Abstract
Preparedness for the ongoing coronavirus disease 2019 (COVID-19) and its spread in India calls for setting up of adequately equipped and dedicated health facilities to manage sick patients while protecting healthcare workers and the environment. In the wake of other emerging dangerous pathogens in recent times, such as Ebola, Nipah and Zika, it is important that such facilities are kept ready during the inter-epidemic period for training of health professionals and for managing cases of multi-drug resistant and difficult-to-treat pathogens. While endemic potential of such critically ill patients is not yet known, the health system should have surge capacity for such critical care units and preferably each tertiary government hospital should have at least one such facility. This article describes elements of design of such unit (e.g., space, infection control, waste disposal, safety of healthcare workers, partners to be involved in design and plan) which can be adapted to the context of either a new construction or makeshift construction on top of an existing structure. In view of a potential epidemic of COVID-19, specific requirements to handle it are also given.
Keywords: Biocontainment, biosafety, coronavirus, designated health facility, infection prevention and control
Introduction
Increasing clusters of people affected with novel severe acute respiratory syndrome coronavirus (SARS-CoV-2)-infected pneumonia (COVID-19) began to be reported from Wuhan, a metropolitan in People's Republic of China, in December 20191,2,3,4. CoVs represent a major family of viruses, which have been implicated in several multi-country outbreaks of severe respiratory illnesses such as the Middle East Respiratory Syndrome CoV (MERS-CoV) and SARS. The World Health Organization (WHO) defines SARS-CoV-2 as 'a new strain of coronavirus that has not been previously identified in humans'5. The SARS-CoV-2 was identified through the ongoing surveillance for 'pneumonia of unknown aetiology', which was initiated in 2003-2004, in the aftermath of the SARS outbreak6. The first four cases of COVID-19 were identified through this routine surveillance programme deployed through the local healthcare facilities and were epidemiologically linked to the Huanan (Southern China) Seafood Wholesale Market7. Since then, there has been a steady increase in the burden of COVID-19, with 60,347 confirmed cases and 1,369 deaths reported globally, as of 13th February 2020. With cases emerging from as many as 29 countries, and travel-related importations also being reported, the global health security implications of COVID-19 have come to the fore4,8,9.
In response, susceptible countries have mounted public health measures to mitigate the threat of proliferation of COVID-19. In India, airport entry screening has been initiated, existing visas from China have been cancelled, and travellers returning from COVID-19-affected areas have been quarantined. Given the implications of importation of cases and the impact thereof on the local epidemiology of COVID-19 in secondary foci, it is essential to break ground on preparing health facilities to combat a local cluster of COVID-19 cases.
A well-equipped dedicated hospital facility (DHF) to deal with these cases with adequate protections for healthcare workers and other patients is the key to the standard of care. Critical care and humane approach to acutely ill patients are essential ingredients of an epidemic control. While the course of the CoV in India is uncertain, setting up of such units is important for future epidemics due to dangerous pathogens and antimicrobial resistant organisms including drug-resistant tuberculosis during the inter epidemic period. Such wards can be used for training of physicians during inter epidemic period.
Here, we focus on establishing a biosecurity ward de novo or in a makeshift manner in a tertiary healthcare facility to manage patients or suspected cases in an effort to combat the spread of COVID-19.
Key partners
A scientific and evidence-based design shall be the key to initiate the process. The designing of DHF should have inputs from the infectious disease department, critical care, engineering and nursing department, departments of microbiology and virology, hospital administration and waste disposal facilities, referral ambulance services, social workers or counsellors for patients' families and situation room with digital connection with national programme. Given the multi-disciplinary clinical needs and the specialized requirements for maintenance of the infrastructure related to DHF, it is vital that all the participating disciplines be brought together under a unified umbrella to identify the existing capacity and infrastructure, and needs, to make a streamlined plan for establishing the unit.
