evaluate their synergistic effects on rice yield, NUE, and economic benefit.
2. Results and Discussion
2.1. Nitrogen Release Rate of CRU in Water and Field Condition
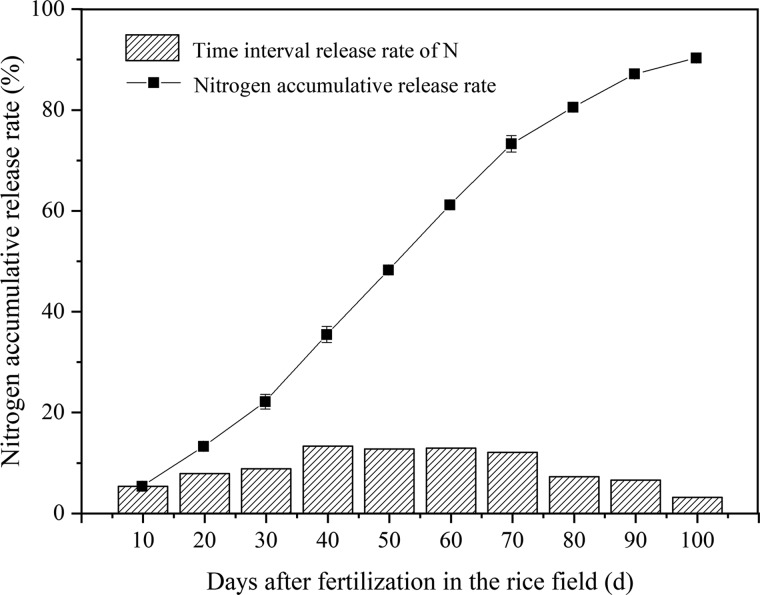
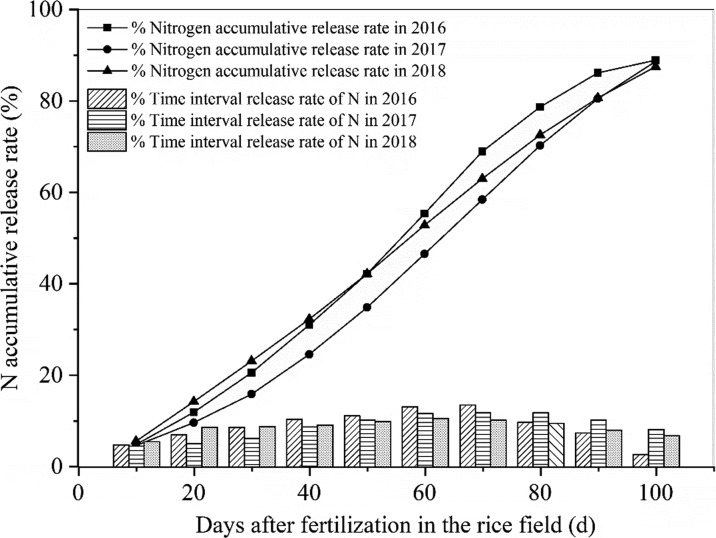
Effective N management is crucial for high-efficiency crop production and sustainable agriculture.25 Measures in N management including the selection of an optimal combination of application rate, source, timing, and placement have been taken to provide adequate N availability to match the crop demand as to maximize N use efficiency, optimize crop production, and minimize the negative impact of N on the environment.26 In this work, N release characteristic of CRU was tested. Under the laboratory condition of 25 °C in water, an initial, relatively slow stage of N release occurred in the first month with only 22.2% (Figure 1); then, followed by an accelerated release rate during the 30th to 70th day, the cumulative N release was 48.4% in this period of time; eventually, a slower release rate occurred at the rest of the duration with 87.2% of the total N released. The N release characteristic of CRU in the natural field conditions was similar with that in water over time (Figure 2). Peak N release occurred from the 30th to 80th day, and the cumulative N release in this period was 58.1, 54.4, and 49.5% in three years, respectively.
Figure 1.
Nitrogen release rate for a period of time and accumulative release rate of CRU in 25 °C water.
Figure 2.
Nitrogen release rate for a period of time and accumulative release rate of CRU in soil.
Synchronized N input with the crop demand is of vital importance for increasing NUE and reducing fertilizer losses.18 Sui et al. observed that the N requirements during the growing season of rice appeared to follow an “S-shaped” curve, low during the early growth period, followed by a high demand from transplanting to the heading stage, and then low from heading to the mature stage.27 Cordero et al. also summarized that rice requires sufficient N input during the tillering stages, thereby maximizing the panicle number and increasing sink size.7 Our present study confirmed that the peak N release of CRU occurred from the 30th to 80th day in the natural field conditions, which precisely matches the growth stage of rice with a high N requirement from the joining stage to the filling stage. Eventually, the annual N accumulative release rate of CRU reached 88.9, 88.7, and 87.5% during three rice growing seasons.
2.2. Effects of the ZNC Dose on Rice Yield and Yield Composition
Three years of data indicated that CRU outperformed urea in increasing rice yields (Table 1). More concretely, the CP0 treatment increased rice yields by 6.9, 21.0, and 14.8% compared to that with the CFF treatment in 2016–2018, respectively, despite applying the same N rate. Ding et al. summarized that rice yield was 7.4% greater on average with the application of CRU as compared to that with application of urea by a meta-analysis.16 Therefore, CRU could substitute for the considerable amounts of urea applied in agriculture for a higher yield.
Table 1. Rice Yield and Yield Components for Different Treatments in 2016–2018a.
| year | treatment | panicles (million ha–1) | spikelets (panicles–1) | 1000-gain weight (g) | yield (kg ha–1) | yield change relative to CFF (%) |
|---|---|---|---|---|---|---|
| 2016 | CK | 3.1d | 79c | 23.2a | 5778e | |
| CFF | 3.7c | 88b | 24.0a | 7641d | ||
| CP0 | 4.3b | 101a | 23.8a | 8171c | 6.9 | |
| CP1 | 5.0a | 97a | 24.0a | 9163a | 19.9 | |
| CP2 | 4.5b | 102a | 23.9a | 8842ab | 15.7 | |
| CP3 | 4.3b | 101a | 24.0a | 8623b | 12.9 | |
| 2017 | CK | 3.0c | 82b | 23.2b | 6287d | |
| CFF | 3.9b | 85b | 24.2a | 6810c | ||
| CP0 | 4.3b | 101a | 23.9ab | 8241b | 21.0 | |
| CP1 | 4.9a | 98a | 24.3a | 9003a | 32.2 | |
| CP2 | 4.4ab | 96a | 24.7a | 8314b | 22.1 | |
| CP3 | 4.18b | 100a | 24.48a | 8155b | 19.8 | |
| 2018 | CK | 3.3d | 80c | 24.4a | 5362d | |
| CFF | 3.8c | 95b | 24.0a | 7133c | ||
| CP0 | 4.5b | 105a | 24.5a | 8186b | 14.8 | |
| CP1 | 4.7a | 105a | 24.3a | 8896a | 24.7 | |
| CP2 | 4.5b | 101a | 24.5a | 8381b | 17.5 | |
| CP3 | 4.4b | 101a | 24.2a | 8148b | 14.2 |
Note: Means followed by the same lowercase letter within each column in the same year were not significantly different (p > 0.05) based on analysis by one-way ANOVAs followed by Duncan multiple range tests.
