Supplemental Digital Content is available in the text.
Abstract
Current evidence suggests that Coronavirus Disease 2019 (COVID-19) spread occurs via respiratory droplets (particles >5 µm) and possibly through aerosol. The rate of transmission remains high during airway management. This was evident during the 2003 severe acute respiratory syndrome epidemic where those who were involved in tracheal intubation had a higher risk of infection than those who were not involved (odds ratio 6.6). We describe specific airway management principles for patients with known or suspected COVID-19 disease for an array of critical care and procedural settings. We conducted a thorough search of the available literature of airway management of COVID-19 across a variety of international settings. In addition, we have analyzed various medical professional body recommendations for common procedural practices such as interventional cardiology, gastroenterology, and pulmonology. A systematic process that aims to protect the operators involved via appropriate personal protective equipment, avoidance of unnecessary patient contact and minimalization of periprocedural aerosol generation are key components to successful airway management. For operating room cases requiring general anesthesia or complex interventional procedures, tracheal intubation should be the preferred option. For interventional procedures, when tracheal intubation is not indicated, cautious conscious sedation appears to be a reasonable approach. Awake intubation should be avoided unless it is absolutely necessary. Extubation is a high-risk procedure for aerosol and droplet spread and needs thorough planning and preparation. As updates and modifications in the management of COVID-19 are still evolving, local guidelines, appraised at regular intervals, are vital in optimizing clinical management.
Suspected or confirmed “Coronavirus Disease 2019” (COVID-19) positive cases due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) present unique challenges to health care workers as the rate of transmission to health care personnel remains very high. In Italy, among the infected cases, 9% were health care workers.1 The transmission occurs by means of respiratory droplets (>5 µm) as well as through hand and surface contamination.2 The viral load in the airway is presumably very high and hence poses significant infective transmission risk during airway management.3,4 Previous experience during the 2003 SARS-CoV outbreak suggests that the virus could be transmitted during aerosol-generating procedures, most commonly during endotracheal intubation.5,6 Recent experimental findings suggest a potential aerosol (<5 µm) transmission of SARS-CoV-2.7 Health care workers involved with tracheal intubation during the 2003 SARS epidemic had a higher risk of contracting the virus (odds ratio 6.6).8 Notably, most of those health care workers were only wearing a standard surgical facemask during intubation. It is considered that there is no strong link between intubation and transmission risk when appropriate airborne precautions are followed.9
SARS-CoV-2 is genetically 85% similar to the previous SARS-CoV. Yet SARS-CoV-2 is a distinctly new coronavirus strain.10 Although the transmission rate is high, the case fatality rate of COVID-19 (varying across regions) seems to be lower than that of SARS (9.5%).11 We aim to outline procedure-specific principles of airway management for suspected or proven COVID-19 patients. These suggestions have been derived from recently published literature, recommendations from various international governing organizations and consensus guidelines. As the COVID-19 situation is dynamically changing with time, this review is presented with an understanding that updates and modifications to clinical practice will continue to evolve. Thus, this review should be read in conjunction with updated local guidelines and protocols.
ENVIRONMENT FOR AIRWAY INTERVENTIONS
Airway management and any subsequent surgical or procedural interventions should preferably be conducted in dedicated negative pressure rooms labeled as “airborne infection isolation rooms” (AIIR) with a minimum of 12 air changes per hour.12 To prevent infection to those outside the room, air is filtered with a high-efficiency particulate air (HEPA) filter and evacuated from these rooms to the external atmosphere.2 The doors should remain closed during the intervention, to optimize the frequency of these air changes. If negative pressure rooms are not available, consultation with the bioengineering departments within an institution should be sought to optimize existing workflows.13 Alternate options to negative pressure room are depicted in Figure 1. Aerosol clearing times after any aerosol-generating medical procedure should be established based on the ventilation flows (air changes per hour)14 (Table 1). A 30-minute waiting period is recommended for rooms with 12 air changes per hour after intubation and extubation.
Figure 1.
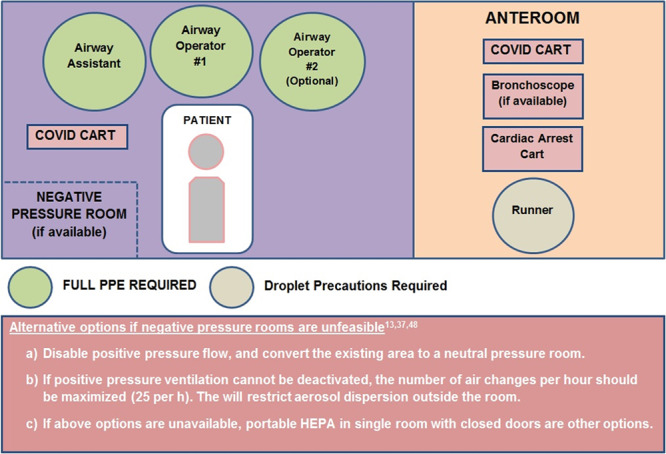
Layout of the airway intervention suite. Adapted and modified from Brewster et al.13 COVID indicates Coronavirus Disease 2019; HEPA, high-efficiency particulate air.
Table 1.
Aerosol Clearance Timea After Aerosol-Generating Interventions Based on the Air Changes per Hour of a Room14
| Air Changesper Hour | Time (min) Needed for 99% Efficacy |
Time (min) Needed for 99.9% Efficacy |
|---|---|---|
| 12 | 23 | 35 |
| 15 | 18 | 28 |
| 20 | 14 | 21 |
| 50 | 6 | 8 |
Institutions may draw up lockdown times based on existing workflows where the doors remain closed for other team members (eg, surgeons). This may not be applicable in emergency situations (eg, an urgent cesarean delivery requiring GETA).
Abbreviation: GETA, general endotracheal tube anesthesia.
aBased on the assumption that aerosol generation has ceased after an intervention,for example, tracheal intubation.
Transfer protocols should be developed and rehearsed with the goal of minimizing interaction with other patients and staff.15 The principles of intrahospital transfer have been reviewed recently by Coccolini et al.16 A decision to transport a stable patient between 2 areas (before airway management) should only be conducted if the new area has better equipment, more experienced staff, and a controlled environment or if transferring to the operating/procedure room.13 A separate anteroom should be established for keeping the essential procedural kits, equipment, and drugs with a set of runners remaining in the anteroom (Figure 1). Alternatively, if a separate anteroom is not available, an adjacent operating room could be used instead.15 Local infectious disease experts should be involved in designing dedicated interventional areas, inclusion of protective equipment, and intrahospital patient flow.
PERSONAL PROTECTIVE EQUIPMENT, AIRWAY EQUIPMENT, AND PREINTUBATION OXYGEN THERAPY
Personal protective equipment (PPE) is a significant component in minimizing the risk of transmission to health care workers (Figure 2). Based on best practice guidelines and advice from local infectious disease experts, health departments should adopt appropriate PPE (Figure 3) and regularly train staff in donning and doffing practice drills and enforce regular monitoring of stock levels. Disposable equipment is preferred to reusable equipment, subject to availability. The regular airway cart based on the local practices should be kept clean outside the operating theatre, and a separate dedicated COVID-airway cart should be organized.13 In addition to airway equipment, there should be close access emergency drugs and devices in a clean area outside the intubating room. Readers are referred to recent guidelines13,18 in relation to preparing the medications and equipment. It is essential to maintain adequate amounts of anesthetic drugs and airway equipment. Critical supply shortages of PPE, medications, and other equipment have been reported across many US hospitals during this outbreak.19 Organizations such as the Centers for Disease Control and Prevention, American Society of Health-System Pharmacists, The Australian Therapeutic Goods Administration, and various other sources have proposed numerous contingency plans.17,20–26 Some of the key measures are outlined in Table 2.
