Abstract
Due to the lack of prospective, randomized, controlled clinical studies on inflammation and cardiovascular involvement, the exact mechanism of cardiac injury among patients with Coronavirus Disease 2019 (COVID-19) still remains uncertain. It was demonstrated that there is a high and significantly positive linear correlation between troponin T and plasma high-sensitivity C-reactive protein levels, biomarkers of cardiac injury and systemic inflammation, respectively. Cardiac injury and inflammation is a relatively common association among patients hospitalized with COVID-19, and it is related to higher risk of in-hospital mortality. In our literature search, we identified several potential mechanisms of myocardial tissue damage, namely, coronavirus-associated acute myocarditis, angiotensin-converting enzyme 2 receptor binding affinity to the virus Spike protein, increased cytokine secretion, and hypoxia-induced cardiac myocyte apoptosis. Elucidation of the disease pathogenesis and prospective histopathological studies are crucial for future proper treatment in case of renewed outbreaks. Of interest is that with hundred of thousands of bodies available for autopsy studies, no prospective investigation has been reported so far. Strong efforts and continued research of the cardiovascular complications and identification of risk factors for poor prognosis in COVID-19 are steadily needed. The high morbidity and mortality of COVID-19, its monumental economic burden and social impact, the despair of a new pandemic outbreak, and the thread of potential utilization of novel severe acute respiratory syndrome coronavirus 2 as biologic weapons make it a preponderant necessity to better comprehend the therapeutic management of this lethal disease. Emerging as an acute infectious disease, COVID-19 may become a chronic epidemic because of genetic recombination. Therefore, we should be ready for the reemergence of COVID-19 or other coronaviruses.
Keywords: biomarkers, cardiac injury, COVID-19, inflammation, mortality
Since the beginning of this year 2020, several studies regarding the epidemiological, physiopathological, and clinical aspects of novel Coronavirus Disease 2019 (COVID-19) have been published in rapid manner. This has greatly contributed to a fast understanding of this novel disease, and rapidly helped physicians, and related-health care workers and policy makers for a better comprehension of COVID-19. This novel disease is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). To date May 5, 2020, this disease has resulted in considerable morbidity and mortality worldwide with 3,679,499 laboratory-confirmed cases and 254,199 deaths. There is increasing evidence of higher mortality risk associated to the presence of cardiovascular involvement in patients with COVID-19.1–5
The novel coronavirus SARS-CoV-2 appears to be easily transmittable, more contagious, with greater infectivity, and somehow similar or higher morbidity and mortality depending on the area considered, as compared with severe acute respiratory syndrome coronavirus (SARS) and Middle East respiratory syndrome coronavirus (MERS).4–9 Until now, there has been a rapidly increasing incidence of patients with SARS-CoV-2 who have developed severe pneumonia, pulmonary edema, acute respiratory distress syndrome, cardiovascular compromise, or multiple organ failure and death. Cardiovascular diseases (CVD) are still the number one killer in the world, and represent the leading noncommunicable epidemic. Since numerous patients with CVD are using angiotensin-converting enzyme inhibitors (ACEI) or angiotensin receptor blockade (ARB) drug agents, clinical studies may be necessary to explore the potential associations of these pharmacological drugs with the susceptibility and prognosis of COVID-19. Despite the theoretical concerns and physiological uncertainties concerning the effect of ACE inhibitors and ARB on angiotensin-converting enzyme 2 (ACE2), ACE inhibitors and ARB should be continued in patients with COVID-19 in otherwise stable condition. This is a position supported by multiple medical and scientific societies that needs to be further explored by randomized clinical trials to search for the potential associations of these pharmacological drugs with the susceptibility and prognosis of COVID-19.
The exact mechanism of cardiac injury among patients with COVID-19 still remains uncertain. However, different possible mechanisms manifested in recent studies can be elaborated.10–19 Nonischemic events and ischemic myocardial involvement are the 2 main pathophysiological mechanisms of acute cardiac injury in COVID-19 patients.3 It is believed that nonischemic myocardial injury is predominant and is secondary to multiple mechanisms that will be analyzed in this article. Huang et al1 reported recently that 12% of patients had COVID-19–associated acute cardiac injury manifesting as myocardial inflammation with troponin I (TnI) elevation and decreased left ventricular systolic function. Wang et al2 recently reported their findings on 138 patients hospitalized with COVID-19. They found that 7% of their patients developed acute cardiac injury, and those patients in the intensive care unit were more likely to have cardiac alterations (22%) than those non-ICU patients. Cardiac arrhythmias were seen in 17%. Patient with known coronary artery disease and heart failure patients were at higher risk than others. There was higher mortality when COVID-19 was associated with acute myocarditis, acute myocardial infarction, and rapid-onset heart failure.1 This observation clearly suggests that cardiovascular involvement and especially cardiac injury is possibly associated with the morbid clinical outcomes of COVID-19.
Therefore, to assess the potential mechanisms of cardiac injury and its association with inflammation in COVID-19 patients, we aim to analyze the effect of different serum biomarkers of inflammation and cardiac involvement on the incidence of clinical outcomes and mortality to delineate their importance in guiding the therapeutic management for better cardiovascular outcome especially in hospitalized patients with COVID-19.
MATERIALS AND METHODS
We conducted an electronic search of PubMed, MEDLINE, Web of Science, and SCOPUS to identify journal articles published in English from December 2019 to April 2020, using the Medical Subject Heading or text words “coronavirus disease 2019” “COVID-19”, or “SARS-CoV-2” or “cardiac injury” or “biomarkers” or “inflammation and cardiac involvement” (Fig. 1). We search for articles that reported on observational studies or clinical trials promoting any relation between cardiac involvement, inflammation, and COVID-19. We considered articles eligible for initial inclusion if they focused on this subject. Citations of relevant articles were reviewed to capture additional studies. We also manually searched the reference of previous reviews and all records that received a full-text review. Few additional articles before our search years were included if they were referenced in existing articles and included pertinent important data for this present article. No randomized trials neither interventional studies, were available at this time in this investigation, hence, observational studies, long-term prospective cohort studies, case-control, or cross-sectional studies were also included in this article. To be eligible for inclusion, studies had to be written in English, be published in a peer-reviewed journal, provides outcome data on cardiac injury, inflammation, and COVID-19. This study was in accordance with the ethical standards of our institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The included studies were first categorized by whether the study compared clinical outcomes between cardiac involvement and inflammation in COVID-19 patients. After the initial search, the authors separately screened all abstracts based on the eligibility criteria. Any abstracts or articles for which there was disagreement or uncertainty were reviewed again and discussed until consensus was reached.
