Abstract
Background:
Specific clinical risk factors linked to transient ischemic attack (TIA) could affect functional ambulatory outcome following thrombolytic therapy in patients having ischemic stroke with a prior TIA (TIA-ischemic stroke). This issue was investigated in this study.
Methods:
We retrospectively analyzed data from 6379 ischemic stroke patients of which 1387 presented with an antecedent TIA prior to onset of stroke. We used logistic regression model to identify demographic and clinical risk factors that are associated with functional ambulatory outcome in patients with TIA-ischemic stroke treated with thrombolytic therapy.
Results:
In a population of TIA-ischemic stroke who received recombinant tissue plasminogen activator, patients with a history of stroke (odds ratio [OR] = 3.229, 95% confidence interval [CI] = 1.494-6.98, P = .003) were associated with increasing odds of improvement in functional ambulation, while the female gender (OR = 0.462, 95% CI = 0.223-0.956, P = .037) was associated with reducing odds of improvement. In the non-TIA group, dyslipidemia (OR = 1.351, 95% CI = 1.026-1.781, P = .032) and blood glucose (OR = 1.003, 95% CI = 1.0-1.005, P = .041) were associated with the increasing odds of improvement while older patients (OR = 0.989, 95% CI = 0.98-0.999, P = .029) with heart failure (OR = 0.513, 95% CI = 0.326-0.808, P = .004) and higher lipid level (OR = 0.834, 95% CI = 0.728-0.955, P = .009) were associated with reducing odds of improvement in ambulation.
Conclusion:
In a population of TIA-ischemic stroke with thrombolytic therapy and a clearly defined TIA without focal ischemic injury, regardless of associated clinical risk factors, a TIA prior to a stroke is not associated with reducing odds of improved ambulatory outcome, except in female patients with TIA-ischemic stroke.
Keywords: ambulation, thrombolytics, ischemic stroke, rtPA
Introduction
The symptoms of a transient ischemic attack (TIA) include motor weakness, gait disturbance, and loss of coordination.1 The presence of motor impairments during a TIA is a high-risk clinical characteristic for a subsequent stroke.2 The association between the presence of motor impairments and a TIA prior to a subsequent stroke has been investigated.1 Findings indicate that motor impairments during a TIA predispose individuals to a greater risk for stroke, and that the odds of a subsequent stroke are greater in individuals who experience motor impairments during a TIA compared with those who do not experience motor impairments.1 Between 7% and 40% of patients who have had a stroke are known to present with an antecedent of a TIA,3 prior to the stroke.4 Although a TIA is clinically defined as a functional, ischemic neurological lesion without structural deficit that may be associated with a predisposing conditioning,5,6 whether or not patients having ischemic stroke with a prior TIA preceding the onset of stroke who received recombinant tissue plasminogen activator (rtPA) show improvement in ambulation is not fully understood.
It has been shown that the assessment of ambulation following stroke may provide some cues on the degree of lesion in the upper motor neurons that maybe associated with a TIA and/or a stroke.5,7,8 For this reason, the ability to accurately predict functional recovery of motor impairments in ischemic stroke with a previous TIA will enhance the development of appropriate motor recovery strategy for post-treatment management. Several prognostic measures have been used to predict functional outcomes following thrombolytic therapy in patients with ischemic stroke.9–13 These measures are based on clinical characteristics of the patient and provide cumulative evaluations for the management of poststroke treatment care. Accurate predictions of ambulatory functions are needed as well, so that clinicians can help patients set motor recovery goals to improve activities of daily living14–18 following thrombolysis therapy.
The presence or absence of specific clinical risk factors in a population of ischemic stroke with a recent TIA is thought to be compelling determinants of the increased odds of motor recovery following thrombolytic therapy.6 Although this clinical proposition requires empirical data for validation, to date, no study has quantified the association of clinical risk factors on population of ischemic stroke with a prior TIA (TIA-ischemic stroke) following thrombolytic therapy. It has been shown that specific comorbidities may influence the benefits of thrombolytic therapy in patients with ischemic stroke.19–25 In addition, we know that some risk factors for TIA and ischemic stroke including hypertension (HTN), diabetes, and heart diseases20,25–31 may interact with thrombolytic therapy to significantly modulate ambulation and affect treatment outcome. In this case, one possibility is that perhaps specific clinical risk factors in patients with TIA-ischemic stroke may increase the odds of good or poor functional ambulation outcome because of the resulting interactive effect with thrombolytic therapy. The objective of this study is to determine whether specific risk factors are associated with an improvement or nonimprovement in functional ambulation in a population of TIA-ischemic stroke. Knowledge of the risk factors for a TIA-ischemic stroke provides the opportunity to develop interventions that could enhance the functional ambulatory outcome following thrombolytic therapy.
Methods
This retrospective study that was institutional review board approved consisted of patients with acute ischemic stroke who were admitted to the Greenville Health System in Greenville, South Carolina, United States between January 2010 and June 2016. Patients with stroke who were included in the analysis presented with an ischemic stroke based on computed tomography (CT) or brain magnetic resonance imaging (MRI). The stroke registry used in this study has been described in previous studies.28,30,32,33 We collected data on patients’ medical history including dyslipidemia, coronary artery disease (CAD), atrial fibrillation/atrial flutter, carotid stenosis, pregnancy, depression, diabetes, drug or alcohol abuse, family history of stroke, congestive heart failure, HTN, migraine, obesity, prior stroke, prior TIA, prosthetic heart valve, peripheral vascular disease (PVD), chronic renal disease, sickle cell, sleep apnea, hormone replacement therapy, and history or smoking. In addition, we collected data on ambulation. Scores ranged from 0 to 3 where patients not able to ambulate were scored “1,” patients who were able to ambulate with assistance were scored “2,” those were able to ambulate independently were scored “3,” and those with no data were scored “0.” This stratification provides the opportunity to analyze functional ambulation with patients demonstrating an improvement or no improvement at discharge compared to ambulation upon admission. The validity and efficacy of this scoring system have been described in a previous study.29
For the data on TIA, we extracted data from patients in which the presence of an acute brain lesion was determined with a combined early diffusion-weighted magnetic resonance imaging (DW-MR) and perfusion-weighted MRI (PW-MR) completed within 24 hours of symptom onset. It identified the presence of a cerebral ischemic lesion in patients who may present with a suspected hemispheric TIA prior to the onset of stroke or who had a history of a prior TIA. Data from patients with ischemic stroke who presented with TIA without focal ischemic lesions were considered for our analysis. This is based on American Heart Association and American Stroke Association guideline of TIA as a transient focal neurological deficit lasting less than 24 hours without acute infarction.34 Only documented cases following neurological examination with brain DW-MR and PW-MR after episode of TIA were included. Patients not fulfilling these criteria or if the symptoms were uncertain were not included in our data analysis as TIA. We used patients with documented evidence of previous TIA prior to the onset of recent ischemic stroke. Data regarding the ambulatory status for each patient were tracked and collected on admission, during admission, and after discharge. Moreover, patient demographic variables were also collected including age, race, and gender. Data were also collected on body mass index, medication history, and stroke severity (National Institutes of Health Stroke Scale [NIHSS]) score.
Statistical Analysis
We used SPSS Statistics Software version 25.0 (Chicago, Illinois) for all our data analysis. Bivariate group comparisons of baseline demographic and clinical characteristics were determined for patients having ischemic stroke with TIA prior to the acute ischemic stroke (patients with TIA-ischemic stroke) and with those who did not have a TIA (non-TIA). In the bivariate analysis, all continuous variables were analyzed using a Student t test, while discrete variables were analyzed using a Pearson chi-square test. The population was then subdivided into patients with TIA-ischemic stroke and non-TIA based on treatment with rtPA. A multivariable logistic regression was then used to examine the clinical factors that are associated with functional ambulatory outcome of patients with TIA-ischemic stroke and non-TIA patients’ population. In this analysis, our primary outcome was demographic or clinical risk factors that are significantly associated with improvements or nonimprovements in ambulation in rtPA-treated patients. This approach allowed us to identify variables that were associated with an improvement or nonimprovement in ambulation among TIA-ischemic stroke group compared with non-TIA group following thrombolytic therapy.