Figure 2.
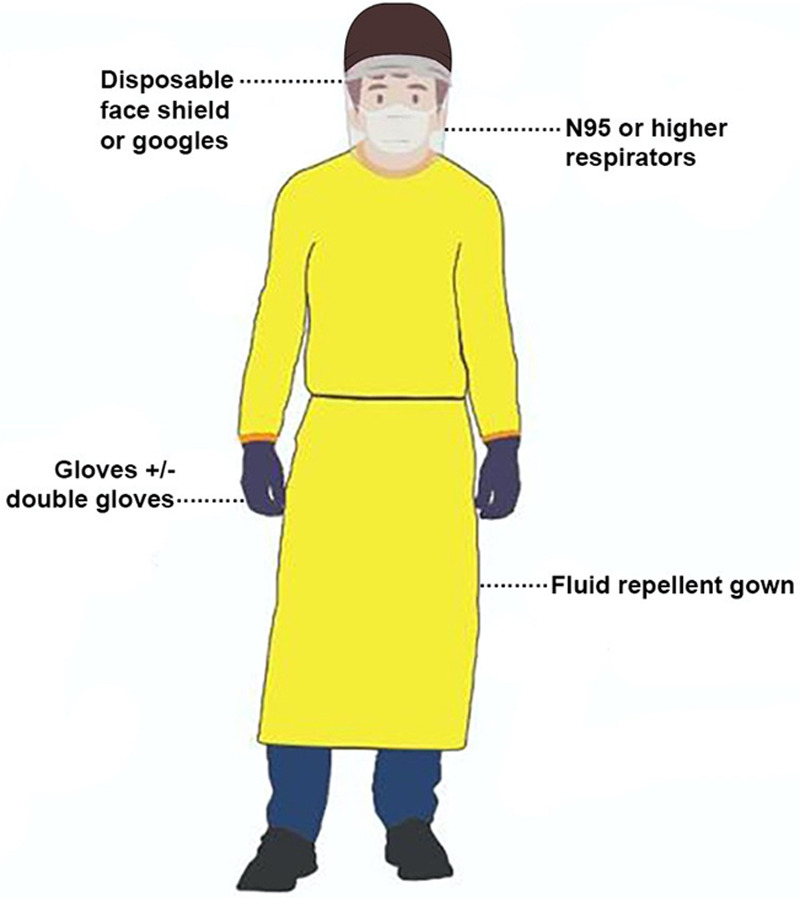
Components of standard airborne precaution personal protective equipment. Adapted and modified from Centers for Disease Control and Prevention.17
Figure 3.
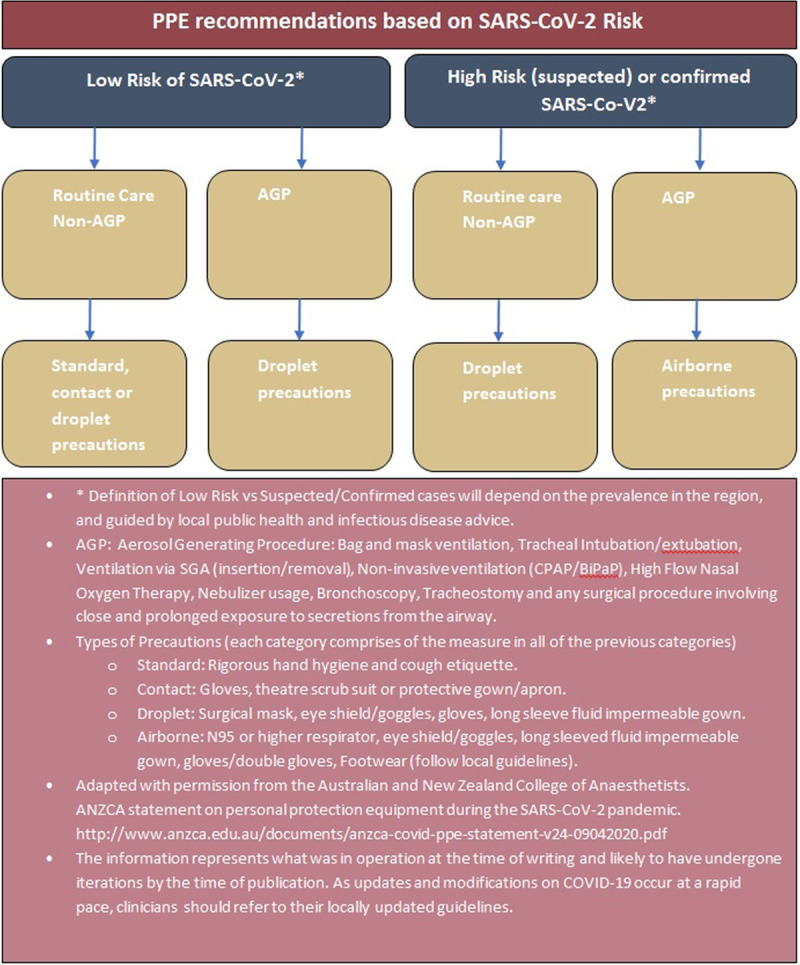
PPE guidelines for anesthesiologists. AGP indicates aerosol-generating procedure; BiPAP, xxx; COVID-19, Coronavirus Disease 2019; CPAP, xxx; PPE, personal protective equipment; SARS-CoV-2, severe acute respiratory syndrome Coronavirus 2; SGA, supraglottic airway.
Table 2.
| General principles |
| - Limit the exposure of health care workers |
| - Reduce nonessential services |
| - Use of physical barriers such as glass or plastic windows |
| PPE |
| - Extended use of N95 respirators without removing between patient encounters, recommended for 8–12 h. A surgical facemask should be applied over N95 to shield from contamination (grosslycontaminated N95 masks should not be used) |
| - Reprocessed masks |
| - During crisis: portable HEPA filters and ventilated headboards |
| - Reusable water-proof gowns, goggles, and face shields |
| Minimizing medication wastage |
| - Reduce contamination of medications |
| - Beyond-use date (as approved by the United States Pharmacopeia) and sterile compounding outsourced to pharmacy compounders |
| - Regular updates made available locally on available alternatives |
| - Adapting to alternative agents and techniques,for example, induced hypotension with volatile agents instead of propofol |
| - Prepare and store prefilled syringes in small aliquots, for example, fentanyl drawn up from a large volume supply |
| - Pharmacy preparation and storage of prefilled syringes in small aliquots |
| Equipment shortage |
| - Reusing airway equipment with approved disinfection process |
| - Modifications of anesthesia machines as ventilators |
| - Using devices outside their intended use,for example, transport ventilators, sleep apnea machines to assist ventilation, and oxygen concentrators for primary supply |
| Other options reviewed by expert panels: some examples |
| - Masks: repurposeprefabricated snorkel, 3D printed masks |
| - Eye/face shields: sportseye protectors, helmets with visors |
| - Gowns: plasticponchos |
| - Use of nonhuman services: drones and robots |
| - Reduce bulk packaging |
For detailed information, readers should refer to the references quoted. Notably, locally updated guidelines are the best resources in many circumstances.