FIGURE 1.
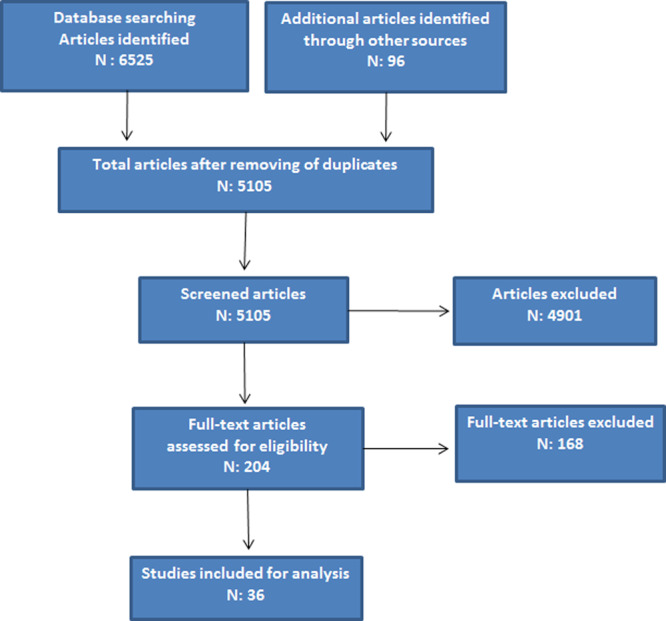
Flow diagram of selected research studies according to the database searching.
MECHANISMS OF CARDIAC INJURY IN PATIENTS WITH COVID-19
Due to the lack of prospective, randomized, controlled clinical studies on inflammation and cardiac involvement, the exact mechanism of cardiac injury among patients with COVID-19 still remains uncertain. However, different possible mechanisms manifested in recent clinical and observational studies can be elaborated and further discussed (Table 1).10–16
TABLE 1.
Potential Factors and Mechanisms for Cardiac Injury in COVID-19
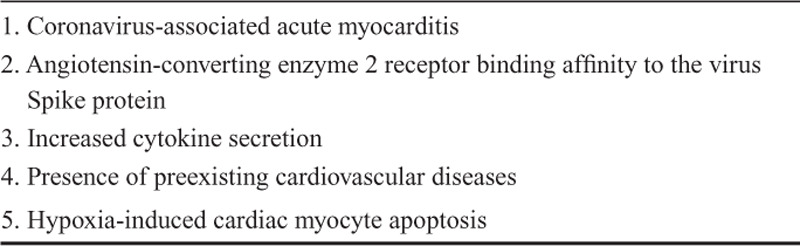
Coronavirus-associated Acute Myocarditis
Alhogbani11 described the evidence from a coronavirus-associated acute myocarditis, manifested as myocardial edema and acute myocardial injury of the apical and lateral walls of the left ventricle. This regional myocardial injury may result from direct viral myocardial infection and inflammation. However, according to a recent pathological study performed by Xu et al,17 there was scarce interstitial mononuclear inflammatory infiltrates in the myocardial tissue without substantial cardiac muscle tissue damage in their patient with COVID-19, suggesting that the coronavirus might not directly impair the heart. Nevertheless, this is just their proposal based on the pathological findings of only 1 patient who acquired COVID-19 in Wuhan. On the other hand, in the study performed by Guo et al,5 plasma troponin T levels were positively linear correlated in a significant manner with plasma high-sensitivity C-reactive protein (CRP) levels, indicating that myocardial injury may be closely associated with inflammatory pathogenesis during the progress of disease. Considering that most of the patients were elderly people with a probable association of coronary heart disease, whether the myocardial injury was directly associated with SARS-CoV-2 infection is still unclear. Direct evidence demonstrating that SARS-CoV-2 infects the myocardial tissue or pericardial effusion liquid in patients with COVID-19 is currently lacking. Of interest is that with hundred of thousands of bodies available for autopsy studies, no prospective investigation has been reported so far. Elucidation of the disease pathogenesis and prospective histopathological studies are crucial for future proper treatment in case of renewed outbreaks.
Angiotensin-converting Enzyme 2
Another plausible mechanism involves the ACE2. The latter is a human cell receptor with a strong binding affinity to the virus Spike protein of SARS-CoV-2. Since ACE2 is also highly expressed in the heart, it is reasonable to hypothesize that COVID-19–induced cardiac injury might be mediated by ACE2.12–16 ACE2 is a key counter regulatory enzyme that degrades angiotensin II to angiotensin-(1–7), thereby attenuating its effects on vasoconstriction, sodium retention, and fibrosis. Although angiotensin II is the primary substrate of ACE2, that enzyme also cleaves angiotensin I to angiotensin-(1–9) and also participates in the hydrolysis of other peptides. ACE2 is expressed broadly, including in the heart and kidneys, and the lung alveolar epithelial cells.20–22
The complexity underlying the renin-angiotensin-aldosterone system (RAAS) clearly shows the different responses to different modulators in the presence of SARS-CoV 2 infection (Fig. 2). The enzyme active sites of ACE and ACE2 are distinct. Therefore, ACE inhibitors in clinical use do not directly affect ACE2 activity.23 Experimental animal studies have shown mixed findings with respect to the effects of ACE inhibitors and ARB on ACE2 levels or activity in tissue.24–30 Some studies have shown that ARB may increase messenger RNA expression or protein levels of ACE2 in tissue24,30 while others showed no effect.27 These ostensibly inconsistent results demonstrate the complex RAAS responses and emphasize the notion that findings from experimental animal studies may not extrapolate to similar physiological responses in humans. This fact shows the importance and relevance of human studies performed on this matter to shed more light for comprehension.