Independent ambulation was analyzed and used to develop a model for functional outcome. This is significant considering that recovery of motor damage after ischemic stroke may be related to the degree of recovery in affected corticospinal tract and general motoric functions after stroke. To compute functional ambulatory improvement, a new variable was determined from the existing data. A value of “1” was associated with an improvement in ambulation from the time of admission to the time of discharge, while “0” was associated with a no improvement. This was used to build a model for improved functional outcomes for patients with stroke who received rtPA. The dependent variable was ambulation while independent variables were clinical risk factors stratified by TIA status (TIA-ischemic stroke and non-TIA) in rtPA-treated patients. In building the logistic model, we did not consider the P values in the univariate analysis, rather our interest was on the clinical knowledge which supersedes univariate statistical analysis of P values in selecting variables for building our logistic model.35 This approach allowed us to consider all factors identified in the univariate analysis including age and sex in the backward selection method. The backward selection method incorporated all our variables and removes the least significant one at each step of the model. We tested the validity of our model using a Hosmer-Lemeshow test, while the overall correct classification percentage and the area under the receiver operating curve (AUROC) for score prediction was determined to test the sensitivity, specificity, and accuracy of the model. Odds ratio (OR) values were considered to predict the increasing or reducing odds of improvements or no improvement in ambulation, and significance was set at the probability level of .05.
Results
Data from a total of 6379 patients with ischemic stroke were collected in the retrospective data analysis. Of this 1387 presented with a TIA, while 4992 did not have a TIA prior to stroke. The demographic and clinical variables for the TIA-ischemic stroke and non-TIA groups are presented in Table 1. The TIA-ischemic stroke group were older (69.41 ± 14.22 vs 66.88 ± 14.79), white (82.6% vs 78.0%), and female (54.0% vs 50.9%). They presented with higher rates of CAD (35.2% vs 29.6%), coronary artery stenosis (10.0% vs 5.3%), depression (15.0% vs 13.0%), and dyslipidemia (60.3% vs 49.1%), but lower rates of alcohol abuse (3.2% vs 6.5%). The TIA-ischemic stroke group were more likely to have HTN (82.6% vs 77.8%), a history of previous stroke (35.5% vs 24.6%), higher rates of PVD (9.8% vs. 7.1%), but lower rates for history of smoking (20.8% vs 27.6%). This group was more likely to be taking an anti-HTN medication (75.8% vs 68.3%), cholesterol reducer (56.1% vs 42.9%), diabetes medication (30.0% vs 27.2%), and antidepressants (15.4% vs 12.8%). They presented with lower NIHSS scores (5.4 ± 6.65 vs 8.33 ± 8.28), lower total cholesterol (168.19 ± 45.59 vs 172.25 ± 52.17), lower low-density cholesterol levels (99.25 ± 38.42 vs 105.06 ± 41.28), lower blood glucose (138 ± 70.36 vs 148.5 ± 82.77), a lower heart rate (78.86 ± 15.95 vs 151.71 ± 29.39), and lower diastolic blood pressure (80.46 ± 18.1 vs 82.21 ± 18.63), but higher high-density cholesterol levels (42.81 ± 14.41 vs 41.73 ± 13.8).
Table 1.
Comparison of Demographics and Clinical Characteristics of Patients Having Acute Ischemic Stroke With a History of TIA and Without a History of TIA.a
| Characteristic | Acute Ischemic Stroke With TIA | Acute Ischemic Stroke Without TIA | P Value |
|---|---|---|---|
| Number of patients | 1387 | 4992 | |
| Age group: No. (%) | |||
| <50 | 134 (9.7) | 625 (12.5) | <.001b,c |
| 50-59 | 198 (14.3) | 933 (18.7) | |
| 60-69 | 327 (23.6) | 1190 (23.8) | |
| 70-79 | 329 (23.7) | 1114 (22.3) | |
| ≥80 | 399 (28.8) | 1130 (22.6) | |
| Mean ± SD | 69.41 ± 14.22 | 66.88 ± 14.79 | <.001b,d |
| Race: No (%) | |||
| White | 1145 (82.6) | 3892 (78.0) | .001b,c |
| Black | 212 (15.3) | 929 (18.6) | |
| Other | 30 (2.2) | 171 (3.4) | |
| Gender: No. (%) | |||
| Female | 749 (54.0) | 2543 (50.9) | .044b,c |
| Male | 638 (46.0) | 2449 (49.1) | |
| Medical history: No. (%) | |||
| Atrial Fib | 223 (16.1) | 836 (16.7) | .554 |
| Coronary artery disease | 488 (35.2) | 1476 (29.6) | <.001b,c |
| Carotid artery stenosis | 139 (10.0) | 266 (5.3) | <.001b,c |
| Depression | 208 (15.0) | 647 (13.0) | .049b,c |
| Diabetes | 507 (36.6) | 1762 (35.3) | .387 |
| Drugs or alcohol | 45 (3.2) | 324 (6.5) | <0.001b,c |
| Dyslipidemia | 837 (60.3) | 2453 (49.1) | <.001b,c |
| Family history of stroke | 141 (10.2) | 443 (8.9) | .140 |
| Heart failure | 145 (10.5) | 540 (10.8) | .699 |
| Hormonal replacement therapy | 17 (1.2) | 73 (1.5) | .509 |
| Hypertension | 1145 (82.6) | 3886 (77.8) | <.001b,c |
| Migraine | 42 (3.0) | 119 (2.4) | .176 |
| Obesity | 572 (41.2) | 2119 (42.4) | .420 |
| Previous stroke | 492 (35.5) | 1228 (24.6) | <.001b,c |
| Prosthetic heart valve | 21 (1.5) | 52 (1.0) | .143 |
| Peripheral vascular disease | 136 (9.8) | 356 (7.1) | .001b,c |
| Chronic renal disease | 117 (8.4) | 405 (8.1) | .698 |
| Sickle cell | 2 (0.1) | 3 (0.1) | .322 |
| Sleep apnea | 55 (4.0) | 155 (3.1) | .112 |
| Smoker | 288 (20.8) | 1378 (27.6) | <.001b,c |
| Medication history: No (%) | |||
| HTN medication | 1052 (75.8) | 3410 (68.3) | <.001b,c |
| Cholesterol reducer | 778 (56.1) | 2143 (42.9) | <.001b,c |
| Diabetes medication | 416 (30.0) | 1357 (27.2) | .039b,c |
| Antidepressant | 213 (15.4) | 637 (12.8) | .012b |
| Initial NIHSS score: No (%) | |||
| 0-9 | 1011 (84.6) | 2983 (71.4) | <.001b,c |
| 10-14 | 103 (8.6) | 461 (11.0) | |
| 15-20 | 52 (4.4) | 467 (11.2) | |
| 21-25 | 29 (2.4) | 268 (6.4) | |
| Mean ± SD | 5.4 ± 6.65 | 8.33 ± 8.28 | <.001b,d |
| Lab values: Mean ± SD | |||
| Total cholesterol | 168.19 ± 45.59 | 172.25 ± 52.17 | .008b,d |
| Triglycerides | 143.88 ± 97.36 | 139.36 ± 105.28 | .181 |
| HDL | 42.81 ± 14.41 | 41.73 ± 13.8 | .020b,d |
| LDL | 99.25 ± 38.42 | 105.06 ± 41.28 | <.001b,d |
| Lipids | 6.99 ± 20.63 | 6.55 ± 2.49 | .468 |
| Blood glucose | 138 ± 70.36 | 148.5 ± 82.77 | <.001b,d |
| Serum creatinine | 1.38 ± 2.78 | 1.29 ± 1.17 | .244 |
| INR | 1.18 ± 0.58 | 1.14 ± 0.47 | .028 |
| BMI | 28.64 ± 6.82 | 28.37 ± 7.01 | .209 |
| Vital signs: Mean ± SD | |||
| Heart rate | 78.86 ± 15.95 | 151.71 ± 29.39 | <.001b,d |
| Blood pressure systolic | 150.73 ± 28.48 | 82.51 ± 19.18 | .273 |
| Blood pressure diastolic | 80.46 ± 18.1 | 82.21 ± 18.63 | <.001b,d |
| Ambulation status prior to event: No. (%) | |||
| Ambulate independently | 1230 (88.7) | 4458 (89.3) | .005b,c |
| Ambulate with assistance | 75 (5.4) | 179 (3.6) | |
| Unable to ambulate | 56 (4.0) | 201 (4.0) | |
| Not documented | 26 (1.9) | 153 (3.1) | |
| Ambulation status on admission: No. (%) | |||
| Ambulate independently | 554 (39.9) | 1201 (24.1) | <.001b,c |
| Ambulate with assistance | 428 (30.9) | 1478 (29.6) | |
| Unable to ambulate | 239 (17.2) | 1607 (32.2) | |
| Not documented | 166 (12.0) | 706 (14.1) | |
| Ambulation status on discharge: No. (%) | |||
| Ambulate independently | 817 (58.9) | 1976 (39.6) | <.001b,c |
| Ambulate with assistance | 378 (27.3) | 1645 (33.0) | |
| Unable to ambulate | 143 (10.3) | 990 (19.8) | |
| Not documented | 49 (3.5) | 381 (7.6) |
Abbreviations: BMI, body mass index; Fib, fibrillation; HDL, high-density cholesterol; LDL, low-density cholesterol; HTN, hypertension; INR, international normalized ratio; SD, standard deviation; TIA, transient ischemic attack.