Following communication of the airway plan to all team members, the number of people in the room during induction should be minimized (Figure 1). The use of cognitive aids aiming to reiterate rescue plans and an intubation checklist may be useful in minimizing hazards associated with airway management under stressful conditions. It is recommended to have a “runner” in the anteroom. This allows any additional equipment to be passed in, and in addition, the runner can act as a PPE checker. An experienced team and clear “closed loop” communication will reduce the time spent in the contaminated environment. The goals of airway intervention are ensuring patient safety, reducing the spread of the infection, and limiting staff exposure.
Oxygen TherapySafetyConsiderations
High-flow nasal cannula (HFNC) therapy has been under immense scrutiny ever since the outbreak, in view of the concerns regarding aerosolization of viral particles. Governing bodies and consensus guidelines differ in their position on using HFNO during the time of intubation.18,27 Recent editorials and other sources have analyzed the available data on bioaerosol generation and dispersion.28,29 A similar risk profile was noted between standard oxygen masks and HFNO.29 Based on this, it has been suggested that HFNC prongs when applied with a superimposed surgical mask may be a reasonable practice in hypoxemic patients where potential intubation may be avoided.29 Reports from Wuhan, China, describe that HFNC-assisted fiberoptic intubation in paralyzed critically ill patients resulted in shorter intubation times and a better oxygenation profile compared to oxygenation through a standard facemask.30 Clinicians should adhere to locally agreed principles based on the transmission risk in the community in usinga HFNC technique. Consensus guidelines recommend low-flow nasal oxygen therapy (flows <5 L/min) in patients at risk of hypoxia during tracheal intubation, in an attempt to extend the apnea time.18 If a nonrebreather mask is used, flow rates should be limited to 15 L·minute−1 and a viral filter should be attached directly to the mask.31 Special facemasks with in-built viral filters such as the Tavish facemask and the HiOx nonrebreather mask are better options if available. A surgical facemask should be applied over any oxygen delivery device.
AIRWAY MANAGEMENT: GENERAL PRINCIPLES
Airway Assessment
Airway assessment is best done without removing the patient’s surgical mask if there are no overt features suggestive of difficult intubation. Patients with a history or features suggestive of difficult intubation will require more extensive assessment. The primary plan and subsequent rescue techniques should be formulated and clearly communicated to all team members including the runners in the anteroom. The need for postoperative ventilatory support should be established. A timely elective or semielective intubation will avoid the additional risks posed to staff by an emergency intubation.
Preoxygenation and GeneralAnesthesiaInduction/IntubationStrategies
Preoxygenation Considerations.
A minimum of 5 minutes preoxygenation is required. The choice of anesthetic circuit is between a circle circuit or a hand-held circuit such as the Mapleson C with the minimum necessary gas flow (≤6 LO2minute−1).13,18 A tight-fitting facemask applied with a 2-handed “vice grip” technique should be used with either option.13 A T-piece or any other semiopen anesthetic circuit without a viral filter should not be used.
It is imperative that a viral filter is fitted between the manual ventilation device and facemask to reduce circuit contamination and reduce aerosolization risks from expired gases. A heat and moisture exchange (HME) filter should be directly attached between the elbow connector and the facemask to reduce the number of connections between the mask and the filter.32 An additional HME filter should be placed at the expiratory limb of the anesthetic circuit toward the machine end. At one of the author’s institutions, viral filters are used on both the inspiratory and expiratory limbs of the circuit at the machine end; as a practice change implemented during the outbreak (Supplemental Digital Content, Figure 1, http://links.lww.com/AA/D131). This is intended to prevent erroneous placement of a single viral filter on the inspiratory limb instead of the expiratory limb.
Preoxygenation aiming for an expired O2 concentration (Eto2) of 90% is achieved with 100% O2 in with a tight mask fit with flow rates limited to 6 L·minute−1. Videolaryngoscopes (VL) should be used when available, as they have been shown to reduce the number of failed intubations.33 A recent airway simulation study showed that VL nearly doubled the mouth-mouth distance from the operator to patient compared with direct laryngoscopy.34 VL also placed the operator’s face above the line of sight to laryngeal inlet.34 A device with a disposable blade and a separate screen can reduce droplet transmission.
Anesthesia Inductionand TrachealIntubation.
A slow titrated administration of opioids and an antisialagogue is suggested before induction, taking into consideration that first time exposure to fentanyl derivatives including alfentanil can induce cough soon after administration.35 Judicious use of either rocuronium 1.5 mg·kg−1 or succinylcholine 1.5 mg·kg−1 is recommended to ensure adequate paralysis.13 Rocuronium is preferable as it can prolong the neuromuscular blockade, thereby reducing aerosol generation in comparison to the possibility of early cough response with succinylcholine.13 Ample time should be allowed before laryngoscopy to ensure complete muscle relaxation so as to avoid patient movement. Rapid sequence intubation is recommended to limit aerosol spread from bag-mask ventilation (BMV). Use of cricoid pressure may cause the operator to move closer to the patient’s airway while also precipitating a cough response secondary to its stimulating nature. Therefore, the risks and benefits of cricoid pressure must be weighed up carefully and will vary for each patient. Manual ventilation at small tidal volumes with a 2-hand mask seal is reserved only for oxygen desaturation. Assistance should be sought for bagging while utilizing as low flows as possible. For rescue BMV, further head end elevation ramping, together with the use of oropharyngeal airway with low flow rates are recommended.13
Oxygen flow should be ceased during intubation and ventilation should commence only after cuff inflation. The use of a stylet or bougie may increase droplet spread, and if required, care should be taken with their removal and subsequent disposal.32 Successful tube placement is better confirmed with end-tidal CO2. Intubation encounters from Wuhan, China, reveal that oxygen saturations did not increase immediately after intubation in critically ill patients as a result of impaired gas exchange.36 Auscultation to confirm correct endotracheal tube (ETT) placement is not advised as it is likely to be difficult with PPE in place.18 Further, it can contaminate the stethoscope and the operator.18 The ETT cuff pressure should be measured to ensure no leak when it is appropriate. If ETT suction is necessary, a closed in-line suction system should be used.37 If suction use is thought to be very likely, it may be advisable to attach this to the anesthetic circuit at the time of preoxygenation. However, the utility of this must be balanced against the need to have a simple circuit and reduce the possibility of a circuit disconnection.
