Abstract
The homeostatic proliferation-differentiation gradient in the esophageal epithelium is perturbed under inflammatory disease conditions such as gastroesophageal reflux disease and eosinophilic esophagitis. Herein we describe the protocols for rapid generation (< 14 d) and characterization of single cell-derived three-dimensional (3D) esophageal organoids from human subjects and mice with normal esophageal mucosa or inflammatory disease conditions. While 3D organoids recapitulate normal epithelial renewal, proliferation and differentiation, non-cell autonomous reactive epithelial changes under inflammatory conditions are evaluated in the absence of the inflammatory milieu. Reactive epithelial changes are reconstituted upon exposure to exogenous recombinant cytokines. These changes are modulated pharmacologically or genetically ex vivo. Molecular, structural and functional changes are characterized by morphology, flow cytometry, biochemistry and gene expression analyses. Esophageal 3D organoids can be translated for the development of personalized medicine in assessment of individual cytokine sensitivity and molecularly-targeted therapeutics in esophagitis patients.
Keywords: esophagus, esophagitis, inflammatory cytokines, organoids, reactive epithelial changes, eosinophilic esophagitis
INTRODUCTION
Epithelium is the barrier between the body and the outside world. A stratified squamous epithelium lines the esophageal mucosa to provide protection against mechanical trauma from food, chemical injury from acids in the luminal content, immunogenic food allergens, as well as invading pathogens(Rosekrans et al., 2015). The stratified squamous epithelium of the esophagus contains a basal layer of proliferative and undifferentiated epithelial cells (basal keratinocytes). These cells undergo post-mitotic terminal differentiation (cornification) within the overlying suprabasal cell layers to form stratified squamous epithelium(Rosekrans et al., 2015). The esophageal epithelium maintains an exquisite balance of proliferation and differentiation through continuous proliferation of basal keratinocytes, migration of differentiated keratinocytes toward the luminal surface, and finally desquamation of cornified and flat keratinocytes, allowing epithelial renewal that occurs within a few weeks(Whelan et al., 2018).
Inflammation, as seen under disease conditions such as gastro-esophageal reflux disease (GERD) and eosinophilic esophagitis (EoE), disrupt the afore-mentioned homeostatic balance of proliferation and differentiation causing subsequent barrier disruption and prolonged mucosal injury. It is therefore imperative to develop a reliable ex vivo model to recapitulate the epithelial differentiation gradient of the esophageal epithelium in humans and mice in order to study these diseases in their physiologically-relevant three-dimensional (3D) context(Whelan et al., 2018).
We have established the single cell-derived esophageal 3D organoid culture system to mimic in vivo tissue architecture to model epithelial homeostasis and reactive changes associated with esophageal disease conditions(Kasagi et al., 2018a). We describe protocols to (1) prepare single cell suspensions from (a) endoscopic biopsies from human subjects or (b) epithelial sheets isolated from murine esophagi as starting materials; (2) grow, passage and cryopreserve 3D organoids; (3) determine growth kinetic of 3D organoids; and (4) perform morphological and functional evaluation of 3D organoids coupled with or without pharmacologic or genetic manipulations.
STRATEGIC PLANNING (optional)
Human Subjects
For human esophageal 3D organoid studies, it is imperative to have (1) the Health Insurance Portability and Accountability Act (HIPAA) authorization, as well as an Institutional Review Board (IRB)-approved protocol; (2) an appropriate clinical care facility (e.g. endoscopy unit); (3) a clinical care team comprising physicians/gastroenterologists, nurses, support staff, research nurses/clinical coordinators who recruit consented human subjects and procure esophageal tissue samples; (4) coordination with laboratory personnel who should be notified in advance to schedule time to initiate 3D organoid culture; and (5) proper safety training of laboratory personnel. IRB protocol should be carefully planned to permit multiple research biopsies for concurrent histopathological analyses and an access for the clinical information (e.g. age, gender, endoscopic findings, pathology reports, therapy outcome).
During routine upper endoscopy (esophagogastroduodenoscopy), an expert gastroenterologist should perform a routine esophageal biopsy with forceps and concurrent photo-documentation of the esophageal mucosa. Biopsy/surgical specimens (submerged in sterile PBS) need to be transported as soon as possible (< 2h) to a research lab for tissue processing and the initiation of 3D organoids culture. Laboratory personnel should have proper knowledge and laboratory safety training about infectious agents including hepatitis and human immunodeficiency viruses that may be potentially present in the starting materials.