FIGURE 2.
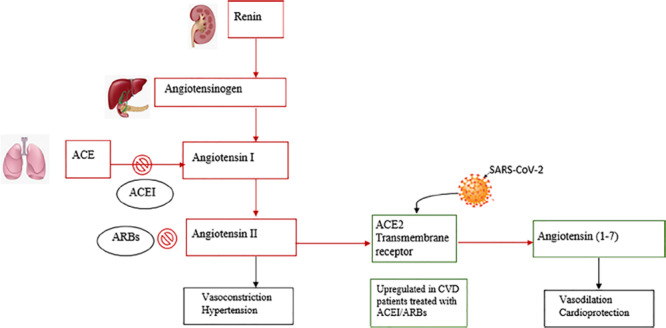
RAS inhibition by ACEI/ARBs and SARS-CoV-2 binding to ACE2 receptors. Red squares indicate RAS actions. Green squares indicate ACE actions. Black squares indicate effects for each component. RAS indicates renin-angiotensin system. Reprinted with permission from Eur J Epidemiol 2020;35:335–337.
At least theoretically, the drug-induced increased ACE2 expression produced by ACEI, or ARB might aggravate lung injury of patients with COVID-19. However, Henry et al,31 in a previous clinical study, showed a beneficial effect of ACEI in patients admitted with viral pneumonia. They retrospectively investigated the impact of ACE inhibitors and statins on the rates of intubation and death in 1055 adult patients with a positive respiratory viral polymerase chain reaction test. They found lower rates of death and intubation in those patients with continued use of ACE inhibitors [odds ratio ¼0.25; 95% confidence interval (CI), 0.09–0.64] during the hospital stay. Moreover, those patients on ACE inhibitors before hospital admission and subsequently discontinued the medication had a higher mortality than those patients who were not on ACE inhibitor before admission. They observed a significantly reduction of the pulmonary inflammatory response and cytokine release caused by virus infection.31 In addition, Mortensen et al32 found a significant decrease in mortality, length of stay, and mechanical ventilation in patients taking ACE inhibitors, or ARB who were hospitalized with pneumonia and compared with a matched cohort.
The study done by Kuba et al33 provided the first genetic proof, a molecular evidence for the severe lung failure and mortality associated with SARS-CoV. They demonstrated that infections with SARS-CoV resulted in ACE2 downregulation through binding of SARS-CoV Spike protein to ACE2 which contributed to the severity of lung pathologies. They further showed that this severity could be attenuated by blocking the renin-angiotensin pathway.33 As the authors mentioned, the fact of providing a molecular link between SARS-CoV pathogenesis and the role of the RAAS in lung failure, envisioned a novel target in the therapeutic management. Recombinant ACE2 protein could not only be a treatment to block virus spreading but modulation of the renin-angiotensin system could also be utilized to protect patients with COVID-19.
The beneficial effect of ACEI/ARB may be related to a compensatory increase in ACE2.18 However, the evidence regarding the use of ACEI/ARB in patients with COVID-19 infection is still emerging, and larger prospective, randomized clinical studies are required. At present, for patients with COVID-19 who previously used ACEI/ARB, the use of these drug agents may not need to be discontinued based on current data.
Increased Cytokine Secretion
Another possible mechanism involved in cardiac injury may be the increased cytokine secretion during COVID-19. In a previous research, 46 patients with established clinical diagnosis of SARS-CoV were prospectively studied by Li et al.18 They found significantly higher left ventricular index of myocardial performance (0.42 ± 0.13 vs. 0.33 ± 0.09; P < 0.001), longer isovolumic relaxation time (102.9 ± 15.7 vs. 81.6 ± 14.7 milliseconds; P < 0.001), lower flow propagation velocity (69.6 ± 15.7 vs. 83.8 ± 19.7 cm/s; P < 0.011), and Doppler-derived cardiac output (4.69 ± 1.01 vs. 5.49 ± 1.04 L/min; P < 0.001) were observed during acute infection when compared with those at 30 days. A decrease in left ventricular ejection fraction correlated moderately with an elevated lactate dehydrogenase level (r = −0.605; P < 0.001), whereas a higher index of myocardial performance correlated weakly with an increase in creatine kinase level (r = 0.38; P = 0.016). In this 30-day echocardiographic follow-up study, they found that reversible, subclinical diastolic left ventricular impairment appears to be a common finding in acute SARS-CoV infection, even among those patients without underlying cardiac disease. They suggested that left ventricular dysfunction in the acute phase of the disease might be attributable to the cytokine storm syndrome resulting from an overaggressive host immune response to SARS-CoV infection.18
Huang et al1 found that severely ill patients with COVID-19 who were admitted to the intensive care unit had higher plasma levels of cytokines, including interleukin (IL)-2, IL-7, IL-10, granulocyte-colony stimulating factor, and IgG-induced protein 10. They also had higher plasma levels of monocyte chemoattractant protein-1, macrophage inflammatory protein 1-alpha, and tumor necrosis factor α. Indeed, this is a serious life-threatening disease with clinical features of systemic inflammation, methemoglobinemia, hemodynamic instability, and multiple organ failure. The trademark clinical endorsement of cytokine storm syndrome is a severe, uncontrolled and, dysfunctional immune response involving the continuous activation and proliferation of lymphocytes and macrophages. Some other markers of inflammatory response, such as CRP, procalcitonin, and leukocytes, were also found to be significantly increased among patients who suffered from cardiac injury.1
The infection-related increased cytokine release is identified by an elevated plasma level of several inflammatory biomarkers. Various inflammatory serum markers that are shown to be elevated in patients with COVID-19–related cardiac injury are CRP, procalcitonin, ferritin, D-dimer, IL-2, IL-7, granulocyte colony-stimulating factor, IgG-induced protein 10, chemokine ligand 3, and tumor necrosis alpha. Therefore, the storming activation and secretion of these inflammatory cytokines can lead to apoptosis or necrosis of myocardial cells in COVID-19.1–3 In this regard, Huang et al1 demonstrated an imbalance of T helper 1 and T helper 2 responses resulting in a cytokine storm, which may have contributed to myocardial injury in patients with COVID-19. The release of inflammatory cytokines after infection may cause reduction in coronary blood flow and decreased oxygen supply.