Pre-requisites for dedicated health facility
The DHF must be a self-contained establishment that can meet most of its daily needs with only essential but limited contact with the outside world. Basic requirements need to be accounted for continuous safe water supply; appropriate cleaning practices; adequate floor space for beds; appropriate handwashing facilities; adequate ventilation for isolation rooms and procedure rooms; adequate isolation facilities for airborne, droplet, contact isolation and protective environment; regulated and rational traffic flow to minimize exposure of high-risk patients and facilitate patient and clinical material transport; precautions to control rodents, pests and other vectors and appropriate waste management facilities/practices must be ensured. The unit can be a standalone facility or can be housed in a tertiary healthcare facility with the equipment and capacity to care for critically ill patients such as those with septic shock requiring vasopressors, bedside surgical procedures, acute respiratory distress syndrome (ARDS) requiring mechanical ventilation, acute kidney injury requiring dialysis and multi-organ failure requiring high degree of quality and multi-disciplinary care with organ support.
Space
An isolation room should be identified within the emergency room. This room will be used to isolate patients who raise any suspicion of COVID-19 infection based on a set of validated, screening questions10. This approach may be adapted for other outbreak-prone infectious diseases as well.
For mapping the patient transfer, the path of transport and specific elevators should be identified. The number of rooms with biocontainment facilities should be mobilized based on the magnitude of the emerging situation. In an epidemic situation, it is ideal to have separate rooms for suspected and confirmed cases11,12.
Within the DHF, two units should be built. The first one will be an isolation space for laboratory confirmed cases. Multiple patients can be kept in the same room. Barrier nursing practices and protective isolation facility will be presented to prevent nosocomial infections.
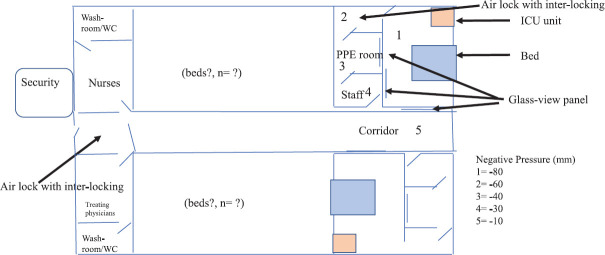
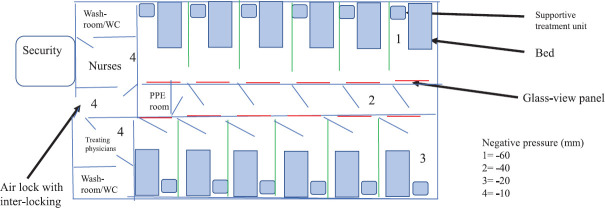
The second unit will be made for suspected cases which will include family and hospital contacts who are suspected to have potential contact with confirmed cases but await laboratory confirmation. This room will be built to include only one suspect per room. Figure 1 is a conceptual drawing of this unit. Figure 2 is a conceptual drawing of multi-bedded isolation room for suspects.
Fig. 1.
Conceptual figure of an isolation unit. WC, water closet; PPE, personal protective equipment; ICU, intensive care unit.
Fig. 2.
Conceptual figure of multi-bedded isolation room for suspects.
Staff
One full-time physician, one paediatrician and one resident should be identified for the management of the unit. Systems should be developed to ensure that an identified clinical rapid response team (RRT) is always available on call. Intensive care unit (ICU) nursing staff should be identified specifically for this DHF, and the ratio of nursing staff in this unit should be similar to ICUs13. Paramedical and housekeeping staff should also be identified. Precautions should be taken to minimize healthcare worker (HCW) exposure. Staff attached to this facility should undergo focused training in infection prevention and control. Mock drills should also be conducted to assure preparedness of the unit and as part of ongoing quality improvement measures.
Training and capacity building
Training will have to be carried out for all the healthcare providers who will participate in the care of the patients including doctors, nurses, paramedical staff, patient transporters, phlebotomists, laboratory technicians and housekeeping staff. This will require both technical training for the physicians and nurses and infection prevention and control training for all the cadres.
