Abstract
Protein C (PC) deficiency is associated with an increased risk for venous thromboembolism (VTE). In daily practice, exclusion of a hereditary PC deficiency is often based on a single determination of PC activity, by either clotting time–based or mostly chromogenic assay. However, diagnosis of hereditary PC deficiency is challenging due to several laboratory and clinical limitations. We compared the potential of PC activity values measured by either chromogenic or clotting time–based assay to predict a variation in the PROC gene. One hundred one (35%) of 287 patients carried variations within the PROC gene, including 2 previously not published variations. In 20 (20%) patients with identified variation, PC activity, determined by chromogenic assay, was within the reference range. For prediction of an underlying genetic defect determined by chromogenic and clotting time–based assay, sensitivity was 80% versus 99%, specificity 75% versus 18%, positive predictive value 64% versus 39%, and negative predictive value (NPV) 88% versus 97%. The lower NPV of chromogenic versus clotting time–based PC assay can be mainly explained by the presence of PC deficiency type IIb. Following our proposed diagnostic algorithm, additional measurement of PC activity by clotting time–based assay in case of a positive VTE history improves detection of this subtype of PC deficiency. Considering potential therapeutic consequences for primary and especially for secondary VTE prophylaxis, genetic analysis is required not only for confirmation but also for clarification of PC deficiency.
Keywords: protein C deficiency type IIb, protein C genotyping, chromogenic and clotting assay, algorithm
Introduction
Deficiencies of natural coagulation inhibitors (antithrombin [AT], protein C [PC], and protein S [PS]) represent an established risk factor for a first venous thromboembolic event1 and are associated with a higher risk of recurrent venous thromboembolism (VTE).2,3 Therefore, the absence or presence of hereditary AT, PC, and PS deficiency has an impact on clinical decision-making when considering risk-associated or permanent anticoagulation for VTE prophylaxis.
Thrombophilia screening includes determination of AT, PC, and PS plasma levels. In cost-conscious clinical everyday practice, this screening is often performed only once. Although a reduced activity of a natural coagulation inhibitor often leads to repeated testing for excluding an acquired deficiency, this is rarely the case for an activity within the reference range, despite potentially false-negative findings. A deductive interpretation of protein plasma levels in terms of a potentially underlying genetic defect can be challenging even for specialized hematologists and/or laboratory physicians. Knowledge of the exact clinical and preanalytical circumstances at time of the blood collection could improve deduction of an underlying genetic defect.
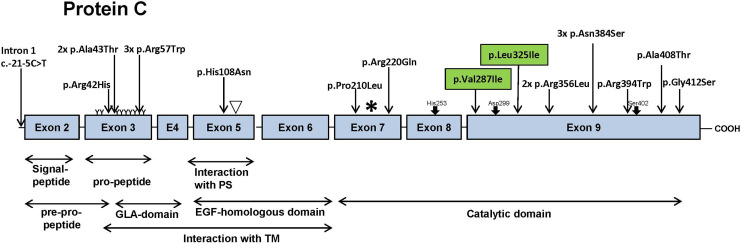
One important natural coagulation inhibitor is the serine protease precursor PC, a vitamin K (VK)-dependent plasma glycoprotein.4 It is encoded by the PROC gene and located on chromosome 2 at position 2q13-q14.5 The PROC gene is 11.2 kb in size and contains a promoter region, 9 exons and 8 intronic regions.6,7 Exon 1 is a noncoding sequence and the start codon is located in exon 2.8 Exons 2 and 3 are pre-pro-peptides including the signal peptide and the pro-peptide sequence (Figure 1). Exons 3 and 4 encode for the Gla domain. This domain contains glutamic acid residues, which are essential for the calcium-dependent binding of the protein. Exons 5 and 6 are located at the N-terminus of PC and encode for the epidermal growth factor (EGF)-homologous domain. Exons 7 to 9 reveal the sequence for the catalytic domain. More than 360 variations in the PROC gene are known to cause PC deficiency.9–11 Most of these variations are single nucleotide polymorphisms.6,12
Figure 1.
Model of Protein C gene (PROC) and variations found in our 20 cases (Table 3). Illustrated are 8 exons of the PROC gene and its various domains which are important for the interaction with protein S (PS) and thrombomodulin (TM). The arrows indicate the localization of the variations found in the PROC gene in our cohort. Most variations were detected in the catalytic domain. Three different variations were found in the propeptide region and one variation in the epidermal growth factor (EGF)-homologous domain. Green box: previously unpublished variations. γ-carboxylated glutamic acid residues: ▿ hydroxyaspartic acid, TM, thrombomodulin; PS, Protein S: site of proteolytic cleavage of the protein into the light (left of *) and into the heavy chain (to the right of *, and a dipeptide): ↓ His235, Asp299, Ser402: amino acid residues of the catalytic domain.
From the laboratory point of view, hereditary PC deficiency can be categorized in 2 types. Type I (found in 75%-80% of the cases) is characterized by a uniform reduction in PC activity and in immunologically measured PC concentration. Type II (in 20%-25% of the cases), on the other hand, is characterized by a reduced PC activity at normal PC concentrations, indicating that determination of PC concentration alone will fail to detect type II.11 Therefore, functional tests measuring PC activity (clotting time–based or chromogenic assays) are additionally used as screening tests for PC deficiencies.13 The first step in both commercially available assays is the activation of PC to activated PC (APC). This reaction is catalyzed by Protac, an enzyme derived from the venom of the Southern Copperhead snake (Agkistrodon contortrix).14 Clotting time–based assays measure the prolongation of activated partial thromboplastin time (APTT) by the degradation of factors Va and VIIIa, while chromogenic assays measure the ability of APC to cleave an amidolytic substrate spectrophotometrically.11
The variability of coagulation end point assays can be attributed to the used APTT reagent and the test specific phospholipid composition by influencing the APC sensitivity or its PS dependence.15,16 Therapy with oral or parenteral direct anticoagulants (= non-vitamin K oral anticoagulants [NOAC] and direct thrombin inhibitors [DTI]) leads to false high PC activity measured by clotting time–based assays.17 Furthermore, the clotting time–based test is influenced by high heparin levels,18 increased factor VIII activity, lupus anticoagulant (LA), or APC resistance.19
In contrast, the chromogenic method is not affected hereby.20,21 Therefore, current guidelines recommend the use of a chromogenic PC assay, as less interferences, inter- and intralaboratory variations are expected,22–24 and it seems to be more cost-effective.25
In the present study, we aimed to evaluate the predictive character of PC activity values gained by both functional assays in terms of identification of underlying variations in the PROC gene.
Materials and Methods
Patients
In this retrospective study, molecular genetic analysis of the PROC gene was performed in 287 mainly Caucasian patients (215 females and 72 males, mean age 37 [2-83]) with suspected thrombophilia at 2 centers in Germany (Bonn and Dortmund) between January 2015 and August 2019. Most of the individuals (n = 269, 94%) were not related, while for 18 individuals from 7 families, a family examination was carried out.