Nevertheless, the only function of CRU is to supply nutrients. Yield gap analyses revealed that rice yields have reached the upper limit of biophysical potential in recent years,28 and more strategies, not just fertilization, are required to boost the rice production.29 In this study, the application of ZNC combined with CRU further improved the rice yield than that of the only application of CRU, and its effects on rice yield have a close relationship with the dose. Compared with the CP0, the CP1 treatment with a dose of 87.5 mL ha–1 ZNC markedly increased the rice yield by 12.1, 9.3, and 8.7% in three years, respectively. However, the yield-increasing effects were decreased with the raising of ZNC dose. Although rice yields of CP2 and CP3 treatments were still higher than that of the CP0 treatment in 2016, no significant difference was observed between CP2/CP3 and CP0 treatments in 2017 and 2018. In general, CRU combined with ZNC at a dose of 87.5 mL ha–1 has the best yield-increasing capacity.
From the perspective of yield components, there was no significant difference between all the treatments with respect to the 1000-grain weight in 2016 and 2018, while in 2017, the 1000-grain weight was obviously lower in the CK treatment as compared to that in CFF, CP1, CP2, and CP3 treatments. The number of panicles per hectare was greater in the CP0 treatment as compared to that in the CFF treatment by 14.8, 18.8, and 18.1% in three years, respectively. Compared with CP0, the CP1 treatment improved effective panicles by 16.7, 15.3, and 5.0% in three years, respectively. Those results indicated that ZNC improved rice yield by increasing the panicles of rice.
A lab experiment verified that 1–10 ng mL–1 was the optimum concentrations of ZNC for boosting Arabidopsis growth,24 and our field experiment results indicated that ZNC were serviceable on the rice production at a dose of 87.5 mL ha–1. In general, μg/mL-mg/mL is the recommended dose of biostimulants for a positive response.20,30,31 The optimal dose of ZNC for a favorable response was considerably lower than that for other traditional biostimulants,24 for instance, the recommended dose of ZNC was 1/50000 lower than chitosan.24 Therefore, ZNC were an ultrahigh activity biostimulant.
2.3. Aboveground Biomass, N Absorption, and N Use Status of Rice at Maturity
Aboveground biomass of rice was strongly affected by different N varieties that occurred during the three growing seasons (Table 2). CRU promoted the growth of rice as evidenced from the aboveground biomass increase by 11.8, 31.5, and 13.6% for three years, compared with the CFF treatment applied urea. Mean NUE in the CFF treatment was below 30% in three years. In other words, over 70% of the N applied in soil was wasted without being utilized by rice. NUE in CP0 was 14.9, 23.8, and 49.2% higher when compared with CFF in three years, respectively. Besides, it demonstrated that CRU outperformed urea in increasing the nitrogen agronomic efficiency (NAE) and nitrogen partial factor productivity (NPFP) of rice.
Table 2. Aboveground Biomass, N Accumulation, NAE, NPFP, and NUE for Different Treatments in 2016–2018a.
| year | treatment | biomass (kg ha–1) | N accumulation (kg ha–1) | NAE (kg N kg–1) | NPFP (kg N kg–1) | NUE (%) | NUE change relative to CP0 (%) |
|---|---|---|---|---|---|---|---|
| 2016 | CK | 11,485d | 83.1d | ||||
| CFF | 14,659c | 139.2c | 10.4d | 42.5d | 31.2c | ||
| CP0 | 16,337b | 147.6b | 13.3c | 45.4c | 35.9b | ||
| CP1 | 17,920a | 157.3a | 18.8a | 50.9a | 41.3a | 15.1 | |
| CP2 | 17,669a | 147.8b | 17.0ab | 49.1ab | 36.0b | 0.3 | |
| CP3 | 16,577b | 148.4b | 15.8b | 47.9b | 36.3b | 1.2 | |
| 2017 | CK | 12,249d | 84.6d | ||||
| CFF | 13,511c | 140.3c | 2.9c | 37.8c | 30.9c | ||
| CP0 | 16,082b | 153.5b | 10.9b | 45.8b | 38.3b | ||
| CP1 | 17,410a | 164.8a | 15.1a | 50.0a | 44.6a | 16.4 | |
| CP2 | 16,651ab | 152.3b | 11.3b | 46.2b | 37.6b | –1.8 | |
| CP3 | 16,392b | 147.6bc | 10.4b | 45.3b | 35.0bc | –8.6 | |
| 2018 | CK | 10,902d | 80.9d | ||||
| CFF | 14,232c | 127.4c | 9.8c | 39.6c | 25.8c | ||
| CP0 | 16,173ab | 150.3b | 15.7b | 45.5b | 38.6b | ||
| CP1 | 17,153a | 164.4a | 19.6a | 49.4a | 46.4a | 20.2 | |
| CP2 | 16,671b | 148.8b | 16.8b | 46.6b | 37.7b | –2.2 | |
| CP3 | 15,993b | 150.3b | 15.5b | 45.3b | 38.5b | –0.1 |
Note: Means followed by same the lowercase letter within each column in the same year were not significantly different (p > 0.05) based on analysis by one-way ANOVAs followed by Duncan multiple range tests.
On the basis of CRU, the CP1 treatment achieved the highest aboveground biomass in each year, which further increased the rice yield by 9.7, 8.3, and 6.1% than that in the CP0 treatment, respectively. Likewise, NUE in the CP1 treatment was further increased by 15.1, 16.4, and 20.2% as compared to that in the CP0 treatment across three years; NAE in CP1 was increased by 41.5, 39.0, and 24.8% as compared to that in the CP0 treatment in 2016–2018; NPFP in CP1 was obviously 12.1, 9.2, and 8.7% higher than that in the CP0 treatment in three years, respectively. The improved total N level in rice by ZNC can be attributed to the upregulated expression of genes associated with the N absorption by ZNC24 and its promotion on total length, surface area, and volume of roots.32,33 However, the mechanisms for increasing yield and promoting nutrient supply by ZNC still need an intensive study at the molecular level.
