Abstract
There is a lack of studies on anticoagulant plus antiplatelet therapy for acute ischemic stroke. The present study made a pilot effort to investigate the efficacy and safety of argatroban plus dual antiplatelet therapy (DAPT) in patients with acute posterior circulation ischemic stroke (PCIS). We retrospectively collected patients diagnosed with acute PCIS according to inclusion/exclusion criteria. According to treatment drugs, patients were divided into an argatroban plus DAPT group and a DAPT group. The primary efficacy end point was the proportion of early neurological deterioration (END). The primary safety outcome was symptomatic intracranial hemorrhage. All outcomes were compared between the 2 groups before and after propensity score matching (PSM). A total of 502 patients were enrolled in the study, including 35 patients with argatroban plus DAPT and 467 patients with DAPT. There was a higher National Institutes of Health Stroke Scale (NIHSS) score in the argatroban plus DAPT group than the DAPT group before PSM (3 vs 2, P = .017). Compared with the DAPT group, the argatroban plus DAPT group had no END (before PSM: 0% vs 6.2%, P = .250; after PSM: 0% vs 5.9%, P = .298). Argatroban plus DAPT yielded a significant decrease in the NIHSS score from baseline to 7 days after hospitalization, compared with that of the DAPT group before PSM (P = .032), but not after PSM (P = .369). No symptomatic intracranial hemorrhage was found in any patient. A short-term combination of argatroban with DAPT appears safe in acute minor PCIS.
Keywords: argatroban, dual antiplatelet, posterior circulation, acute ischemic stroke
Introduction
Acute posterior circulation ischemic stroke (PCIS) accounts for approximately 20% of acute ischemic stroke, and its prognosis is worse, with higher disability and higher mortality.1 Early intravenous thrombolysis or endovascular treatment is the most effective method for the treatment of acute ischemic stroke including PCIS and is strongly recommended by relevant guidelines.2,3 On the one hand, most of the patients with PCIS did not receive intravenous thrombolysis or endovascular intervention, due to time and technical limitations. On the other hand, the treatment is easily delayed due to the slight symptom and atypical sign in this population. For patients not receiving reperfusion treatment, all guidelines recommend antiplatelet treatment.3 However, the antiplatelet treatment is not satisfied due to the high rate of early neurological deterioration (END) and poor prognosis in acute PCIS,4 and the prognosis would be worse once END occurs.
Acute anticoagulant therapy for ischemic stroke has been controversial: Anticoagulant therapy can reduce the recurrence rate of ischemic stroke, the incidence of pulmonary embolism, and deep vein thrombosis but is offset by the increase in symptomatic intracranial hemorrhage (sICH).5–7 In this population of patients with PCIS, anticoagulant therapy also remains controversial. Several subgroup studies showed the efficacy of anticoagulant,8–10 but such efficacy was not observed in another subgroup study.11 Argatroban is a direct thrombin inhibitor with the advantages of rapid onset, short duration of action, low bleeding tendency, and no immunogenicity. Several studies had revealed its anticoagulant efficacy and safety in patients with acute ischemic stroke.12,13 Two recent clinical trials demonstrated the efficacy and safety of dual antiplatelet therapy (DAPT) in patients with acute minor stroke.14,15 However, there is a lack of study about DAPT for acute PCIS.
In contrast with the anterior circulation stroke, the PCIS has several characteristics such as difficult diagnosis, poor treatment effect, inadequate collateral circulation, and easy deterioration, leading to poor outcomes.16 It is reasonable that intensive antithrombotic treatment such as antiplatelet plus anticoagulant agents should be beneficial for treating acute ischemic stroke if there is no increasing bleeding risk, given that the intensive treatment can more effectively prevent the progression and reoccurrence of stroke. However, there is a lack of combination of antithrombotic studies for acute ischemic stroke. In our stroke center, DAPT or DAPT combined with argatroban was used for acute PCIS with minor or moderate neurological deficits as off-label therapy. The aim of this retrospective cohort study was to investigate the effect of argatroban in combination with DAPT versus DAPT in patients with acute PCIS.
