Abstract
INTRODUCTION:
Elevated liver enzyme levels are observed in patients with coronavirus disease 2019 (COVID-19); however, these features have not been characterized.
METHODS:
Hospitalized patients with COVID-19 in Zhejiang Province, China, from January 17 to February 12, 2020, were enrolled. Liver enzyme level elevation was defined as alanine aminotransferase level >35 U/L for men and 25 U/L for women at admission. Patients with normal alanine aminotransferase levels were included in the control group. Reverse transcription polymerase chain reaction was used to confirm severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, and patients symptomatic with SARS-CoV-2 infection were defined as patients with COVID-19. Epidemiological, demographic, clinical, laboratory, treatment, and outcome data were collected and compared.
RESULTS:
Of 788 patients with COVID-19, 222 (28.2%) patients had elevated liver enzyme levels (median [interquartile range {IQR}] age, 47.0 [35.0–55.0] years; 40.5% women). Being male, overweight, and smoking increased the risk of liver enzyme level elevation. The liver enzyme level elevation group had lesser pharyngalgia and more diarrhea than the control group. The median time from illness onset to admission was 3 days for liver enzyme level elevation groups (IQR, 2–6), whereas the median hospitalization time for 86 (38.7%) discharged patients was 13 days (IQR, 11–16). No differences in disease severity and clinical outcomes were noted between the groups.
DISCUSSION:
We found that 28.2% of patients with COVID-19 presented with elevated liver enzyme levels on admission, which could partially be related to SARS-CoV-2 infection. Male patients had a higher risk of liver enzyme level elevation. With early medical intervention, liver enzyme level elevation did not worsen the outcomes of patients with COVID-19.
INTRODUCTION
Since late December 2019, the outbreak of coronavirus disease 2019 (COVID-19) in Wuhan, China (1), has caused a huge amount of infections and spread across the world (2,3). Its virological, epidemiological, and clinical features had been widely discussed, from which a subset of patients with elevated liver enzyme levels could be observed. The first few studies of COVID-19 showed significantly higher alanine aminotransferase (ALT) in intensive care unit (ICU) patients, although the median of ALT in this group was close to the upper limit of normal (4,5). Chen et al. (6) reported that the rate of increased ALT was 28% in COVID-19 patients, as well as severe liver function damage in one patient (ALT 7590 U/L, AST 1445 U/L). A much larger study by Guan et al. (7) revealed that patients with COVID-19 with elevated liver enzyme levels sustained not only a higher probability of severe pneumonia but also higher risks of ICU admission, mechanical ventilation, and death. However, the clinical characteristics and outcome of patients with COVID-19 with elevated liver enzyme levels have not been well described until now.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) shares 79.5% of its genome sequence with severe acute respiratory syndrome coronavirus (SARS-CoV) (8). Previous study on SARS demonstrated that approximately 34% of patients with SARS had elevated liver enzyme levels (9), and SARS coronavirus N protein and RNA polymerase gene fragments were detected in hepatocytes near the central vein (10). It is reasonable to speculate that SARS-CoV-2 might cause liver damage with a similar pathological mechanism to SARS-CoV (11,12).
It has been proven that SARS-CoV-2 used the same cell entry receptor, angiotensin-converting enzyme 2 (ACE2), as in SARS-CoV (13,14). A previous study revealed that ACE2 was abundantly expressed on the surface of the lungs and the small intestine epithelium, and occasionally on the bile ducts (15). A recent study, using a single cell RNA-seq technique, demonstrated that 59.7% of cholangiocytes and 2.6% hepatocytes expressed ACE2 (16). ACE2 expression in the liver was a structural prerequisite of direct liver damage by SARS-CoV-2. A recent autopsy case also found moderate microvesicular steatosis and mild lobular activity in the liver, indicating either SARS-CoV-2 infection or drug-induced liver injury (17). Both virologic and pathologic evidence indicated that SARS-CoV-2 infection was related to liver damage.