To compare esophageal 3D organoids grown from different individuals for parameters such as organoid formation rate and growth rate, it is important to include a quality control cell line (e.g. immortalized normal human esophageal cell line such as EPC2-hTert)(Harada et al., 2003; Muir et al., 2013) to minimize the influence of variables such as cell culture medium components, cell culture incubator conditions, and experience levels of experimenters.
Mice
For murine esophageal 3D organoid studies, experiments must be planned and performed in accordance with regulations and an approved protocol under a local regulatory body (e.g. Institutional Animal Care and Use Committee and Animal Ethics Committee). Mice should be housed at a proper animal care facility that ensures humane treatment of mice and provides appropriate veterinary care of mice and laboratory safety training of laboratory personnel.
Biological replicates include sets of 3D organoids generated from independent mice. As organoids can be manipulated ex vivo pharmacologically or genetically for evaluation, a minimal number of mice (n=2) is generally sufficient per experiment to ensure multiple technical replicates (n=3–6). If necessary, pilot studies should be performed to estimate appropriate sample size of mice permitting detection of medium to large effects with 80% power in experiments planned.
BASIC PROTOCOL 1
Generation of esophageal organoids from biopsy or murine esophageal epithelial sheets
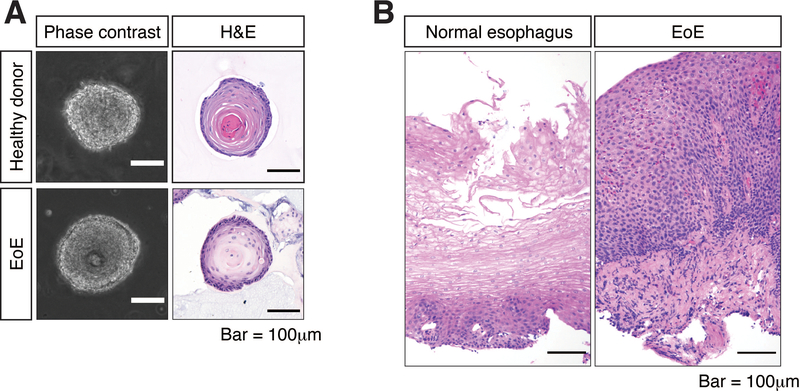
Tissue specimens are obtained either via biopsy (for human patients) or necropsy (for murine esophagi) and dissociated enzymatically and mechanically, followed by embedding of single cells in Matrigel. The organoids are cultured for 11 days, resulting in formation of 3D spherical structures recapitulating the original tissue. Representative images of esophageal organoids generated from EoE patient and a healthy control donor can be found in Figure 1. Murine organoids can be cultured in the same medium as human patient-derived organoids.
Figure 1: Morphological characteristics of organoids derived from normal esophagus and EoE biopsies.
(A) Representative images of normal esophageal and EoE organoids. Organoid growth can be monitored via phase contrast microscopy while in culture, and histopathological analysis can be conducted on hematoxylin-eosin (H&E)-stained organoids after fixation, paraffin embedding and sectioning. Expansion of basaloid cell compartment (basal cell hyperplasia) is evident in the EoE organoid. (B) H&E-stained sections of biopsies from which the organoids were derived.
Materials
All tools should be properly cleansed and sterilized prior to use. All plasticware and glassware should be cell culture grade and disposable. Water and DMSO used to dissolve or dilute reagents should be ultrapure and sterile. Standard equipment and tools for cell culture (CO2 incubator, tissue culture hoods, liquid nitrogen cell storage tank, vacuum aspirator/collection system, centrifuges, pipettors, etc.) are needed. While alternatives are available, we will list key items used routinely in our laboratories.