Analyzed clinical data included information on positive personal or family history of VTE and vascular pregnancy complications (eg, recurrent miscarriages, preeclampsia, etc). Venous blood samples were collected in citrate plasma (Sarstedt, citrate buffer 3.2%). Protein C activity testing was performed on ACL Top 750 CTS (Werfen, Spain) using a clotting time based (Hemoclot; CoaChrom Diagnostica, Vienna, Austria; and HemosIL; Werfen, Barcelona, Spain) and a chromogenic assay (HemosIL), respectively (reference range in both assays 70%-140%). Protein C concentration was determined by an enzyme immunoassay on Euroimmun Analyzer (Zymotest; CoaChrom Diagnostica, reference range 60%-140%) or by immunodiffusion (reference range: 1.62-3.14 mg/L).
Statistical Analysis
For the calculation of the predictive values, a “positive” and “negative” results for PC activity were defined as results below and within the reference range, respectively (specified by the reagent manufacturer [70%-140%]). Sensitivity, specificity, positive, and negative predictive values (PPV and NPV) were calculated in patients with and without a variation in the PROC gene, using a 4-fold table.
Genetic Analysis
Genomic DNA was isolated from EDTA-treated whole blood following the manufacturer’s instructions, using Wizard genomic DNA purification kit (Promega, Mannheim, Germany). The coding regions and intron/exon boundaries of the PROC gene (NG 016323.1; NM_000312.3) were amplified by polymerase chain reaction (PCR) using a touchdown 55°C method, previously described by Korbie and Mattick.26 In-house primer sets are listed in Table 1. After amplification, the PCR products were sequenced by Sanger method (ABI 3730xl Analyzer) and analyzed.
Table 1.
In-House Primer List PROC Sequencing.
| Exon | Forward | Reverse |
|---|---|---|
| Exon 2 | ACAGGGACAGCCCTTTCATT | GGCTCAGAGAGATGGTGGAA |
| Exon 3 | CCAGCTCTGCTTCCTCAGAC | TCAGCATTGAGTCCCCACCT |
| Exon 4 | CCTAGCAGCCAACGACCATC | GCTCCAAGGGCAAGACCAAG |
| Exon 5 | CTGGCCTTCTGGTCCAA | CCCACCTCCTCTAGGCAGTA |
| Exon 6 | GTGAGGGGGAGAGGTGGAT | TGTTCCTCGCTCCCTCCCTA |
| Exon 456 | TCGGGCGTCGATCCCTGTTT | CCGCTGCCCCAAGGCTCAACT |
| Exon 7 | TGACTGGAGGGGGTTCATAG | CATAGCTGCCAGGATGGACT |
| Exon 8 | GGAACCCAGGAAAGTGCATA | CTCTGGCAGCCCCCTTCT |
| Exon 9a | AGTCTCCGGGTGAACCTTCT | ATGACCTCGCTGCACTCATT |
| Exon 9b | CTACCACAGCAGCCGAGAGA | TCCCTTTAATGTCCCATCCA |
Results
Clinical and Laboratory Findings
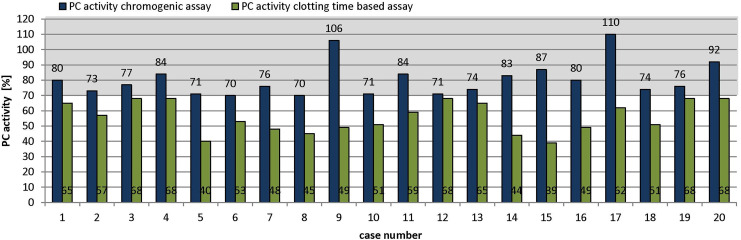
In total, we analyzed 287 patients and found a variation in the PROC gene in 101 (35%) patients (Table 2). Interestingly, 20 (20%) of those patients carrying a variation showed PC activities within the reference range when measured by chromogenic assay (Table 3). In these cases, PC activity ranged from 70% to 110%, while the PC activity, measured by clotting time–based assay, was below the reference range in all cases (39%-68%, Figure 2). Fourteen (70%) of these 20 patients were female. Eight patients (cases 4, 5, 10, 12, 14, 16, 18, and 20, Table 3) had personal history of VTE, while all other patients (except case 12) had a positive family history of VTE. Two patients also had vascular pregnancy complications (case 8: preeclampsia and case 17: early pregnancy loss).
Table 2.
Characteristics of Patients With and Without PC Variation.
| Patient Characteristics | Included Patients (n = 287) | ||
|---|---|---|---|
| Patients: PC Variation, Chromogen PC Activity ≥70% | Patients: PC Variation, Chromogen PC Activity <70% | Patients: Without PC Variation | |
| Number of patients | 20 | 81 | 186 |
| Females | 14 | 47 | 154 |
| Males | 6 | 34 | 32 |
| Age (years) | 40.9 ± 19.2 | 36.8 ± 16.8 | 37.1 ± 15.5 |
| PC activity chromogen (%) | 80.5 ± 11.3 | 50.8 ± 11.2 | 77.3 ± 14.7 |
| PC activity clotting (%) | 55.9 ± 10.2 | 44.0 ± 12.0 | 62.7 ± 15.8 |
| PC antigen (%) | 69.9 ± 14.8 | 48.4 ± 16.2 | 65.3 ± 14.1 |
| PC antigen (mg/L) | 2.4 ± 0.3 | 1.9 ± 0.6 | 2.4 ± 0.3 |
| F VIII activity (%) | 116.3 ± 40.6 | 114.9 ± 37.2 | 135.7 ± 53.8 |
| Lupus anticoagulant (lac screen ratio) | 1.3 ± 0.5 | 1.0 ± 0.3 | 1.0 ± 0.2 |
| Deep vein thrombosis | 7 (35%) | 26 (32%) | 64 (34%) |
| Pulmonary embolism | 3 (15%) | 8 (10%) | 21 (11%) |
| Factor V Leiden | 6 (30%) | 10 (12%) | 44 (24%) |
| Women with pregnancy complications | 2 (10%) | 9 (19%) | 42 (27%) |
Abbreviation: PC, protein C.
Table 3.
Clinical Data of 20 Cases of Patients With “Normal” PC Activity (by Chromogenic Assay) Despite Variation in the PROC Gene.