Because early reports tended to enroll more severe cases due to the shortage of healthcare resources in Wuhan after an outburst, there may be a slight selection bias in evaluating the incidence and prognosis of complications such as liver enzyme elevation in patients with COVID-19. As one of the major affected provinces with sufficient medical resources in China, the Zhejiang Province represents a good platform to investigate the clinical characteristics and outcomes of patients with COVID-19 with elevated liver enzyme level complications.
METHODS
Study design and participants
A retrospective study investigating the clinical characteristics and outcomes of patients with COVID-19 with elevated liver enzyme levels in the Zhejiang Province was conducted. The diagnosis of COVID-19 was according to the WHO interim guidance (18). Confirmed patients with COVID-19 were admitted in designated hospitals by the Health Commission of Zhejiang Province from January 17 to February 12, 2020, and then, they were enrolled and divided into 2 groups depending on whether they presented with liver enzyme level elevation within 48 hours of admission. Liver enzyme level elevation was defined as patients having ALT levels greater than 35 U/L for men and 25 U/L for women, according to the American Association for the Study of Liver Diseases definitions (19). The final date of follow-up was February 12, 2020. The patients with normal ALT levels on admission were included in the control group.
This study was approved by the Clinical Research Ethics Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University (NO. IIT20200005C). Written informed consent was waived by the ethics commission of the designated hospital, and oral consent was obtained from patients.
Data collection
Data were uniformly collected by the Health Commission of Zhejiang Province in designated hospitals with data collection forms from original medical records and reviewed separately by 2 clinicians. Missing or vague dates were confirmed by direct communication with healthcare providers. Each patient was assigned a unique number, and corresponding demographic data, exposure history, medical history, underlying medical conditions, laboratory results, and chest computed tomography scans were recorded within 48 hours of admission. Treatment measures and outcomes were recorded based on the final follow-up on February 12, 2020.
The date of onset of illness was defined as the day when the first symptom was noticed. Acute respiratory distress syndrome was defined according to the WHO interim guidance (18). Acute kidney injury was defined according to the Kidney Disease: Improving Global Outcomes definition (20). All confirmed patients with COVID-19 were divided into 4 severity subtypes according to the diagnosis and treatment scheme for novel coronavirus pneumonia (sixth edition) by the National Health Commission of China on admission and by the last follow-up. In brief, mild type was defined as having slight clinical symptoms with no signs of pneumonia by radiography. Common type was defined as fever and/or respiratory symptoms plus pneumonia from radiography. Severe type was diagnosed according to any dyspnea (respiratory rate ≥30 breaths/minute), resting peripheral oxygen saturation ≤93%, or arterial PaO2/FiO2 ≤300 mm Hg (1 mm Hg = 0.133 kPa). Critical type was determined by any respiratory failure requiring mechanical ventilation, septic shock, or ICU admission for organ failure other than of the lungs.
Statistical analysis
Continuous variables were described using medians (with interquartile ranges [IQRs]) and compared using the Mann-Whitney U test. Categorical variables were described as frequencies (percentages) and compared using the χ2 test or Fisher exact test where appropriate. A P value < 0.05 was considered statistically significant. Associations with gender, weight, smoking, exposure history to Wuhan or confirmed patients, pharyngalgia, diarrhea, leukocytes, neutrophils, hemoglobin, blood urine nitrogen, C-reactive protein, and procalcitonin with elevated liver enzyme levels were analyzed using a univariable logistic regression. The statistical analyses described above were performed using SPSS (version 19.0).
The Kaplan-Meier method was used to estimate hospitalization time, and the log rank test was applied for comparisons between the liver enzyme elevation group and control groups. The Kaplan-Meier analysis was conducted in R (version 3.6.2) using the “survfit” function in the R package “survival.” The Sankey diagram was used to visualize the patient status at the end of follow-up and was conducted in R using the “sankeyNetwork” function in the R package “networkD3.”