Systems
Screening systems will have to be identified in the triage area to isolate patients who present with: (i) Fever and cough with a history of travel to COVID-19 affected countries; (ii) HCWs with fever and cough caring for patients with pneumonia whose cause of pneumonia is unknown; (iii) Patients with fever and cough with contact history with someone with known SARS-CoV-2 infection; and (iv) Patients with fever or cough with a history of visit to a healthcare facility with a case of COVID-19 infection.
Once a patient has been isolated, RRT should be activated. Simultaneously, it should be reported to the nodal officers in the Ministry of Health and Family Welfare, State Department of Health, for notifying the WHO in accordance with International Health Regulations (2005)14. Specimens for transport must be placed in leak-proof specimen bags, which have a separate sealable pocket for the specimen (i.e., a plastic biohazard triple-layered specimen bag). The hospital which is receiving the patient should be informed ahead of time to initiate the necessary precautions. All specimens are to be delivered by hand if possible. Any shipment of specimens should be in accordance with the prevailing International Air Transport Association15 and UN/WHO norms16.
Food should be served on disposable crockery and should be consumed with disposable cutlery. Non-disposable crockery and cutlery should be washed using hot water (70°C) and detergent, rinsed and dried. Where possible, eating utensils should be cleaned in a dishwasher using a hot water cycle (reaching at least 70°C). A record of all patients or staff entering the room should be maintained.
Infection prevention and control
Standard hygiene: Standard precautions must be followed by all HCWs, for all patients and at all times17. This comprises broadly of hand hygiene practices, proper donning, doffing and disposal of the personal protective equipment (PPE) and maintaining respiratory hygiene and cough etiquettes18.
Hand hygiene is recommended with alcohol-based handrubs (ABHR) or soap and water. Printed posters of both the methods should be pasted near all hand hygiene units. Adequate supply of ABHR (60-80% ethanol is recommended) and antiseptic soap solution (chlorhexidine gluconate 2% and alcohol combination) has been seen to be synergistic19.
Personal protective equipment (PPE): All HCWs involved should have the knowledge of the correct donning and doffing steps, along with appropriate disposal of PPE and be trained in this procedure. Non-powdered latex-free gloves should be used by all HCWs. Eye protection and face shield should be used20. Respiratory hygiene and cough etiquette must be followed.
Gowns should be long-sleeved and made of non-absorbable (fluid-resistant) materials. The same gown should not be worn for all patients. In case gowns are not available, waterproof aprons should be used.
The WHO recommends the use of medical masks (surgical or procedure masks which may be flat or pleated and are affixed with head straps) and particulate respirators (NIOSH-certified N95, EU standard FFP2 or equivalent) for contact and airborne precautions as well as aerosol-generating procedures (AGPs), respectively18,21. The mask has to be worn before entering the patient's room and has to be removed once outside the room. If the patient is under droplet precautions and requires to be moved or transported, he/she should wear a mask. Hand hygiene should be ensured after removing the masks. Each room should have dedicated equipment such as sphygmomanometer, thermometer and stethoscopes.
Patient transport and stay: The movement of patients should be minimized within the hospital premises. If such transport is necessary, patients must don either medical masks or particulate respirators, whichever is available. The area to which they are being transported should be alerted about their arrival. A separate corridor should be preferred. In case the patients meet any surfaces, they must be disinfected. Complete inactivation of the virus is seen with 70 per cent ethanol and povidone-iodine with an exposure time of one minute or 2.5 per cent glutaraldehyde with an exposure time of five minutes. The Central Drugs Standard Control Organization registered disinfectant or 1:100 dilution of household bleach and water will suffice for disinfection of surface of non-critical patient care equipment. High-level disinfection and sterilization of semi-critical and critical devices, respectively, does not need to be altered for patients with known or suspected COVID-19.