| Case | Gender | Age at Time of Investigation | PC Activity (Chromogen), %, Ref. value: 70-140 | PC Activity (Clotting), %, Ref. Value: 70-140 | Additional Relevant Thrombophilia | Ratio PC Activity (Clotting), %/PC Activity (Chromogen), % | PC Antigen, %, Ref. Value: 60-140 | PC Antigen (mg/L), Ref. Value: 1.62-3.14 | Ratio PC Activity (Clotting), %/PC Antigen, % | Type of PC Deficiency | Clinical Findings | Pregnancy | NOAC | Exon | Nucleotide Exchange (HGVS) | Protein Exchange (HGVS) | Domains | References | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Personal History of VTE/Pregnancy Complications | Family History | |||||||||||||||||||
| 1 | Female | 22 | 80 | 652) | None | 0.81 | NA | NA | NA | NA | None | Daughter of case 11 and granddaughter of case 12, respectively | None | None | 9 | Heterozygous | c.1151A>G | p.Asn384Ser | Catalytic domain | 27 |
| 2 | Female | 18 | 73 | 572) | None | 0.78 | NA | NA | NA | NA | None | Grandmother with DVT at the age of 56 | None | None | 5 | Heterozygous | c.322C>A | p.His108Asn | Hya (EGF homolog domain) | 27 |
| 3 | Female | 17 | 77 | 681) | Heterozygous FVL | 0.88 | 69 | NA | 0.99 | I | None | Mother and grandmother with DVT at the age of 34 and 40, respectively | None | None | 9 | Heterozygous | c.859G>A | p.Val287Ile | Catalytic domain | Not published |
| 4 | Male | 62 | 76 | 681) | Heterozygous FVL | 0.87 | 47 | NA | 1.45 | I | Spontaneous PE and prox. DVT at athe age of 62 | Father: postoperative PE | None | Last NOAC intake 17 hours before (Apixaban level of 31.52 ng/mL) | 1 | Heterozygous | c.-21-5C>T | 28 | ||
| 5 | Female | 26 | 84 | 681) | Positive LA: DRVV ratio 1.33 | 0.81 | 69 | NA | 0.99 | I | Postpartal UEDVT and DVT at the age of 24 and 26, respectively | Grandmother maternal (in young age) and paternal with DVT and long-term VKA treatment | None | Last NOAC intake 3 days before (Apixaban level of 2.73 ng/mL) | 9 | Heterozygous | c.1222G>A | p.Ala408Thr | Catalytic domain | 29 |
| 6 | Female | 45 | 71 | 401) | None | 0.56 | 62 | NA | 0.64 | II | None | Daughter with DVT under COC | None | None | 3 | Heterozygous | c.169C>T | p.Arg57Trp | Gla-domain/propeptide | 27 |
| Son with spontaneous bilateral DVT | ||||||||||||||||||||
| Mother and maternal grandmother with signs of PTS | ||||||||||||||||||||
| 7 | Female | 59 | 70 | 531) | None | 0.76 | 67 | NA | 0.79 | II | None | Daughter with PE under COC at the age of 24, carrier of same mutation, PC activity 50 (chromogen assay) and 31% (clotting), PC antigen 1.46 mg/L 52 days after cessation of VKA therapy | None | None | 9 | Heterozygous | c.973C>A | p.Leu325Ile | Catalytic domain | Not published |
| 8 | Female | 33 | 76 | 481) | Heterozygous FVL | 0.63 | 59 | NA | 0.81 | II | Preeclampsia in first pregnancy | Grandmother died of PE at the age of 56 | 7 weeks after delivery | None | 9 | Heterozygous | c.1234G>A | p.Gly412Ser | Catalytic domain | 30 |
| 9 | Female | 31 | 70 | 451) | None | 0.64 | 67 | NA | 0.67 | II | None | Mother with pregnancy associated DVT at the age of 28 and SVT at the age of 36 | None | None | 3 | Heterozygous | c.169C>T | p.Arg57Trp | Gla-domain/propeptide | 27 |
| Maternal grandmother PE at the age of 30 and DVT at the age of 40 | ||||||||||||||||||||
| Sister of the mother: travel DVT | ||||||||||||||||||||
| 10 | Male | 37 | 106 | 492) | None | 0.46 | NA | 3.26 | NA | II | PE and proximal DVT after long-distance flight at the age of 31 | Sister of the father with DVT after achilles tendon rupture | None | Last NOAC intake 3 days before (Rivaroxaban level of 0.76 ng/mL) | 3 | Heterozygous | c.125G>A | p.Arg42His | Propeptide | 31 |
| Prox. DVT after trauma and immobilization at the age of 36 | ||||||||||||||||||||
| 11 | Male | 57 | 71 | 511) | None | 0.72 | 103 | NA | 0.50 | II | None | Son of case 12 | None | None | 9 | Heterozygous | c.1151A>G | p.Asn384Ser | Catalytic domain | 27 |
| 12 | Female | 83 | 84 | 592) | On spec APS | 0.70 | NA | 2.7 | NA | II | Spontaneous prox. DVT left at the age of 73 | None | None | Last NOAC intake 26 hours before (Apixaban level of 37.21 ng/mL) | 9 | Heterozygous | c.1151A>G | p.Asn384Ser | Catalytic domain | 27 |
| DRVV ratio 1.65 | Spontaneous prox. DVT right at the age of 82 | |||||||||||||||||||
| 13 | Female | 29 | 71 | 682) | None | 0.96 | NA | 2.18 | NA | II | Spontaneous prox. DVT right at the age of 83 | Grandfather with PE and DVT | None | None | 7 | Heterozygous | c.659 G>A | p.Arg220Gln | Catalytic domain | 32 |
| 14 | Male | 34 | 74 | 651) | Heterozygous PTM | 0.88 | 76 | NA | 0.86 | II | Spontaneous prox. DVT at the age of 32 | Father with stroke at the age of approximately 50-55 | None | Last NOAC intake 25 hours before (Rivaroxaban level of 8.82 ng/mL) | 7 | Heterozygous | c.629C>T | p.Pro210Leu | Catalytic domain | 27.33 |
| recurrent DVT at the age of 33 | ||||||||||||||||||||
| 15 | Female | 27 | 83 | 441) | Heterozygous FVL | 0.53 | 77 | NA | 0.57 | II | None | Grandfather with recurrent DVT | 10th week of gestation | None | 9 | Heterozygous | c.1067G>T | p.Arg356Leu | Catalytic domain | 27.34 |
| 16 | Female | 52 | 87 | 391) | None | 0.45 | NA | 3.10 | NA | II | Car travel–related PE under COC requiring resuscitation at the age of 37 | Mother with multiple revascularization of PAD and stroke at the age >70 | None | None | 9 | Heterozygous | c.1180C>T | p.Arg394Trp | Catalytic domain | 35 |
| 17 | Female | 27 | 74 | 511) | None | 0.69 | 55 | NA | 0.92 | II | 2 early pregnancy losses | Father at the age of 20 PE during inpatient stay due to pneumonia | 10th week of gestation | None | 3 | Heterozygous | c.169C>T | p.Arg57Trp | Gla-domain/propeptide | 27 |
| 18 | Male | 80 | 80 | 491) | Heterozygous FVL | 0.61 | 68 | NA | 0.72 | II | Recurrent DVT (first DVT at the age of 42 | Father: fatal posttraumatic PE | None | Last NOAC intake 12 hours before (Rivaroxaban level of 264.72 ng/mL) | 9 | Heterozygous | c.1067G>T | p.Arg356Leu | Catalytic domain | 27.34 |
| 19 | Female | 28 | 92 | 681) | Heterozygous FVL | 0.74 | 73 | NA | 0.93 | II | None | Sister: DVT at the age of 30 mother: DVT at the age of 54 | None | None | 3 | Heterozygous | c.127G>A | p.Ala43Thr | Gla-domain/propeptide | 36 |
| 20 | Male | 47 | 110 | 621) | None | 0.56 | 96 | NA | 0.64 | II | PST after recurrent provoked DVT at the age of 19 and 28 | Father and 2 sister: DVT (1 sister at the age of 18) | None | None | 3 | Heterozygous | c.127G>A | p.Ala43Thr | Gla-domain/propeptide | 36 |
| Spontaneous PE at the age of 47 | ||||||||||||||||||||
Abbreviations: APC, activated protein C; APS, antiphospholipid syndrome; COC, combined oral contraceptives; CVO, central vein occlusion; DRVV, diluted Russell viper venom; DVT, deep vein thrombosis; EGF, epidermal growth factor; FVL, factor V Leiden; GFR, glomerular filtration rate; HGVS, Human Genome Variation Society; LA, lupus anticoagulant; NA, not available; NOAC, non-vitamin K antagonist oral anticoagulants; PC, protein C; PE, pulmonary embolism; PST, postthrombotic syndrome Prox., proximal; PTM, prothrombin G20210A mutation; Ref., reference; SVT, superficial vein thrombosis; UEDVT, upper extremity deep vein thrombosis; VK, vitamin K; VKA, vitamin K antagonist; VTE, venous thromboembolism.