a Results for continuous variables are presented as mean ± SD, while discrete data are Presented as percentage frequency. Pearson chi-square is used to compare differences between demographic and clinical characteristics in groups with a past TIA and without a history of TIA.
b P value < .05.
c Pearson chi-square test.
d Student t test.
The demographic and clinical variables associated with improvement and nonimprovement after rtPA treatment for TIA-ischemic stroke and non-TIA groups are presented in Table 2. Patients in the TIA-ischemic stroke group that received rtPA were more likely to be females (45.6% vs. 61.2%), present with a history of previous stroke (48.1% vs 22.4%), and have no improvement in ambulation. The non-TIA group was younger (64.15 ± 14.87 vs 66.23 ± 14.7), less likely to present with heart failure (8.0% vs 12.8%), lower NIHSS scores (9.7 ± 7.18 vs 10.33 ± 8.57) and is more likely to present with an improvement in ambulation.
Table 2.
Demographic and Clinical Characteristics of Patients Having Ischemic Stroke With a TIA History or Without a TIA History on Improvement in Ambulation.a
| Characteristic | Acute Ischemic Stroke With rtPA and TIA | Acute Ischemic Stroke With rtPA and Without TIA | ||||
|---|---|---|---|---|---|---|
| No Improvement | Improvement | P Value | No Improvement | Improvement | P Value | |
| Number of patients | 85 | 79 | 485 | 616 | ||
| Age group: No. (%) | ||||||
| <50 | 10 (11.8) | 16 (20.3) | 0.534 | 63 (13.0) | 106 (17.2) | .110 |
| 50-59 | 14 (16.5) | 16 (20.3) | 99 (20.4) | 122 (19.8) | ||
| 60-69 | 24 (28.2) | 18 (22.8) | 121 (24.9) | 150 (24.4) | ||
| 70-79 | 16 (18.8) | 13 (16.5) | 97 (20.0) | 136 (22.1) | ||
| >=80 | 21 (24.7) | 16 (20.3) | 105 (21.6) | 102 (16.6) | ||
| Mean ± SD | 67.49 ± 14.27 | 63.62 ± 15.39 | 0.096 | 66.23 ± 14.75 | 64.15 ± 14.87 | .021b,c |
| Race: No (%) | ||||||
| White | 75 (88.2) | 61 (77.2) | 0.128 | 392 (80.8) | 484 (78.6) | .562 |
| Black | 9 (10.6) | 14 (17.7) | 82 (16.9) | 113 (18.3) | ||
| Other | 1 (1.2) | 4 (5.1) | 11 (2.3) | 19 (3.1) | ||
| Gender: No. (%) | ||||||
| Female | 52 (61.2) | 36 (45.6) | 0.045b,d | 253 (52.2) | 290 (47.1) | .094 |
| Male | 33 (38.8) | 43 (54.4) | 232 (47.8) | 326 (52.9) | ||
| Medical history: No. (%) | ||||||
| Atrial Fib | 10 (11.8) | 9 (11.4) | 0.941 | 84 (17.3) | 81 (13.1) | .054 |
| Coronary artery disease | 34 (40.0) | 29 (36.7) | 0.665 | 144 (29.7) | 177 (28.7) | .729 |
| Carotid artery stenosis | 8 (9.4) | 9 (11.4) | 0.678 | 12 (2.5) | 23 (3.7) | .237 |
| Depression | 22 (25.9) | 17 (21.5) | 0.512 | 74 (15.3) | 94 (15.3) | .999 |
| Diabetes | 25 (29.4) | 24 (30.4) | 0.892 | 160 (33.0) | 183 (29.7) | .243 |
| Drugs or alcohol | 2 (2.4) | 6 (7.6) | 0.119 | 23 (4.7) | 45 (7.3) | .079 |
| Dyslipidemia | 53 (62.4) | 50 (63.3) | 0.901 | 235 (48.5) | 327 (53.1) | .127 |
| Stroke family history | 10 (11.8) | 13 (16.5) | 0.387 | 41 (8.5) | 67 (10.9) | .180 |
| Heart failure | 7 (8.2) | 9 (11.4) | 0.496 | 62 (12.8) | 49 (8.0) | .008b,d |
| Hormonal replacement therapy | 3 (3.5) | 0 (0) | 0.092 | 12 (2.5) | 15 (2.4) | .967 |
| Hypertension | 70 (82.4) | 67 (84.8) | 0.672 | 377 (77.7) | 474 (76.9) | .758 |
| Migraine | 7 (8.2) | 7 (8.9) | 0.886 | 11 (2.3) | 24 (3.9) | .126 |
| Obesity | 45 (52.9) | 43 (54.5) | 0.848 | 250 (51.5) | 328 (53.2) | .575 |
| Previous stroke | 19 (22.4) | 38 (48.1) | 0.001b,d | 104 (21.4) | 125 (20.3) | .640 |
| Prosthetic heart valve | 0 (0) | 1 (1.3) | 0.298 | 2 (0.4) | 5 (0.8) | .408 |
| Peripheral vascular disease | 8 (9.4) | 9 (11.4) | 0.678 | 26 (5.4) | 31 (5.0) | .807 |
| Chronic renal disease | 6 (7.1) | 6 (7.6) | 0.895 | 30 (6.2) | 30 (4.9) | .340 |
| Sleep apnea | 6 (7.1) | 1 (1.3) | 0.067 | 19 (3.9) | 20 (3.2) | .550 |