Whenever there is a need to disconnect, to prevent aerosolization, the adjustable pressure limiting (APL) valve should be opened fully, fresh gas flows turned-off and positive pressure ventilation (PPV) should be ceased, and the ventilator bellow should be at end-expiration.37 Mechanical ventilation should only be restarted once the circuit is reconnected and a closed system is reestablished. Clamping the ETT during disconnections has been suggested,37 but accidental damage to the tube and the pilot balloon assembly is an unwelcome possibility. A simpler alternative is to disconnect the circuit proximal to the viral filter. If a disconnection distal to the filter at the patient end becomes inevitable (eg, attaching an in-line suction later during the procedure), it is recommended to clamp the ETT close to the tube connector over a gauze or tape. This method can preserve a decent length of the ETT in the event of an accidental damage.
If the primary intubation attempt fails, a second-generation supraglottic airway (SGA) that allows direct adult ETT insertion guided by a fiberoptic bronchoscope (eg, LMA® Protector™a, i-gel, AmbuAuraGain, Air-Q) should be preferred.38,39 Repeated intubation attempts could potentiate virus aerosolization; hence, SGA should be considered early.36 A second-generation SGA offers better seal pressure and reduces virus aerosolization during PPV if there is a need to prolong oxygenation.13 Devices with a small laryngeal lumen, for example, LMA® Supreme™b are not suitable. Although airway exchange catheter-guided tracheal intubation is an option, it increases the contamination risk as it involves multiple steps: SGA removal, ETT insertion, and subsequent airway exchange catheter removal before connecting the ventilator. Notably, for devices that allow direct placement of ETT, only a few steps are involved and the SGA can be left in situ.38,39 This process is much quicker and there is less chance of contamination. When performed in a fully paralyzed patient without PPV or suction through the bronchoscope, this procedure should not generate any aerosols.13 For urgent front of neck access, a scalpel-bougie technique as described by the Difficult Airway Society guidelines40 is recommended in view of aerosol generation with high-pressure oxygen delivery related to cannula techniques.13,18,32 Avoid any attempts to deliver oxygen above the site of tracheal puncture during a cricothyroidotomy procedure to avoid aerosolization of virus-containing fluid. A nasogastric tube (if required) should be inserted after intubation to avoid any further close contact with the patient’s airway. Sites other than the nasopharynx should be chosen for temperature monitoring to avoid further contamination risk from the upper airway.
Role of AirwayTents/AerosolBoxes/AirwayShields
During the SARS outbreak, special airway intervention and resuscitation (AIR) tents have been described to reduce droplet and aerosol transmission to health care workers. Various adaptations (aiming to reduce aerosolization) of tents, boxes with arm holes, shield, and tent combinations and a negative pressure intubating tent (where a suction port with an attached viral filter is left inside the tent) have been described. Their merits have been discussed across various online COVID-19 airway discussion forums and social media. A clear plastic sheet attached over a frame/right angle extension bar/ surgical tray, or a rectangular screen or rigid box with fixed shapes and arm holes are some of the more popular modalities. Currently, the evidence for these strategies in limiting aerosol spread is limited to benchtop studies.41 While it might offer extra protection, these interventions can add an extra layer of complexity and pose an infection risk.32 To be successful, it must be a simple system which allows good visibility, seal, free arm movements, and poses no contamination risk either during or after its use. A recent simulation study assessed the efficacy of specialist anesthetists performing intubation using both early (2 holes for the person doing intubation) and late generation (with extra holes for assistant’s hands, and for bougie insertion). The results revealed that aerosol boxes increased intubation times and pose infection risk to clinicians as a result of breaches of their PPE.42 First-hand experiences from Wuhan emphasize intubating using VL under a transparent disposable sheet.36 It should be replaced after securing the airway, but it can be left over the patient’s head, neck, and upper part of chest during the procedure.
Extubation/Emergence Strategies
Extubation may pose an even greater aerosolization risk and the same principles that guide intubation must apply to extubation. There is an additional consideration that some patients may become agitated in the periextubation period,thereby increasing the risk of droplet spread. Antiemetics are strongly recommended to prevent postprocedure droplet spread from vomiting (Figure 4). Deep extubation may help in attenuating the airway response but leaves the issue of an anesthetized patient and an unprotected airway. Recent consensus guidelines on this topic do not suggest SGA exchange as a first-line option in view of the need for a second procedure and the likelihood of airway difficulty after its insertion.18 If sugammadex reversal is used, practitioners should be aware that the sudden return of the upper airway tone has induced laryngospasm or straining on the ETT in some patients.43 Pharmacological measures such as intravenous lidocaine, opioids, and dexmedetomidine can be considered to reduce the cough response.18 Applying a head-up tilt with the operators positioned behind the head end of the patient may assist in reducing droplet exposure. A new set of masks and airway supporting devices are recommended. Extubating and transitioning to facemasks under clear plastic sheets may be a viable approach to reduce droplet/aerosol dispersion. In a recent manikin model, clear plastic sheets have been shown to reduce cough-induced droplet spread.44 An anesthetic facemask with an attached viral filter can be held over the airway as the ETT is withdrawn. To further reduce droplet spread, a soft cloth/gauze can be used to wipe any secretions from the ETT while it is being removed and disposed of. When it is appropriate, patients can be transitioned to either a standard facemask or low-flow nasal cannula. Facemasks with in-built viral filters are good options if available. As a minimum, a surgical mask (preferable with ear loops) should be placed over the supplemental oxygen delivery device. It is prudent not to attempt use of any unfamiliar techniques and pharmacological agents.
Figure 4.
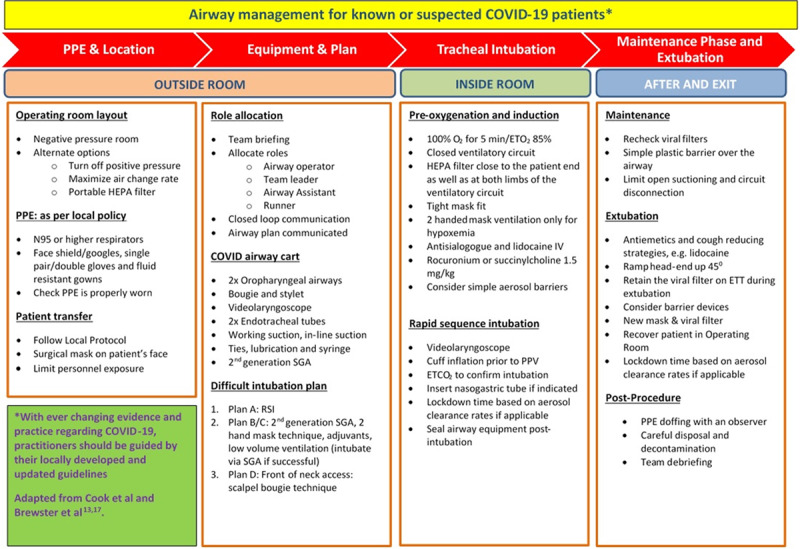
Airway management for known or suspected COVID-19 patients. COVID indicates Coronavirus Disease 2019; Etco2, xxx; Eto2, expired O2 concentration; ETT, endotracheal tube; HEPA, high-efficiency particulate air; IV, xxx; PPE, personal protective equipment; PPV, positive pressure ventilation; RSI, xxx; SGA, supraglottic airway.