1)Hemoclot.
2)HemosIL.
Figure 2.
Protein C activity in patients of Table 3. Blue column: chromogenic assay, green column: clotting time–based assay Gray box: reagent-specific reference range of 70% to 140%.
In these 20 patients (Table 3), PC deficiency could be formally classified as type I in 3 (15%) patients and as type II in 15 (75%) patients. For case 1 and case 2, the subtype of PC deficiency could not be classified, as no PC concentration had been determined. Anyways, case 1 is likely to be classified as PC deficiency type II deducing from results of the investigated relatives (cases 11 and 12).
In the 15 patients, we categorized as type II, the ratio between clotting time–based and chromogenic assay varied from 0.45 (case 16) to 0.96 (case 13). Considering the underlying variation and the high ratio between clotting time–based and chromogenic assay, case 13 could possibly also be classified as type I. Taking PC activities of 58% to 63% in the chromogenic assay from previous examinations into account, case 8 should be classified as PC deficiency type I. We assume pseudonormalization of the PC activity due to postdelivery-related hemostasis activation, leading to “false” classification following a “snapshot mode.”
Treatment with NOAC was stopped in 5 patients more than 17 hours before blood collection (cases 4, 5, 10, 12, and 14, Table 3). Therefore, an impact of NOAC therapy on clotting time–based PC activities can be ruled out, indicated by their corresponding low trough levels. The PC activity measured by clotting time–based assay in case 18 might even be overestimated, indicated by the high NOAC level (measured by anti-FXa activity), which is explained by the used therapeutic dosage of rivaroxaban (15 mg twice daily), renal impairment (glomerular filtration rate of 27 mL/min), and stopping NOAC treatment for only 12 hours before blood sampling.
We found positive LA in case 5 and case 12 (diluted Russell viper venom ratio of 1.33 and 2.00, respectively) and APC resistance due to heterozygous factor V Leiden mutations in cases 3, 4, 8, 15, 18, and 19, which might have contributed to an increased PC activity, measured by clotting time–based assay.
Genetic Findings
In our collective, we found 9 different variations located in the catalytic domain (exons 7 and 9) of the PROC gene (Figure 1). Furthermore, 3 different variations were detected in the pro-peptide region and 1 in exon 5. Exon 5 is the EGF-homologous domain of the PROC gene and interacts with PS and thrombomodulin. Cases 3 and 7 carry variations which have not been described in the international PC variation register (Human Genetic Variation Database [HGVD]) previously (exon 9: p.Val287Ile and p.Leu325Ile, respectively; Figure 1 and Table 3). All other variations were described and associated with hereditary PC deficiency27–36 (Table 3).
One relative of the family carrying the p.Asn348Ser variation (case 21, Supplement Figure 1, Supplement Table 1) was not listed in Table 3 because his PC activity of 66%, determined by chromogenic assay, was below the reference value of 70%. Additional 3 patients (cases 22, 23, and 24 [Supplement Table 1]) are carriers of PC variations also found in our 20 cases (p.Arg220Gln, p.Gly412Ser, and p.Arg57Trp, respectively) and showed relatively high residual PC activities of 65%, 66%, and 69% in the chromogenic assay, while corresponding clotting time–based PC activities were 57%, 50%, and 35%, respectively. An additional, probably nutritive VK deficiency was found in case 23, carrying the p.Gly412Ser variation (case 23, Supplement Table 1), indicating that in absence of VK deficiency this case should have been listed in Table 3 as well.
Predictive Values
The predictive values for identification of an underlying PC variation on the basis of the respective PC activity performed by chromogenic versus clotting time–based assay were 64% versus 39% for PPV, 88% versus 97% for NPV, 80% versus 99% for sensitivity, and 75% versus 18% for specificity (Table 4).
Table 4.
Predictive Values for PC Activity Obtained by Chromogenic and Clotting Time–Based Assays.
| Four-fold table for calculation of predictive values | ||||
|---|---|---|---|---|
| Patient Characteristics | PC Activity Screening Test | |||
| <70% (= “Positive”) | ≥70 (= “Negative”) | |||
| Chromogenic Assay | Clotting Time–Based Assay | Chromogenic Assay | Clotting Time–Based Assay | |
| Genetic testing | ||||
| Negative | n = 46 | n = 151 | n = 140 | n = 34 |
| Positive | n = 81 | n = 95 | n = 20 | n = 1 |
| Predictive values by assay | ||||
| Assay | Sensitivity | Specificity | NPV | PPV |
| Chromogenic | 0.80 | 0.75 | 0.88 | 0.64 |
| Clotting time–based assay | 0.99 | 0.18 | 0.97 | 0.39 |
Abbreviations: NPV, negative predictive value; PC, protein C; PPV, positive predictive value.
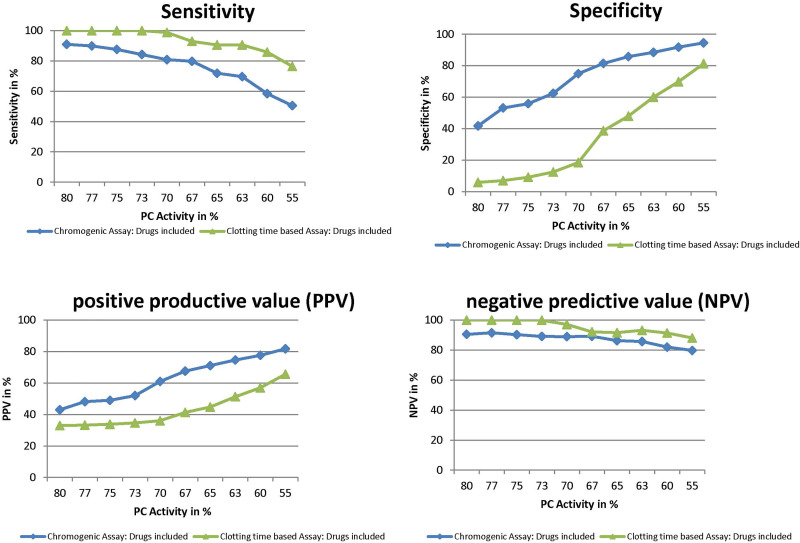
In order to increase the NPV of the chromogenic assay, it would seem obvious to advance the lower reference range for PC activity. However, advancing the lower reference range would also increase sensitivity, but decrease PPV and specificity (Figure 3). Assuming for example a higher lower limit of the reference range of 75% (instead of 70%), the predictive values for PC activity performed by chromogenic versus clotting time–based assay would be 52% versus 36% for PPV, 89% versus 100% for NPV, 88% versus 100% for sensitivity, and 56% versus 9% for specificity. The lower reference range value for chromogenic assay was 73% for our in-house calculation (for patients without anticoagulants).
Figure 3.
Changes in predictive values by varying the cutoff for protein C (PC) activity.