Materials and Methods
Participants
We continuously collected patients diagnosed with PCIS at the Department of Neurology, General Hospital of Northern Theater Command from January 2014 to August 2018. Posterior circulation ischemic stroke was defined as a symptomatic infarct in the territory of the vertebral arteries, cerebellar or posterior cerebral arteries, or basilar artery, which was confirmed by head computed imaging (CT) or magnetic resonance imaging. The inclusion and exclusion criteria were as follows. Patients were enrolled if they met the following criteria: (1) age ≥18 years, (2) acute PCIS within 48 hours of onset, (3) the National Institutes of Health Stroke Scale (NIHSS) ≤10 at admission, and (4) antithrombotic regimen consisting of either argatroban combined with DAPT (aspirin and clopidogrel) or a dual antiplatelet combination (aspirin and clopidogrel). Patients were excluded if they met one of the following criteria: (1) received intravenous thrombolysis, endovascular intervention, and surgical treatment during hospitalization; (2) used defibration (such as batroxobin), other anticoagulation (such as dabigatran, rivaroxaban), or other platelet aggregation agents (such as ticagrelor, cilostazol); and (3) incomplete clinical data. According to the treatment regimen, the patients were divided into 2 groups: the argatroban combined with DAPT group and the DAPT group. Argatroban treatment was as follows: continuous infusion with a dose of 60 mg/d for 2 days, followed by 20 or 30 mg per day for 2 to 5 days. Dual antiplatelet therapy treatment was as follows: clopidogrel at an initial dose of 300 mg, followed by 75 mg daily plus 100 mg aspirin daily.
Data Collection
The following data were obtained from the electronic database: age; gender; smoking status (continuously smoking ≥1 cigarette a day for 6 months); alcohol consumption (drinking >2 U/d on average for men or >1 U/d on average for women); history of hypertension, diabetes, coronary heart disease, stroke, atrial fibrillation, or hyperlipidemia; NIHSS score at admission and 7 days after hospitalization; time of onset; Trial of Org 10172 in Acute Stroke Treatment classification17; gastrointestinal bleeding; and intracranial hemorrhage.
Efficacy and Safety Outcomes
The primary efficacy outcome was END, which is defined as a 2 points or greater increase in NIHSS score at 7 days after hospitalization, compared with baseline.18,19 The secondary efficacy outcome was NIHSS change at baseline, compared with that at 7 days after hospitalization and the proportion of modified Rankin Scale (mRS) scores of 0 to 2 at 7 days after hospitalization.
The primary safety outcome was sICH, which is defined as imaging evidence of any hemorrhage on CT combined with an NIHSS score increase of ≥4 points.20 Secondary safety outcomes included gastrointestinal bleeding and asymptomatic intracranial hemorrhage (aICH). Gastrointestinal hemorrhage was defined as any evidence of gastrointestinal bleeding. Asymptomatic intracranial hemorrhage is defined as imaging evidence of hemorrhage on CT (hemorrhagic transformation), but without clinical deterioration.21
Statistical Analysis
We compared the baseline characteristics, safety, and efficacy of the 2 groups with SPSS 20.0 statistical software analysis. Continuous variables are presented as mean ± standard deviation (normal distribution) and as the median with interquartile range (skewed distribution). The Kolmogorov-Smirnov test was used to test the normality of the data. The normal distribution was tested by the t test, while the skewed distribution was tested by the Mann-Whitney rank-sum test. Categorical variables are presented as percentages. Statistical comparisons between the 2 groups were performed using the χ2 test, or Fisher exact test was performed at a small expected frequency.
We used a propensity score matching (PSM) algorithm including all baseline characteristics to calculate the propensity score for each patient. Then the patients with argatroban combined with the DAPT were matched with the dual antiplatelet patients using the nearest neighbor 1:2 matching algorithm to obtain baseline data balance and compare the safety and efficacy of the 2 groups again. P <.05 was considered statistically significant.