| Smoker | 26 (30.6) | 25 (31.6) | 0.884 | 136 (28.0) | 188 (30.5) | .370 |
| Medication history: No (%) | ||||||
| HTN medication | 63 (74.1) | 59 (74.7) | 0.934 | 351 (72.4) | 423 (68.7) | .182 |
| Cholesterol reducer | 50 (58.8) | 49 (62.0) | 0.675 | 218 (44.9) | 286 (46.4) | .625 |
| Diabetes medication | 22 (25.9) | 20 (25.3) | 0.934 | 132 (27.2) | 144 (23.4) | .144 |
| Antidepressant | 25 (29.4) | 18 (22.8) | 0.335 | 73 (15.1) | 106 (17.2) | .336 |
| Initial NIHSS score: No (%) | ||||||
| 0-9 | 54 (70.1) | 49 (64.5) | 0.438 | 274 (6.9) | 366 (61.6) | .014b,d |
| 10-14 | 10 (13.0) | 13 (17.1) | 63 (14.0) | 103 (17.3) | ||
| 15-20 | 8 (10.4) | 12 (15.8) | 64 (14.2) | 91 (15.3) | ||
| 21-25 | 5 (6.5) | 2 (2.6) | 49 (10.9) | 34 (5.7) | ||
| Mean ± SD | 9.19 ± 8.68 | 9.38 ± 6.62 | 0.875 | 10.33 ± 8.57 | 9.7 ± 7.18 | .192 |
| Lab values: Mean ± SD | ||||||
| Total cholesterol | 166.16 ± 46.54 | 165.6 ± 50.1 | 0.942 | 171.05 ± 48.42 | 168.17 ± 44.46 | .320 |
| Triglycerides | 143.99 ± 90.13 | 154.35 ± 116.43 | 0.529 | 146.02 ± 112.92 | 138.25 ± 108.34 | .256 |
| HDL | 41.63 ± 11.81 | 39.59 ± 11.27 | 0.265 | 41.67 ± 13.66 | 42.12 ± 14.03 | .604 |
| LDL | 101.06 ± 40.22 | 99.4 ± 40.32 | 0.794 | 104.31 ± 40.27 | 102.02 ± 37.82 | .342 |
| Lipids | 6.29 ± 1.61 | 5.99 ± 1.46 | 0.236 | 6.3 ± 1.57 | 6.2 ± 1.61 | .321 |
| Blood glucose | 133.65 ± 65.78 | 126.76 ± 50.32 | 0.457 | 141.53 ± 71.72 | 140.08 ± 78.9 | .754 |
| Serum creatinine | 1.23 ± 1.14 | 1.26 ± 0.87 | 0.862 | 1.14 ± 0.88 | 1.1 ± 0.52 | .355 |
| INR | 1.03 ± 0.11 | 1.03 ± 0.12 | 0.993 | 1.06 ± 0.15 | 1.05 ± 0.12 | .085 |
| BMI | 28.08 ± 5.57 | 29.41 ± 8.13 | 0.228 | 28.9 ± 6.49 | 29.2 ± 7.16 | .478 |
| Vital signs: Mean ± SD | ||||||
| Heart rate | 78.49 ± 14.52 | 78.66 ± 13.94 | 0.941 | 82.32 ± 16.65 | 81.26 ± 17.46 | .309 |
| Blood pressure systolic | 150.94 ± 25.55 | 149.92 ± 24.03 | 0.794 | 150.38 ± 25.72 | 151.98 ± 27.83 | .329 |
| Blood pressure diastolic | 82.47 ± 18.57 | 82.59 ± 17.84 | 0.965 | 82.1 ± 18.06 | 83.74 ± 18.08 | .138 |
| Ambulation status prior to event: No. (%) | ||||||
| Ambulate independently | 76 (89.4) | 76 (96.2) | 0.122 | 446 (92.0) | 605 (98.2) | <.001b,d |
| Ambulate with assistance | 3 (3.5) | 3 (3.8) | 13 (2.7) | 3 (0.5) | ||
| Unable to ambulate | 3 (3.5) | 0 (0.0) | 17 (3.5) | 1 (0.2) | ||
| Not documented | 3 (3.5) | 0 (0.0) | 9 (1.9) | 7 (1.1) | ||
| Ambulation status on admission: No. (%) | ||||||
| Ambulate independently | 48 (56.5) | 0 (0.0) | <0.001b,d | 192 (39.6) | 0 (0.0) | <.001b,d |
| Ambulate with assistance | 16 (18.8) | 22 (27.6) | 109 (22.5) | 162 (26.3) | ||
| Unable to ambulate | 20 (23.5) | 17 (21.5) | 183 (37.7) | 194 (31.5) | ||
| Not documented | 1 (1.2) | 40 (50.6) | 1 (0.2) | 260 (42.2) | ||
| Ambulation status on discharge: No. (%) | ||||||
| Ambulate independently | 45 (52.9) | 55 (69.6) | 0.001b,d | 168 (34.6) | 414 (67.2) | <.001b,d |
| Ambulate with assistance | 18 (21.2) | 21 (26.6) | 127 (26.2) | 190 (30.8) | ||
| Unable to ambulate | 21 (24.7) | 3 (3.8) | 187 (38.6) | 12 (1.9) | ||
| Not documented | 1 (1.2) | 0 (0.0) | 3 (0.6) | 0 (0.0) | ||
Abbreviations: BMI, body mass index; Fib, fibrillation; HDL, high-density cholesterol; LDL, low-density cholesterol; HTN, hypertension; INR, international normalized ratio; NIHSS, National Institutes of Health Stroke Scale; rtPA, recombinant tissue plasminogen activator; SD, standard deviation; TIA, transient ischemic attack.
a Results for continuous variables are presented as mean ± SD, while discrete data are presented as percentage frequency. Pearson chi-square is used to compare differences between demographic and clinical characteristics in groups with or without a TIA history on improvement in ambulation.
b P value < .05.
c Student t test.
d Pearson chi-square test.