Discussion
Limitations of Defining a Reference-Based Cutoff Value
Routine determination of natural clotting inhibitors is usually performed as a step-by-step approach. In case of a reduced PC activity, the PC concentration is measured to distinguish between PC deficiency types I and II. Type I is characterized by a uniform reduction in PC activity and concentration, while type II is characterized by a normal concentration and a disproportionately reduced activity. The diagnostic workflow is completed by genetic analysis for confirmation and family-specific investigations. In case of a PC activity within the reference range, genetic analysis often seems to be dispensable in clinical practice, as it is usually provided by only few specialized laboratories.
However, clinical practice frequently lacks detailed information on analytical conditions like the performed assay for PC activity determination. Additionally, clinicians are often not aware of the limitations of the various tests, bearing the risk of an interpretation solely based on the reference range. Therefore, better definitions of clinically irrelevant low activity values of natural coagulation inhibitors are required to improve deduction of a genotype from a laboratory phenotype. Thus, there is a need to define a cutoff value of “too low” PC activity, which leads to subsequent genetic testing.
Caspers et al found PC activity levels ranging from 17% to 79% in patients with detectable PC variations. However, the authors stated that genotyping is not advisable for PC activities >70%.37 Their statement provides limited information on the PC activity assay used. Our results showed that the chromogenic determination of PC activity fails the identification of hereditary PC deficiency in a relevant proportion of patients (20%) and this is consistent with prior findings of Allaart et al.38 Furthermore, this proportion might even be underestimated due to additional VK deficiency and/or the intra-assay-specific variation of the chromogenic assay (coefficient of variation 15% according to the manufacturer of the reagent). This is further supported by those 3 cases (cases 22, 23, and 24 [Supplement Table 1]) with relatively high residual PC activities (65%-69%) carrying the same variations as individuals described in Table 3.
The comparably lower NPV for the chromogenic versus clotting time–based PC assay (88% vs 97%) indicates that the lower reference range of the manufacturer’s reagent does not meet the requirements of a suitable cutoff for PC activity, which is illustrated in Figure 2. A recent comparison between the chromogenic and clotting time–based PC assay showed a good correlation (R = 0.94 and r2 = 0.88) but also a significant bias, measuring on average 7.8% more PC by chromogenic than by clotting time–based PC assay,39 which might be more relevant for patients with borderline results than with extreme alterations of PC activity. A recent study in a Spanish cohort found 4 individuals carrying a PC variation with PC activity ranging from 72% to 77% and 10 relatives ranging from 75% to 94%.40 Therefore, the authors suggested genetic testing in families with PC activity around the lower limit of the reference range. However, simply increasing the cutoff of PC activity would of course increase NPV and sensitivity but at the expense of lowering PPV and specificity (see Figure 3).
Limitations of Laboratory Classification of PC Deficiency
The main explanation for the comparably lower NPV of the chromogenic compared to the clotting time–based assay is the presence of PC deficiency type IIb, which is not considered in the abovementioned diagnostic workflow. Protein C deficiency type IIb is characterized by a normal PC activity in the chromogenic assay, a low PC activity in the clotting time–based assay and a normal PC concentration. The incidence of this subtype is considered to be rare,41–43 which could imply negligibility. However, a case report of an intractable cerebral vein thrombosis caused by qualitative PC deficiency has recently been described (PROC. c.577_579delAAG), which was not detected by chromogenic assays,44 thereby stressing the importance of additional tests.
In our study, we identified 15 patients (cases 6-20) which could be formally classified as PC deficiency type IIb. However, one of these patients (case 8) met the criteria of PC deficiency type IIb only in a snapshot mode (= without knowing/considering of previous PC activities) explained by pseudonormalization due to pregnancy-related activation of hemostasis.45 According to the definition of PC type IIb, the ratio between clotting time–based and chromogenic assay should be low. However, following an arbitrarily chosen ratio below 0.6, this subtype was only found in 5 patients (cases 6, 10, 15, 16, and 20, Table 3). Furthermore, we classified 3 patients (cases 3-5, Table 3) with PC activity within the reference range, measured by chromogenic assay, but with identified variation, as PC deficiency type I.
These data suggest that a clear classification of PC deficiency type I or type II is often challenging and might lead to different interpretations, depending on available prevalues of the patients or affected family members. For PC deficiency type IIb, PC activities between the 2 assays differ per definition. Consequently, the assessment of a disproportional reduction compared to the PC concentration is only feasible by calculation of the clotting time–based assay, but of course not of the chromogenic assay, which has to be normal in PC deficiency type IIb.
Laboratory and genotype phenotype correlation
For membrane-bound thrombin–thrombomodulin, activation of PC Gla domain is essential.46 Thus, Gla-domainless PC could be one explanation for the overestimation of measured PC activity by chromogenic assay, which could be demonstrated in patients receiving vitamin K antagonist (VKA).47 We found 5 patients (cases 6, 9, 17, 19, and 20) with heterozygous nucleotide exchange affecting the Gla domain (Figure 1). The nonrelated cases 19 and 20 are carrier of the same variation of the Gla domain (p.Ala43Thr) and have both a positive family history for VTE. Case 20 has a lower PC ratio in comparison to case 19 and reveals an additional personal VTE history, whereas the coinheritance of factor V Leiden mutation in case 19 potentially could have both contributed to a falsely decreased PC activity measured by chromogenic assay and the VTE in her family. Unfortunately, there are no data of the affected family members of case 19, so this cannot be discussed in more detail.
Additionally, it has been assumed that variations affecting the interaction with thrombin, endothelial PC receptor, phospholipids, and PS are the reason for the failed detection in chromogenic assays.13 One patient (case 2) had an abnormal interaction with the PS binding site, which was discussed to be a functional alteration resulting in PC deficiency type IIb.48,49 Furthermore, we found variations in the propeptide sequence in 3 cases (case 6, 9, and 10). In 12 (60%) patients, we detected variations in the catalytic domain of PC, indicating a wide variation in functional but not uniform molecule defects. In this context, homology modeling could improve our knowledge on variations which cause PC deficiency.
Finally, we found 2 previously unpublished variations. The laboratory phenotype of one of these variations (p.Leu325Ile in exon 9, chr2:128186109C>A (hg19)) was classified as PC deficiency type II in case 7, while her clinically affected daughter would rather be classified as type I (case 27, Supplement Table 1) 52 days after ending therapy with VKAs. The same variation was also observed in 2 other patients, case 28 with and case 29 without personal VTE. Case 29 was classified as PC deficiency type II, whereas his son (case 28) could not be classified due to missing data of PC antigen. Interestingly, this variation was not found in the daughter of case 29 (case 30), even though she had a deep vein thrombosis under combined estrogen contraceptives and after air traveling of 11 hours. We are the first to find the heterozygous PC variation p.Leu325Ile in 2 independent families. Due to personal and family history of thrombotic events, it can be assumed that this variation plays a role in the promotion of thrombotic events. Furthermore, in silico calculations (calculated via combined annotation–dependent depletion50) indicated that an amino acid exchange of Leu to Ile at protein position 325 might be likely pathologic. This variation is located in the catalytic domain of PC and 10 bases around this variation, there are published variations associated with PC deficiency. Due to personal and family history of VTE, as well as in silico calculation and location, it can be assumed that this variation plays a role in the promotion of VTE.
The second unpublished variation (c.859G>A, p.Val287Ile, chr2:128185995G>A (hg19), case 3) is probably a non-disease-causing polymorphism (calculated in silico via combined annotation–dependent depletion). This suggestion is supported by 2 other carriers of this variation (case 25 and case 26, Supplement Table 1) who presented with PC activities over 100% in both assays. Both patients had positive personal and family histories for VTE, probably associated by their heterozygous factor V Leiden mutation. In case 3, a known APC resistance might have influenced PC activity measured by clotting time–based assay. Therefore, clinical significance of this variation remains unclear.