Results
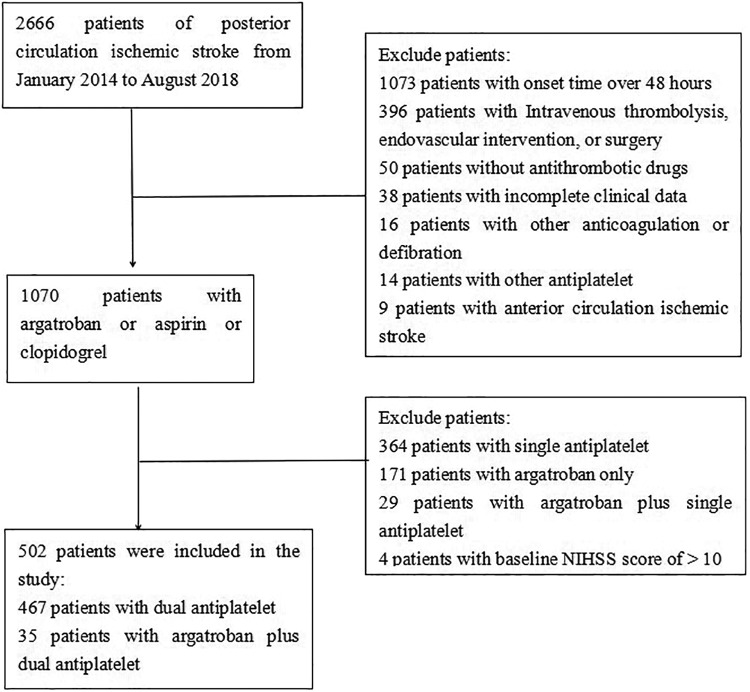
A total of 2666 cases of PCIS were screened. As shown in Figure 1, we excluded 2164 patients. Finally, 502 patients were recruited to the current study, including 35 patients with argatroban plus DAPT and 467 patients with DAPT.
Figure 1.
Flow diagram of the participants selection.
The main baseline characteristics are presented in Table 1. The patients in the argatroban plus DAPT group had a higher mean baseline NIHSS score than the dual antiplatelet group (P = .017). As shown in Table 2, there was no END rate in the argatroban plus DAPT group, compared with the DAPT group (0% vs 6.2%, P = .250). The argatroban plus DAPT produced a significant decrease in NIHSS score from baseline to 7 days after hospitalization, compared with the NIHSS score of the DAPT group (P = .032). There was no significant difference in the proportion of mRS scores of 0 to 2 at 7 days after hospitalization between the 2 groups (77.1% vs 78.2%, P = .889). No sICH was found in any patient. There were no significant differences in gastrointestinal bleeding (0% vs 1.7%, P = 1.000) or aICH (0% vs 0.9%, P = 1.000) between the 2 groups.
Table 1.
Baseline Characteristics of Patients Prior to Matching.
| Argatroban + DAPT (n = 35) | DAPT (n = 467) | P Value | |
|---|---|---|---|
| Age, years, mean ± SD | 61.7 ± 9.7 | 61.2 ± 12.2 | .788 |
| Male, n (%) | 26 (74.3) | 344 (73.7) | .936 |
| Hypertension, n (%) | 24 (68.6) | 303 (64.9) | .659 |
| Diabetes, n (%) | 13 (37.1) | 144 (30.8) | .438 |
| History of CAD, n (%) | 5 (14.3) | 70 (15.0) | .910 |
| History of AF, n (%) | 1 (2.9) | 23 (4.9) | .887 |
| History of IS/TIA, n (%) | 12 (34.3) | 113 (34.2) | .183 |
| History of HS, n (%) | 0 (0) | 11 (2.1) | 1.000 |
| Hypercholesterolemia, n (%) | 18 (51.4) | 204 (43.7) | .374 |
| Smoking, n (%) | 15 (42.9) | 188 (40.3) | .762 |
| Drinking, n (%) | 14 (40.0) | 158 (33.8) | .458 |
| Time of onset (hours), median (IQR) | 18.0 (10.0-29.0) | 24.0 (13.0-34.0) | .117 |
| Baseline NIHSS, median (IQR) | 3 (2-6) | 2 (1-4) | .017 |
| SBP, mm Hg, mean ± SD | 154.9 ± 20.2 | 151.8 ± 18.6 | .513 |
| DBP, mm Hg, mean ± SD | 90.1 ± 15.0 | 86.4 ± 10.8 | .159 |
| BMI, kg/m2, mean ± SD | 25.94 ± 2.11 | 25.11 ± 3.20 | .199 |
| BG, mmol, mean ± SD | 6.91 ± 1.97 | 8.73 ± 26.5 | .703 |