RESULTS
Characteristics of patients with COVID-19 with elevated liver enzyme levels
This study enrolled 788 confirmed patients with COVID-19 from January 17, 2020, to February 12, 2020, in the Zhejiang Province. Among them, 222 (28.2%) patients presented with liver enzyme elevation according to the American Association for the Study of Liver Diseases definition on admission. Medication history and alcohol use was documented on admission. Among the 222 liver enzyme elevation patients, 5 (2.3%) patients were taking medications associated with intrinsic or idiosyncratic drug-induced liver injury, as defined by the European Association for the Study of the Liver guidelines (21). Four cases had taken statins, and 1 case took antituberculosis treatment including isoniazid/pyrazinamide. Other medications that may affect the ALT levels included acarbose (3 cases), febuxostat (2 cases), and oxcarbazepine (1 case). Of the 222 liver enzyme elevation patients, 10 (4.5%) patients had a history of long-term alcohol use higher than the upper limit of safe drinking, especially one standard drink per day for women and 2 standard drinks for men (22). Acute infection with viral hepatitis, Epstein-Barr virus, Cytomegalovirus, and herpes simplex virus were tested for and excluded by clinical manifestation and laboratory tests in patients with elevated liver enzyme levels. No patient reported jaundice, pruritus, nor acholic stools on admission.
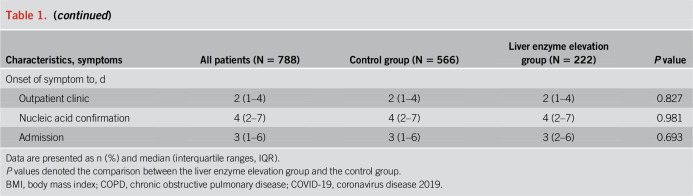
Of the 222 liver enzyme elevation patients, with a median age of 47.0 years (IQR: 35.0–55.0), 90 (40.5%) were women and 22 (9.9%) were smokers. The median body mass index (BMI) for patients was 24.3 kg/m2, which fell into the range of the overweight threshold for Chinese people (24.0–27.9 kg/m2). Of the 222 patients, 71 (32.0%) had 1 or more coexisting medical conditions. Hypertension (39 [17.6%]), diabetes (17 [7.7%]), and chronic liver disease (15 [6.8%]) were the most common coexisting conditions. There were 94 (43.7%) patients who had traveled to Wuhan. The most common symptoms at illness onset were fever (184 [82.9%]), cough (141 [63.5%]), sputum production (80 [36.0%]), fatigue (39 [17.6%]), diarrhea (25 [11.3%]), and headache (25 [11.3%]). Less common symptoms were hemoptysis, dyspnea, nausea, and vomiting. The median duration from the onset of the first symptoms to the first outpatient clinic visit, polymerase chain reaction confirmation, and hospital admission was 2 days (IQR, 1–4), 4 days (IQR, 2–7), and 3 days (IQR, 2–6), respectively (Table 1).
Table 1.
Characteristics of 222 liver enzyme elevation patients in a cohort of 788 patients with COVID-19
Compared with the control group, there were less women (40.5% vs 51.4%) and a higher BMI (24.3 vs 23.1) in the liver enzyme elevation group. More patients had diarrhea and less patients had pharyngalgia in the liver enzyme elevation group compared with the control group. The pre-existing medical conditions were comparable between the liver enzyme elevation group and control group except for chronic liver disease. Of the 222 liver enzyme elevation patients, 15 (6.8%) patients had chronic liver disease, which was higher than the 16 (2.8%) of the control group (P = 0.011). Of the 15 patients with pre-existing chronic liver disease in the liver enzyme elevation group, 13 (86.7%) suffered from chronic hepatitis B, including one case that also suffered from fatty liver disease and one case that also had cirrhosis of the liver. The other 2 cases of pre-existing chronic liver disease had cirrhosis of unknown origin. Of the 444 patients whose BMI was available, the BMIs of 184 (41.4%) patients surpassed the overweight threshold for Chinese people (24.0 kg/m2). There were more overweight patients in the liver enzyme elevation group than in the control group (72 [54.5%] vs 112 [35.9%], P < 0.001). ALT levels were significantly higher in the overweight patients (median [IQR]: 26 [19–42] vs 19 [14–28], P < 0.001). Of the 260 patients with a normal BMI, 60 (23%) patients had elevated liver enzyme levels.