Presence of Preexisting CVDs
Another interesting factor to consider is the presence of preexisting CVDs might also render patients to be more susceptible to COVID-19–induced heart injury. Shi et al3 demonstrated that patients with cardiac injury had a history of coronary heart disease (30%) and hypertension (60%), which were significantly more prevalent than in those patients without cardiac injury. Wang et al2 found very similar percentages in their patients who were critically ill with COVID-19, 25% had underlying heart diseases and 58% had hypertension. It is reasonable to assume that patients with predisposing CVDs such as coronary artery disease or heart failure are susceptible to cardiac injury, and once such patients are infected with COVID-19, myocardial ischemia or infarction, and left ventricular systolic dysfunction are more likely to occur, ultimately leading to a sudden deterioration. The intrinsic inflammatory process within the atherosclerotic plaques in the coronary walls is exacerbated during the systemic inflammatory response in COVID-19, making them susceptible to fissure initiating an acute coronary syndrome.19
Hypoxia-induced Cardiac Myocyte Apoptosis
Altered myocardial demand/supply ratio in association with increased cardiometabolic demand in the myocardial tissue can result in myocyte hypoxia. This alteration in the context of systemic infection associated with acute respiratory illness-related hypoxia can further impair myocardial oxygen demand/supply relationship and lead to acute myocardial injury.16 In addition, the systemic inflammation also causes endothelial dysfunction and increases the procoagulant activity of the blood, which can further contribute to the formation of occlusive thrombi over a ruptured coronary plaque. In the context of inflammation and hypoxemic state in COVID-19, atrial fibrillation may supervene worsening the prognosis and developing heart failure.19
CARDIOVASCULAR FINDINGS IN COVID-19 RECENT CLINICAL STUDIES
Several observational studies reported evidence of higher morbidity and mortality risk associated to the findings of inflammation and cardiovascular involvement and especially cardiac injury in patients with COVID-19 (Table 2).
TABLE 2.
Inflammation, Cardiac Injury, and Mortality in Observational Studies With COVID-19

Shi et al3 studied a total of 416 hospitalized patients with COVID-19 with the median age of 64 years (range, 21–95 years), and 211 (50.7%) were female. They found a total of 82 patients (19.7%) with cardiac injury, and compared them with patients without cardiac injury. The patients with cardiac involvement were older 74 versus 60 years; P < 0.001; and had more comorbidities (hypertension in 59.8% vs. 23.4%; P < 0.001). They also had higher levels of CRP {median [IQR (interquartile range)], 10.2 vs. 3.7 mg/dL}, procalcitonin (median [IQR], 0.27 vs. 0.06 ng/mL), creatinine kinase–myocardial band (median [IQR], 3.2 vs. 0.9 ng/mL), myohemoglobin (median [IQR], 128 vs. 39 μg/L), and high-sensitivity TnI (median [IQR], 0.19 vs. <0.006 μg/L). The authors also found that greater proportions of patients with cardiac injury required noninvasive mechanical ventilation (46.3% vs. 3.9%; P < 0.001) or invasive mechanical ventilation (22.0% vs. 4.2%; P < 0.001) than those without cardiac injury. Regarding compromise of target organs, they observed that complications were more common in patients with cardiac injury than those without cardiac injury and included acute respiratory distress syndrome (58.5% vs. 14.7%; P < 0.001), acute kidney injury (8.5% vs. 0.3%; P < 0.001), electrolyte disturbances (15.9% vs. 5.1%; P = 0.003), hypoproteinemia (13.4% vs. 4.8%; P = 0.01), and coagulation disorders (7.3% vs. 1.8%; P = 0.02). Regarding mortality, the authors found that patients with cardiac injury had higher mortality than those without cardiac injury (51.2% vs. 4.5%; P < 0.001). In a Cox regression model, patients with cardiac injury, as compared with those patients without it, were at a higher risk of death, both during the time from symptom onset [hazard ratio, 4.26 (95% CI, 1.92–9.49)] and from admission to end point [hazard ratio, 3.41 (95% CI, 1.62–7.16)]. Therefore, they concluded that cardiac injury is a common condition among hospitalized patients with COVID-19, and it is associated with higher risk of in-hospital mortality.3 This is the first study showing that cardiac injury was independently associated with an increased risk of mortality in patients with COVID-19. Unfortunately, this study lacks imaging evidence from nuclear magnetic resonance, CT scans, or echocardiography to determine the features of myocardial injury and inflammation. Hence, due to the current limited evidence based on clinical investigation of observational studies, the question of whether this novel coronavirus can directly injure the cardiac tissue requires further investigation and demonstration. In this context, there are 3 recent case reports documenting myocardial inflammation and damage with MRI and histological studies in patients with COVID-19.36–38 These 3 recent case reports highlight the importance of clinical surveillance and laboratory testing directly targeting serum troponin levels, to guarantee appropriate rapid identification of atypical cases with acute illness of COVID-19 to proceed with prompt isolation of patients and eventually to reduce further transmission.