In summary, classification of PC deficiency into different laboratory subtypes is not sufficient to identify patients at risk of VTE. It is assumed that the 2 laboratory types of PC deficiency do not differ in their clinical manifestation,23,49 indicating that both types—quantitatively or qualitatively—contribute to the risk of VTE. This observation and our data raise the question, how PC levels correlate with clinical manifestations and variations in the PROC gene (Supplemental Figure 2): while intersections A and C undoubtedly are associated with an increased risk of VTE, this association might be limited for intersection B and D. One explanation for the lack of correlation between the clinical phenotype, the underlying genotype, and PC plasma levels is the varying prevalence of PC deficiency with and without VTE, while the prevalence of heterozygous PC deficiency in healthy individuals is ∼0.3% and 6% in families with hereditary thrombophilia, but it is only ∼3% in unselected patients with a first VTE.51–53
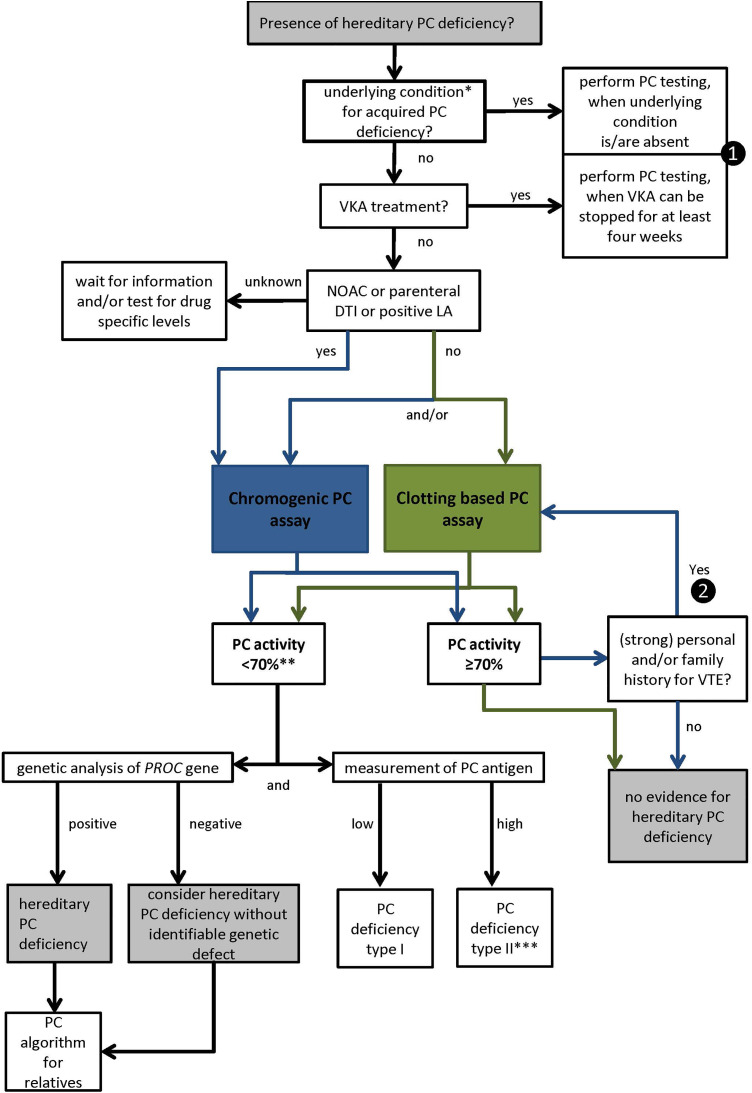
Our findings indicate that a deduction of an underlying genotype from the laboratory phenotype is limited. For PC activity, we have to recognize that the term “normal” is not synonymous to the term “within the reference range” and that in particular neither can be used synonymously for the absence of a variation. Consequently, we have to distinguish precisely between the various levels of laboratory phenotype and genotype to come to a final interpretation of relevant or not relevant PC deficiency. In this context, the assessment of the clinical phenotype seems indispensable. Therefore, we propose an algorithm (Figure 4) combining preanalytical and clinical data as a guideline for clinical decision-making. All of our 20 cases of hereditary PC deficiency were detectable by following this algorithm.
Figure 4.
Proposal of an algorithm for diagnosis of hereditary PC deficiency in adults combining clinical and laboratory data. First, acquired conditions for PC deficiency should be excluded. If the patient is on NOAC/DTI therapy displays a positive LA or the chromogenic assay is the first assay chosen, the algorithm follows the blue line. In case of a strong personal and/or family history for VTE additional performance of the clotting time–based PC assay, even for PC activities close to the lower limit of the reference range should be considered. For patients without NOAC/DIT therapy or known positive LA, the algorithm follows the green lines. *for example, VTE < 1 month, pregnancy, DIC, reduced liver dysfunction, VK deficiency, l-asparaginase therapy. **if confirmed by a second plasma sample. ***when PC chromogenic assay is available and normal, consider PC deficiency type IIb. ❶ if not possible and knowledge of hereditary PC deficiency would influence therapeutic decision-making perform testing with careful interpretations of PC activity in relation to VK-dependent procoagulant clotting factors. ❷ if NOAC therapy can be interrupted for >48 hours/parenteral DTI therapy can be stopped. DTI indicates direct thrombin inhibitor; LA, lupus anticoagulant; NOAC, nonvitamin K antagonist oral anticoagulant; PC, protein C; VK, vitamin K; VTE, venous thromboembolism.
In conclusion, we should be aware that there is no “ideal” PC activity test so far, with which the genotype can be reliably deduced from the laboratory phenotype. Apart from low interlaboratory variability, this “ideal” test should not be influenced by APC resistance and should be insensitive to NOAC treatment. In this context, innovative new assays might be promising.54 In absence of this “ideal PC assay,” we recommend using the clotting time–based PC assay for patients with strong personal and/or family history for VTE. For patients without NOAC or parenteral DTI use, the clotting time–based PC assay should be preferred over the chromogenic test because of its higher sensitivity. However, providing 2 different tests for one parameter might not be feasible in clinical and laboratory practice. Therefore, our algorithm suggests choosing the assay based on the preanalytical and clinical circumstances (Figure 4). Thus, indicating a single determination of PC activity might not be sufficient for the exclusion of a relevant hereditary PC deficiency. The key clinical information is (strong) personal and/or family history for VTE. Considering potential therapeutic consequences for primary and especially for secondary prophylaxis, genetic analysis is necessary for confirmation and should not be waived in case of uncertainty.