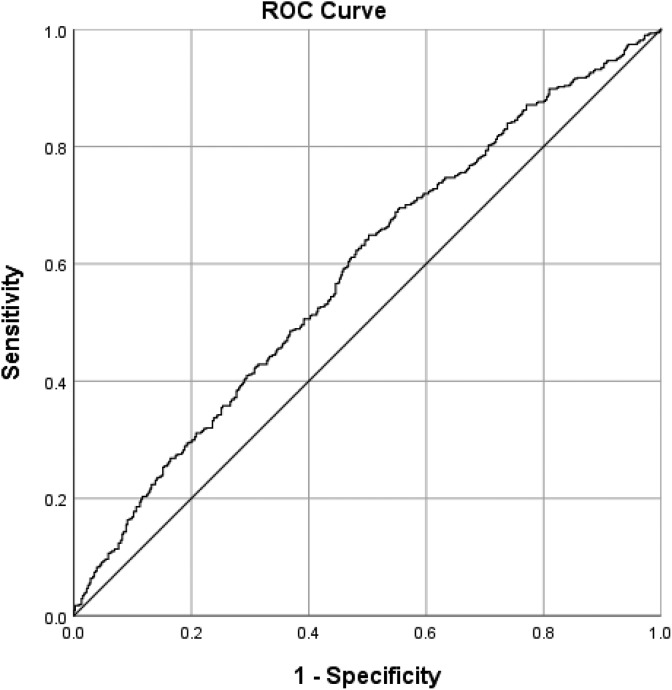
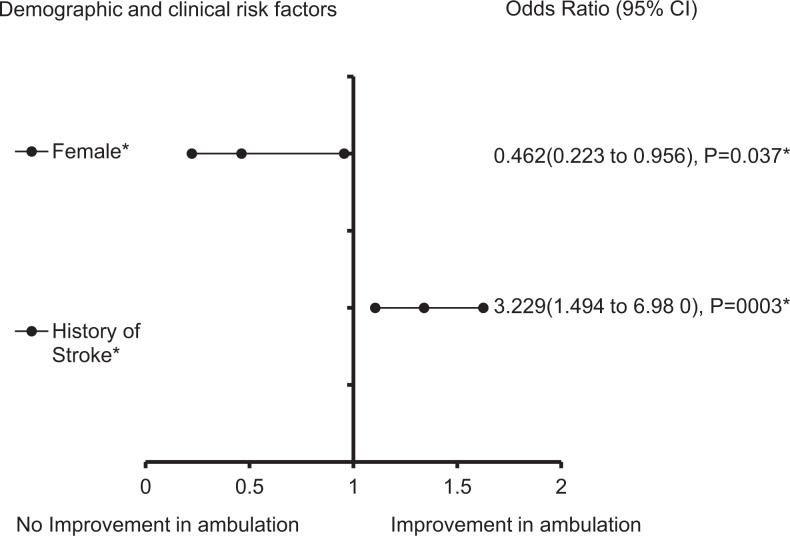
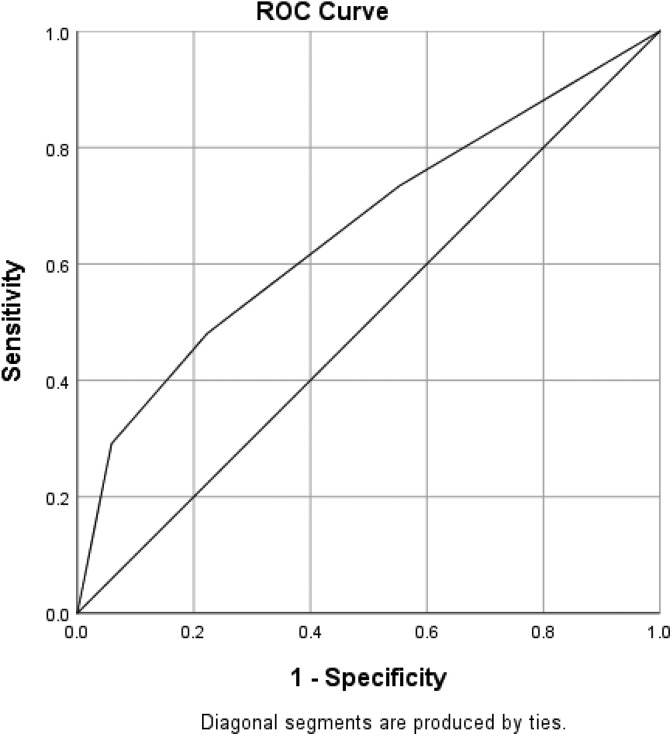
After using a multivariate analysis to adjust for the confounding effects of nonsignificant variables in the non-TIA group that received rtPA (Table 3 and Figure 1), we found that dyslipidemia (OR = 1.351, 95% confidence interval [CI] = 1.026-1.781, P = .032) and blood glucose (OR = 1.003, 95% CI = 1.0-1.005, P = .041) were significantly associated with the increasing odds of improvement in ambulation in the non-TIA group that received rtPA. Increasing age (OR = 0.989, 95% CI = 0.98-0.999, P = .029), heart failure (OR = 0.513, 95% CI = 0.326-0.808, P = .004), and higher lipid level (OR = 0.834, 95% CI = 0.728-0.955, P = .009) were significantly associated with reducing odds of improvement in functional ambulation. The predictive power of the logistic regression was good (Figure 2) as with the AUROC = 0.586 (95% CI = 0.511-0.621, P < .001). The clinical factors that were associated with an improvement in ambulation for TIA-ischemic stroke group with rtPA is presented in Table 4 and Figure 3. The result reveals that a history of stroke (OR = 3.229, 95% CI = 1.494-6.98, P = .003) was associated with increasing odds of improvement in functional outcome, while being a female (OR = 0.462, 95% CI = 0.223-0.956, P = .037) was associated with reducing odds of improvement ( also see Table 3). The discriminating capability of the model was good (Figure 4) with AUROC = 0.660 (95% CI = 0.576-0.744, P < .001).
Table 3.
Stepwise Regression Model for Functional Outcome in Acute Ischemic Stroke Population With rtPA and Without TIA.a
| Variables | B Value | Wald | Odds Ratio | 95% CI | P Value |
|---|---|---|---|---|---|
| Increasing age | −0.011 | 4.79 | 0.989 | 0.98-0.999 | .029b |
| Female | −0.244 | 3.119 | 0.784 | 0.598-1.027 | .077 |
| CAS | 0.819 | 3.671 | 2.269 | 0.981-5.244 | .055 |
| Drugs or alcohol | 0.507 | 2.645 | 1.661 | 0.901-3.061 | .104 |
| Dyslipidemia | 0.301 | 4.579 | 1.351 | 1.026-1.781 | .032b |
| Heart failure | −0.667 | 8.282 | 0.513 | 0.326-0.808 | .004b |
| Migraine | 0.753 | 3.107 | 2.123 | 0.919-4.904 | .078 |
| Lipids | −0.182 | 6.884 | 0.834 | 0.728-0.955 | .009b |
| Blood glucose | 0.003 | 4.193 | 1.003 | 1-1.005 | .041b |
Abbreviations: CI, confidence interval; OR, odds ratio; rtPA, recombinant tissue plasminogen activator; TIA, transient ischemic attack; CAS, Carotid artery stenosis.
a Clinical factors that were associated with an improvement in ambulation for ischemic stroke population with rtPA and without TIA. Adjusted OR < 1 denote factors that are associated without an improvement in ambulation, while OR > 1 denote factors that are associated with an improvement in ambulation. Hosmer-Lemeshow test (P = .921), Cox and Snell (r2 = 0.041). The overall classified percentage of 58.4% was applied to check for fitness of the logistic regression model. Backward Stepwise model based on Likelihood Ratio was applied. Model assumptions were fulfilled. Multicollinearity and interactions among independent variables were checked and no significant interactions were found.
b Statistical significance (P < .05) with a 95% confidence interval.
Figure 1.
Forest plot representation of Table 4. Confidence interval band below 1 denotes factors that are associated with poor functional outcome while confidence interval band above 1 denotes factors that are associated with improved functional outcome. Confidence interval bands that cross 1 cannot be associated with poor or improved functional outcome. *Statistical significance (P < .05) with a 95% confidence interval. ^The data were adjusted by taking the fourth square root.
Figure 2.
Receiver operating curve associated with prediction of functional outcome for acute ischemic stroke population with tissue plasminogen activator (rtPA) and without TIA. Higher area under the curve (AUC) values in ROC analysis indicate better discrimination of the score for the measured outcome. Classification table (overall correctly classified percentage = 58.4%) and area under the ROC curve (AUC = 0.586, 0.511-0.621) were applied to check model fitness.
Table 4.
Stepwise Regression Model for Functional Outcome in Acute Ischemic Stroke Population With rtPA and TIA.a
| Variables | B Value | Wald | Odds Ratio | 95% CI | P Value |
|---|---|---|---|---|---|
| Female | −0.773 | 4.336 | 0.462 | 0.223-0.956 | .037b |
| History of stroke | 1.172 | 8.88 | 3.229 | 1.494-6.98 | .003b |
Abbreviations: CI, confidence interval; OR, odds ratio; rtPA, recombinant tissue plasminogen activator; TIA, transient ischemic attack.
a Clinical factors that were associated with an improvement in ambulation for ischemic stroke population with rtPA and TIA. Adjusted OR < 1 denote factors that are associated without an improvement in ambulation, while OR > 1 denote factors that are associated with an improvement in ambulation. Hosmer-Lemeshow test (P = .522), Cox and Snell (R 2 = .101). The overall classified percentage of 63.4% was applied to check for fitness of the logistic regression model. Backward stepwise model based on likelihood ratio was applied. Model assumptions were fulfilled. Multicollinearity and interactions among independent variables were checked and no significant interactions were found
b Statistical significance (P < .05) with a 95% confidence interval.