Biomarkers of Inflammation and Cardiac Injury
Viral diseases can harm myocardial cells through direct myocyte damage by the virus, and by systemic inflammatory responses. Han et al34 studied and analyzed in detail the main laboratory markers associated to cardiac injury in COVID-19 patients and investigated the correlation between heart injury and severity of the disease. They specifically analyzed serum levels of creatine kinase isoenzyme-MB, myohemoglobin (MYO), cardiac TnI (ultra-TnI), and N-terminal probrain natriuretic peptide (NT-proBNP), in 273 COVID-19 patients. Their patients were divided into 3 groups according to strict diagnostic criteria of their clinical condition: mild (198 cases), severe (60 cases), and critical (15 cases). The positive rate of MYO, ultra-TnI, and NT-proBNP was higher in severe cases and critical cases as compared with mild cases, the differences among the groups were statistically significant (P < 0.05).These findings showed that higher concentrations in venous blood of creatine kinase isoenzyme-MB, MYO, ultra-TnI, and NT-proBNP were associated with the severity and mortality of COVID-19. Of note, the authors analyzed these 4 cardiac injury parameters between the death (n = 24) and alive patients (n = 249). All 4 parameters were significantly higher in the death than in the alive group (P < 0.001). Therefore, the authors concluded that careful monitoring of the myocardial enzyme profiles is of great importance in reducing the complications and mortality in COVID-19 patients.34 In this context, an interesting meta-analysis was performed by Lippi et al39 in a total number of 341 patients. They found that TnI plasma values were significantly increased in patients with severe COVID-19 compared with those patients with milder forms of the disease. Therefore, it seems rational to assume that initial measurements of serum biomarkers of cardiac injury should be performed immediately after hospitalization for COVID-19 patients, and further in-hospital monitoring may help identifying a subset of severe cases with possible cardiac involvement and consequently predict progression towards a worse clinical scenario.
Guo et al5 performed a retrospective single-center case series study to analyzed 187 patients with confirmed COVID-19. The mean age was 58.50 ± 14.66 years. They evaluated the association of underlying CVD and myocardial injury with fatal outcomes in patients with COVID-19. Of 187 patients, 144 patients (77%) were discharged from the hospital and 43 patients (23%) died. Overall, 66 (35.3%) patients had underlying CVD including hypertension, coronary heart disease, and cardiomyopathy, and 52 (27.8%) exhibited myocardial injury as indicated by elevated troponin T (TnT) levels. The mortality during hospitalization was 7.6% for patients without underlying CVD and normal TnT levels, 13.3% for those with underlying CVD and normal TnT levels, 37.5% for those without underlying CVD but elevated TnT levels, and 69.4% for those with underlying CVD and elevated TnTs (Fig. 3). Patients with underlying CVD were more likely to exhibit elevation of TnT levels compared with the patients without CVD (54.5% vs. 13.2%). Plasma TnT levels demonstrated a high and significantly positive linear correlation with plasma high-sensitivity CRP levels (β = 0.530; P < 0.001) and NT-proBNP levels (β = 0.613; P < 0.001). During hospitalization, patients with elevated TnT levels had more frequent malignant arrhythmias, and the use of glucocorticoid therapy (71.2% vs. 51.1%) and mechanical ventilation (59.6% vs. 10.4%) were higher compared with patients with normal TnT levels. The mortality rates of patients with and without use of ACEI/angiotensin receptor blockers was 36.8% and 25.6%. Therefore, the authors concluded that myocardial injury is significantly associated with fatal outcome of COVID-19, while the prognosis of patients with underlying CVD but without myocardial injury is relatively favorable. Myocardial injury is associated with cardiac dysfunction and arrhythmias. Inflammation may be a potential mechanism for myocardial injury. Aggressive treatment may be considered for patients at high risk of myocardial injury. Interestingly, these authors demonstrated a high and significantly positive linear correlation between troponin T and plasma high-sensitivity CRP levels, biomarkers of cardiac injury and systemic inflammation, respectively (Fig. 4). Guo et al5 observed 52 (27.8%) patients who exhibited myocardial injury as demonstrated by elevation of troponin T levels, and the mortality was markedly higher in these patients with elevated TnT levels than in those with normal TnT levels (59.6% vs. 8.9%). There was nearly 70% mortality in those COVID-19 patients who had underlying CVD and elevated troponin T levels. In addition, their data on NT-proBNP showed that its serum elevation and the presence of malignant arrhythmias were significantly more common in patients with elevated TnT levels. Moreover, the serum levels of NT-proBNP were significantly correlated with TnT levels. In this respect, Gao et al35 studying 54 patients with COVID-19 reported that the best cutoff value of NT-proBNP was 88 pg/mL for predicting in-hospital death. They found it to have an excellent sensitivity and good specificity (Fig. 5). Patients with high NT-proBNP values had a significantly increased risk of death comparing to those with low values. After adjustment for potential risk factors, NT-proBNP was independently correlated with in-hospital death.35 The results of these 2 latter studies argue in favor of the concept that the greater the cardiac injury, the greater the ventricular dysfunction in COVID-19 patients. Also, patients with underlying CVD are more prone to experience myocardial injury during the course of their COVID-19.
FIGURE 3.

Mortality of patients with COVID-19 with/without CVD and with/without elevated TnT levels. Reprinted with permission from JAMA Cardiol 2020;e201017.
FIGURE 4.

Correlation between plasma TnT with hsCRP. Plasma TnT, hsCRP, collected on admission. hsCRP indicates high-sensitivity C-reactive protein. Reprinted with permission from JAMA Cardiol 2020;e201017.
FIGURE 5.

The NT-proBNP for in-hospital death of COVID-19 by receiver operating characteristic curves. The AUC of NT-proBNP was 0.909. The best cutoff of NT-proBNP for prediction in-hospital death was 88.64 pg/mL with the sensitivity of 100% and the specificity of 66.67%. AUC indicates area under the curve. Reprinted with permission from Respir Res 2020;21:83.