Laboratory parameters of patients with COVID-19 with elevated liver enzyme levels
On admission, 36 (16.2%) and 10 (4.5%) of patients with COVID-19 with elevated liver enzyme levels had lymphopenia and thrombocytopenia, respectively. Compared with the control group, the level of hemoglobin, aspartate aminotransferase, total bilirubin, lactate dehydrogenase, blood urea nitrogen, C-reactive protein, and procalcitonin were higher than that of the control group. Of the patients with elevated liver enzyme levels, 87 (39.2%) patients showed bilateral pneumonia, whereas 63 (28.4%) showed multiple mottling and ground-glass opacity. The radiographic presentations were similar between patients with COVID-19 with and without liver enzyme elevation (Table 2).
Table 2.
Laboratory and radiographic findings of patients with COVID-19 on admission
Complication, treatment, and outcome
Until the last follow-up on February 12, 2020, 16 (7.2%) patients with elevated liver enzyme levels developed acute respiratory distress syndrome and 4 (1.8%) were mechanically ventilated and admitted to the ICU (Table 3). All patients were treated in isolation with supportive treatment. Because SARS-CoV-2 is a newly emerging infectious disease, no effective antiviral drugs have been established. Therefore, lopinavir/ritonavir and arbidol that are effective against other coronaviruses were used. Of the 222 patients, 191 (86.0%) received empirical antivirus treatment in which 155 (69.8%) and 109 (49.1%) patients received regimens containing lopinavir/ritonavir or arbidol, respectively. The median time from illness onset to antiviral therapy was 3 days (IQR, 1–6). The complications and treatment measures were generally comparable between the liver enzyme elevation group and the control group.
Table 3.
Complications, treatment, and outcomes of patients with COVID-19 with liver enzyme elevation
According to the diagnosis and treatment scheme for novel coronavirus pneumonia by the National Health Commission of China (sixth edition), 187 (84.2%) patients were diagnosed with common type and 17 (7.7%) and 3 (1.4%) were diagnosed as severe type and critical type, respectively, on admission. On the final follow-up, 86 (38.7%) patients were discharged after marked improvement of symptoms and radiography as well as 2 consecutive negative nucleic acid tests for respiratory specimens. The median hospitalization time of discharged patients was 13 days (IQR, 11–16). Kaplan-Meier analysis showed no differences in hospitalization time between the liver enzyme elevation group and the control group (Figure 1). The status flow of all 788 patient is demonstrated in Figure 2.
Figure 1.
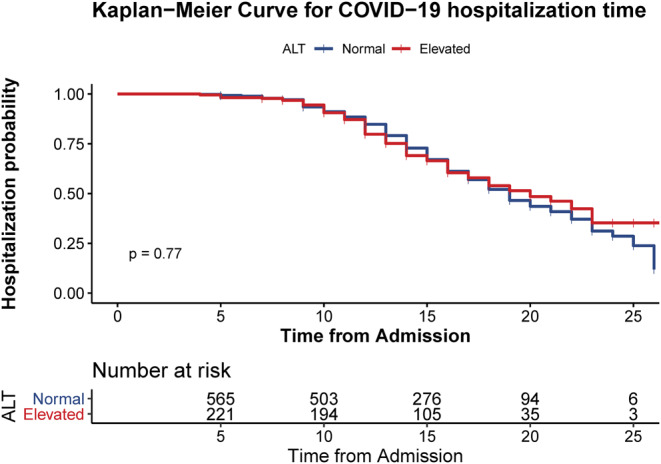
Kaplan-Meier analysis showed no differences in hospitalization time between the liver enzyme elevation group and the control group.
Figure 2.

The Sankey diagram showing the status flow of all 788 patients with COVID-19 by the final follow-up. Columns on the left stand for disease severity and corresponding number of patients with COVID-19 on admission, “L” stands for the liver enzyme elevation group, “N” stands for control group. Columns on the right stand for patient status and relative number by the final follow-up on February 12, 2020. COVID-19 = coronavirus disease 2019.