When transmission-based precautions are being practiced, the ideal condition is to have single occupancy rooms for every patient. Since that may not be feasible in every scenario, the practice of cohort isolation can be followed with a spatial separation of ≥3 feet (or 1 m). There are certain environmental cleaning procedures one needs to keep in mind. Dusting should be avoided; floors should not be carpeted, and rooms should not have upholstery. For environmental cleaning of areas needing airborne precautions, high touch surfaces should be cleaned every day, and as needed, while low touch surfaces can be cleaned on a scheduled basis. Primary focus should remain in adherence to required PPE and additional entry/exit procedures. For undertaking droplet and/or contact precautions, cleaning may be done twice a day, or as needed, for high touch surfaces, with a focus on all surfaces within the patient zone, non-critical patient care equipment, and any surface visibly soiled with blood or body fluids22.
What to do with the body of the deceased, when transmission-based precautions are being observed: Dead bodies are categorized into three categories based on the level of PPE being observed. All tubings attached to the bodies, such as nasogastric tubes, Foley's catheter, and any others, are to be removed, keeping biosafety in place. All orifices are to be cleaned and disinfected and plugged to prevent any fluid leakage. All wounds must be redressed after disinfection with impermeable dressings.
All PPE must be removed immediately, and hand hygiene must be performed immediately. Once the body reaches the mortuary, the following precautions must be taken: (i) All bodies must be categorized and identified correctly; (ii) Bodies found to be soiled with blood/body fluids should be placed in disposable plastic bags; (iii) Dead bodies must be kept in cold temperatures of −4°C; and (iv) All staff posted in the mortuary should follow strict PPE guidelines.
Funeral workers must also observe strict adherence to PPE and follow strict environmental cleaning precautions.
Ventilation: The overall risk of infection in a room can be mitigated by ventilation through two principles: dilution and removal. Clean air, when added to a room, dilutes the airborne contaminants present in the room, thus reducing the chances of inhalation of infectious droplets. For outbreak-prone and epidemic-prone infectious diseases such as COVID-19, the WHO recommends the institution of contact and droplet precautions18. The Centers for Disease Control and Prevention and WHO guidelines provide the following recommendations for preventing airborne infections18,23: (i)To help prevent airborne infections, adequate ventilation in healthcare facilities in all patient-care areas is necessary; (ii) For natural ventilation, the following minimum hourly averaged ventilation rates should be provided: (a)160 l/s/patient (hourly average ventilation rate) for airborne precaution rooms (with a minimum of 80 l/sec/patient for new health care facilities and major renovations); and (b) 2.5 l/sec/m3 for corridors and other transient spaces without a fixed number of patients; however, when patient care is undertaken in corridors during emergency or other situations, the same ventilation rate requirements for airborne precaution rooms or general wards will apply.
The design should consider fluctuations in ventilation rate.
For aerosol generating procedures (AGPs) (Supplementary Table I (available from http://www.ijmr.org.in/articles/2020/151/2/images/IndianJMedRes_ 2020_151_2_177_280781_sm3.pdf)), adequate airflow is at least 160 l/sec/patient or in negative pressure rooms at least 12 air changes per hour (ACH) and controlled direction of air flow when using mechanical ventilation.
Supplementary Table I.
List of aerosol generating procedures (AGPs) and non-aerosol generating procedures (Non-AGPs)
| AGPs | Non-AGPs |
|---|---|
| Endotracheal intubation Open respiratory and airway suctioning Tracheostomy care Cardiopulmonary resuscitation Sputum induction Bronchoscopy Aerosolized administration of pentamidine or other medications Pulmonary function testing Autopsy, clinical, surgical, and laboratory procedures that may aerosolize Pathogens, such as operating bone saws, centrifuges, blenders, and Aspiration equipment |
Closed suctioning with invasive ventilation Non-invasive positive pressure ventilation Bi-level positive airway pressure Nasopharyngeal aspiration Nebulization (but this procedure should be performed in an are physically separate from other patients) Chest physiotherapy is not considered an AGP but a surgical mask Should be worn by the patient as well as HCWs |
HCWs, healthcare workers
Negative pressure rooms: Negative pressure rooms include mechanical ventilation systems which maintain the pressure of the room at a slightly lower level than the pressure of the entry area so that it allows air to flow into the isolation room but not escape from the room, as air naturally flows from areas with higher pressure to areas with lower pressure, thereby preventing contaminated air from the isolation room to escape outwards.