Figure 3.
Forest plot representation of Table 3. Confidence interval band below 1 denotes factors that are associated with poor functional outcome while confidence interval band above 1 denotes factors that are associated with improved functional outcome. Confidence interval bands that cross 1 cannot be associated with poor or improved functional outcome. *Statistical significance (P < .05) with a 95% confidence interval.
Figure 4.
Receiver operating curve associated with prediction of functional outcome for acute ischemic stroke population with TIA <24 hours. Higher area under the curve (AUC) values in ROC analysis indicate better discrimination of the score for the measured outcome. Classification table (overall correctly classified percentage = 63.4%) and area under the ROC curve (AUC = 0.660, 0.576-0.744) were applied to check model fitness.
Discussion
The purpose of the current study was to determine whether specific clinical risk factors are associated with improvement or nonimprovement in ambulatory outcomes in TIA-ischemic stroke and non-TIA stroke population receiving rtPA therapy. We found that dyslipidemia and blood glucose were associated with the increasing odds of improvement in ambulation, while increasing age, heart failure, and a higher lipid level were associated with reducing odds of improvement in non-TIA stroke population that received rtPA. Similar findings for dyslipidemia,36,37 blood glucose,38 age,39 heart failure,40 and a higher lipid level41 have been reported for functional outcomes in other studies. In addition, we found that patients with TIA-ischemic stroke who received rtPA and presented with a history of previous stroke more than 3 months are more likely to be associated with increased odds of improvement in functional ambulation. Moreover, being a female patient with TIA-ischemic stroke was the only variable that was associated with a reducing odd of improvement in functional ambulatory outcome.
Our finding that patients having TIA-ischemic stroke with a history of previous stroke more than 3 months was associated with increased odds of improvement in functional ambulation has been reported in other studies for ischemic stroke populations.29,42–44 Although the history of an established previous stroke more than 3 months prior or a TIA prior may not be formal contraindications for rtPA therapy in patients with ischemic stroke, a TIA within 24 hours preceding an acute ischemic stroke may influence outcome after rtPA therapy.4,5 This is because some patients may have invisible lesions on a CT scan, both ischemic and intracerebral lesions.4 Moreover, it has been shown that motor impairments during a TIA are often associated with structural brain lesions.45 For the TIA data used in this study, the presence of acute brain lesions was determined with a combined early DW-MRI and PW-MR, which has the capability to identify the presence of a cerebral ischemic lesion in patients who may present with a suspected hemispheric TIA prior to the onset of stroke.46 This provides the opportunity to rule out a TIA with imaging evidence of focal infarction that represents a severe unstable condition with early risk of stroke which is more than 20 times higher than the risk after.47 However, a TIA without tissue damage is the focus of the current study. The clinical symptoms of TIA without tissue damage mostly last less than 24 hours, with no apparent nonvascular cause.48
The nonsignificant association of clinical risk factors with reducing odds of improvement in ambulation and significant increased odds of improvement with previous stroke indicate different possibilities: (1) that the use of rtPA in patients with ischemic stroke after a TIA preceding the current stroke in our population does not appear to have major adverse effects on functional ambulation and (2) the imaging approach helped to exclude patients with significant ischemic tissue lesion. In this context, our sample was comprised of an ischemic stroke population with a TIA preceding an acute ischemic stroke with clinical TIA symptoms and without focal lesions and a population with a history of a previous TIA who received rtPA. This provided a TIA-ischemic population without concerns regarding the risk of bleeding as a result of thrombolytic therapy.49 Therefore, the already established population of ischemic stroke patients without TIA-related focal lesions within 24 hours prior to an ischemic stroke reveal the safety of rtPA without the risk of intracerebral bleeding in TIA less than 24 hours for ischemic stroke patients qualified for rtPA therapy.
We found that female patients with TIA-ischemic stroke presented with reducing odds of improved ambulatory outcome following rtPA therapy. Our finding is supported by existing studies that women display poorer functional outcomes than men after an ischemic stroke,50 and this was sustained even after the adjustments for age and gender in the current study. Previous studies indicate that women display significantly poorer locomotor function as compared to men after ischemic stroke,51 indicating that the reducing odds of improved ambulation observed in the current study can be linked to the typical features of female patients with stroke, who are known to present with a stroke at an older age than male patients with stroke,50 rather than a TIA.
The use of rtPA in ischemic stroke with a TIA 24 hours prior to the onset of a stroke or a history of previous TIA is a significant clinical issue with risk of bleeding complications that increased in patients with prolonged symptom duration and a DWI lesion.4,48,52,53 It has been shown that the prevalence of a prior TIA in patients presenting with ischemic stroke ranges from 7% to 40%,7,54–57 and the effect and safety of rtPA therapy in patients with a previous TIA on functional ambulatory outcome are not fully understood. A higher symptomatic intracerebral hemorrhage rate was reported in clinical trials of rtPA in an ischemic stroke population.5,58–61 The increased rate of intracerebral bleeding in ischemic stroke with a previous history of TIA was observed in older patients (mean age 68).3,6,62 Our patients with TIA-ischemic stroke who received rtPA therapy and had an improvement in ambulation were younger (63.62 ± 15.39) than in similar studies.5,6
Motor impairments during a TIA itself is a compelling factor of the increased odds of a subsequent stroke,5 and the assumed transient nature of initial motor impairments may not be so transient as thought since other clinical risk factors could complicate motor impairment. The resulting stroke suggests the pressing need to be able to identify clinical risk factors that may be associated with functional motor recovery following rtPA therapy. Moreover, the most common deficit after a stroke is motor impairment and this could happen due to a direct lesion of the cerebral blood vessel resulting in the disruption of signal transmission from the cerebral cortex to the lower or upper extremities.63 Although the clinical diagnosis of motor impairment may be enough to identify residual motor deficits following a TIA-ischemic stroke,64 the ability to accurately identify and predict different comorbidities that are associated with an improvement or nonimprovement in functional motor outcome following rtPA therapy is important to understand the extent of motor recovery following rtPA therapy. In a group of homogenous patients having acute ischemic stroke with a clearly defined TIA prior without focal ischemic injury, we found that regardless of associated clinical risk factors, a recent TIA does not appear to be associated with reducing odds of improved ambulatory functional outcome, except in female patients with TIA-ischemic stroke.
The small number of TIAs among the ischemic stroke population in our data could be considered a limitation. Data for patients’ information may be prone to selection bias due to the retrospective nature of the study. In addition, we do not have data on ischemic stroke cases subtyped into cardioembolic, large-vessel, and small-vessel, making it difficult to examine the effects of ischemic stroke subtypes. A major strength in the current study is the opportunity to use a homogenous sample of patients who presented with a TIA with no focal lesions to determine functional ambulation outcome in an population of ischemic stroke with rtPA therapy. Our findings provide additional information on the safety of rtPA therapy in patients with TIA and previous stroke history, indicating that rtPA is safe to use despite risk factors in patients with TIA.
Conclusion
After controlling for associated clinical risk factors, we found that patients presenting with an ischemic stroke with a prior TIA who were treated with rtPA are not associated with reducing odds of improved ambulatory functional outcome, except in female patients. Additionally, we found that a patient having TIA-ischemic stroke with a history of stroke within the past 3 months is more likely to be associated with increasing odds of improvement in functional ambulatory outcome after receiving rtPA. This study provides important information for clinical decisions, especially when deciding to administer rtPA in patients with TIA-ischemic stroke.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by the Fullerton Foundation Grant.