- Equipment:
- ThermoMixer C (Thermo Fisher Scientific, cat. No. 14-285-562 PM))
- Centrifuge Sorval ST 16R (Thermo Fisher Scientific, cat. No. 75004380)
- Microcentrifuge Minispin (Eppendorf, cat. No. 022620100)
- Countess II FL Automated Cell Counter (Invitrogen, cat. No. AMQAX1000)
- Tissue culture-grade plasticware and tools:
- Forceps (VWR, cat. No. 82027-386)
- 1 mL Tuberculin Syringe (BD, cat. No. 309659)
- 70-μm cell strainer (Thermo Fisher Scientific, cat. No. 22363548)
- 40-μm cell strainer (Thermo Fisher Scientific, cat. No. 22363547)
- 50 mL conical tube (Thermo Fisher Scientific, cat. No. 12-565-270)
- 5-mL round bottom polystyrene tube with cell strainer snap cap (BD, cat. No. 352235)
- 24 well plate (Thermo Fisher Scientific, cat. No. 12-556-006)
- 60mm cell culture dish (Thermo Fisher Scientific, cat. No. 12-556-001)
- Buffers and media components:
- Penicillin-Streptomycin (Thermo Fisher Scientific, cat. No. 15140122)
- Keratinocyte SFM medium (KSFM) (Life Technologies, cat. No 10724-011), supplemented with 1 ng/ml epidermal growth factor, 5 μg/ml bovine pituitary extract and Penicillin/Streptomycin
- Dispase (50U/mL, stored undiluted at −20oC in 1mL aliquots) (Corning, cat. No. 354235)
- Hank’s Balanced Salt Solution (HBSS) (Thermo Fisher Scientific, cat. No. 14175079)
- Dulbecco’s Phosphate-Buffered Saline (1x PBS) (Thermo Fisher Scientific, cat. No. 14190250)
- 0.05% Trypsin-EDTA (Thermo Fisher Scientific, cat. No. 25-300-054)
- 250 μg/mL Soybean Trypsin Inhibitor (Sigma-Aldrich, cat. No. T9128)
- Trypan Blue Cell Stain (Thermo Fisher Scientific, cat. No. T10282)
- Matrigel basement membrane matrix (Corning, cat. No. 354234)
- 10 μM Y27632 (Selleck Chemicals, cat. No. S1049)
- 0.5 μg/ml Fungizone (Thermo Fisher Scientific, cat. No. 15290018)
- 4% Paraformaldehyde (Sigma-Aldrich, cat. No. 158127-500G)
- Calcium chloride, anhydrous (Thermo Fisher Scientific, cat. No. AC349615000))
- CaCl2, 300mM solution: dissolve 33.3g of calcium chloride in 1L of water, filter-sterilize and store at 4°C
- KSFMC: add 1mL of 300mM CaCl2 solution to 500 mL KSFM supplemented with BPE, EGF and Penicillin/Streptomycin
Procurement of tissue and dissociation into a single cell suspension
Place the tissue fragment (a single human patient biopsy punch) in a 1.5mL microcentrifuge tube containing 750μL cold KSFM medium and transfer to lab on ice.
- Remove KSFM media, incubate biopsy in 1 mL dispase (10 U/ml) for 10 min at at 37°c in thermomixer (700–800 RPM)
- To prepare working solution of dispase, dilute the 50 U/ml stock in HBSS at 1:5 ratio
Remove dispase and wash the tissue three times with 1 ml of 1x PBS
- Incubate biopsy with 500 μl of 0.05% Trypsin (heated prior to addition) at 37°c for 10 min in thermomixer (700–800 RPM)
- When generating murine esophageal organoids, begin at this step (please see Support Protocol 1 For preparation of murine esophageal epithelial sheets)
Place 70 μm cell strainer on top of a 50 mL conical tube
Pre-load cell strainer with 2 mL of 250 μg/mL Soybean Trypsin Inhibitor
Pipette to transfer the isolated cells and add dissociated tissue to strainer
- Use plunger head of 1 mL TB Syringe to force remaining cells and tissue fragments through the strainer
-
Placed the strainer in a 60 mm cell culture dish to prevent membrane damage
-
Rinse the strainer with 1 mL of 250 μg/mL Soybean Trypsin Inhibitor
Filter cell suspension into a 5-mL round bottom polystyrene tube with a cell strainer cap
Pellet the cell suspension at 500g for 5 min (Sorval ST centrifuge)
Aspirate 2.5 mL of supernatant, re-suspend the pellet in KSFM and transfer to a 1.5 mL microcentrifuge tube
Pellet in the mini centrifuge at 500g for 3 min
Re-suspend pellet in 50–100 μL KSFM, keep on ice and count the cells
Organoid seeding and culture in 24-well plates
-
2
Thaw and keep Matrigel on ice
-
3
Pre-warm a 24-well plate at 37°C
-
4
Plate 2000 cells per well of a 24-well plate in 50 μL 1:1 Matrigel and KSFMC droplet
-
5
Incubate droplets at 37°c for 15 min
-
6Add 500 μL of KSFMC media to each well
- Supplement KSFMC media on day 0 with 0.5 μg/ml Fungizone and 10 uM Y27632 (Addition of Y27632 improves viability of esophageal keratinocytes in single-cell suspension)
-
7
Change media on day 1, 4, 6, 8, and 10
-
8
The organoids are ready for harvest and/or passage on day 11.