Of the 788 patients, liver failure was not observed. Paired ALT data on admission and discharge were available for 209 (64.9%) of 322 patients discharged by the final follow-up. The median of ALT level was 20 (14–30) U/L on admission compared with 22 (15–40) U/L on discharge. The ALT levels did not elevate significantly during the admission course, confirmed using a paired Wilcoxon test (P = 0.069). Of the 209 patients with paired ALT levels, 56 were in the liver enzyme elevation group on admission and their ALT levels returned to normal in 28 (50%) patients; by contrast, the ALT levels elevated in 49 (32%) patients of 153 patients who had normal ALT levels on admission. Four patients in each group developed jaundice, which had improved significantly by the time they were discharged.
The risk factors of liver enzyme elevation
With univariable logistic regression analysis, we found increasing odds of liver enzyme elevation associated with male gender (odds ratio, 1.552 [95% confidence interval, 1.133–2.126], P = 0.006), being overweight (2.143 [1.417–3.240], P < 0.001), a history of smoking (1.836 [1.042–3.235], P = 0.036), contact with confirmed patients (1.410 [1.032–1.927], P = 0.031), diarrhea (1.814 [1.065–3.092], P = 0.029), hemoglobin (1.021 [1.011–1.031], P < 0.001), and procalcitonin (10.811 [1.151–101.505], P = 0.037) on admission, whereas patients with Wuhan traveling history (0.708 [0.518–0.967], P = 0.030) and pharyngalgia (0.449 [0.264–0.763] P = 0.003) had lower risk of liver enzyme elevation (Table 4).
Table 4.
Univariable analysis of factors significantly associated with liver enzyme elevation

DISCUSSION
In this study, we reported the epidemiological, clinical, and outcome features of 222 patients with COVID-19 with elevated liver enzyme levels from the Zhejiang Province. This report, to our knowledge, is the first study to date on liver enzyme elevation in COVID-19. Previous studies focused on the characteristics of severe cases of COVID-19 in a small sample size. Our data were collected by the Health Commission of the Zhejiang Province in designated hospitals for the purpose of disease surveillance, which allows our data to be a useful, unbiased sample. Overall, the liver enzyme elevation was mild, and no liver failure was observed on the follow-up.
Based on the constant mutation nature of coronaviruses (23–25), concerns on adaptive mutations and enhanced virulence of SARS-CoV-2 virus during interhuman communication have been raised (12). Because 49.9% of patients in our study had a traveling history to Wuhan who had very high probability of being infected there, they represented an earlier generation of patients compared with local patients without a Wuhan traveling history. Liver enzyme level elevation was a good index to observe whether there were any adaptive mutations during the human-to-human transmission. In our study, although the rate of Wuhan traveling history was lower in the liver enzyme elevation group than the control group, the ALT and total bilirubin levels were comparable in patients with and without a Wuhan traveling history. The adaptive evolution of SARS-CoV-2 cannot be established at this time. The conclusion in our clinical study was consistent with a previous genomic analysis that declared that adaptive evolution cannot be confirmed currently (26).
The common causes for liver enzyme elevation need to be differentiated in COVID-19. In our study, the incidence rate of pre-existing chronic liver diseases was higher in the liver enzyme elevation group. The incidence rate of chronic liver disease was only 6.8%, and most of the chronic liver diseases were chronic hepatitis B with consistent anti-HBV treatment, so pre-existing chronic diseases cannot explain all the liver enzyme abnormalities. In addition, the albumin levels in the liver enzyme level elevation group and control group were similar and they were both in the middle of the normal range, which did not support the conclusion that the liver damage was caused by chronic liver diseases. Similarly, approximately only 5% of patients were taking medications that might affect the ALT levels and 4.5% of patients had a history of long-term alcohol use higher than the upper limit of safe drinking. Acute infection of viral hepatitis, EB virus, cytomegalovirus, and herpes simplex virus were tested and excluded by clinical manifestation and laboratory tests in patients with elevated liver enzyme levels. There were more overweight patients in the liver enzyme elevation group than in the control group. Being overweight was a risk factor of fatty liver, and being overweight may contribute to liver enzyme elevation either by steatohepatitis or by increasing the susceptibility of hepatocytes during SARS-CoV-2 infection. Because the confounding liver injury causes could not be excluded comprehensively in our study, the liver enzyme elevation cannot be fully associated to the SARS-CoV-2 infection.