Nevertheless, the aboveground biomass, N uptake, NAE, NPFP, and NUE increased at a low ZNC dose (87.5 mL ha–1) and decreased thereafter, illustrating that the effect of ZNC was promoted initially and restrained afterward. Lu et al. found that ZNC promoted the growth of plants at 1 and 10 ng/mL but inhibited at a higher concentration (100 ng/mL).24 A similar study also reported by other researchers states that strong induction of a defense response is often accompanied by growth inhibition, limiting the usefulness of these biostimulants in the field.34 The application of ZNC by spraying on the surface of CRU is an effective measure to magnify the advantages of CRU and can avoid the risk of crop yield reduction by controlling the application dose of the biostimulant.
2.4. Content of NO3−−N, NH4+–N, and Available P and K in Soil at Maturity
At maturity, the content of soil NO3−−N at the 0–20 cm profile in the treatments applied with CRU was 36.8–46.0% higher than that in the CFF treatment in 2016, 37.5–55.4% higher in 2017, and 29.5–44.6% higher in 2018 (Table 3). Similarly, the NH4+–N content of the treatments applied with CRU was 16.4–34.4% higher than that of the CFF treatment in 2016, 26.4–44.0% higher in 2017, and 40.6–56.9% higher in 2018. Adequate nutrient supply is the guarantee for the successful completion of rice life activities represented by filling. CRU could slowly release N to keep soil N at a relatively appropriate level and meet the nutrient demand of crops,17 and our result also indicated that CRU application possessed better results than the urea in the heightening inorganic N content of rice at the maturing stage. Some studies believe that the N release of CRU enables the soil with a relatively stable C/N value and continuously stimulates the mineralization of soil organic matter, which also effectively stabilizes the soil P pool and promote the release of K in soil. However, no prominent difference about soil available P and K was observed among all the treatments in this work.
Table 3. Content of NO3−−N, NH4+–N, and Available P and K in the 0–20 cm Soil Layer from Different Treatments in 2016–2018 at Maturitya.
| year | treatment | NO3−−N (mg kg–1) | NH4+–N (mg kg–1) | available P (mg kg–1) | available K (mg kg–1) |
|---|---|---|---|---|---|
| 2016 | CK | 19.1c | 10.5c | 27.4a | 200.0a |
| CFF | 25.0b | 12.8bc | 28.1a | 216.8a | |
| CP0 | 34.2a | 16.3a | 28.3a | 210.1a | |
| CP1 | 36.5a | 17.1a | 28.2a | 198.3a | |
| CP2 | 34.6a | 17.2a | 32.5a | 200.0a | |
| CP3 | 36.5a | 14.9ab | 32.9a | 186.5a | |
| 2017 | CK | 13.6b | 16.3b | 30.8a | 161.6a |
| CFF | 16.8b | 18.2b | 26.8a | 169.6a | |
| CP0 | 23.4a | 23.8a | 31.0a | 161.6a | |
| CP1 | 23.5a | 24.0a | 30.1a | 156.8a | |
| CP2 | 23.1a | 23.0a | 28.8a | 145.5a | |
| CP3 | 26.1a | 26.2a | 27.5a | 155.2a | |
| 2018 | CK | 16.1b | 10.3b | 23.3a | 189.3a |
| CFF | 20.9b | 11.4b | 23.0a | 174.6a | |
| CP0 | 27.4a | 16.9a | 27.2a | 165.5a | |
| CP1 | 27.0a | 17.4a | 23.6a | 177.8a | |
| CP2 | 28.0a | 17.9a | 21.6a | 172.0a | |
| CP3 | 30.1a | 16.0a | 23.0a | 157.7a |
Note: Means followed by the same lowercase letter within each column in the same year were not significantly different (p > 0.05) based on analysis by one-way ANOVAs followed by Duncan multiple range tests.
Moreover, the dose of ZNC had no obvious effects on the soil inorganic N content, and this conclusion is precisely consistent with the concept of biostimulants defined by the European Biostimulant Industry Council (EBIC): “Biostimulants operate through different mechanisms than fertilizers, regardless of the presence of nutrients in the products”.9,20
2.5. Revenue and Expenditure Analysis of Different Treatments
Economic benefit is the most concerned index in the actual production of farmers. The income and expenditure link were analyzed in the whole production process (Table 4). Compared with the most widely applied urea, the application of CRU exhibits many advantages in labor, time, and energy saving. The net returns in the CP0 treatment were increased by 7.2, 26.4, and 17.7% as compared to that in the CFF treatment, respectively. This is attributed to the reduced labor cost and increased grain yield in the former as compared to that in the latter treatment.
Table 4. Annual Total Revenue, Cost, and Net Returns of Crop Production with Different Treatments in 2016–2018a.