BASIC PROTOCOL 2
Propagation and cryopreservation of esophageal organoids
Once the organoids are established, esophageal keratinocytes can be isolated from the primary culture by enzymatic digestion and used for subsequent passaging and propagation. The isolated esophageal keratinocytes can be cryopreserved for long-term storage.
Materials
All culture equipment, centrifuges, plasticware, pipettors, reagents are the same as in Basic Protocol 1, unless otherwise stated below.
0.25% Trypsin-EDTA (Thermo Fisher Scientific, cat. No. 25–200-056)
DNAse I (Sigma-Aldrich, cat. No. 10104159001)
Fetal Bovine Serum (FBS) (HyClone, cat. No. SH30071.03)
Dimethyl sulfoxide (DMSO) (Sigma-Aldrich, cat. No. D4540)
Nalgene general long-term storage cryogenic tubes (Thermo Fisher Scientific, cat. No. 03–337-7D)
CoolCell LX freezing container (Corning, cat. No. 432002)
Growing culture of esophageal organoids from Basic Protocol 1
Organoid disintegration and preparation of single cell suspension
-
1
Digest Matrigel by adding 400 ul dispase per well and incubating for 15 min at 37°C
-
2
Transfer the digested suspension to 1.5 mL microcentrifuge tube
-
3
Pellet suspension at 500g for 5 min, remove supernatant
-
4Incubate with 0.25 % Trypsin supplemented with 10 μM Y27632 and 0.5 u/mL DNase I for 10 min at 37°C in thermomixer
- DNAse I prevents clumping of cells by DNA strands released from dying cells.
-
5
Place 40 μm cell strainer on top of a 50 mL conical tube
-
6
Pre-load the cell strainer with 1 mL of 250 μg/mL Soybean Trypsin Inhibitor
-
7
Filter the cell suspension through the strainer
-
8
Rinse the strainer with 1 mL of 250 μg/mL Soybean Trypsin Inhibitor
-
9
Pellet the cells at 500g for 5 min
-
10
Aspirate the supernatant, resuspend in 1mL KSFM and transfer to a 1.5 mL microcentrifuge tube
-
11
Pellet the cells using mini centrifuge at 500g for 3 min
-
12
Resuspend the pellet in 50–100 μL KSFMC, keep on ice and count cells
Passaging to 24-well plates
-
13
Thaw and keep Matrigel on ice
-
14
Pre-warm a 24-well plate at 37°C
-
15
Plate 2000 cells per well of a 24-well plate in 50 μL 1:1 Matrigel and KSFMC droplet
-
16
Incubate droplets at 37°C for 15 min
-
17Add 500 μL of KSFMC media to each well
- Supplement KSFMC media on day 0 with 0.5 ug/ml Fungizone and 10 uM Y27632
-
18
Change media on day 1, 4, 6, 8, and 10
Cryopreservation of esophageal organoids
-
19Prepare freezing medium by mixing 9 mL FBS with 1 mL DMSO. This medium can be stored at −20°C.
-
Any brand of FBS can be used for this purpose.
-
-
20
Resuspend the cell pellet from step 11 in freezing medium to the final concentration of 105 cells/mL
-
21
Dispense 1 mL of suspension from step 20 per cyovial
-
22
Place the cryovials in a freezing container and keep it at −80°C overnight
-
23
Transfer the frozen vials into a liquid nitrogen storage tank
Recovery after cryopreservation
-
2
Thaw and keep Matrigel on ice
-
3
Pre-warm a 24-well plate at 37°C
-
4
Thaw the cryovial in a 37°C water bath for about 30 sec (until the contents are liquid)
-
5
Transfer the cell suspension to a microcentrifuge tube
-
6
Pellet the cells in the mini centrifuge at 500x g for 3 min, remove the supernatant
-
7
Resuspend the cell pellet in 1 mL 1xPBS
-
8
Pellet the cells in the mini centrifuge at 500x g for 3 min, remove the supernatant
-
9
Resuspend the pellet in 1mL KSFM, assess cell density and viability
-
10
Pellet the cells in the mini centrifuge at 500x g for 3 min, remove the supernatant
-
11
Resuspend the cell pellet in 1:1 Matrigel and KSFMC to final concentration of 4×105 cells/mL and plate droplets of 50 μL /well of the 24-well plate
BASIC PROTOCOL 3
Harvesting of esophageal organoids for RNA isolation, immunohistochemistry and evaluation of 3D architecture
Histological evaluation and gene expression profiling are essential tools in research. Here we provide a protocol for fixation of esophageal organoids for embedding into paraffin blocks, as well as for RNA isolation from esophageal organoids.