In our study, although several clinical features including total bilirubin and blood urea nitrogen were higher than in the control group, no differences in the severity, discharge rate, and median hospitalization time were observed in the liver enzyme elevation group. This was different from a previous study by Guan et al. (7), and the main reason was the generally lower level of disease severity compared with previously published cohorts. Another point we want to emphasize was that the median time from onset of illness to admission in the Zhejiang Province was 3 days, much shorter than the 7 days mentioned by Huang et al. in Wuhan (4). Early medical intervention likely neutralized the effects resulting from liver enzyme elevation. Moreover, the early medical intervention originated from both rigorous early surveillance of potentially infected people by the local government and health institutions, and increased availability of medical resources in Zhejiang than in Wuhan. The Zhejiang Province was the first province that raised the highest response level for COVID-19 and quarantined every suspected patient, followed by immediate virus detection. This could be determined from the time of illness onset to hospital admission. Hospital admission and polymerase chain reaction confirmation was 3 days and 4 days, respectively, in Zhejiang, which meant that suspected cases were admitted to the hospital for quarantine even before laboratory confirmation.
Although there was evidence of ACE2 expression in the liver, the mechanism of liver injury in COVID-19 could be caused either by direct virus infection or by a systemic inflammatory response syndrome reaction. Based on the nature of this cross-sectional study, the mechanism cannot be addressed at this time. Previous studies demonstrated that renal and cardiac ACE2 distribution were regulated by sex hormones (27,28), and male subjects had higher ACE2 expression. The higher risk of liver damage in male patients could be explained by either higher ACE2 expression in male liver or a more severe systemic inflammatory response syndrome reaction induced by higher ACE2 expression in other organs in men. For the latter, a higher rate of complication and worse outcomes should be observed, which not seen in our study. Thus, the speculation that male patients had higher ACE2 expression in their liver than female patients leads to the higher risk of liver damage.
This study has several limitations. First, the pathogenesis of liver enzyme elevation could not be fully associated to SARS-CoV-2 infection in our study because of difficulties in excluding all confounding liver injury causes. Second, the retrospective design of this study may decrease its credibility, and future perspective cohort studies should be considered to validate the risk factors of liver enzyme elevation during COVID-19. Third, the data regarding the outcomes need to be further investigated because more than half of the patients were still under treatment. Finally, the mechanism of liver injury during COVID-19 and the mechanisms of its risk factors were speculative and should be validated in future studies.
In summary, we reported the epidemiological, clinical, and prognostic features of patients with COVID-19 with elevated liver enzyme levels. Patients who are male, overweight, and smoke had a higher risk of liver enzyme elevation. The liver enzyme elevation group had less pharyngalgia and more diarrhea than the control group. Early medical intervention likely neutralized the effects caused by liver enzyme elevation. Attention should be paid to elevated liver enzyme levels in patients with COVID-19, whereas in-depth studies that help to understand the SARS-CoV-2 infection mechanism and clinical management of COVID-19 is urgently needed.
CONFLICTS OF INTEREST
Guarantor of the article: Shao-Rui Hao, MD, Shan-Yan Zhang, MD, Jiang-Shan Lian, MD, Xi Jin, MD, and Cheng-Yin Ye, PhD, contributed equally to this article. Yi-Da Yang, MD, accepts full responsibility for the conduct of the study and had full access to the data and control of the decision to publish.
Specific author contributions: Y.-D.Y., T.-B.L., J.-F.S., and L.-J.L. designed the study. S.-R.H. and S.-Y.Z. managed the full deidentified data set. S.-R.H. and C.-Y.Y. performed the statistical analyses. S.-R.H. and J.-S.L. authored the initial draft of the manuscript. X.J. and Y.-D.Y. critically revised the manuscript. All authors were involved in the review and interpretation of data and approved the final copy.
Financial support: This work was supported by National Natural Science Foundation of China (81700549) and National Major Science and Technology Research Projects for the Control and Prevention of Major Infectious Diseases in China (2017ZX10202202).