QT Prolongation and Cardiac Arrhythmias in COVID-19
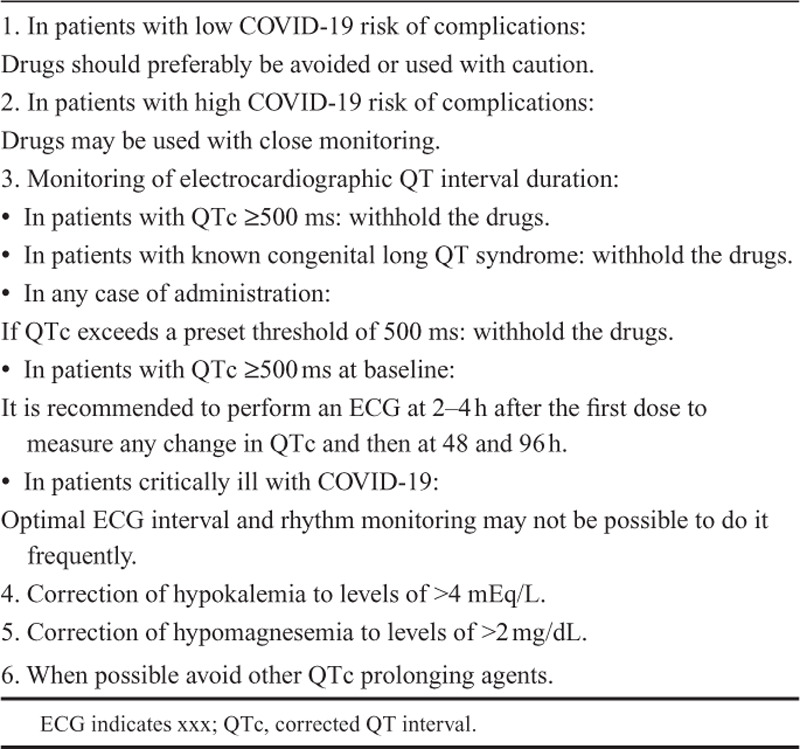
There is no known direct association between the SARS-CoV-2 itself and the QT interval prolongation in the electrocardiogram of COVID-19 patients. However, there is known association to prolonged QT interval and cardiac arrhythmias among certain drug agents that may be utilized in the therapeutic management of COVID-19. Hydroxychloroquine and azithromycin have been utilized as potential pharmacological agents in the prophylaxis or therapeutic management in COVID-19. Both drug agents are known to provoke malignant ventricular arrhythmias in certain patients.40–42 Hydroxychloroquine acts on the entry and postentry stages of SARS-CoV-2 via effects on endosomal pH and the resulting underglycosylation of ACE 2 receptors that are required for viral entry. Since it may prolong the QT interval and develop ventricular arrhythmias, it should be given with extreme cautious in association with other QT interval prolonging drugs (Fig. 6). There are case reports of patients with systemic lupus erythematosus showing hydroxychloroquine-induced QT interval prolongation and developing torsade de pointes.43,44 Azithromycin is increasingly recognized as antibiotic that may cause QT prolongation, malignant ventricular arrhythmias, non–pause-dependent polymorphic ventricular tachycardia, torsade des points, and increased risk for sudden cardiac death.45 There are very limited data evaluating the safety and clinical outcome of combination therapy with these 2 pharmacological agents, and the effect of their combination on QT interval or arrhythmia risk has not been studied. Therefore, multiple randomized clinical trials are currently ongoing studies. Table 3 shows certain care and safety considerations to consider.
FIGURE 6.

Hydroxychloroquine therapy according to cardiovascular risk. HCQ, hydroxychloroquine; QTc, corrected QT interval. Reprinted with permission from Ind Pac Electrophysiol J 2020.
TABLE 3.
Cardiac Involvement Comparison of COVID-19 With SARS and MERS
The novel coronavirus SARS-CoV-2 appears to be more transmittable, with greater infectivity, and somehow similar or higher morbidity and mortality depending on the area considered, as compared with SARS and MERS.4–9 So far, there is a greater incidence of patients with SARS-CoV-2 who have developed severe pneumonia, pulmonary edema, acute respiratory distress syndrome, cardiovascular compromise, or multiple organ failure and death. Oudit et al46 previously demonstrated that the SARS-CoV genome was positively detected in the myocardial tissue in 35% of the patients with SARS-CoV infection. This raises the possibility of direct damage of cardiomyocytes by the virus itself. SARS-CoV-2 may share the same mechanism with SARS-CoV because the 2 viruses are highly homologous in genome.47,48 Animal experimental studies have shown that some strains of coronavirus infection may seriously affect the heart. For example, coronavirus infection may induce cardiomyopathy that results in cardiac chamber dilatation and impairment of systolic function simulating dilated cardiomyopathy in rabbits.49 In this context of cardiomyopathy, it is interesting to note that Dong et al50 reported 4 end-stage heart failure patients who were infected with COVID-19, 2 of them with severe illness presentation. All 4 patients were male, had typical lymphopenia, and had significantly increased CRP level. Three patients had elevated TnI in the later evolution period, and TnI increased significantly a few days before death. Moreover, the levels of CRP and brain natriuretic peptide were significantly higher. An interesting finding was that the TnI level of the 2 critically ill patients increased significantly by more than 20-fold, indicating important myocardial injury. Patients with end-stage heart failure seemed to be older, have more comorbidity, poor general condition, a greater myocardial injury, and higher mortality rate after COVID-19.