Materials
All culture equipment, centrifuges, plasticware, pipettors, reagents are the same as in Basic Protocol 1, unless otherwise stated below.
Bacto-agar (Beckton-Dickinson, cat. No. 214010)
Gelatin (Thermo Fisher Scientific, cat. No. G7–500)
15 mL conical tubes (Thermo Fisher Scientific, cat. No.14–959-53A)
Embedding gel (2% bacto-agar – 2.5% gelatin): prepare the gel by resuspending 1 g of bacto-agar and 1.25 g of gelatin in 50 mL water, swirl the suspension and let it sit for 30–60 min at room temperature, autoclave for 20 min and aseptically dispense 5 mL per 15 mL conical tube, then store aliquots at room temperature
Embedding pipette tips: cut the end of a 200 μL pipette tip to make a bevel
Parafilm M Wrap (Thermo Fisher Scientific, cat. No. S37440)
Microcentrifuge tube rack (Southern Labware, cat. No. 0061)
Embedding mold surface: wrap a microcentrifuge rack in parafilm to make a hydrophobic surface
Tissue Cassette (Thermo Fisher Scientific, cat. No. 22–272416)
Ethanol 200 Proof (Thermo Fisher Scientific, cat. No. 22–032-601)
0.25% Trypsin-EDTA (Thermo Fisher Scientific, cat. No. 25–200-056)
Growing culture of esophageal organoids from Basic Protocol 1
Organoid Harvesting
-
1
Aspirate media from wells
-
2
Use 1250 μL pipette tip to loosen Matrigel droplet attachment to well
-
3
Scrape loose Matrigel droplet to bottom of well
-
4
Transport Matrigel droplet to microcentrifuge tube
-
5
Combine 3 droplets into 1 microcentrifuge tube
-
6
Add 1x PBS and dispase (250 μL of PBS mixed with 100 μL dispase)
-
7
Pipette vigorously 50 times to break up Matrigel droplets
-
8
Vortex for 15 seconds
-
9
Centrifuge for 3 minutes at 500g in microcentrifuge and rinse pellet with 1 mL of 1x PBS
-
10
Resuspend by vortexing briefly and pellet again
RNA/protein isolation
-
11
For isolation of RNA or protein from esophageal organoids, add appropriate lysis buffer directly to pellet from step 10
Fixation for Immunohistochemistory and evaluation of 3D architecture
-
12
Fix the esophageal organoid pellet from step 10 by resuspending in 500 uL of 4% Paraformaldehyde (PFA) and incubating at 4°C overnight.
-
13
Discard the 4% PFA, pellet the organoids for 3 minutes at 1500 RPM in microcentrifuge.
-
14Wash the pellet with 1 mL 1x PBS, pellet the organoids for 3 minutes at 1500 RPM in microcentrifuge, and aspirate as much liquid as possible with a pipette without disturbing pellet.
- Small amounts of residual liquid (e.g. 10 uL) are acceptable, however larger volumes will dilute the embedding gel and can complicate sample processing.
-
15Liquify the embedding gel: place the 15 mL conical tube with solid gel into a 150 mL beaker containing 100 mL water and microwave on highest power setting for 1 min or until the water starts boiling. Confirm that embedding gel is liquid.
- Loosen the cap on conical tube with the gel!
-
16
Slowly add 30 μL of embedding gel down the tube wall to cover the pellet from step 14.
-
17
Without disrupting the pellet, push with the embedding tip to dislodge the mix from the tube wall.
-
18
Transfer the pellet suspended in embedding gel embedding mold surface, forming a dome-shaped droplet.
-
19
Incubate the droplet at 4°C for 1 hour.
-
20
Transfer the droplet to sectioning cassette lined with construction paper, store in 70% ethanol at 4°C for up to 1 month.
-
21
Proceed with paraffin embedding via routine histological processing to prepare paraffin blocks.