Potential competing interests: All the authors have no conflict of interest to declare.
Study Highlights.
WHAT IS KNOWN
✓ Liver enzyme elevation was observed in patients with COVID-19.
✓ The features of the liver enzyme elevation subset in COVID-19 remain unknown.
WHAT IS NEW HERE
✓ Of patients with COVID-19, 28.2% presented with elevated liver enzyme levels on admission.
✓ Most liver enzyme elevations were mild, and no liver failures were observed.
✓ Being male, overweight, and with a history of smoking were risk factors of liver enzyme elevation.
✓ The liver enzyme elevation group had less pharyngalgia and more diarrhea.
✓ Early medical intervention likely neutralized the effects that resulted from the liver enzyme elevation.
ACKNOWLEDGMENTS
We thank the Health Commission of Zhejiang Province, China, for coordinating the data collection and all the frontline medical staff of Zhejiang Province for their bravery and efforts in COVID-19 prevention and control. We would like to thank Editage (www.editage.com) for English language editing.
REFERENCES
- 1.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020;382:727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med 2020;382:929–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silverstein WK, Stroud L, Cleghorn GE, et al. First imported case of 2019 novel coronavirus in Canada, presenting as mild pneumonia. Lancet 2020;395:734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020;323:1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020;395:507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020;579:270–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peiris JS, Lai ST, Poon LL, et al. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet 2003;361:1319–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ding Y, He L, Zhang Q, et al. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: Implications for pathogenesis and virus transmission pathways. J Pathol 2004;203:622–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gu J, Gong E, Zhang B, et al. Multiple organ infection and the pathogenesis of SARS. J Exp Med 2005;202:415–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perlman S. Another decade, another coronavirus. N Engl J Med 2020;382:760–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu X, Chen P, Wang J, et al. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci 2020;63:457–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020;395:565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamming I, Timens W, Bulthuis ML, et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 2004;203:631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chai X, Hu L, Zhang Y, et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. bioRxiv 2020:2020.02.03.931766. [Google Scholar]
- 17.Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;8:420–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO. Clinical Management of Severe Acute Respiratory Infection When Novel Coronavirus (nCoV) Infection is Suspected: Interim Guidance. 2020. (https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected) (2020). Accessed February 20, 2020. [Google Scholar]
- 19.Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018;67:1560–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kellum JA, Lameire N, Aspelin P, et al. Kidney disease: Improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Supplements 2012;2:1–138. [Google Scholar]
- 21.European Association for the Study of the Liver. EASL clinical practice guidelines: Drug-induced liver injury. J Hepatol 2019;70:1222–61. [DOI] [PubMed] [Google Scholar]
- 22.Crabb DW, Im GY, Szabo G, et al. Diagnosis and treatment of alcohol-associated liver diseases: 2019 practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2020;71:306–33. [DOI] [PubMed] [Google Scholar]
- 23.Cui J, Li F, Shi ZL. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol 2019;17:181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Letko M, Miazgowicz K, McMinn R, et al. Adaptive evolution of MERS-CoV to species variation in DPP4. Cell Rep 2018;24:1730–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chinese SARS Molecular Epidemiology Consortium. Molecular evolution of the SARS coronavirus during the course of the SARS epidemic in China. Science 2004;303:1666–9. [DOI] [PubMed] [Google Scholar]
- 26.Cui J, Li X, Wei X. Evolutionary perspectives on novel coronaviruses identified in pneumonia cases in China. Natl Sci Rev 2020;7:239–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu J, Ji H, Zheng W, et al. Sex differences in renal angiotensin converting enzyme 2 (ACE2) activity are 17beta-oestradiol-dependent and sex chromosome-independent. Biol Sex Differ 2010;1:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dalpiaz PL, Lamas AZ, Caliman IF, et al. Sex hormones promote opposite effects on ACE and ACE2 activity, hypertrophy and cardiac contractility in spontaneously hypertensive rats. PLoS One 2015;10:e0127515. [DOI] [PMC free article] [PubMed] [Google Scholar]