Supplemental Material
Supplemental Material, supplement_Table_1 for Laboratory Limitations of Excluding Hereditary Protein C Deficiency by Chromogenic Assay: Discrepancies of Phenotype and Genotype by Holger Seidel, Bianca Haracska, Jennifer Naumann, Philipp Westhofen, Moritz Sebastian Hass and Johannes Philipp Kruppenbacher in Clinical and Applied Thrombosis/Hemostasis
Footnotes
Authors’ Note: Our institution does not require ethical approval for reporting individual cases or case series. Written informed consent was obtained from the patients for their anonymized information to be published in this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Holger Seidel  https://orcid.org/0000-0002-5337-3797
https://orcid.org/0000-0002-5337-3797
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Bovill EG, Bauer KA, Dickerman JD, Callas P, West B. The clinical spectrum of heterozygous protein C deficiency in a large New England kindred. Blood. 1989;73(3):712–717. [PubMed] [Google Scholar]
- 2. Dentali F, Gianni M. VTE recurrence in patients with inherited deficiencies of natural anticoagulants. Thromb Haemost. 2009;101(1):5–6. [PubMed] [Google Scholar]
- 3. Ho WK, Hankey GJ, Quinlan DJ, Eikelboom JW. Risk of recurrent venous thromboembolism in patients with common thrombophilia: a systematic review. Arch Intern Med. 2006;166(7):729–736. doi:10.1001/archinte.166.7.729. [DOI] [PubMed] [Google Scholar]
- 4. Kisiel W. Human plasma protein C: isolation, characterization, and mechanism of activation by alpha-thrombin. J Clin Invest. 1979;64(3):761–769. doi:10.1172/JCI109521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Foster DC, Yoshitake S, Davie EW. The nucleotide sequence of the gene for human protein C. Proc Natl Acad Sci U S A. 1985;82(14):4673–4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Plutzky J, Hoskins JA, Long GL, Crabtree GR. Evolution and organization of the human protein C gene. Proc Natl Acad Sci U S A. 1986;83(3):546–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Patracchini P, Aiello V, Palazzi P, Calzolari E, Bernardi F. Sublocalization of the human protein C gene on chromosome 2q13-q14. Hum Genet. 1989;81(2):191–192. [DOI] [PubMed] [Google Scholar]
- 8. Foster DC, Rudinski MS, Schach BG, et al. Propeptide of human protein C is necessary for gamma-carboxylation. Biochemistry. 1987;26(22):7003–7011. [DOI] [PubMed] [Google Scholar]
- 9. Tsay W, Lee YM, Lee SC, Shen MC, Chen PJ. Characterization of human protein C gene promoter: insights from natural human mutants. DNA Cell Biol. 1996;15(11):907–919. doi:10.1089/dna.1996.15.907. [DOI] [PubMed] [Google Scholar]
- 10. Alhenc-Gelas M, Gandrille S, Aubry ML, Aiach M. Thirty-three novel variations in the protein C gene. French INSERM network on molecular abnormalities responsible for protein C and protein S. Thromb Haemost. 2000;83(1):86–92. [PubMed] [Google Scholar]
- 11. Khor B, Van Cott EM. Laboratory tests for protein C deficiency. Am J Hematol. 2010;85(6):440–442. doi:10.1002/ajh.21679. [DOI] [PubMed] [Google Scholar]
- 12. Foster D, Davie EW. Characterization of a cDNA coding for human protein C. Proc Natl Acad Sci U S A. 1984;81(15):4766–4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kottke-Marchant K, Comp P. Laboratory issues in diagnosing abnormalities of protein C, thrombomodulin, and endothelial cell protein C receptor. Arch Pathol Lab Med. 2002;126(11):1337–1348. doi:10.1043/0003-9985(2002)126<1337: LIIDAO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 14. Stocker K, Fischer H, Meier J, Brogli M, Svendsen L. Protein C activators in snake venoms. Behring Inst Mitt. 1986(79):37–47. [PubMed] [Google Scholar]
- 15. Cooper PC, Siddiq S, Morse C, Nightingale J, Mumford AD. Marked discrepancy between coagulometric protein C activity assays with the pro-thrombotic protein C Asn2Ile substitution. Int J Lab Hematol. 2011;33(5):451–456. doi:10.1111/j.1751-553X.2011.01311.x. [DOI] [PubMed] [Google Scholar]
- 16. D’Angelo SV, Gilardoni F, D’Angelo A. Evaluation of coagulometric assays in the assessment of protein C anticoagulant activity; variable sensitivity of commercial APTT reagents to the cofactor effect of protein S. Thromb Haemost. 1989;62(3):861–867. [PubMed] [Google Scholar]
- 17. Walenga JM, Fasanella AR, Iqbal O, et al. Coagulation laboratory testing in patients treated with argatroban. Semin Thromb Hemost. 1999;25(suppl 1):61–66. [PubMed] [Google Scholar]
- 18. Funk DM. Coagulation assays and anticoagulant monitoring. Hematology Am Soc Hematol Educ Program. 2012;2012:460–465. doi:10.1182/asheducation-2012.1.460. [DOI] [PubMed] [Google Scholar]
- 19. Ireland H, Bayston T, Thompson E, et al. Apparent heterozygous type II protein C deficiency caused by the factor V 506 Arg to Gln variation. Thromb Haemost. 1995;73(4):731–732. [PubMed] [Google Scholar]
- 20. de Moerloose P, Reber G, Bouvier CA. Spuriously low levels of protein C with a Protac activation clotting assay. Thromb Haemost. 1988;59(3):543. [PubMed] [Google Scholar]
- 21. Jennings I, Kitchen S, Cooper PC, Rimmer JE, Woods TA, Preston FE. Further evidence that activated protein C resistance affects protein C coagulant activity assays. Thromb Haemost. 2000;83(1):171–172. [PubMed] [Google Scholar]
- 22. Baglin T, Gray E, Greaves M, et al. Clinical guidelines for testing for heritable thrombophilia. Br J Haematol. 2010;149(2):209–220. doi:10.1111/j.1365-2141.2009.08022.x. [DOI] [PubMed] [Google Scholar]
- 23. Mackie I, Cooper P, Lawrie A, et al. Guidelines on the laboratory aspects of assays used in haemostasis and thrombosis. Int J Lab Hematol. 2013;35(1):1–13. doi:10.1111/ijlh.12004. [DOI] [PubMed] [Google Scholar]
- 24. Meijer P, Kluft C, Haverkate F, De Maat MP. The long-term within- and between-laboratory variability for assay of antithrombin, and proteins C and S: results derived from the external quality assessment program for thrombophilia screening of the ECAT Foundation. J Thromb Haemost. 2003;1(4):748–753. [DOI] [PubMed] [Google Scholar]
- 25. Marlar RA, Gausman JN. Laboratory testing issues for protein C, protein S, and antithrombin. Int J Lab Hematol. 2014;36(3):289–295. doi:10.1111/ijlh.12219. [DOI] [PubMed] [Google Scholar]
- 26. Korbie DJ, Mattick JS. Touchdown PCR for increased specificity and sensitivity in PCR amplification. Nat Protoc. 2008;3(9):1452–1456. doi:10.1038/nprot.2008.133. [DOI] [PubMed] [Google Scholar]
- 27. Reitsma PH, Bernardi F, Doig RG, et al. Protein C deficiency: a database of variations, 1995 update. On behalf of the Subcommittee on Plasma Coagulation Inhibitors of the Scientific and Standardization Committee of the ISTH. Thromb Haemost. 1995;73(5):876–889. [PubMed] [Google Scholar]