| TG, mmol, mean ± SD | 2.23 ± 2.26 | 2.10 ± 6.82 | .909 |
| TC, mmol, mean ± SD | 4.38 ± 1.37 | 4.52 ± 1.17 | .488 |
| LDL-C, mmol, mean ± SD | 2.56 ± 0.85 | 3.06 ± 7.43 | .693 |
| HDL-C, mmol, mean ± SD | 1.04 ± 0.24 | 1.04 ± 0.26 | .999 |
| TOAST classification, n (%) | .252 | ||
| Large-artery atherosclerosis | 18 (51.4) | 162 (34.7) | |
| Cardioembolism | 1 (2.9) | 10 (2.1) | |
| Small-artery occlusion | 11 (31.4) | 200 (42.8) | |
| Undetermined cause | 5 (14.3) | 95 (20.3) |
Abbreviations: AF, atrial fibrillation; BG, blood glucose; BMI, body mass index; CAD, coronary artery disease; DAPT, dual antiplatelet therapy; DBP, diastolic blood pressure; HDL-C, high density lipoprotein cholesterol; HS, hemorrhagic stroke; IQR, interquartile range; IS, ischemic stroke; LDL-C, low-density lipoprotein cholesterol; NIHSS, National Institute of Health Stroke Scale; SBP, systolic blood pressure; SD, standard deviation; TC, total cholesterol; TG, triglyceride; TIA, transient ischemic attack; TOAST, Trial of Org 10172 in Acute Stroke Treatment.
Table 2.
Outcomes of Unmatched Patients in the 2 Groups.
| Argatroban + DAPT (n = 35) | DAPT (n = 467) | P Value | |
|---|---|---|---|
| END, n (%) | 0 (0) | 29 (6.2) | .250 |
| Change of NHISS (IQR) | 1 (0-2) | 0 (0-1) | .032 |
| mRS scores of 0-2, n (%), 7 days after hospitalization | 27 (77.1) | 365 (78.2) | .889 |
| Gastrointestinal bleeding, n (%) | 0 (0) | 8 (1.7) | 1.000 |
| sICH, n (%) | 0 (0) | 0 (0) | 1.000 |
| aICH, n (%) | 0 (0) | 4 (0.9) | 1.000 |
Abbreviations: aICH, asymptomatic intracranial hemorrhage; DAPT, dual antiplatelet therapy; END, early neurological deterioration; IQR, interquartile range; mRS, modified Rankin Scale; sICH, symptomatic intracranial hemorrhage.
After PSM, 34 patients receiving argatroban plus DAPT were matched to 68 patients receiving DAPT, without any difference in the baseline characteristics between groups (Table 3). As shown in Table 4, the END rate of the argatroban plus DAPT group was lower than that of the DAPT group but did not reach statistical significance (0% vs 5.9%, P = .298). Additionally, there was no difference in the change in the NIHSS score (P = .369), the proportion of mRS scores of 0 to 2 at 7 days after hospitalization (76.5% vs 73.5%, P = .748), gastrointestinal bleeding (0% vs 1.5%, P = 1.000), and aICH (P = 1.000).
Table 3.