According to the novel knowledge acquired from recent studies on COVID-19, those patients who manifested cardiac injury presented with more severe acute illness requiring noninvasive or invasive ventilation compared with patients without cardiac injury. They also manifested clearly abnormal laboratory, radiographic findings, and mortality in half of the hospitalized patients. It is worth comparing these cardiovascular findings in COVID-19 with those of SARS of about 15 years ago.4–9 Yu et al10 studied the cardiovascular complications of SARS in 121 hospitalized patients. They observed that hypertension occurred in 50.4%. Of these patients, 71.9% developed persistent tachycardia, including 40% with continued tachycardia during outpatient follow-up. Therefore, tachycardia could not be explained by fever which had already subsided by then. It was neither explained by coexisting hypotension, which was only present in a comparatively small number of patients. Although tachycardia-related cardiovascular complications were common in patients with SARS, they were generally self-limiting and not associated with mortality. Other types of cardiac arrhythmias were uncommon in patients with SARS. Transient atrial fibrillation occurred in only 1 patient who had no history of cardiac disease. Therefore, the cardiac arrhythmia–associated effect of the coronavirus that causes SARS is low. In total, 47 (39%) patients developed arterial desaturation that needed oxygen therapy. However, only 18 (15%) patients were admitted into intensive care unit. Only 6 (5%) patients died and, all of them due to pneumonia.10 In contrast with that from SARS findings, more than half of the patients with cardiac injury experienced in-hospital death with COVID-19 indicating that this novel coronavirus–induced cardiac injury is associated with major adverse clinical outcomes and worse prognosis.3 Indeed, cardiac injury was found to be independently associated with an increased risk of mortality in patients with COVID-19.
CONCLUSIONS
Myocardial injury and common pathways of inflammation are significantly associated with a higher risk of in-hospital mortality and overall fatal outcome of COVID-19. We identified several potential mechanisms of myocardial tissue damage, namely, coronavirus-associated acute myocarditis, ACE2 receptor binding affinity to the virus Spike protein, increased cytokine secretion, and hypoxia-induced cardiac myocyte apoptosis. Myocardial injury was found to be related to cardiac dysfunction, ventricular arrhythmias, and it was independently associated with an increased risk of mortality in patients with COVID-19. Inflammation may be a potential mechanism for myocardial injury. There was a high and significantly positive linear correlation between troponin T and plasma high-sensitivity CRP levels, biomarkers of cardiac injury and systemic inflammation, respectively. Although, there are several possible mechanisms of myocardial tissue damage, the exact mechanism of cardiac injury needs to be further explored in well-designed clinical studies. Strong efforts and continued research of the cardiovascular complications and identification of risk factors for poor prognosis in COVID-19 are steadily needed. Given the high infectivity and lethality of COVID-19 and the enormous economic and social impact of the worldwide outbreak, elucidation of the disease pathogenesis and prospective histopathological studies are crucial for future treatment in case of renewed outbreaks.
CVDs are still the number one killer in the world, and represent the leading noncommunicable epidemic. Since numerous patients with CVD are using ACEI/ARB drug agents, clinical studies may be necessary to explore the potential associations of these pharmacological drugs with the susceptibility and prognosis of COVID-19. Despite the theoretical concerns and physiological uncertainties concerning the effect of RAAS inhibitors on ACE2, ACE inhibitors and ARB drug agents should be continued in patients with COVID-19 in otherwise stable condition.51–53 This is a position supported by multiple medical and scientific societies that needs to be explored by randomized clinical trials to search for the potential associations of these pharmacological drugs with the susceptibility and prognosis of COVID-19.
The high morbidity and mortality of COVID-19, its monumental economic burden and social impact, the despair of a new pandemic outbreak, and the thread of potential utilization of novel SARS-CoV-2 as biologic weapons make it a preponderant necessity to better comprehend the therapeutic management of this lethal disease. Emerging as an acute infectious disease, COVID-19 may become a chronic epidemic because of genetic recombination. Therefore, we should be ready for the reemergence of COVID-19 or other coronaviruses in the near future.
DISCLOSURES
Nothing to declare.
ACKNOWLEDGMENTS
We thank Dr. Angelica Helga Neumann for her excellent technical assistance and constant support in the making of this article, and Miss Felicita Torales for her help with the literature search.
REFERENCES
- 1.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 inWuhan, China. JAMA Cardiol. 2020:e200950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Z, McGoogan JM. Characteristics of and important lessons from the Coronavirus Disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020. [DOI] [PubMed] [Google Scholar]
- 5.Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020:e201017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Wit E, van Doremalen N, Falzarano D, et al. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14:523–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yin Y, Wunderink RG. MERS, SARS and other coronaviruses as causes of pneumonia. Respirology. 2018;23:130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song Z, Xu Y, Bao L, et al. From SARS to MERS, thrusting coronaviruses into the spotlight. Viruses. 2019;11:E59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu CM, Wong RS, Wu EB, et al. Cardiovascular complications of severe acute respiratory syndrome. Postgrad Med J. 2006;82:140–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alhogbani T. Acute myocarditis associated with novel Middle East respiratory syndrome coronavirus. Ann Saudi Med. 2016;36:78–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wrapp D, Wang N, Corbett KS, et al. Cryo-EM structure of the 2019-nCoV spike in the perfusion conformation. Science. 2020;367:1260–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xin Z, Ke C, Zou J, et al. The single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to Wuhan 2019-nCoV infection. Front Med. 2020;14:185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel VB, Zhong JC, Grant MB, et al. Role of the ACE2/angiotensin 1-7 axis of the renin-angiotensin system in heart failure. Circ Res. 2016;118:1313–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li W, Moore MJ, Vasilieva N, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Padro T, Manfrini O, Bugiardini R, et al. ESC Working Group on coronary pathophysiology and microcirculation position paper on ‘coronary microvascular dysfunction in cardiovascular disease’. Cardiovasc Res. 2020;116:741–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li SS, Cheng CW, Fu CL, et al. Left ventricular performance in patients with severe acute respiratory syndrome: a 30-day echocardiographic follow-up study. Circulation. 2003;108:1798–1803. [DOI] [PubMed] [Google Scholar]