AIRWAY MANAGEMENT IN SPECIFIC SCENARIOS
Awake Intubation
Awake intubation techniques carry a high risk for aerosolization and should be avoided except for specific situations such as a neck abscess compromising the airway. Antisialagogue and anxiolytic/sedative (eg, dexmedetomidine) administration may help reduce restlessness and droplet generation. Airway topicalization using nebulizers or aerosol-generating atomizers such as the DeVilbiss is not recommended.45 Mucosal atomizers should be used instead.45 Local anesthetic impregnated swabs and cotton pledges and nerve blocks may be preferable when used by experienced operators.45 Single-use fiberoptic bronchoscopes with a separate screen also help reduce contamination.45 Application of an endoscopy facemask can reduce the exposure of the surrounding to the patient.46 Use of a smaller size ETT might reduce arytenoid impingement and subsequent coughing.45 VL-assisted awake intubation can also be considered if appropriate.45 If awake intubation fails, an awake tracheostomy under local anesthetic may be the only option taking into account the risk of aerosolization.45
Tracheostomy
Tay et al47 have recently reviewed perioperative considerations of tracheostomy during the COVID-19 outbreak. Drawing on the experience gained during the 2003 SARS outbreak (23 procedures of open tracheostomies) combined with their own understanding, the authors describe certain considerations when planning for an open tracheostomy.47 First, enhanced PPE measures ranging from face shields over N95 masks to powered air-purifying respirators (PAPRs) were used in those procedures across 5 institutions and all members of the surgical team remained free of infection. Second, the location of the procedure should be carefully evaluated. During the SARS outbreak, most open tracheostomies were performed at the bedside in negative pressure rooms in intensive care units (ICU). This avoided unnecessary transport and the associated risk of ventilatory circuit disconnections. Early clinical judgment in identifying patients at risk and placing them in rooms that are tailor-made or modified to handle aerosol-generating procedures can minimize unnecessary transfers within the ICU. If the procedure needs to be performed in the operating room, it should be done in a dedicated negative pressure theatre suite, if available. Third, aerosol exposure should be minimized by ensuring adequate paralysis, ceasing mechanical ventilation before entering the trachea and limiting the use of suctioning during the procedure. A closed in-line suction unit is recommended. Finally, it is the authors’ opinion is that percutaneous tracheostomy involves more extensive airway manipulation, thereby increasing infectious risk, and thus open tracheostomy that allows for faster entrance into the trachea is the preferred technique.
AIRWAY MANAGEMENT OUTSIDE THE OPERATING ROOM: INTERVENTIONAL SUITES
Interventional PulmonologyProcedures
Bronchoscopy is one of the highest risk procedures for aerosol generation.48 The American Society of Bronchology and interventional Pulmonology (AABIP) has recently issued a statement on bronchoscopy during the COVID-19 outbreak.49 The AABIP strongly recommends deferring elective interventions in suspected or confirmed cases of COVID-19 until full recovery and viral clearance. The indications for these elective interventions and in the setting of COVID-19 diagnosis are listed in their statement. Urgent bronchoscopy (rigid or flexible) in known or suspected cases is indicated only for life-threatening indications such as massive hemoptysis. Other indications include investigation of severe airway stenosis or identifying the etiology of an endobronchial obstruction.49 Clearly, the goal is to minimize aerosol generation and droplet dispersion during and after the intervention. The procedure should be performed only in negative pressure rooms. An antisialagogue along with an anxiolytic may be helpful. A major concern with any anesthetic technique is the leak around the oropharyngeal cavity and the need for frequent suctioning. A general endotracheal tube anesthesia (GETA) with a large-sized ETT and neuromuscular blockade may be the best option. Closed in-line suctioning should be used. Both in-line suction and the bronchoscopic port/adapter should be connected at the same time to avoid multiple exposures. Ventilation should be ceased; fresh gas flows should be turned-off and the APL valve should be fully opened during disconnections and suctioning. Higher flow rates to compensate leaks should be avoided if possible. Adequate lubricant applied to the bronchoscope port can often seal a leak. A procedure completed quickly with minimal suctioning attempts will result in less overall risk of leakage. Close communication with the pulmonologist is essential to reduce the contamination risk. For patients not requiring postprocedure ventilatory support, dexmedetomidine, opioids, and intravenous lidocaine are suitable options for cough suppression during extubation.50,51
For nonurgent procedures in unsuspected patients, the prevalence rate of COVID-19 infections should be considered in deciding the best management strategies.49 In regions with a high prevalence, proceeding in asymptomatic patients should be in designated isolation rooms with full precautions for aerosol generation.49 The aforementioned principles apply for bronchoscopy in the ICU setting as well.
Interventional Gastroenterology
Upper gastrointestinal endoscopies are also deemed aerosol-generating interventions, and there are suggestions that the virus may be present in fecal and gastrointestinal secretions.48,52 Aerosolization may happen during scope insertion into the pharynx as well as during manipulation of instruments through the endoscope’s channel.53 Esophageal intubation with the endoscope often requires positive gas insufflation, which is also a risk factor for aerosol generation.54 Only urgent or semiurgent interventions are likely to be performed during the COVID-19 pandemic as per the position statements from governing organizations.55
In known or suspected cases, GETA should be the first choice. Practitioners from Wuhan have described using wet gauze to cover the area around the nose and mouth while intubating confirmed cases to reduce droplet spread.36 This technique can be adapted during endoscopy whereby a wet soft cloth/gauze could be used to gently seal the space between the endoscope and the bite block without hindering the proximal manipulation of the endoscope. Ensuring adequate neuromuscular paralysis and administration of an antisialagogue and antiemetics can help reduce droplet spread and possible virus aerosolization. Other aerosol limiting considerations include esophageal intubation without gas insufflation (if feasible), sealing the biopsy channel with a soft cloth or air suctioning56 while removing instruments and stopping gas insufflation as the endoscope is withdrawn from the oropharynx.
Patients without suspicion of COVID-19 may present for interventions where tracheal intubation is not indicated, for example, biliary sepsis/obstruction for an endoscopic retrograde cholangiopancreatography without aspiration/difficult airway risk. In such instances, the prevalence rate in the community and opinion from local infectious disease specialists should be considered in choosing the appropriate technique. Sedation techniques with adequate airborne PPE precautions may be an appropriate choice for less complex cases.
Interventional CardiologyProcedures
Cardiology organizations have issued statements regarding deferring all nonurgent interventions.57 Transesophageal echocardiography(TEE) has a high risk of aerosolization and droplet spread similar to upper gastrointestinal endoscopic procedures.57 It is indicated only as an urgent in-patient diagnosis that will alter clinical management (eg, high suspicion of endocarditis).57 If warranted for known or suspected cases, the procedure is best conducted under GETA either within the negative pressure rooms in ICU or at a dedicated operating room. GETA is a safe option not only to minimize aerosol generation from TEE but also in having a secured airway in the event of an unforeseen cardiopulmonary resuscitation (CPR)and intubation requirement.58 A small number of urgent transcatheter cardiac valve replacements, device closure interventions, and percutaneous coronary interventions are also likely to be encountered. If the diagnosis is unknown, a vigilant sedation technique with full airborne PPE may be an appropriate choice. If the intervention suite is not conducive for airway interventions, it is best to secure the airway in a specifically designated operating room if located in close proximity. This may not be feasible, and airway interventions may need to be performed in the suite, with adaptations to existing workflow as per local guidelines.