The negative pressure room should have a minimum of 12 ACH in a high-risk area for airborne transmission, compared to six ACH per hour in a low-risk area. Negative pressure differential between airflow from adjacent spaces to the patient room should be >2.5 Pascal. An airflow differential >125 cfm (56 l/sec) should be maintained between exhaust and supply. Sealing of the room (entry) should have provision of allowing approximately 0.5 square feet (0.046 m2) leakage. Clean air entering the room should flow first to the area of the room where staff or visitors are likely to be present and then flow across the bed area to the exhaust. Direction of airflow and patient beds should not be in the same direction. The air from inside the room should be allowed to flow out via an exhaust fan or a high-efficiency particulate air (HEPA) filter. There should be an anteroom outside of the negative pressure room. The pressure of which should be negative to the hallway and positive to the patient room so that unidirectional airflow is maintained24.
HEPA filter should be placed in each room, and if not available, adequate ventilation should be ensured. Exhaust fans must be properly installed closely fitting to the window. HEPA filter is useful in small volume settings such as bronchoscopy suites, laboratories or individual TB patient rooms. Careful attention should be given to the equivalent ACH the filter requires as most filters clean very little air per hour. Maintenance of HEPA filters with timely replacement of the membrane is necessary to ensure its functionality.
The efficiency of filters and sustainability of negative pressure must be validated before DHF is open to patients. Subsequently, validation must be undertaken regularly and at pre-decided intervals through a certified agency.
Renovating or converting a room: While establishing an isolation room or a Class N room (negative pressure room) in an existing facility, available infrastructure and financial implications for carrying out changes must be considered. It is rarely possible to create an ideal room. There is an extensive list of requirements given in 'Guidelines for the classification and design of isolation rooms in healthcare facilities' which are the basic requirements to be met before any conversion25.
Some of the salient requirements are the presence of a clinical handwash basin with non-touch, fixed tap, wall mounted soap dispensers, handrub dispensers, disposable towel holders, glove dispensers, clean waste bins in accordance to 'the Bio-Medical Waste Management (Amendment) Rules, 2018'26 and provision of two-way intercommunication system between the patient's room and the nurses' station. This document also gives insight into what considerations an institute needs to consider before converting a room into a class N room. The recirculating air system needs to be disconnected. It would be a preferred option to adjust the building ventilation system to create a permanent negative pressure room or to add a HEPA filtration unit as a supplement or install it permanently in the hospital ventilation system. One should be able to seal the room adequately; dampers should be adjusted.
Supply
Since clinical manifestations of SARS-CoV-2 infection range from septic shock, pneumonia, ARDS, acute kidney injury to multi-organ dysfunction, the unit should be equipped like an ICU. A detailed list of disease commodity package for novel CoV has been published by the WHO27. Additional material is shown in Supplementary Table II (available from http://www.ijmr.org.in/articles/2020/151/2/images/IndianJMedRes_2020_151_2_177_280781_sm4.pdf).
Supplementary Table II.