- 19.Tersalvi G, Vicenzi M, Callabreta D, et al. Elevated troponin in patients with Coronavirus Disease 2019 (COVID-19): possible mechanisms. J Cardiac Fail. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vickers C, Hales P, Kaushik V, et al. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem. 2002;277:14838–14843. [DOI] [PubMed] [Google Scholar]
- 21.Hamming I, Timens W, Bulthuis ML, et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Serfozo P, Wysocki J, Gulua G, et al. Ang II (Angiotensin II) conversion to angiotensin-(1-7) in the circulation is POP (prolyloligopeptidase)-dependent and ACE2 (angiotensin-converting enzyme 2)-independent. Hypertension. 2020;75:173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rice GI, Thomas DA, Grant PJ, et al. Evaluation of angiotensin-converting enzyme (ACE), its homologue ACE2 and neprilysin in angiotensin peptide metabolism. Biochem J. 2004;383(Pt 1):45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamming I, van Goor H, Turner AJ, et al. Differential regulation of renal angiotensin-converting enzyme (ACE) and ACE2 during ACE inhibition and dietary sodium restriction in healthy rats. Exp Physiol. 2008;93:631–638. [DOI] [PubMed] [Google Scholar]
- 25.Ferrario CM, Jessup J, Chappell MC, et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–2610. [DOI] [PubMed] [Google Scholar]
- 26.Ocaranza MP, Godoy I, Jalil JE, et al. Enalapril attenuates downregulation of angiotensin-converting enzyme 2 in the late phase of ventricular dysfunction in myocardial infarcted rat. Hypertension. 2006;48:572–578. [DOI] [PubMed] [Google Scholar]
- 27.Gonzalez-Jaramillo N, Low N, Franco OH. The double burden of disease of COVID-19 in cardiovascular patients: overlapping conditions could lead to overlapping treatments. Eur J Epidemiol. 2020;35:335–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burrell LM, Risvanis J, Kubota E, et al. Myocardial infarction increases ACE2 expression in rat and humans. Eur Heart J. 2005;26:369–375. [DOI] [PubMed] [Google Scholar]
- 29.Vuille-dit-Bille RN, Camargo SM, Emmenegger L, et al. Human intestine luminal ACE2 and amino acid transporter expression increased by ACE-inhibitors. Amino Acids. 2015;47:693–705. [DOI] [PubMed] [Google Scholar]
- 30.Soler MJ, Ye M, Wysocki J, et al. Localization of ACE2 in the renal vasculature: amplification by angiotensin II type 1 receptor blockade using telmisartan. Am J Physiol Renal Physiol. 2009;296:F398–F405. [DOI] [PubMed] [Google Scholar]
- 31.Henry C, Zaizafoun M, Stock E, et al. Impact of angiotensin-converting enzyme inhibitors and statins on viral pneumonia. Proc (Bayl Univ Med Cent). 2018;31:419–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mortensen EM, Nakashima B, Cornell J, et al. Population-based study of statins, angiotensin II receptor blockers, and angiotensin-converting enzyme inhibitors on pneumonia-related outcomes. Clin Infect Dis. 2012;55:1466–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuba K, Imai Y, Rao S, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han H, Xie L, Liu R, et al. Analysis of heart injury laboratory parameters in 273 COVID-19 patients in one hospital in Wuhan, China. J Med Virol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao L, Jiang D, Wen XS, et al. Prognostic value of NT-proBNP in patients with severe COVID-19. Respir Res. 2020;21:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim IC, Kim JY, Kim HA, et al. COVID-19-related myocarditis in a 21-year-old female patient. Eur Heart J. 2020;41:1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sala S, Peretto G, Gramegna M, et al. Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur Heart J. 2020;41:1861–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Inciardi RM, Lupi L, Zaccone G, et al. Cardiac involvement in a patient with Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lippi G, Lavie CJ, Sanchis-Gomar F. Cardiac troponin in COVID-2019. Progress Cardiovasc Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sapp JL, Alqarawi W, MacIntyre CJ, et al. Guidance on minimizing risk of drug-induced ventricular arrhythmia during treatment of COVID-19: a statement from the Canadian Heart Rhythm Society. Can J of Cardiol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kapoor A, Pandurangi U, Arora V, et al. Cardiovascular risks of hydroxychloroquine in treatment and prophylaxis of COVID-19 patients: a scientific statement from the Indian Heart Rhythm Society. Ind Pac Electrophysiol J. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giudicessi JR, Noseworthy PA, Friedman PA, et al. Urgent guidance for navigating and circumventing the QTc prolonging and torsadogenic potential of possible pharmacotherapies for COVID-19. Mayo Clin Proc. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Laughlin JP, Mehta PH, Wong BC. Life threatening severe QTc prolongation in patient with systemic lupus erythematosus due to hydroxychloroquine. Case Rep Cardiol. 2016;2016:4626279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Olano J, Howland MA, Su MK, et al. Toxicokinetics of hydroxychloroquine following a massive overdose. Am J Emerg Med. 2019;37:2264.e5–2264.e8. [DOI] [PubMed] [Google Scholar]
- 45.Ray WA, Murray KT, Hall K, et al. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366:1881–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oudit GY, Kassiri Z, Jiang C, et al. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Invest. 2009;39:618–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu N, Zhang D, Wang W, et al. China novel coronavirus investigating and research team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu X, Chen P, Wang J, et al. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci. 2020;63:457–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alexander LK, Small JD, Edwards S, et al. An experimental model for dilated cardiomyopathy after rabbit coronavirus infection. J Infect Dis. 1992;166:978–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dong N, Cai J, Zhou Y, et al. End-stage heart failure with COVID-19: strong evidence of myocardial injury by 2019-nCoV. JACC: Heart Failure. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheng H, Wang Y, Wang GQ. Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19. J Med Virol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bourgonje AR, Abdulle AE, Timens W, et al. Angiotensin-converting enzyme-2 (ACE2), SARS-CoV-2 and pathophysiology of coronavirus disease 2019 (COVID-19). J Pathol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mali SN, Thorat BR, Chopade AR. A viewpoint on angiotensin-converting enzyme 2, anti-hypertensives and Coronavirus Disease 2019 (COVID-19). Infect Disord Drug Targets. 2020. [DOI] [PubMed] [Google Scholar]