Diagnostic and InterventionalRadiology
An anteroom may need to be established in the radiology suite. For known or suspected cases, GETA should be an appropriate choice. Prolonged interventions may benefit from judicious use of muscle relaxants. For urgent cases where the diagnosis is unknown, sedation techniques can be carefully used under airborne PPE precautions. Existing workflows may need to be utilized, for example, to avoid lengthy intrahospital transfers, and altered as per local recommendations.
Electroconvulsive Therapy
BMV and cough during electroconvulsive therapy (ECT) can potentially disperse droplets and aerosols. The procedure should not be performed on confirmed cases. New treatment or an interrupted treatment cycle should only be commenced if the patient becomes asymptomatic after a waiting period of 14 days and a subsequent negative test result (as per institutional guidelines), and if ECT is deemed as a life-saving procedure.59 Adequate preoxygenation and apneic oxygenation using nasal prongs are options to reduce mask ventilation. Hyperventilation using BMV should be avoided unless other means to improve seizure quality are ineffective. If hyperventilation is warranted, a SGA can be considered instead of BMV.59 Options to reduce periprocedural secretions and cough include intravenous glycopyrrolate, lidocaine, and remifentanil.59
OTHER DISCIPLINES/SCENARIOS
Obstetric Anesthesia
This topic has recently been reviewed in great detail.60 Neuraxial techniques avoid general anesthesia and are recommended. Both early epidural analgesia and spinal analgesia for lower segment cesarean sections are considered safe in COVID-19 patients. Currently, information is limited regarding cleaning, filtering, and possible aerosolization with the use of nitrous oxide (as per theSociety of Obstetric Anesthesia and Perinatology [SOAP]).61 Based on this, SOAP suggests that individual labor units should review the risks and benefits and consider suspending the use of nitrous oxide.61 Cesarean deliveries should be performed in operating rooms modified for COVID-19 patients. If general anesthesia is warranted, general principles of airway management applicable for a known COVID-19 case should be followed.60 After delivery, the baby should be temporarily separated from the mother.62 Currently, there is a lack of evidence to support vertical transmission occurring63; however, the theoretical risk of transmission after birth via respiratory droplets is feasible. Simulating the workflows and creating dedicated kits with equipment and drugs required for labor analgesia and cesarean deliveries are recommended to prevent contamination.37
Cardiopulmonary Resuscitation
CPR involves chest compressions, mask ventilation, suctioning, and intubation, all of which are aerosol generating.8 PPE applicable to aerosol-generating procedures should be worn, and minimal number of personnel should be involved.64 Chest compression-only CPR should be commenced initially.64 Mouth-to-mouth ventilation and expired air resuscitation masks are not recommended.64 BMV should be minimized with apneic oxygenation techniques used instead if feasible.10 It is recommended to secure the airway (tracheal intubation) early in resuscitation to minimize aerosol generation. Existing supplemental oxygen delivering devices should be left in place. If they are not in place, a facemask applied beneath a surgical mask has been recommended.64 Chest compressions should be ceased during intubation or SGA insertion.10,12 Use of mechanical external chest compression devices such as the Autopulse (Zoll Medical, Chelmsford, MA) or LUCAS Chest Compression System (Physio-Control, Redmond, VA) may help reduce the number of health care workers in proximity to the patient.10,65 Familiarity, accessibility, and postcare disinfection are limiting factors of these devices.
CONCLUSIONS
SARS-CoV-2 is likely to remain in many communities for the foreseeable future. Although the daily new cases had reduced in many countries, some regions are witnessing a second wave of infection. Airway management is one of the highest risk procedures for aerosol and droplet dispersion. Adhering to strict PPE practice is an essential approach in mitigating the infection risk. GETA should be the preferred option for known or suspected COVID-19 cases, and awake intubation should be avoided unless absolutely indicated. Extubation strategies should be planned in advance to attenuate the emergence response of airway irritation and agitation. Monitored anesthesia care with sedation may be an appropriate option if carefully performed. For interventional procedures performed outside the operating room setting,clear communication, appreciating the implications, and advanced planning will improve the outcome. Each and every adaptation based on available resources to limit aerosol generation should be followed throughout the periprocedural period. National/society guidelines have been published to guide safe approaches to airway management. However, the locally adapted guidelines that are updated regularly by a committed team are often the best sources that clinicians should rely on. Efforts tailored to the local environment will not only help overcome the current COVID-19 crisis but also help in the events of similar outbreaks in the future.
ACKNOWLEDGMENTS
We thank Dr Adrian Chung, Department of Gastroenterology and Hepatology, Flinders Medical Centre, South Australia, for his valuable inputs toward the section interventional gastroenterology.
DISCLOSURES
Name: Venkatesan Thiruvenkatarajan, MD, DA, DNB, FANZCA.
Contribution: This author helped in review conception, literature review, drafting and revising the manuscript.
Name: David T. Wong, MD.
Contribution: This author helped in manuscript writing, critical review, and revision.
Name: Harikrishnan Kothandan, DNB, DA, FANZCA.
Contribution: This author helped in manuscript writing and critical review.
Name: Vimal Sekhar, MBBS, MClinSci.
Contribution: This author helped in manuscript writing, review, revision.
Name: Sanjib Das Adhikary, MD.
Contribution: This author helped in manuscript writing and critical review.
Name: John Currie, MBChB, FFARCSI.
Contribution: This author helped in critical review, editing, and expert opinion.
Name: Roelof Van Wijk, MD, PhD, FANZCA, FFPMANZCA.
Contribution: This author helped in critical review, editing, and expert opinion.
This manuscript was handled by: Narasimhan Jagannathan, MD, MBA.
Supplementary Material
FOOTNOTES
GLOSSARY
- 3D
- 3-dimensional
- AABIP
- American Society of Bronchology and Interventional Pulmonology
- AGP
- aerosol-generating procedure
- AIIR
- airborne infection isolation room
- AIR
- airway intervention and resuscitation
- APL
- adjustable pressure limiting
- BiPAP
- xxx
- BMV
- bag-mask ventilation
- COVID-19
- Coronavirus Disease 2019
- CPAP
- xxx
- CPR
- cardiopulmonary resuscitation
- ECT
- electroconvulsive therapy
- Etco2
- xxx
- Eto2
- expired O2 concentration
- ETT
- endotracheal tube
- GETA
- general endotracheal tube anesthesia
- HEPA
- high-efficiency particulate air
- HFNC
- high-flow nasal cannula
- HFNO
- xxx
- HME
- heat and moisture exchange
- ICU
- intensive care unit
- IV
- xxx
- PAPRs
- powered air-purifying respirators
- PPE
- personal protective equipment
- PPV
- positive pressure ventilation
- RSI
- xxx
- SARS-CoV
- severe acute respiratory syndrome coronavirus
- SGA
- supraglottic airway
- SOAP
- Society of Obstetric Anesthesia and Perinatology
- TEE
- transesophageal echocardiography
- VL
- videolaryngoscopes
Funding: None.
The authors declare no conflicts of interest.
Abbreviations: 3D, 3-dimensional; HEPA, high-efficiency particulate air; PPE, personal protective equipment.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website.