| year | treatment | total revenue($ ha–1 year–1) | N fertilizer cost | ZNC cost | labor cost of fertilization | other cost | net returns | net return change relative to CFF (%) |
|---|---|---|---|---|---|---|---|---|
| 2016 | CK | 2666.7 | 0.0 | 0.0 | 30.8 | 615.4 | 2020.6 | |
| CFF | 3526.6 | 120.4 | 0.0 | 92.3 | 615.4 | 2698.5 | ||
| CP0 | 3771.1 | 231.8 | 0.0 | 30.8 | 615.4 | 2893.1 | 7.2 | |
| CP1 | 4229.0 | 231.8 | 11.5 | 30.8 | 615.4 | 3339.5 | 23.8 | |
| CP2 | 4080.9 | 231.8 | 23.1 | 30.8 | 615.4 | 3179.8 | 17.8 | |
| CP3 | 3979.9 | 231.8 | 34.6 | 30.8 | 615.4 | 3055.7 | 13.2 | |
| 2017 | CK | 2901.9 | 0.0 | 0.0 | 30.8 | 615.4 | 2255.7 | |
| CFF | 3143.0 | 120.4 | 0.0 | 92.3 | 615.4 | 2314.9 | ||
| CP0 | 3803.4 | 231.8 | 0.0 | 30.8 | 615.4 | 2925.4 | 26.4 | |
| CP1 | 4155.0 | 231.8 | 11.5 | 30.8 | 615.4 | 3265.4 | 41.1 | |
| CP2 | 3837.4 | 231.8 | 23.1 | 30.8 | 615.4 | 2936.4 | 26.9 | |
| CP3 | 3763.6 | 231.8 | 34.6 | 30.8 | 615.4 | 2839.5 | 22.7 | |
| 2018 | CK | 1828.6 | 0.0 | 0.0 | 30.8 | 615.4 | 1828.6 | |
| CFF | 2464.2 | 120.4 | 0.0 | 92.3 | 615.4 | 2464.2 | ||
| CP0 | 2900.1 | 231.8 | 0.0 | 30.8 | 615.4 | 2900.1 | 17.7 | |
| CP1 | 3216.3 | 231.8 | 11.5 | 30.8 | 615.4 | 3216.3 | 30.5 | |
| CP2 | 2967.2 | 231.8 | 23.1 | 30.8 | 615.4 | 2967.2 | 20.4 | |
| CP3 | 2836.5 | 231.8 | 34.6 | 30.8 | 615.4 | 2836.5 | 15.1 |
Note: Typical prices in China (USD): rice, $461.5 t–1; CRU, $533.9 t–1; urea, $307.7 t–1; calcium superphosphate, $123.1 t–1; potassium chloride, $307.7 t–1; labor cost of fertilization for one time, $30.8 ha–1. Other cost included machinery, irrigation, pesticides, insecticides, seeds, P fertilizer, K fertilizer, and other materials and expenses.
Under the premise of applying CRU uniformly, the annual net returns in CP1 were increased by 15.4, 11.6, and 10.9% relative to the CP0 treatment, attributing to the improvement in rice yield with the application of ZNC. ZNC improved rice yield at a relatively lower dose, and the cost of ZNC at an optimal dose is only $11.5 ha–1 year–1. Accordingly, the economic return with an optimal dose of ZNC is much greater than the cost of input for ZNC. Furthermore, in most agricultural production practices, fertilizers and biostimulants are commonly applied separately, which increases the cost of labor. In this work, the spray of ZNC on the surface of CRU achieved the simultaneous application in the field, and this facilitates no extra cost associated with its application. Whereas with the increase in ZNC addition, the reduction of the yield caused the reduction of economic benefits. The economic performance in CP2 was decreased by 4.8, 10.1, and 7.7% in three years compared to CP1. In addition, the economic performance in CR3 was further decreased by 3.9, 3.3, and 4.4% in three years compared to CP2, respectively.
2.6. Chemical Analysis of ZNC
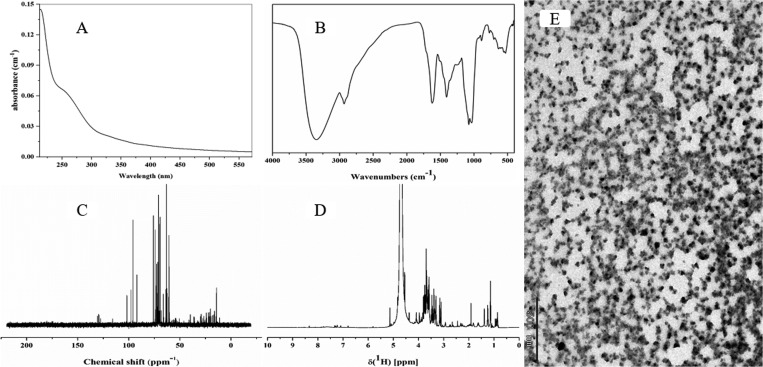
The mechanism of biostimulants is undiscovered and hard to identify because they originate mainly from complex sources containing multiple bioactive components together that may contribute to specific effect on plants.35 For instance, many biostimulants contain hormones, such as auxins,36 gibberellins,37 and cytokines,38 which are recognized as the main active components responsible for the beneficial effects on plant growth. Consequently, the chemical structure and material composition of ZNC are necessary to be verified to make full use of it. The UV–vis absorbance spectra of ZNC sharply decreased with the increase in wavelength, and an absorption peak appeared at 240–280 nm (Figure 3A), which implies the existence of the aromatic structure in ZNC. The partial chemical structure of ZNC was determined by the FTIR (Figure 3B), and the results indicated that ZNC present a higher content of carbohydrate structures and aliphatic structures. NMR spectroscopy also revealed that ZNC have a higher content of carbohydrates, amino acid, and aliphatic structures (Figure 3C,D). Furthermore, ZNC contained a large number of amino acids that existed in the form of granules, leaves, and flakes, respectively (Figure 3E), and we speculated they might be the main active substances in ZNC.
Figure 3.
(A) UV–vis absorbance spectra of ZNC. (B) FTIR spectra (400–4000 cm–1) of ZNC. (C) Liquid-state 13C NMR of ZNC. (D) Liquid-state 1H NMR of ZNC. (E) Staining of amino acid in ZNC.
In the present study, CRU enriched by ZNC (at a concentration of 87.5 mL ha–1) significantly increased rice yield by 12.1, 9.3, and 8.7% than the treatment that applied CRU only in three years, which is attributed to the promotion on panicles and N uptake of rice. Numerous studies have also demonstrated that biostimulants represented by amino acids may play a signaling role in regulating N acquisition by the roots, promoting N assimilation in plants and coordinating the regulation of C and N metabolism,20,39,40 and partial amino acids were confirmed to increase the activity of enzymes in the tricarboxylic acid cycle, N reduction and assimilation, and enhance gene expression of TCA cycle enzymes.41
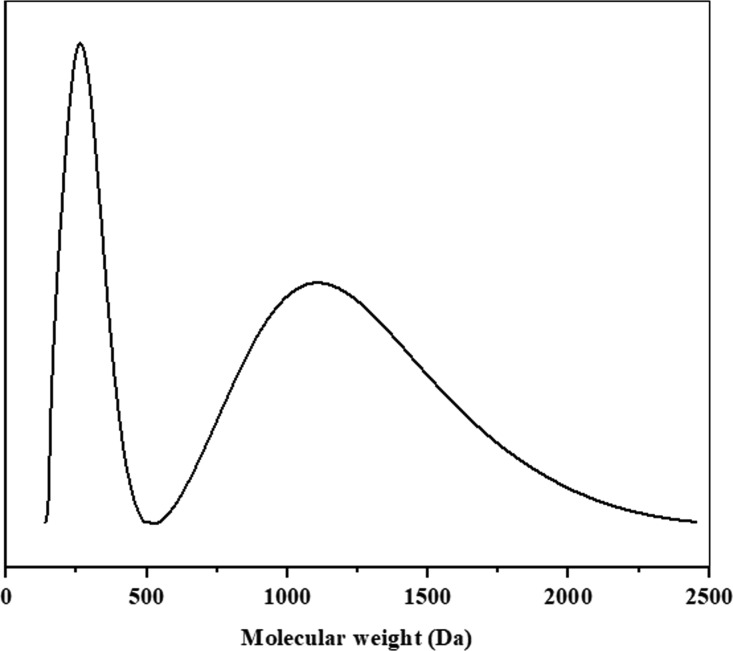
Due to the distinct sources and complex composition of biostimulants, the optimal dose of biostimulants is also different.42,43 Researches using isotope labeling confirmed that plant roots could readily take up amino acids and peptides.44,45 The SEC results showed that ZNC have two well-separated peaks with the molecular weight (Mw) ranging from 140 to 2507 Da (Figure 4). The ratio of low Mw fraction (Mw < 500 Da) was 28.9%, and the relative higher molecular weight (Mw > 500 Da) was 71.1%. Overall, ZNC are a biostimulant with a very low molecular weight, which is more easily absorbed by crops than other biostimulants.