List of provisions necessary
| Medications |
| Sedatives - Propofol, midazolam, fentanyl |
| Vasopressors - Norepinephrine, dopamine, dobutamine, phenylephrine |
| Diuretics - Furosemide |
| Proton pump inhibitors - Pantoprazole |
| Heparin |
| Crystalloids - Ringer’s lactate, normal saline |
| Colloids - Albumin |
| Pain medications - Morphine, NSAIDs |
| Insulin |
| Kits - Central line kits, arterial line kits, haemodialysis catheter kits |
| Standardized ACLS cart |
| Hospital supplies |
| Bed |
| Sheets |
| iv stand |
| iv sets and cannula |
| Patient gowns |
| Band aid |
| Foley’s catheter, Foley’s bag |
| Tegaderm |
| Kits - Central line kits, arterial line kits, haemodialysis catheter kits |
| Infection prevention and control |
| HEPA filter |
| Personal protective equipment |
| Surgical face masks |
| N95 mask |
| Face shield |
| Cap |
| Isolation gowns |
| Overshoes |
| Gloves |
| Stethoscope in each room |
| Sphygmomanometer in each room |
| Thermometer in each room |
| Decontamination of equipment and environmental cleaning |
| Single use devices to be used wherever possible. Single use device reprocessing policies of the institute to be followed |
| Appropriate soiled linen disposal |
| Environmental cleaning – Equipment and procedures |
NSAIDs, non-steroidal anti-inflammatory drug; iv, Intravenous; ACLS, advanced cardiac life support; HEPA, high-efficiency particulate air
Conclusion
Developing the infrastructure and mobilizing the human resources needed to counter a rapidly emerging outbreak of a highly contagious or lethal disease needs responsive systems, mounting multi-disciplinary response, one critical part of which is enabling key healthcare facilities in providing high-quality clinical care. This not only helps in limiting the loss of lives from the onslaught of the contagions, but also interrupts the transmission by excluding the infective patients from the general population. As such, biocontainment provides value for money in the longer term, even if it is a resource-intensive affair in the short term. These actions will substantially contribute to global health security.
Footnotes
Supplementary material available from http://www.ijmr.org.in/article.asp?issn=0971-5916;year=2020;volume=151;issue=2;spage=177;epage=183;aulast=Agarwal
Financial support & sponsorship: None
Conflicts of Interest: None.
References
- 1.Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001316. doi: 101056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. doi: 101001/jama20201585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395:507–13. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim JY, Choe PG, Oh Y, Oh KJ, Kim J, Park SJ, et al. The first case of 2019 novel coronavirus pneumonia imported into Korea from Wuhan, China: Implication for infection prevention and control measures. J Korean Med Sci. 2020;35:e61. doi: 10.3346/jkms.2020.35.e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. Frequently asked questions on novel coronavirus - update. WHO; 2020. [accessed on February 13, 2020]. p. 3. Available from: https://wwwwhoint/csr/disease/coronavirus_infections/faq_dec12/en/ [Google Scholar]
- 6.Xiang N, Havers F, Chen T, Song Y, Tu W, Li L, et al. Use of national pneumonia surveillance to describe influenza A (H7N9) virus epidemiology, China, 2004-2013. Emerg Infect Dis. 2013;19:1784–90. doi: 10.3201/eid1911.130865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Munster VJ, Koopmans M, van Doremalen N, van Riel D, de Wit E. A novel coronavirus emerging in China - Key questions for impact assessment. N Engl J Med. 2020;382:692–4. doi: 10.1056/NEJMp2000929. [DOI] [PubMed] [Google Scholar]
- 8.Johns Hopkins CS. Coronavirus COVID-19 (2019-nCoV) Coronavirus COVID-19 global cases by Johns Hopkins CSSE. Wuhan: WHO; 2020. [accessed on February 13, 2020]. p. 1. Available from: https://gisanddatamapsarcgiscom/apps/opsdashboard/indexhtml #/bda7594740fd40299423467b48e9ecf6 . [Google Scholar]
- 9.Rodríguez-Morales AJ, MacGregor K, Kanagarajah S, Patel D, Schlagenhauf P. Going global - Travel and the 2019 novel coronavirus. Travel Med Infect Dis. 2020;33:101578. doi: 10.1016/j.tmaid.2020.101578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. Protocol for assessment of potential risk factors for 2019-novel coronavirus (2019-nCoV) infection among health care workers in a health care setting. Geneva: WHO; 2020. p. 32. [Google Scholar]