Baseline Characteristics of Propensity Score-Matched Patients.
| Argatroban + DAPT (n = 34) | DAPT (n = 68) | P Value | |
|---|---|---|---|
| Age, years, mean ± SD | 61.7 ± 9.8 | 61.7 ± 10.9 | .989 |
| Male, n (%) | 25 (73.5) | 49 (72.1) | 1.000 |
| Hypertension, n (%) | 23 (67.6) | 44 (64.7) | .941 |
| Diabetes, n (%) | 13 (38.2) | 23 (33.8) | .826 |
| History of CAD, n (%) | 5 (14.7) | 5 (7.4) | .450 |
| History of AF, n (%) | 1 (2.9) | 1 (1.5) | 1.000 |
| History of IS/TIA, n (%) | 11 (32.4) | 17 (25) | .582 |
| History of HS, n (%) | 0 | 0 | |
| Hypercholesterolemia, n (%) | 17 (50) | 38 (55.9) | .726 |
| Smoking, n (%) | 14 (41.2) | 30 (44.1) | .944 |
| Drinking, n (%) | 14 (41.2) | 30 (44.1) | .944 |
| Time of onset (hours), mean ± SD | 21.4 ± 14.0 | 21.5 ± 12.1 | .952 |
| Baseline NIHSS, mean ± SD | 3.6 ± 2.4 | 3.6 ± 2.4 | .954 |
| TOAST classification, n (%) | .943 | ||
| Large-artery atherosclerosis | 17 (50) | 37 (54.4) | |
| Cardioembolism | 1 (2.9) | 1 (1.5) | |
| Small-artery occlusion | 11 (32.4) | 21 (30.9) | |
| Undetermined cause | 5 (14.7) | 9 (13.2) |
Abbreviations: AF, atrial fibrillation; CAD, coronary artery disease; DAPT, dual antiplatelet therapy; HS, hemorrhagic stroke; IS, ischemic stroke; IQR, interquartile range; NIHSS, National Institute of Health Stroke Scale; SD, standard deviation; TIA, transient ischemic attack.
Table 4.
Outcomes of Propensity Score-Matched Patients in the 2 Groups.
| Argatroban + DAPT (n = 34) | DAPT (n = 68) | P Value | |
|---|---|---|---|
| END, n (%) | 0 | 4 (5.9) | .298 |
| mRS scores of 0-2, n (%), 7 days after hospitalization | 26 (76.5) | 50 (73.5) | .748 |
| Change of NHISS (IQR) | 1 (0-1.3) | 0 (0-2) | .369 |
| Gastrointestinal bleeding, n (%) | 0 | 1 (1.5) | 1.000 |
| sICH, n (%) | 0 | 0 | 1.000 |
| aICH, n (%) | 0 | 0 | 1.000 |
Abbreviations: aICH, asymptomatic intracranial hemorrhage; DAPT, dual antiplatelet therapy; END, early neurological deterioration; IQR, interquartile range; mRS, modified Rankin Scale; sICH, symptomatic intracranial hemorrhage.
Discussion
The present study determined the efficacy and safety of argatroban plus DAPT versus DAPT in the treatment of acute minor PCIS. To the best of our knowledge, this is the first study to investigate the treatment with argatroban plus DAPT for acute PCIS. The results suggest that short-term application of argatroban combined with dual antiplatelet seems safe and may more effectively prevent END, compared with dual antiplatelet alone in this population.
Regarding the antithrombotic treatment for PCIS, there is no best recommendation from the guidelines. Some studies have investigated anticoagulant therapy, but the results remain controversial. For example, several studies have shown the possible benefit of anticoagulation therapy for PCIS: The subgroup results of the Fraxiparine in Ischemic Stroke (FISS-tris) trial showed that low-molecular-weight heparin is associated with better outcomes than those of aspirin treatment in patients with posterior circulation stenosis8; compared with aspirin, low-molecular-weight heparin reduced END and improved the 6-month outcome of patients with posterior circulation or symptomatic basilar artery stenosis.9,10 However, the Trial of Tinzaparin in Acute Ischemic Stroke Trial (TAIST) found that treatment with tinzaparin within 48 hours of acute ischemic stroke did not improve functional outcomes of patients compared with aspirin therapy.11 Recently, clopidogrel in high-risk patients with acute non-disabling cerebrovascular Events (CHANCE)14 and platelet-oriented inhibition in new TIA and minor ischemic stroke (POINT)15 studies showed that DAPT can benefit patients with minor stroke, but it is not clear whether DAPT is beneficial to patients with PCIS. Several studies have shown the possible efficacy of argatroban combined with antiplatelet drugs in the treatment of ischemic stroke. A retrospective study showed that argatroban combined with aspirin improved the discharge NIHSS score of patients with acute ischemic stroke without significant symptomatic cerebral hemorrhage.22 Another study showed that, compared with argatroban plus aspirin, argatroban combined with DAPT may prevent the incidence of progressing stroke.23 It is reasonable that intensive antithrombotic treatment should be beneficial to acute ischemic stroke if there is no increasing bleeding risk, given that intensive treatment can more effectively prevent the progression and reoccurrence of stroke.