Figure 4.
Size exclusion chromatography of ZNC.
3. Conclusions
The three-year field study demonstrated that the use of CRU as a N source resulted in greater rice yield as compared to that with the use of urea. Moreover, CRU enriched by the new biostimulant ZNC (87.5 mL ha–1) significantly increased rice yield by 12.1, 9.3, and 8.7% with positive increases in panicles; NUE by 15.0, 16.4, and 20.2% with positively increased in N absorption; and average net returns by 15.4, 11.6, and 10.9% as compared to that with the use of only CRU. Hence, “CRU + ZNC” is a promising model, which could take a respective advantage and generate synergetic performance to obtain sustainable increase in yields, NUE, and net returns of rice. The focus of our future work is to validate whether the amount of fertilizer can be reduced by adding ZNC.
4. Materials and Methods
4.1. Study Site
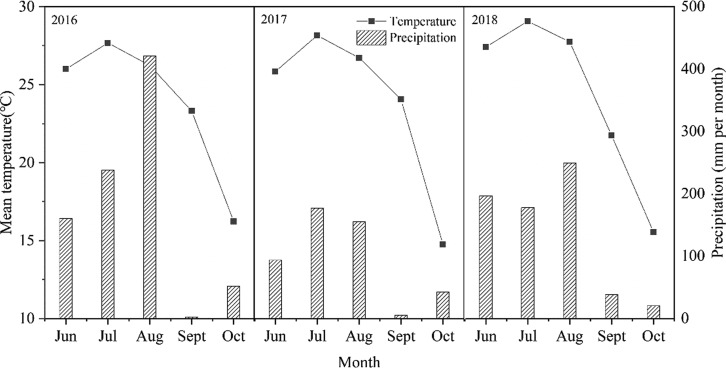
The field experiment was conducted during three rice growing seasons from June 2016 to October 2018 using rice cultivar Shengdao14. This study was conducted in the North China Plain close to the Yellow River, located in Gaolou village, Jiyang county, Shandong Province of China (117°23′17″ E, 37°04′21″ N). Monthly mean temperatures and total precipitation at the experimental site during June to October are described in Figure 5. Basic properties for the top soil layer (0–20 cm) before rice was planted are presented in Table 5.
Figure 5.
Monthly mean temperatures and total precipitation at the experimental site from June to October in 2016, 2017, and 2018.
Table 5. Select Properties of the Soil in the Experimental Site before Rice Planting.
| year | pH | organic matter (g kg–1) | total N (g kg–1) | NO3−−N (mg kg–1) | NH4+–N (mg kg–1) | available P (mg kg–1) | available K (mg kg–1) |
|---|---|---|---|---|---|---|---|
| 2016 | 7.3 | 12.1 | 1.2 | 15.8 | 8.2 | 24.3 | 90.6 |
| 2017 | 7.2 | 13.0 | 1.3 | 17.0 | 10.9 | 23.7 | 101.8 |
| 2018 | 7.1 | 13.5 | 1.3 | 15.4 | 12.3 | 19.7 | 113.5 |
4.2. Biostimulant and Fertilizers
ZNC were the ethanol crude extract of Paecilomyces variotii produced by the Shandong Pengbo Biotechnology Co., LTD, China.24 Standard fertilizers used in the experiment included urea (46% N), calcium superphosphate (15.5% P2O5), and potassium chloride (60% K2O). The polymer-coated urea (43% N) was designated as the three-month release product produced by the National Engineering Research Center for Slow or Controlled-Release Fertilizers of Shandong Agricultural University. The same products were used throughout three years. The N content and longevity of CRU in 25 °C water were measured by the method of “State Standard of the People’s Republic of China-Slow Release Fertilizer”.17 The N cumulative release curves of CRU under field conditions were determined by the method of “weight loss”.17
ZNC in different treatments were mixed with CRU before their application in the field. For CP1, CP2, and CP3 treatments, 0.14, 0.28, and 0.42 mL ZNC were diluted in water (100 mL) in advance with three replicates, respectively. Then, CRU was placed in the drum granulator with the rotating speed set at 30 rpm and temperature at 25 °C. While the drum was rotating, ZNC were sprayed evenly on the CRU using a sprinkling can, air-dried, and then packaged separately.
4.3. Experimental Design
There were six treatments with three replicates in the present experiment. Treatments included the following: (1) control without N application (CK), (2) urea was applied in three split doses at a ratio of 6:2:2 (basal/seeding/jointing) according the local fertilization habit (CFF), (3) CRU was basally applied once before rice planting, (4) CRU and ZNC (at a dose of 87.5 mL ha–1) were simultaneously applied before rice planting (CP1), (5) CRU and ZNC (at a dose of 175 mL ha–1) were simultaneously applied before rice planting (CP2), and (6) CRU and ZNC (at a dose of 262.5 mL ha–1) were simultaneously applied before rice planting (CP3). All plots received an application of 180 kg ha–1 N (expect CK), 90 kg ha–1 P2O5, and 150 kg ha–1 K2O similar with the rates used by most farmers in this region.