ORCID iD: Thomas Nathaniel  https://orcid.org/0000-0003-0954-5050
https://orcid.org/0000-0003-0954-5050
References
- 1. Lodha N, Harrell J, Eisenschenk S, Christou EA. Motor impairments in transient ischemic attack increase the odds of a subsequent stroke: a meta-analysis. Front Neurol. 2017;8:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Asimos AW, Rosamond WD, Rose KM, et al. MRI use in TIA patients: variations by joint commission stroke center certification status and implications for a revised tissue-based definition of TIA. Stroke. 2008;39(2):584–584. [Google Scholar]
- 3. Hill MD, Yiannakoulias N, Jeerakathil T, Tu JV, Svenson LW, Schopflocher DP. The high risk of stroke immediately after transient ischemic attack - A population-based study. Neurology. 2004;62(11):2015–2020. [DOI] [PubMed] [Google Scholar]
- 4. Sobolewski P, Brola W, Wiszniewska M, et al. Intravenous thrombolysis with RT-PA for acute ischemic stroke within 24 h of a transient ischemic attack. J Neurol Sci. 2014;34(1-2):44–49. [DOI] [PubMed] [Google Scholar]
- 5. de Lecinana MA, Fuentes B, Masjuan J, et al. Thrombolytic therapy for acute ischemic stroke after recent transient ischemic attack. Int J Stroke. 2012;7(3):213–218. [DOI] [PubMed] [Google Scholar]
- 6. McKinney JS, Masjuan J, Purroy F, Calvet D, Ay H, Cucchiara BL. Safety of thrombolytic therapy for acute ischemic stroke after recent transient ischemic attack. J Stroke Cerebrovasc Dis. 2012;21(7):551–554. [DOI] [PubMed] [Google Scholar]
- 7. Tursunov D, Akbarkhodjaeva Z. Risk factors of developing transient ischemic attack. J neurol sci. 2017;381:1115–1115. [Google Scholar]
- 8. Wang ZL, van Veluw SJ, Wong A, et al. Risk factors and cognitive relevance of cortical cerebral micro infarcts in patients with ischemic stroke or transient ischemic attack. Stroke. 2016;47(10):2450–2455. [DOI] [PubMed] [Google Scholar]
- 9. Fahey M, Crayton E, Wolfe C, Douiri A. Clinical prediction models for mortality and functional outcome following ischemic stroke: a systematic review and meta-analysis. PloS one. 2018;13(1):e0185402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Drozdowska BA, Singh S, Quinn TJ. . Thinking about the future: a review of prognostic scales used in acute stroke. Front Neurol. 2019;10:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hodgson K, Adluru G, Richards LG, et al. predicting motor outcomes in stroke patients using diffusion spectrum MRI microstructural measures. Front Neurol. 2019;10:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shea-Shumsky NB, Schoeneberger S, Grigsby J. Executive functioning as a predictor of stroke rehabilitation outcomes. Clin Neuropsychol 2019;33(5):854–872. [DOI] [PubMed] [Google Scholar]
- 13. Wu DM, Wang S, Wen X, et al. Impact of serum omentin-1 levels on functional prognosis in nondiabetic patients with ischemic stroke. Am J Transl Resear. 2019;11(3):1854–1863. [PMC free article] [PubMed] [Google Scholar]
- 14. Bhatt T, Dusane S, Patel P. Does severity of motor impairment affect reactive adaptation and fall-risk in chronic stroke survivors? J Neuroeng Rehabil. 2019;16(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hayward KS, Neva JL, Mang CS, et al. Interhemispheric pathways are important for motor outcome in individuals with chronic and severe upper limb impairment post stroke. Neural Plast. 2017;2017:4281532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hirakawa Y, Takeda K, Tanabe S, et al. Effect of intensive motor training with repetitive transcranial magnetic stimulation on upper limb motor function in chronic post-stroke patients with severe upper limb motor impairment. Top Stroke Rehabil. 2018;25(5):321–325. [DOI] [PubMed] [Google Scholar]
- 17. Jorgensen HS, Nakayama H, Pedersen PM, Kammersgaard L, Raaschou HO, Olsen TS. Epidemiology of stroke-related disability—the Copenhagen stroke study. Cli Geriat Med. 1999;15(4):785–800. [PubMed] [Google Scholar]
- 18. Kwakkel G, Kollen BJ. Predicting activities after stroke: what is clinically relevant? Int J Stroke. 2013;8(1):25–32. [DOI] [PubMed] [Google Scholar]
- 19. Heldner MR, Mattle HP, Jung S, et al. Thrombolysis in patients with prior stroke within the last 3 months. Eur J Neurol. 2014;21(12):1493–1499. [DOI] [PubMed] [Google Scholar]
- 20. Nathaniel TI, Gainey J, Blum B, Montgomery C, Ervin L, Madeline L. Clinical risk factors in thrombolysis therapy: telestroke versus nontelestroke. J Stroke Cerebrovasc Dis. 2018;27(9):2524–2533. [DOI] [PubMed] [Google Scholar]
- 21. Blum B, Brechtel L, Nathaniel T. Thrombolysis therapy in specialized and non-specialized stroke units. Arch Med Res. 2018;49(8):588–597. [DOI] [PubMed] [Google Scholar]
- 22. Blum B, Penwell A, Wormack L, Walker B, Lari S, Nathaniel TI. Gender and thrombolysis therapy in acute ischemic stroke patients with incidence of obesity. Neurol Sci. 2019;40(9):1829–1839. [DOI] [PubMed] [Google Scholar]
- 23. Blum B, Wormack L, Holtel M, et al. Gender and thrombolysis therapy in stroke patients with incidence of dyslipidemia. Bmc Womens Health. 2019;19(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fredwall M, Sternberg S, Blackhurst D, Lee A, Leacock R, Nathaniel TI. Gender differences in exclusion criteria for recombinant tissue-type plasminogen activator. J stroke cerebrovasc dis: offic J Nat Stroke Assoc. 2016;25(11):2569–2574. [DOI] [PubMed] [Google Scholar]
- 25. Nathaniel TI, Cochran T, Chaves J, et al. Co-morbid conditions in use of recombinant tissue plasminogen activator (rt-PA) for the treatment of acute ischaemic stroke. Brain injury. 2016;30(10):1261–1265. [DOI] [PubMed] [Google Scholar]
- 26. Nathaniel IT, Williams J, Fazzone F, et al. Contraindications and exclusion criteria in guidelines for RT-pa in acute ischemic stroke: Can the new AHA/ASA guideline expand the use of RT-pa? Hypertension. 2016:245. [Google Scholar]
- 27. Brecthel L, Gainey J, Penwell A, Nathaniel TI. Predictors of thrombolysis in the telestroke and non telestroke settings for hypertensive acute ischemic stroke patients. BMC neurol. 2018;18(1):215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gainey J, Brechtel L, Konklin S, et al. In a stroke cohort with incident hypertension; are more women than men likely to be excluded from recombinant tissue-type plasminogen activator (rtPA)? J neurol sci. 2018;387(15):139–146. [DOI] [PubMed] [Google Scholar]
- 29. Gainey J, Brecthtel J, Blum B, et al. Functional outcome measures of recombinant tissue plasminogen activator–treated stroke patients in the telestroke technology. J Exp Neurosci. 2018;12:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gainey J, Blum B, Bowie B, et al. Stroke and dyslipidemia: clinical risk factors in the telestroke versus non-telestroke. Lipids Health Dis. 2018;17:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brian Fazzone GM, Black L-A, Williams J-A, Leacock R, Sternberg S, Blackhurst D, Nelson A, Nathaniel TI. Exclusion and inclusion criteria for thrombolytic therapy in an ischemic stroke population. J Neurol Disord Stroke. 2016;4(2):1112. [Google Scholar]
- 32. Gainey J, Wormack L, Brechtel L, Nathaniel IT. A functional outcome model for a telestroke-guided tissue plasminogen activator treatment of stroke patients. Stroke. 2018;49(1):P89. [Google Scholar]