Based on this thought, we performed a retrospective study. The present results show that, compared with DAPT alone, short-term application of argatroban plus DAPT for acute PCIS may safely prevent END. This study is different from the abovementioned investigations. First, argatroban plus DAPT versus DAPT alone was used in the present study. Argatroban is a selective thrombin inhibitor. It can directly inhibit thrombin in blood clots and has the potential advantages of rapid onset, short duration of action, low bleeding tendency, no immunogenicity, and clinical monitoring of anticoagulation levels.24,25 We argue that the combination should produce a better antithrombotic effect. The results show that END was 0% in the combination group versus 6.2% in the DAPT group. It is interesting to note that the rate of END in the DAPT group was relatively low, compared with that in previous reports,9,10 which may be due to the dual antiplatelet effect. Anticoagulation combined with antiplatelet therapy was chosen based on more possible etiology of artery atherosclerosis in PCIS.26,27 This kind of combined therapy has been routinely used for patients with acute coronary artery syndrome, in whom the etiology is always arterial atherosclerosis.28 In this study, nearly half of the large-artery atherosclerosis etiology was observed in the argatroban plus DAPT group, with nearly one-third in the DAPT group. Second, the study participants were patients with PCIS. Posterior circulation ischemic stroke is different from anterior circulation stroke, with the former showing the more misdiagnosis, delayed treatment, poor collateral circulation, and higher END leading to poor outcomes.16 Studies of antithrombotic treatment of PCIS are relatively few. A previous study showed a low risk of intracranial hemorrhage after intravenous thrombolysis in PCIS, compared with the anterior circulation ischemic stroke.29 Based on these facts, we argue that combined antithrombotic treatment should be suitable and safe in acute PCIS. Third, patients with minor to moderate PCIS (NIHSS score ≤10 points) were enrolled in the study, which is different from the minor stroke population in CHANCE and POINT studies.14,15 Fourth, we chose patients within 48 hours after onset. It is well known that most recurrence or deterioration of stroke occurs in the initial 48 to 72 hours after symptom onset,30–33 and early intervention in patients with transient ischemic attack or minor stroke produces better outcomes than delayed intervention.34 In the present study, the 48-hour time window covered more patients who potentially benefited from the dual antiplatelet plus argatroban, which is different from the CHANCE and POINT studies.14,15 Finally, a short-term combination was used in the present study. We argue that on the one hand, the combination treatment administered for 3 to 5 days is enough to cover the time window of high END, and on the other hand, the short-term combination will decrease the risk of bleeding events. Based on the above discussion, we believe that a short-term treatment with argatroban plus dual antiplatelet agents may be a suitable choice for populations with minor to moderate PCIS who have missed reperfusion therapy.
Our study has several limitations. First, it was a single-center retrospective study, and the sample size of the argatroban plus DAPT group was relatively small. Second, there is a possible selection bias because doctors may be more likely to apply argatroban plus DAPT to patients with a higher risk of stroke progression based on clinical experience. Third, the dose of argatroban was adjusted to a target-activated partial thromboplastin time (APTT) of 1.75 × baseline. This study did not investigate the proportion of patients with APTT compliance or report the total dose and time of argatroban administration. The dosage and time of argatroban administration was determined according to the patient’s condition. Fourth, the neurological deterioration might be biased due to choosing patients with NIHSS scores below 10, which excluded some patients with higher risk of intracranial hemorrhage. Finally, long-term stroke outcomes were not evaluated due to the retrospective nature of the study.