Plots were arranged randomly with an area of 16 m2 (4 m × 4 m). Before rice planting, soil ridges (30 cm height, 30 cm width) were built around the main plots to ensure independent irrigation and drainage between adjacent plots. Rice was planted in late June and harvested in mid-October with 13 rows per plot, and the distance between each row was 30 cm. Irrigation and plant protection measures were identical with the planting habit by most farmers in this area. During the blank period of two-crop rice cultivation, wheat was cultivated to balance the soil fertility without any fertilization.
4.4. Chemical Characterization of ZNC
Liquid-state CP-MAS 13C NMR spectroscopy and 1H NMR spectroscopy were acquired with an Avance 600 MHz (Bruker, Karlsruhe, Germany) spectrometer. The size exclusion chromatography (SEC) was performed on solutions of the sample using a Sephadex G-100 medium gel (Code No. 17-0060-02 Pharmacia Biotech AB). The morphology of amino acid in ZNC was dyed by uranyl acetate and observed by a JEM 1200EX (Japan) transmission electron microscope.
4.5. Soil Sampling and Analysis
At maturity, three soil cores from the 0–20 cm soil layers were collected and mixed in a single composite sample in each plot. Every soil sample per plot was divided into two parts, the one part was used to determine NO3−−N and NH4+-N in fresh, and the other part was air dried and ground to pass through a 2.0 mm sieve for further analysis. Soil NO3−−N and NH4+–N concentrations were extracted by 0.01 M CaCl246 and analyzed by the AA3 Auto-analyzer (Model AA3-A001-02E, Bran-Luebbe, Germany). The soil-available phosphorus (P) concentration was extracted by 0.5 M NaHCO3 at pH 8.546 and analyzed by the Discrete Auto-analyzer (Smart Chem 200, Alliance, France). The soil-available potassium (K) concentration was extracted by 1 M CH3COONH4 at pH 723 and analyzed by a flame photometer (Model 410, Sherwood, England).
4.6. Yield Determination and Plant Analysis
At the end of the growing season, the middle three rows were entirely harvested and weighed to determine grain yields for each plot. Plant samples were pruned at 3 cm above the soil surface, separated into a straw and grain, dried at 105 °C to deactivate enzymes for 0.5 h, and then dried at 75 °C until a constant weight was reached. Plant samples were determined by H2SO4–H2O2 digestion,47 concentrations of the total N and P were determined using a Smart Chem 200 Auto-analyzer, and that of K was measured by a flame photometer.
4.7. Data Analysis
NUE, NAE and NPFP were calculated by the following formulas48,49
NUE (%) = (N accumulative uptake of plant from the N treatment – N accumulative uptake from the no N treatment)/total applied N of fertilizer in the N treatment × 100%
NAE (kg N kg–1) = (the yield in the N treatment – the yield in the no N treatment)/N application
NPFP (kg N kg–1) = the yield in the N treatment/N application
Net return = rice gross profit – N fertilizer cost – ZNC cost – labor cost – other cost
The response parameters were subjected to analysis of variance (ANOVA) and mean separation test using Statistical Analysis System package version 9.2 (2010, SAS Institute Cary, NC). Means and standard error values were assessed to assemble graphs using SigmaPlot software version 10 (MMIV Systat Software, Inc., San Jose, CA).
Acknowledgments
This work was supported by the National Key Research and Development Program of China (grant nos. 2017YFD0200706 and 2017YFD0200705), the National Natural Science Foundation of China (grant no. 41571236), and the Key Research and Development Program of Shandong Province (project no, 2018GNC110001).
The authors declare no competing financial interest.
References
- Jiang Q.; Du Y.; Tian X.; Wang Q.; Xiong R.; Xu G.; Yan C.; Ding Y. Effect of panicle nitrogen on grain filling characteristics of high-yielding rice cultivars. Eur. J. Agron. 2016, 74, 185–192. 10.1016/j.eja.2015.11.006. [DOI] [Google Scholar]
- Cao Y.; Sun H.; Zhang J.; Chen G.; Zhu H.; Zhou S.; Xiao H. Effects of wheat straw addition on dynamics and fate of nitrogen applied to paddy soils. Soil Tillage Res. 2018, 178, 92–98. 10.1016/j.still.2017.12.023. [DOI] [Google Scholar]
- Prasad R. Fertilizer urea, food security, health and the environment. Curr. Sci. 1998, 75, 667–683. [Google Scholar]
- Hu X.-F.; Cheng C.; Luo F.; Chang Y.-Y.; Teng Q.; Men D.-Y.; Liu L.; Yang M.-Y. Effects of different fertilization practices on the incidence of rice pest and diseases: a three year case study in Shanghai in subtropical southeastern China. Field Crops Res. 2016, 196, 33–50. 10.1016/j.fcr.2016.06.004. [DOI] [Google Scholar]
- Wu W.; Shah F.; Shah F.; Huang J. Rice sheath blight evaluation as affected by fertilization rate and planting density. Australas. Plant Pathol. 2015, 44, 183–189. 10.1007/s13313-014-0338-z. [DOI] [Google Scholar]
- Dong Z.; Wu L.; Chai J.; Zhu Y.; Chen Y.; Zhu Y. Effects of nitrogen application rates on rice grain yield, Nitrogen Use Efficiency and water quality in paddy field. Commun. Soil Sci. Plant Anal. 2015, 46, 1579–1594. 10.1080/00103624.2015.1045595. [DOI] [Google Scholar]
- Cordero E.; Moretti B.; Miniotti E. F.; Tenni D.; Beltarre G.; Romani M.; Sacco D. Fertilisation strategy and ground sensor measurements to optimise rice yield. Eur. J. Agron. 2018, 99, 177–185. 10.1016/j.eja.2018.07.010. [DOI] [Google Scholar]