- 33. Nathaniel IT, Gainey J, Blum B, Montgomery C. Clinical risk factors in thrombolysis therapy: telestroke versus nontelestroke. J stroke Cerebrovasc Dis. 2018;27(9):2524–2533. [DOI] [PubMed] [Google Scholar]
- 34. Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(7):2160–2236. [DOI] [PubMed] [Google Scholar]
- 35. Sauerbrei W, Royston P, Binder H. Selection of important variables and determination of functional form for continuous predictors in multivariable model building. Statist Med. 2007;26(30):5512–5528. [DOI] [PubMed] [Google Scholar]
- 36. Menet R, Bernard M, ElAli A. Hyperlipidemia in stroke pathobiology and therapy: insights and perspectives. Front Physiol. 2018;9:488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gainey J, Blum B, Bowie B, et al. Stroke with dyslipidemia: clinical risk factors in the telestroke versus non-telestroke. Lipids Health Dis. 2018;4(87):123–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Osei E, Fonville S, Zandbergen AAM, Koudstaal PJ, Dippel DWJ, den Hertog HM. Impaired fasting glucose is associated with unfavorable outcome in ischemic stroke patients treated with intravenous alteplase. J Neurol. 2018;265(6):1426–1431. [DOI] [PubMed] [Google Scholar]
- 39. Sharobeam A, Cordato DJ, Manning N, Cheung A, Wenderoth J, Cappelen-Smith C. Functional outcomes at 90 days in octogenarians undergoing thrombectomy for acute ischemic stroke: a prospective cohort study and meta-analysis. Front Neurol. 2019;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Burkot J, Kopec G, Pera J, Slowik A, Dziedzic T. Decompensated heart failure is a strong independent predictor of functional outcome after ischemic stroke. J Card Fail. 2015;21(8):642–646. [DOI] [PubMed] [Google Scholar]
- 41. Yaghi S, Elkind MSV. Lipids and cerebrovascular disease research and practice. Stroke. 2015;46(11):3322–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kongsawasdi S, Klaphajone K, Wivatvongvana P, Watcharasaksilp P. prognostic factors of functional outcome assessed by using the modified Rankin scale in subacute ischemic stroke. J Clin Med Res 2019: 11(5):75–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kissela B, Lindsell CJ, Kleindorfer D, et al. Clinical prediction of functional outcome after ischemic stroke the surprising importance of periventricular white matter disease and race. Stroke. 2009;40(2):530–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Thompson DD, Murray GD, Sudlow CLM, Dennis M, Whiteley WN. Comparison of statistical and clinical predictions of functional outcome after ischemic stroke. PloS one. 2014;9(10):e110189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Redgrave JNE, Coutts SB, Schulz UG, Briley D, Rothwell PM. Systematic review of associations between the presence of acute ischemic lesions on diffusion-weighted imaging and clinical predictors of early stroke risk after transient ischemic attack. Stroke. 2007;38(5):1482–1488. [DOI] [PubMed] [Google Scholar]
- 46. Lee SH, Nah HW, Kim BJ, et al. Role of perfusion-weighted imaging in a diffusion weighted-imaging-negative transient ischemic attack. J Clin Neurol. 2017;13(2):129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sorensen AG, Ay H. Transient ischemic attack: definition, diagnosis, and risk stratification. Neuroimaging Clin N Am. 2011;21(2):303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Abbott AL, Silvestrini M, Topakian R, et al. Optimizing the definitions of stroke, transient ischemic attack, and infarction for research and application in clinical practice. Front Neurol. 2017;8:537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Morsund AH, Naess H. Factors influencing employment and quality of life 12 months after a minor stroke. Eur J Neurol. 2019;26:66–66.30063100 [Google Scholar]
- 50. Park SJ, Shin SD, Ro YS, Song KJ, Oh J. Gender differences in emergency stroke care and hospital outcome in acute ischemic stroke: a multicenter observational study. Am J Emerg Med. 2013;31(1):178–184. [DOI] [PubMed] [Google Scholar]
- 51. Fukuda M, Kanda T, Kamide N, Akutsu T, Sakai F. Gender differences in long-term functional outcome after first-ever ischemic stroke. Intern Med. 2009;48(12):967–973. [DOI] [PubMed] [Google Scholar]
- 52. Edlow BL, Hurwitz S, Edlow JA. Diagnosis of DWI-negative acute ischemic stroke. A meta-analysis. Neurology. 2017;89(3):256–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Engelter ST, Wetzel SG, Bonati LH, Fluri F, Lyrer PA. The clinical significance of diffusion-weighted MR imaging in stroke and TIA patients. Swiss Med Wkly. 2008;138(49-50):729–740. [DOI] [PubMed] [Google Scholar]
- 54. Bose P, Wilson A, Mistri A. Diagnosis and management of transient ischemic attacks in primary care: a systematic review. J Prim Health Care. 2017;9(2):114–130. [DOI] [PubMed] [Google Scholar]
- 55. Siket MS, Edlow JA. Transient ischemic attack reviewing the evolution of the definition, diagnosis, risk stratification, and management for the emergency physician. Emerg Med Clin North Am. 2012;30(3):745–770. [DOI] [PubMed] [Google Scholar]
- 56. Furuta Y, Hata J, Mukai N, et al. Secular trends in the incidence, risk factors, and prognosis of transient ischemic attack in Japan: the Hisayama study. Atherosclerosis. 2018;273:84–90. [DOI] [PubMed] [Google Scholar]
- 57. Kapadia S, Agarwal S, Miller DC, et al. Insights into timing, risk factors, and outcomes of stroke and transient ischemic attack after trans catheter aortic valve replacement in the partner trial (placement of aortic trans catheter valves). Circ-Cardiovasc Interv. 2016;9(9):e002981. [DOI] [PubMed] [Google Scholar]
- 58. Liu MY, Pan YS, Zhou LC, Wang YJ. Predictors of post-thrombolysis symptomatic intracranial hemorrhage in Chinese patients with acute ischemic stroke. PloS one. 2017;12(9):e0184646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chenna V, Kaul S, Tandra S, et al. Predictors of intracerebral hemorrhage in acute stroke patients receiving intravenous recombinant tissue plasminogen activator. Ann Indian Acad of Neurol. 2018;21(3):214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Fang CW, Tsai YT, Chou PC, et al. Intravenous thrombolysis in acute ischemic stroke after idarucizumab reversal of dabigatran effect: analysis of the cases from Taiwan. J Stroke Cerebrovasc Dis. 2019;28(3):815–820. [DOI] [PubMed] [Google Scholar]
- 61. Modrego PJ. The risk of symptomatic intracranial hemorrhage after thrombolysis for acute stroke: current concepts and perspectives. Ann Indian Acad of Neurol. 2019;22(3):336–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chandratheva A, Lasserson DS, Geraghty OC, Rothwell PM, Oxford Vasc S. Population-based study of behavior immediately after transient ischemic attack and minor stroke in 1000 consecutive Patients lessons for public education. Stroke. 2010;41(6):1108–1114. [DOI] [PubMed] [Google Scholar]
- 63. Zhao LR, Willing A. Enhancing endogenous capacity to repair a stroke-damaged brain: An evolving field for stroke research. Prog Neurobiol. 2018;163:5–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Strommen AM, Christensen T, Jensen K. Quantitative measurement of physical activity in acute ischemic stroke and transient ischemic attack. Stroke. 2014;45(12):3649–3655. [DOI] [PubMed] [Google Scholar]