Conclusion
The short-term use of argatroban combined with DAPT for acute minor PCIS seems safe. The study should be helpful for further research to investigate the efficacy and safety of this treatment regimen.
Footnotes
Authors’ Note: H.S.C. designed the study and revised the manuscript. L.S.Z. drafted the manuscript. L.S.Z., X.Q.L., and Z.H.Z. collected the data. All authors read and approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The work was supported by grant from the Science and Technology Project Plan of Liaoning Province [grant number 2018225023].
ORCID iD: Hui-Sheng Chen  https://orcid.org/0000-0002-7486-1992
https://orcid.org/0000-0002-7486-1992
References
- 1. Schonewille WJ, Wijman CA, Michel P, et al. Treatment and outcomes of acute basilar artery occlusion in the Basilar Artery International Cooperation Study (BASICS): a prospective registry study. Lancet Neurol. 2009;8(8):724–730. [DOI] [PubMed] [Google Scholar]
- 2. Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49(3):e46–e99. [DOI] [PubMed] [Google Scholar]
- 3. Jauch EC, Saver JL, Adams HP, Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870–947. [DOI] [PubMed] [Google Scholar]
- 4. Kim SH, Lee J-Y, Kim DH, et al. Factors related to the initial stroke severity of posterior circulation ischemic stroke. Cerebrovasc Dis. 2013;36(1):62–68. [DOI] [PubMed] [Google Scholar]
- 5. Sandercock PA, Counsell C, Kamal AK. Anticoagulants for acute ischaemic stroke. Cochrane Database Syst Rev. 2008;(4):CD000024. [DOI] [PubMed] [Google Scholar]
- 6. Whiteley WN, Adams HP, Jr, Bath PM, et al. Targeted use of heparin, heparinoids, or low-molecular-weight heparin to improve outcome after acute ischaemic stroke: an individual patient data meta-analysis of randomised controlled trials. Lancet Neurol. 2013;12(6):539–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sajldercock PA, Counsell C, Kane EJ. Anticoagulants for acute ischaemic stroke. Cochrane Database Syst Rev. 2015;(3):CD000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang QS, Chen C, Chen XY, et al. Low-molecular-weight heparin versus aspirin for acute ischemic stroke with large artery occlusive disease: subgroup analyses from the Fraxiparin in Stroke Study for the treatment of ischemic stroke (FISS-tris) study. Stroke. 2012;43(2):346–349. [DOI] [PubMed] [Google Scholar]
- 9. Yi X, Chi W, Wang C, Zhang B, Lin J. Low-molecular-weight heparin or dual antiplatelet therapy is more effective than aspirin alone in preventing early neurological deterioration and improving the 6-month outcome in ischemic stroke patients. J Clin Neurol. 2015;11(1):57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yi X, Lin J, Wang C, Zhang B, Chi W. Low-molecular-weight heparin is more effective than aspirin in preventing early neurologic deterioration and improving six-month outcome. J Stroke Cerebrovasc Dis. 2014;23(6):1537–1544. [DOI] [PubMed] [Google Scholar]
- 11. Bath PM, Lindenstrom E, Boysen G, et al. Tinzaparin in acute ischaemic stroke (TAIST): a randomised aspirin-controlled trial. Lancet. 2001;358(9283):702–710. [DOI] [PubMed] [Google Scholar]
- 12. Andrew D, Andrei V, Lyden P, et al. The argatroban and tPA stroke study: final results of a pilot safety study. Stroke. 2012;43(3):770–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Andrew D, Gary A, Shen L, et al. Randomized, multicenter trial of ARTSS-2 (Argatroban With Recombinant Tissue Plasminogen Activator for Acute Stroke). Stroke. 2017;48(6):1608–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369(1):11–19. [DOI] [PubMed] [Google Scholar]