- Wang X.; Fan J.; Xing Y.; Xu G.; Wang H.; Deng J.; Wang Y.; Zhang F.; Li P.; Li Z. The effects of mulch and nitrogen fertilizer on the soil environment of crop plants. Adv. Agron. 2019, 153, 121–173. 10.1016/bs.agron.2018.08.003. [DOI] [Google Scholar]
- Yao Y.; Wang X.; Chen B.; Zhang M.; Ma J. Seaweed extract improved yields, leaf photosynthesis, ripening time, and net returns of tomato (Solanum lycopersicum Mill.). ACS Omega 2020, 5, 4242–4249. 10.1021/acsomega.9b04155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J.; Liu X. J.; Zhang Y.; Shen J. L.; Han W. X.; Zhang W. F.; Christie P.; Goulding K. W. T.; Vitousek P. M.; Zhang F. S. Significant acidification in major Chinese croplands. Science 2010, 327, 1008–1010. 10.1126/science.1182570. [DOI] [PubMed] [Google Scholar]
- Abraham J.; Rajasekharan Pillai V. N. Membrane-encapsulated controlled-release urea fertilizers based on acrylamide copolymers. J. Appl. Polym. Sci. 1996, 60, 2347–2351. . [DOI] [Google Scholar]
- Kiran J. K.; Khanif Y. M.; Amminuddin H.; Anuar A. R. Effects of controlled release urea on the yield and nitrogen nutrition of flooded rice. Commun. Soil Sci. Plant Anal. 2010, 41, 811–819. 10.1080/00103621003592333. [DOI] [Google Scholar]
- Xie J.; Yang Y.; Gao B.; Wan Y.; Li Y. C.; Cheng D.; Xiao T.; Li K.; Fu Y.; Xu J.; Zhao Q.; Zhang Y.; Tang Y.; Yao Y.; Wang Z.; Liu L. Magnetic-sensitive nanoparticle self-assembled superhydrophobic biopolymer-coated slow-release fertilizer: fabrication, enhanced performance, and mechanism. ACS Nano 2019, 13, 3320–3333. 10.1021/acsnano.8b09197. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Zhang M.; Li Y. C.; Fan X.; Geng Y. Controlled release urea improved nitrogen use efficiency, activities of leaf enzymes, and rice yield. Soil Sci. Soc. Am. J. 2012, 76, 2307–2317. 10.2136/sssaj2012.0173. [DOI] [Google Scholar]
- Geng J.; Ma Q.; Chen J.; Zhang M.; Li C.; Yang Y.; Yang X.; Zhang W.; Liu Z. Effects of polymer coated urea and sulfur fertilization on yield, nitrogen use efficiency and leaf senescence of cotton. Field Crops Res. 2016, 187, 87–95. 10.1016/j.fcr.2015.12.010. [DOI] [Google Scholar]
- Ding W.; Xu X.; He P.; Ullah S.; Zhang J.; Cui Z.; Zhou W. Improving yield and nitrogen use efficiency through alternative fertilization options for rice in China: A meta-analysis. Field Crops Res. 2018, 227, 11–18. 10.1016/j.fcr.2018.08.001. [DOI] [Google Scholar]
- Geng J.; Sun Y.; Zhang M.; Li C.; Yang Y.; Liu Z.; Li S. Long-term effects of controlled release urea application on crop yields and soil fertility under rice-oilseed rape rotation system. Field Crops Res. 2015, 184, 65–73. 10.1016/j.fcr.2015.09.003. [DOI] [Google Scholar]
- Tian X.; Li C.; Zhang M.; Li T.; Lu Y.; Liu L. Controlled release urea improved crop yields and mitigated nitrate leaching under cotton-garlic intercropping system in a 4-year field trial. Soil Tillage Res. 2018, 175, 158–167. 10.1016/j.still.2017.08.015. [DOI] [Google Scholar]
- Zheng W.; Liu Z.; Zhang M.; Shi Y.; Zhu Q.; Sun Y.; Zhou H.; Li C.; Yang Y.; Geng J. Improving crop yields, nitrogen use efficiencies, and profits by using mixtures of coated controlled-released and uncoated urea in a wheat-maize system. Field Crops Res. 2017, 205, 106–115. 10.1016/j.fcr.2017.02.009. [DOI] [Google Scholar]
- Calvo P.; Nelson L.; Kloepper J. W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. 10.1007/s11104-014-2131-8. [DOI] [Google Scholar]
- Bulgari R.; Cocetta G.; Trivellini A.; Vernieri P.; Ferrante A. Biostimulants and crop responses: a review. Biol. Agric. Hortic. 2014, 31, 1–17. 10.1080/01448765.2014.964649. [DOI] [Google Scholar]
- du Jardin P. Plant biostimulants: definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. 10.1016/j.scienta.2015.09.021. [DOI] [Google Scholar]
- Sharma H. S. S.; Fleming C.; Selby C.; Rao J. R.; Martin T. Plant biostimulants: a review on the processing of macroalgae and use of extracts for crop management to reduce abiotic and biotic stresses. J. Appl. Phycol. 2014, 26, 465–490. 10.1007/s10811-013-0101-9. [DOI] [Google Scholar]
- Lu C.; Liu H.; Jiang D.; Wang L.; Jiang Y.; Tang S.; Hou X.; Han X.; Liu Z.; Zhang M.; Chu Z.; Ding X. Paecilomyces variotii extracts (ZNC) enhance plant immunity and promote plant growth. Plant Soil 2019, 441, 383–397. 10.1007/s11104-019-04130-w. [DOI] [Google Scholar]
- Yuan Z.; Ata-Ul-Karim S. T.; Cao Q.; Lu Z.; Cao W.; Zhu Y.; Liu X. Indicators for diagnosing nitrogen status of rice based on chlorophyll meter readings. Field Crops Res. 2016, 185, 12–20. 10.1016/j.fcr.2015.10.003. [DOI] [Google Scholar]
- Malhi S. S.; Grant C. A.; Johnston A. M.; Gill K. S. Nitrogen fertilization management for no-till cereal production in the Canadian Great Plains: a review. Soil Tillage Res. 2001, 60, 101–122. 10.1016/S0167-1987(01)00176-3. [DOI] [Google Scholar]
- Sui B.; Feng X.; Tian G.; Hu X.; Shen Q.; Guo S. Optimizing nitrogen supply increases rice yield and nitrogen use efficiency by regulating yield formation factors. Field Crops Res. 2013, 150, 99–107. 10.1016/j.fcr.2013.06.012. [DOI] [Google Scholar]
- van Wart J.; Kersebaum K. C.; Peng S.; Milner M.; Cassman K. G. Estimating crop yield potential at regional to national scales. Field Crop Res. 2013, 143, 34–43. 10.1016/j.fcr.2012.11.018. [DOI] [Google Scholar]
- Mi W.; Zheng S.; Yang X.; Wu L.; Liu Y.; Chen J. Comparison of yield and nitrogen use efficiency of different types of nitrogen fertilizers for different rice cropping systems under subtropical monsoon climate in China. Eur. J. Agron. 2017, 90, 78–86. 10.1016/j.eja.2017.07.013. [DOI] [Google Scholar]