- 11.Ameli J. Communicable diseases and outbreak control. Turk J Emerg Med. 2015;15(Suppl 1):20–6. doi: 10.5505/1304.7361.2015.19970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siegel JD, Rhinehart E, Jackson M, Chiarello L Health Care Infection Control Practices Advisory Committee. 2007 guideline for isolation precautions: Preventing transmission of infectious agents in health care settings. Am J Infect Control. 2007;35:S65–164. doi: 10.1016/j.ajic.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arora M. Minimum standards for ICUS (intensive care units) to be adopted throughout the country. New Delhi: All India Institute of Medical Sciences; 2012. [Google Scholar]
- 14.World Health Organization. International Health Regulations (2005) 3rd ed. [accessed on February 13, 2020]. Available from: https://wwwwhoint/ihr/publications/9789241580496/en/
- 15.IATA. Infectious substances shipping guidelines. [accessed on February 13, 2020]. Available from: https://wwwiataorg/en/publications/store/infectious-substances-shipping-guidelines/
- 16.UN3373 medical packaging. UN3373: Biological substances category B. [accessed on February 13, 2020]. Available from: https://wwwun3373com/category-biological-substances/category-b/
- 17.Juneja D, Nasa P, Singh O. Physician staffing pattern in intensive care units: Have we cracked the code? World J Crit Care Med. 2012;1:10–4. doi: 10.5492/wjccm.v1.i1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization. Infection prevention and control of epidemic-and pandemic prone acute respiratory diseases in health care WHO/CDS/EPR/20076. Geneva: WHO; 2007. [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. Guideline for hand hygiene in health-care settings: Recommendations of the healthcare infection control practices advisory committee and the HICPAC/SHEA/APIC/IDSA hand hygiene task force. In: Gerberding JL, Fleming D, Snider DE Jr, editors. Morbidity and mortality weekly report. 1st ed. Atlanta: CDC; 2002. pp. 3–56. [Google Scholar]
- 20.Lu CW, Liu XF, Jia ZF. 2019-nCoV transmission through the ocular surface must not be ignored. Lancet. 2020;395:e39. doi: 10.1016/S0140-6736(20)30313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization. Home care for patients with suspected novel coronavirus (nCOV) infection presenting with mild symptoms and management of contacts. Geneva: WHO; 2020. [Google Scholar]
- 22.Centers for Disease Control and Prevention. Best practices for environmental cleaning in healthcare facilities in resource-limited settings. 1st ed. Atlanta, GA: US Department of Health and Human Services, CDC; Cape Town, South Africa: Infection Control Africa Network; 2019. [Google Scholar]
- 23.Sehulster LM, Chinn RYW, Arduino MJ, Carpenter J, Donlan R, Ashford D, et al. Guidelines for environmental infection control in health-care facilities Recommendations from CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC) Chicago, IL: American Society for Healthcare Engineering/American Hospital Association; 2004. [Google Scholar]
- 24.Centers for Disease Control and Prevention. Morbidity and mortality weekly report. Atlanta: CDC; 2003. Guidelines for environmental infection control in health-care facilities recommendations of CDC and the healthcare infection control practices advisory committee (HICPAC) pp. 3–48. [PubMed] [Google Scholar]
- 25.Victorian Advisory Committee on Infection Control. Guidelines for the classification and design of isolation rooms in health care facilities Victorian advisory committee on infection control. Melbourne: Victorian Advisory Committee on Infection Control; 2007. [Google Scholar]
- 26.Press Information Bureau. The bio-medical waste management (amendment) rules, 2018. New Delhi: Ministry of Environment Forest and Climate Change, Government of India; 2018. [Google Scholar]
- 27.World Health Organization. Disease commodity package for novel coronavirus. WHO; 2009. [accessed on February 13, 2020]. p. 2. Available from: https://wwwwhoint/publications-detail/disease-commodity-package-for-novel-coronavirus . [Google Scholar]