- 15. Johnston SC, Easton JD, Farrant M, et al. Clopidogrel and aspirin in acute Ischemic stroke and high-risk TIA. N Engl J Med. 2018;379(3):215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Merwick Á, Werring D. Posterior circulation ischaemic stroke. BMJ. 2014;348:g3773. [DOI] [PubMed] [Google Scholar]
- 17. Adams HP, Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. Stroke. 1993;24(1):35–41. [DOI] [PubMed] [Google Scholar]
- 18. Kwon HM, Lee YS, Bae HJ, Kang DW. Homocysteine as a predictor of early neurological deterioration in acute ischemic stroke. Stroke. 2014;45(3):871–873. [DOI] [PubMed] [Google Scholar]
- 19. Kanamaru T, Suda S, Muraga K, et al. Albuminuria predicts early neurological deterioration in patients with acute ischemic stroke. J Neurol Sci. 2017;372:417–420. [DOI] [PubMed] [Google Scholar]
- 20. Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet. 1998;352(9136):1245–1251. [DOI] [PubMed] [Google Scholar]
- 21. Goyal N, Tsivgoulis G, Zand R, et al. Systemic thrombolysis in acute ischemic stroke patients with unruptured intracranial aneurysms. Neurology. 2015;85(17):1452–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen L, Cao S, Yang J. Argatroban plus aspirin versus aspirin in acute ischemic stroke. Neurol Res. 2018;40(10):862–867. [DOI] [PubMed] [Google Scholar]
- 23. Nishi R, Mano T, Kobayashi Y, Matsuo K, Kobayashi Y. Argatroban, aspirin, and clopidogrel combination therapy for acute penetrating artery infarction: a pilot study. Brain Nerve. 2016;68(2):181–189. [DOI] [PubMed] [Google Scholar]
- 24. Kobayashi S, Tazaki Y. Effect of the thrombin inhibitor argatroban in acute cerebral thrombosis. Semin Thromb Hemost. 1997;23(6):531–534. [DOI] [PubMed] [Google Scholar]
- 25. Ikoma H. Development of argatroban as an anticoagulant and antithrombin agent in Japan. Pathophysiol Haemost Thromb. 2002;32(suppl 3):23–28. [DOI] [PubMed] [Google Scholar]
- 26. Gorelick PB, Wong KS, Bae HJ, Pandey DK. Large artery intracranial occlusive disease: a large worldwide burden but a relatively neglected frontier. Stroke. 2008;39(8):2396–2399. [DOI] [PubMed] [Google Scholar]
- 27. Suh DC, Lee SH, Kim KR, et al. Pattern of atherosclerotic carotid stenosis in Korean patients with stroke: different involvement of intracranial versus extracranial vessels. AJNR Am J Neuroradiol. 2003;24(2):239–244. [PMC free article] [PubMed] [Google Scholar]
- 28. Davies MJ. The pathophysiology of acute coronary syndromes. Heart. 2000;83(3):361–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tong X, Liao X, Pan Y, et al. Intravenous thrombolysis is more safe and effective for posterior circulation stroke: data from the Thrombolysis Implementation and Monitor of Acute Ischemic Stroke in China (TIMS-China). Medicine (Baltimore). 2016;95:e38–e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Johnston SC, Gress DR, Browner WS, Sidney S. Short-term prognosis after emergency department diagnosis of TIA. JAMA. 2000;284(22):2901–2906. [DOI] [PubMed] [Google Scholar]
- 31. Lovett JK, Dennis MS, Sandercock PA, et al. Very early risk of stroke after a first transient ischemic attack. Stroke. 2003;34(8):e138–e140. [DOI] [PubMed] [Google Scholar]
- 32. Hill MD, Yiannakoulias N, Jeerakathil T, et al. The high risk of stroke immediately after transient ischemic attack: a population-based study. Neurology. 2004;62(11):2015–2020. [DOI] [PubMed] [Google Scholar]
- 33. Coull AJ, Lovett JK, Rothwell PM; Oxford Vascular Study. Population based study of early risk of stroke after transient ischaemic attack or minor stroke: implications for public education and organisation of services. BMJ. 2004;328(435):326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rothwell P M, Giles MF, Chandratheva A, et al. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet. 2007;370(9596):1432–1442. [DOI] [PubMed] [Google Scholar]