- 28. Tait RC, Walker ID, Reitsma PH, et al. Prevalence of protein C deficiency in the healthy population. Thromb Haemost. 1995;73(1):87–93. [PubMed] [Google Scholar]
- 29. Speker M, Balogh G, Oláh Z. Genetic heterogeneity of protein C deficiency in Hungary; genotype-phenotype correlations. Poster presented at ISTH2017; 2017. https://www.postersessiononline.eu/173580348_eu/congresos/ISTH2017/aula/-PB_1538_ISTH2017.pdf
- 30. Gandrille S, Goossens M, Aiach M. Scanning method to establish the molecular basis of protein C deficiencies. Hum Mutat. 1994;4(1):20–30. doi:10.1002/humu.1380040104. [DOI] [PubMed] [Google Scholar]
- 31. Gandrille S, Alhenc-Gelas M, Gaussem P, et al. Five novel variations located in exons III and IX of the protein C gene in patients presenting with defective protein C anticoagulant activity. Blood. 1993;82(1):159–168. [PubMed] [Google Scholar]
- 32. Reitsma PH, Poort SR, Allaart CF, Briet E, Bertina RM. The spectrum of genetic defects in a panel of 40 Dutch families with symptomatic protein C deficiency type I: heterogeneity and founder effects. Blood. 1991;78(4):890–894. [PubMed] [Google Scholar]
- 33. Conard J, Horellou MH, van Dreden P, et al. Homozygous protein C deficiency with late onset and recurrent coumarin-induced skin necrosis. Lancet. 1992;339(8795):743–744. [DOI] [PubMed] [Google Scholar]
- 34. Gandrille S, Aiach M. Identification of mutations in 90 of 121 consecutive symptomatic French patients with a type I protein C deficiency. The French INSERM Network on Molecular Abnormalities Responsible for protein C and protein S deficiencies. Blood. 1995;86(7):2598–2605. [PubMed] [Google Scholar]
- 35. Doig RG, Begley CG, McGrath KM. Hereditary protein C deficiency associated with variations in exon IX of the protein C gene. Thromb Haemost. 1994;72(2):203–208. [PubMed] [Google Scholar]
- 36. Dodojacek R, Hofler G, Leschnik B, Muntean W. A novel type of mutation at the propeptide cleavage site (AlA+1Thr) causing symptomatic protein C type II deficiency. Thromb Res. 2000;100(1):109–113. doi:10.1016/s0049-3848(00)00291-7. [DOI] [PubMed] [Google Scholar]
- 37. Caspers M, Pavlova A, Driesen J, et al. Deficiencies of antithrombin, protein C and protein S—practical experience in genetic analysis of a large patient cohort. Thromb Haemost. 2012;108(2):247–257 doi:10.1160/TH11-12-0875. [DOI] [PubMed] [Google Scholar]
- 38. Allaart CF, Poort SR, Rosendaal FR, Reitsma PH, Bertina RM, Briet E. Increased risk of venous thrombosis in carriers of hereditary protein C deficiency defect. Lancet. 1993;341(8838):134–138. [DOI] [PubMed] [Google Scholar]
- 39. Roshan TM, Stein N, Jiang XY. Comparison of clot-based and chromogenic assay for the determination of protein c activity. Blood Coagul Fibrinolysis. 2019;30(4):156–160. doi:10.1097/MBC.0000000000000806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Martos L, Fernandez-Pardo A, Lopez-Fernandez MF, et al. Identification of 58 variations (26 novel) in 94 of 109 symptomatic Spanish probands with protein C deficiency. Thromb Haemost. 2019;119(9):1409–1418. doi:10.1055/s-0039-1692440. [DOI] [PubMed] [Google Scholar]
- 41. Chalmers E, Cooper P, Forman K, et al. Purpura fulminans: recognition, diagnosis and management. Arch Dis Child. 2011;96(11):1066–1071. doi:10.1136/adc.2010.199919. [DOI] [PubMed] [Google Scholar]
- 42. Bertina RM. Protein C activity and antigen In: Jespersen J, Bertina RM, Haverkate F, eds. Laboratory Techniques in Thrombosis—A Manual. Dordrecht, the Netherlands: Springer; 1999:129–139. [Google Scholar]
- 43. Wojcik EG, Simioni P, d Berg M, Girolami A, Bertina RM. Mutations which introduce free cysteine residues in the Gla-domain of vitamin K dependent proteins result in the formation of complexes with alpha 1-microglobulin. Thromb Haemost. 1996;75(1):70–75. [PubMed] [Google Scholar]
- 44. Wang V, Vo KH, Mahajerin A. Qualitative protein C deficiency due to PROC c.577_579delAAG variation not detected by chromogenic assays: a case of intractable cerebral sinovenous thrombosis. Pediatr Blood Cancer. 2019;66(1): e27443 doi:10.1002/pbc.27443. [DOI] [PubMed] [Google Scholar]
- 45. Szecsi PB, Jorgensen M, Klajnbard A, Andersen MR, Colov NP, Stender S. Haemostatic reference intervals in pregnancy. Thromb Haemost. 2010;103(4):718–727. doi:10.1160/TH09-10-0704. [DOI] [PubMed] [Google Scholar]
- 46. Bovill EG, Tomczak JA, Grant B, et al. Protein CVermont: symptomatic type II protein C deficiency associated with two GLA domain mutations. Blood. 1992;79(6):1456–1465. [PubMed] [Google Scholar]
- 47. Guglielmone HA, Vides MA. A novel functional assay of protein C in human plasma and its comparison with amidolytic and anticoagulant assays. Thromb Haemost. 1992;67(1):46–49. [PubMed] [Google Scholar]
- 48. Vasse M, Borg JY, Monconduit M., Protein C: Rouen, a new hereditary protein C abnormality with low anticoagulant but normal amidolytic activities. Thromb Res. 1989;56(3):387–398. [DOI] [PubMed] [Google Scholar]
- 49. Marlar RA, Adcock DM, Madden RM. Hereditary dysfunctional protein C molecules (type II): assay characterization and proposed classification. Thromb Haemost. 1990;63(3):375–379. [PubMed] [Google Scholar]
- 50. Rentzsch P, Witten D, Cooper GM, Shendure J, Kircher M. CADD: predicting the deleteriousness of variants throughout the human genome. Nucleic Acids Res. 2019;47(D1):D886–D94. doi:10.1093/nar/gky1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Haemostasis and Thrombosis Task Force, British Committee for Standards in Haematology. Investigation and management of heritable thrombophilia. Br J Haematol. 2001;114(3):512–528. [DOI] [PubMed] [Google Scholar]
- 52. Whitlatch NL, Ortel TL. Thrombophilias: when should we test and how does it help? Semin Respir Crit Care Med. 2008;29(1):25–39. doi:10.1055/s-2008-1047560. [DOI] [PubMed] [Google Scholar]
- 53. Wypasek E, Undas A. Protein C and protein S deficiency—practical diagnostic issues. Adv Clin Exp Med. 2013;22(4):459–467. [PubMed] [Google Scholar]
- 54. Reda S. WJ, Rühl H, Müller J, Oldenburg J, Pötzsch B. Protac based measurement of protein C (PC) activity levels corresponds to thrombin-thrombomodulin-induced generation of activated PC (APC) in hereditary PC deficiency. Trans Med Hemother. 2018;45:73–74. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, supplement_Table_1 for Laboratory Limitations of Excluding Hereditary Protein C Deficiency by Chromogenic Assay: Discrepancies of Phenotype and Genotype by Holger Seidel, Bianca Haracska, Jennifer Naumann, Philipp Westhofen, Moritz Sebastian Hass and Johannes Philipp Kruppenbacher in Clinical and Applied Thrombosis/Hemostasis