Reprints will not be available from the authors.
REFERENCES
- 1.Paterlini M. On the front lines of coronavirus: the Italian response to COVID-19. BMJ. 2020;368:m1065. [DOI] [PubMed] [Google Scholar]
- 2.Bowdle A, Munoz-Price LS. Preventing infectionof patientsand healthcare workers should bethe new normalin the eraof novel coronavirus epidemics. Anesthesiology. 2020;132:1292–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Q, Guan X, Wu P. Early transmission dynamicsin Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scales DC, Green K, Chan AK. Illness in intensive care staff after brief exposure to severe acute respiratory syndrome. Emerg Infect Dis. 2003;9:1205–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loeb M, McGeer A, Henry B. SARS among critical care nurses, Toronto. Emerg Infect Dis. 2004;10:251–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Doremalen N, Bushmaker T, Morris DH. Aerosol and surface stabilityof SARS-CoV-2 as comparedwith SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tran K, Cimon K, Severn M, Pessoa-Silva CL, Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One. 2012;7:e35797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenland JR, Michelow MD, Wang L, London MJ. COVID-19 infection: implicationsfor perioperativeand critical care physicians. Anesthesiology. 2020;132:1346–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang G, Chan AKM. Perioperative management of suspected/ confirmed cases of COVID-19. World Federation of Societies of Anaesthesiologists; Available at: https://www.wfsahq.org/components/com_virtual_library/media/1c4ec5c64b9aaacf7c47f76a61fb6edc-atow-422-01.pdf. Published April 6, 2020. Accessed April 7, 2020. [Google Scholar]
- 11.Rajgor DD, Lee MH, Archuleta S, et al. The many estimates of the COVID-19 case fatality rate. Lancet Infect Dis. 2020. Published ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peng PWH, Ho PL, Hota SS. Outbreak of a new coronavirus: what anaesthetists should know. Br J Anaesth. 2020;124:497–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brewster DJ, Chrimes N, Do TB. Consensus statement: Safe Airway Society principles of airway management and tracheal intubation specific to the COVID-19 adult patient group. Med J Aust. 2020;212:472–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sehulster L, Chinn RY; CDC; HICPAC CDC; HICPAC. Guidelines for environmental infection control in health-care facilities. Recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep. 2003;52:1–42. [PubMed] [Google Scholar]
- 15.Bajwa SJ, Sarna R, Bawa C, et al. Peri-operative and critical care concerns in coronavirus pandemic. Indian J Anaesth 2020;64:267–274.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coccolini F, Perrone G, Chiarugi M, et al. Surgery in COVID-19 patients: operational directives. World J Emerg Surg 2020;15:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. Interim infection prevention and control recommendations for patient swith suspectedor confirmed coronavirus disease 2019 (COVID-19) in healthcare settings. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html. Published April 13, 2020. Accessed April 30,2020
- 18.Cook TM, El-Boghdadly K, McGuire B, et al. Consensus guidelines for managing the airway in patients with COVID-19: guidelinesfrom the Difficult Airway Society, the Association of Anaesthetists the Intensive Care Society, the Faculty of Intensive Care Medicine and the Royal College of Anaesthetists. Anaesthesia. 202075785–799.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ranney ML, Griffeth V, Jha AK. Critical supply shortages—the need for ventilators and personal protective equipment during the Covid-19 pandemic. New Eng J Med. 2020;382:e41. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. Strategies to optimizethe supplyof PPE and equipment. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/index.html. Published April 22, 2020. Accessed April 30,2020.
- 21.Centers for Disease Control and Prevention. Recommended guidance for extended use and limited reuse of N95 filtering face piece respirators in healthcare settings. Available at: https://www.cdc.gov/niosh/topics/hcwcontrols/recommendedguidanceextuse.html. Published March 27, 2020. Accessed April 30,2020.
- 22.American Society of Anesthesiologists. Minimizing medication waste during the Coronavirus-19 global pandemic. Available at: https://www.asahq.org/about-asa/newsroom/news-releases/2020/04/minimizing-medication-waste-during-the-coronavirus-19-global-pandemic. Published April 22, 2020. Accessed April 30, 2020.
- 23.Hall R, Chisholm R, Cheng D, Murphy M, Campbell D. Drug shortages in anesthesia and perioperative medicine: Canada needs a better supply system. Can J Anaesth. 2012;59:629–635. [DOI] [PubMed] [Google Scholar]
- 24.Livingston E, Desai A, Berkwits M. Sourcing personal protective equipment duringthe COVID-19 pandemic. JAMA. 2020. Published ahead of print. [DOI] [PubMed] [Google Scholar]
- 25.Ip V, Özelsel TJP, Sondekoppam RV, et al. COVID-19 pandemic: greater protectionfor health care providersin the hospital“Hot Zones”?. Anesth Analg. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Australian Government Department of Health: Therapeutic Goods Administration. Information for clinicians on ventilators and alternative strategies when in short supply during COVID-19. Available at: https://www.tga.gov.au/information-clinicians-ventilators-and-alternative-strategies-when-short-supply-during-covid-19. Published April 17, 2020. Accessed April 30,2020.
- 27.World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: interim guidance. Available at: https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Published March 13, 2020. Accessed April 30,2020.