- Colla G.; Rouphael Y.; Canaguier R.; Svecova E.; Cardarelli M. Biostimulant action of a plant-derived protein hydrolysate produced through enzymatic hydrolysis. Front Plant Sci. 2014, 5, 1–6. 10.3389/fpls.2014.00448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengasamy K. R. R.; Kulkarni M. G.; Stirk W. A.; Van Staden J. Eckol - a new plant growth stimulant from the brown seaweed Ecklonia maxima. J. Appl. Phycol. 2015, 27, 581–587. 10.1007/s10811-014-0337-z. [DOI] [Google Scholar]
- Wang X.; Yao Y.; Liu Z.; Chen B.; Zhang M.; Ma J.; Wang Q. Effects of Paecilomyces variotii extracts on the physiological adaptability of rice seedlings under salt stress. J. Agric. Resour. Environ. 2020, 37, 98–107. 10.13254/j.jare.2018.0349. [DOI] [Google Scholar]
- Wang X.; Yao Y.; Chen B.; Zhang M.; Liu Z.; Ma J.; Wang Q. Optimum levels of Paecilomyces variotii extracts in regulating resistance of rice seedlings to low temperature stress. J. Plant. Nutr. Fert. 2019, 25, 2133–2141. [Google Scholar]
- Shirano Y.; Kachroo P.; Shah J.; Klessig D. F. A gain-of-function mutation in an Arabidopsis Toll Interleukin1 receptor-nucleotide binding site-leucine-rich repeat type R gene triggers defense responses and results in enhanced disease resistance. Plant Cell 2002, 14, 3149–3162. 10.1105/tpc.005348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertani A.; Francioso O.; Tugnoli V.; Righi V.; Nardi S. Effect of commercial lignosulfonate-humate on Zea mays L. metabolism. J. Agric. Food Chem. 2011, 59, 11940–11948. 10.1021/jf202473e. [DOI] [PubMed] [Google Scholar]
- Jindo K.; Martim S. A.; Navarro E. C.; Pérez-Alfocea F.; Hernandez T.; Garcia C.; Aguiar N. O.; Canellas L. P. Root growth promotion by humic acids from composted and non-composted urban organic wastes. Plant Soil 2012, 353, 209–220. 10.1007/s11104-011-1024-3. [DOI] [Google Scholar]
- Stirk W. A.; Tarkowská D.; Turečová V.; Strnad M.; Van Staden J. Abscisic acid, gibberellins and brassinosteroids in Kelpak®, a commercial seaweed extract made from Ecklonia maxima. J. Appl. Phycol. 2014, 26, 561–567. 10.1007/s10811-013-0062-z. [DOI] [Google Scholar]
- Pizzeghello D.; Francioso O.; Ertani A.; Muscolo A.; Nardi S. Isopentenyladenosine and cytokinin-like activity of different humic substances. J. Geochem. Explor. 2013, 129, 70–75. 10.1016/j.gexplo.2012.10.007. [DOI] [Google Scholar]
- Ertani A.; Schiavon M.; Muscolo A.; Nardi S. Alfalfa plant-derived biostimulant stimulate short-term growth of salt stressed Zea mays L. plants. Plant Soil 2013, 364, 145–158. 10.1007/s11104-012-1335-z. [DOI] [Google Scholar]
- Nardi S.; Pizzeghello D.; Schiavon M.; Ertani A. Plant biostimulants: physiological responses induced by protein hydrolyzed-based products and humic substances in plant metabolism. Sci. Agric. 2016, 73, 18–23. 10.1590/0103-9016-2015-0006. [DOI] [Google Scholar]
- Schiavon M.; Ertani A.; Nardi S. Effects of an alfalfa protein hydrolysate on the gene expression and activity of enzymes of the tricarboxylic acid (TCA) cycle and nitrogen metabolism in Zea mays L. J. Agric. Food Chem. 2008, 56, 11800–11808. 10.1021/jf802362g. [DOI] [PubMed] [Google Scholar]
- Khan W.; Rayirath U. P.; Subramanian S.; Jithesh M. N.; Rayorath P.; Hodges D. M.; Critchley A. T.; Craigie J. S.; Norrie J.; Prithiviraj B. Seaweed extracts as biostimulants of plant growth and development. J. Plant Growth Regul. 2009, 28, 386–399. 10.1007/s00344-009-9103-x. [DOI] [Google Scholar]
- Rose M. T.; Patti A. F.; Little K. R.; Brown A. L.; Roy Jackson W.; Cavagnaro T. R. Chapter Two - A meta-analysis and review of plant-growth response to humic substances: practical implications for agriculture. Adv. Agron. 2014, 124, 37–89. 10.1016/B978-0-12-800138-7.00002-4. [DOI] [Google Scholar]
- Miller A. J.; Fan X.; Shen Q.; Smith S. J. Amino acids and nitrate as signals for the regulation of nitrogen acquisition. J. Exp. Bot. 2007, 59, 111–119. 10.1093/jxb/erm208. [DOI] [PubMed] [Google Scholar]
- Nacry P.; Bouguyon E.; Gojon A. Nitrogen acquisition by roots: physiological and developmental mechanisms ensuring plant adaptation to a fluctuating resource. Plant Soil 2013, 370, 1–29. 10.1007/s11104-013-1645-9. [DOI] [Google Scholar]
- Zheng W.; Zhang M.; Liu Z.; Zhou H.; Lu H.; Zhang W.; Yang Y.; Li C.; Chen B. Combining controlled-release urea and normal urea to improve the nitrogen use efficiency and yield under wheat-maize double cropping system. Field Crops Res. 2016, 197, 52–62. 10.1016/j.fcr.2016.08.004. [DOI] [Google Scholar]
- Douglas L. A.; Riazi A.; Smith C. J. A semi-micro method for determining total nitrogen in soils and plant material containing nitrite and nitrate. Soil Sci. Soc. Am. J. 1980, 44, 431–433. 10.2136/sssaj1980.03615995004400020047x. [DOI] [Google Scholar]
- Devkota M.; Martius C.; Lamers J. P. A.; Sayre K. D.; Devkota K. P.; Vlek P. L. G. Tillage and nitrogen fertilization effects on yield and nitrogen use efficiency of irrigated cotton. Soil Tillage Res. 2013, 134, 72–82. 10.1016/j.still.2013.07.009. [DOI] [Google Scholar]
- Hartmann T. E.; Yue S.; Schulz R.; He X.; Chen X.; Zhang F.; Müller T. Yield and N use efficiency of a maize-wheat cropping system as affected by different fertilizer management strategies in a farmer’s field of the North China Plain. Field Crops Res. 2015, 174, 30–39. 10.1016/j.fcr.2015.01.006. [DOI] [Google Scholar]