- 28.Lyons C, Callaghan M. The use of high-flow nasal oxygen in COVID-19. Anaesthesia. 2020;75:843–847. [DOI] [PubMed] [Google Scholar]
- 29.Li J, Fink JB, Ehrmann S. High-flow nasal cannula for COVID-19 patients: low risk of bio-aerosol dispersion. Eur Respir J. 2020552000892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu CN, Xia LZ, Li KH, et al. High-flow nasal-oxygenation-assisted fibreoptic tracheal intubation in critically ill patients with COVID-19 pneumonia: a prospective randomised controlled trial. Br J Anaesth. 2020. Published ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Australian and New Zealand Intensive Care Society. ANZICS COVID-19 guidelines. Melbourne: ANZICS; Available at: https://www.anzics.com.au/wp-content/uploads/2020/03/ANZICS-COVID-19-Guidelines-Version-1.pdf. 2020. Accessed April 5, 2020. [Google Scholar]
- 32.Australian Society of Anaesthetists. Anaesthesia and caring for patients during the COVID-19 outbreak. Melbourne: ASA; Available at: https://www.asa.org.au/wordpress/wp-content/uploads/News/eNews/covid-19/ASA-summary-of-anaesthesia-management.pdf. Published March 2020. Accessed April 5, 2020. [Google Scholar]
- 33.Lewis S, Butler A, Parker J, et al. Videolaryngoscopy versus direct laryngoscopy for adult patients requiring tracheal intubation: a Cochrane systematic review. Br J Anaesth. 2020;119:369–383.. [DOI] [PubMed] [Google Scholar]
- 34.Hall D, Steel A, Heij R, et al. Videolaryngoscopy increases ‘mouth-to-mouth’ distance compared with direct laryngoscopy. Anaesthesia. 202075822–823.. [DOI] [PubMed] [Google Scholar]
- 35.Cho HB, Kwak HJ, Park SY, Kim JY. Comparison of the incidence and severity of cough after alfentanil and remifentanil injection. Acta Anaesthesiol Scand. 2010;54:717–720. [DOI] [PubMed] [Google Scholar]
- 36.Luo M, Cao S, Wei L, et al. Precautions for intubating patientswith COVID-19. Anesthesiology. 20201321616–1618.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong J, Goh QY, Tan Z. Preparing for a COVID-19 pandemic: a review of operating room outbreak response measures in a large tertiary hospital in Singapore. Can J Anaesth. 2020;67:732–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wong DT, Yang JJ, Mak HY, et al. Use of intubation introducers through a supraglottic airway to facilitate tracheal intubation; a brief review. Can J Anaesthes. 2012;59:704–715.. [DOI] [PubMed] [Google Scholar]
- 39.Wong DT, Wang J, Venkatraghavan L. Awake bronchoscopic intubation through an air-Q® with the application of BIPAP. Can J Anaesth. 2012;59:915–916. [DOI] [PubMed] [Google Scholar]
- 40.Frerk C, Mitchell VS, McNarry AF; Difficult Airway Society intubation guidelines working group. Difficult Airway Society 2015 guidelines for management of unanticipated difficult intubation in adults. Br J Anaesth. 2015;115:827–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Canelli R, Connor CW, Gonzalez M, Nozari A, Ortega R. Barrier enclosureduring endotracheal intubation. N Engl J Med. 2020;382:1957–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Begley JL, Lavery KE, Nickson CP, et al. The aerosol box for intubation in COVID-19 patients: an in-situ simulation crossover study. Anaesthesia. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu TS, Tseng WC, Lai HC, Huang YH, Wu ZF. Sugammadex and laryngospasm. J Clin Anesth. 2019;56:52. [DOI] [PubMed] [Google Scholar]
- 44.Matava CT, Yu J, Denning S. Clear plastic drapes may be effective at limiting aerosolization and droplet spray during extubation: implications for COVID-19. Can J Anaesth. 202067902–904.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sorbello M, El-Boghdadly K, Di Giacinto I, et al. The Italian coronavirus disease 2019 outbreak: recommendations from clinical practice. Anaesthesia. 2020. [DOI] [PubMed] [Google Scholar]
- 46.Zuo MZ, Huang YG, Ma WH, et al. Expert recommendationsfor tracheal intubationin criticallyill patientswith novel coronavirus disease2019. Chin Med Sci J. 2020. [Google Scholar]
- 47.Tay JK, Khoo ML, Loh WS. Surgical considerationsfor tracheostomy duringthe COVID-19 pandemic: lessons learnedfrom the severe acute respiratory syndrome outbreak. JAMA Otolaryngol Head Neck Surg. 2020. [DOI] [PubMed] [Google Scholar]
- 48.Wax RS, Christian MD. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can J Anaesth. 2020:1–9.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wahidi MM, Lamb C, Murgu S, et al. American Association for Bronchology and Interventional Pulmonology (AABIP) statementon the useof bronchoscopyand respiratory specimen collectionin patientswith suspectedor confirmedCOVID-19 infection. J Bronch ology Interv Pulmonol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Clivio S, Putzu A, Tramèr MR. Intravenous lidocainefor the preventionof cough: systematic reviewand meta-analysis of randomized controlled trials. Anesth Analg. 2019;129:1249–1255. [DOI] [PubMed] [Google Scholar]
- 51.Salim B, Rashid S, Ali MA, Raza A, Khan FA. Effect of pharmacological agents administeredfor attenuatingthe extubation responseon the qualityof extubation: a systematic review. Cureus. 2019;11:e6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.American College of Gastroenterology. COVID-19 clinical insights for our community of gastroenterologists and gastroenterology care providers. American College of Gastroenterology Website; Available at: https://gi.org/2020/03/15/joint-gi-society-message-on-covid-19/. Published March 2020. Accessed April 5, 2020. [Google Scholar]
- 53.Sultan S, Lim JK, Altayar O, et al. AGA institute rapid recommendationsfor gastrointestinal procedures duringthe COVID-19 pandemic. Gastroenterology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chiu PWY, Ng SC, Inoue H. Practice of endoscopy during COVID-19 pandemic: position statements of the Asian Pacific Society for Digestive Endoscopy (APSDE-COVID statements). Gut. 2020;69:991–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gastroenterological Society of Australia. Considerations of Australian endoscopy units during the Covid-19 pandemic. Available at: https://www.gesa.org.au/public/13/files/COVID-19/COVID-19%20GESA%20ENDOSCOPY%20STATEMENT%2020Mar20_2.pdf. Published March 20, 2020. Accessed April 5, 2020.
- 56.Vavricka SR, Tutuian R, Imhof A. Air suctioning during colon biopsy forceps removal reduces bacterial air contamination in the endoscopy suite. Endoscopy. 2010;42:736–741. [DOI] [PubMed] [Google Scholar]
- 57.Wahi S, Thomas L. CSANZ imaging council position statement on echocardiography services during the COVID-19 pandemic. Cardiac Society of Australia and New Zealand; Available at: https://www.csanz.edu.au/wp-content/uploads/2020/03/CSANZ-Imaging-Council-Position-Statement-on-Echocardiography-Services-During-the-COVID-19-Pandemic.pdf. Published March 30, 2020. Accessed April 5, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lo S, Yong AS, Sinhal A. Consensus guidelines for interventional cardiology services delivery during COVID-19 pandemic in Australia and New Zealand. Cardiac Society of Australia and New Zealand; Available at: https://www.csanz.edu.au/wp content/uploads/2020/03/CSANZ_CONSENSUS_GUIDELINES_FOR_INVASIVE_CARDIOLOGY_SERVICES_DELIVERY_DURING_COVID_PANDEMIC_29-March_2020.pdf. Published March 29, 2020. Accessed April 5, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Flexman AM, Abcejo A, Avitisian R, et al. Neuroanesthesia practice duringthe COVID-19 pandemic: recommendationsfrom Society for Neuroscience in Anesthesiology & Critical Care (SNACC). J Neurosurg Anesthesiol. 202032 Published ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bauer M, Bernstein K, Dinges E, et al. Obstetric anesthesia duringthe COVID-19 pandemic. Anesth Analg. 2020. Published Ahead of Print [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Society for Obstetric Anesthesia and Perinatology. Labor and delivery COVID-19 considerations. Available at: https://s3.amazonaws.com/cdn.smfm.org/media/2277/SMFM-SOAP_COVID_LD_Considerations_3-27-20_(final)_PDF.pdf. Published March 27, 2020. Accessed April 9, 2020.
- 62.Favre G, Pomar L, Qi X, et al. Guidelines for pregnant women with suspected SARS-CoV-2 infection. Lancet Infect Dis. 202020652–653.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen H, Guo J, Wang C. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Resuscitation Council UK. Statement on COVID-19 in relation to CPR and resuscitation in healthcare settings. Available at: https://www.resus.org.uk/media/statements/resuscitation-council-uk-statements-on-covid19-coronavirus-cpr-and-resuscitation/covid-healthcare/. Published March 2020. Accessed April 5, 2020.
- 65.Poole K, Couper K, Smyth MA, Yeung J, Perkins GD. Mechanical CPR: who? When? How? Crit Care. 2018;22:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