BASIC PROTOCOL 4
Modeling of reactive epithelium in esophageal organoids
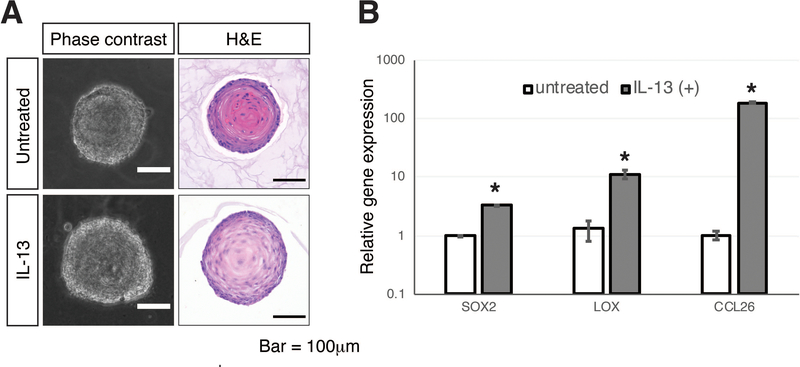
Reactive changes in esophageal epithelium are induced by multiple soluble factors secreted by immune cells and fibroblasts. The function of these factors in disease development can be modeled and evaluated in esophageal organoids. Here we provide an example of such experiment by treating normal esophageal organoids with interleukin 13 (IL-13) to model reactive changes induced in esophageal epithelium by eosinophils, however, any bioactive compounds (cytokines, antibodies, small molecule inhibitors) can be utilized to model pathologic conditions and/or treatment strategies. Representative images of esophageal organoids treated with IL-13, as well as gene expression profiles of select EoE-relevant genes(Kasagi et al., 2018b; Blanchard et al., 2007) can be found in Figure 2.
Figure 2: Modeling reactive epithelium in esophageal organoids.
(A) Representative images of organoids derived from normal esophageal keratinocytes and treated with EoE-relevant cytokine IL-13, compared to untreated organoids. Basal cell hyperplasia is evident in IL-13-treated organoid. (B) Relative gene expression profiles of EoE-relevant genes SOX2, LOX and CCL26 (eotaxin) in IL-13-treated organoids, compared to untreated controls (n=3 per group; error bars = SEM; asterisk denotes statistical significance).
Materials
All culture equipment, centrifuges, plasticware, pipettors, reagents are the same as in Basic Protocol 1, unless otherwise stated below.
- IL-13 (R&D systems, cat. No. 213-ILB-005)
- Reconstitute lyophilized IL-13 to 100 μg/mL in PBS, aliquot and store at −80°C, avoid repeating freeze-thaw cycles
- Growing culture of esophageal organoids from Basic Protocol 1
- Remove spent medium.
- Dispense 500μL of KSFMC containing 10 ng/mL IL-13.
- Observe organoid growth under a phase-contrast microscope.
- Harvest organoids for histology and RNA isolation, as described in Basic Protocol 3.
- Evaluate changes in morphology and gene expression.
Support Protocol 1
Procurement of murine esophageal epithelial sheets
Herein we describe the procedure for isolation of epithelial sheets from murine esophagus. After completion of this protocol, proceed to Basic Protocol 1, step 4 for generation of murine esophageal organoids.
Materials
All culture equipment, centrifuges, plasticware, pipettors, reagents are the same as in Basic Protocol 1, unless otherwise stated below.
CO2 gas chamber
Sterile dissection-grade scissors (VWR, cat. No. 25870–002)
Sterile forceps (VWR, cat. No. 82027–386)
Sterile iris microdissecting scissors (Carolina Biological Supply, cat. No. 623555)
Petri dish (Thermo Fisher Scientific, Rochester, NY)
Fungizone, 0.5 μg/mL (Thermo Fischer Scientific, cat. No. 15290018)
Dulbecco’s Modified Eagle’s Medium (DMEM) (Corning, cat. No. MT10013CV)
Dissection and establishment of single cell suspension
Sacrifice mice according to your IACUC-approved euthanasia protocol.
Use sterile forceps to remove the esophagus and place it in a petri dish containing HBSS.
Open esophagus with sterile iris microdissecting scissors in the longitudinal direction, rinse in tissue in HBSS.
Place the opened esophagus in a microcentrifuge tube containing 500 μl of dispase and incubate at 37°C in the thermomixer at 800 RPM for 10 min
Peel the epithelium away from the submucosa (and discard submucosa).
Proceed with organoid generation as described in Basic Protocol 1, beginning at step 4.
COMMENTARY
Background Information
Historically, in vitro analysis of benign disorders of the esophageal epithelium involved stimulation of 2D cultures(Muir et al., 2013; Lim et al., 2009). However, the ability to assess the functional properties of the barrier as well as proliferation and differentiation are limited in flat cultures. To fill this void, organotypic culture (OTC) was adopted from methods of differentiating dermal keratinocytes (Kalabis et al., 2012). In this method, esophageal keratinocytes are seeded on top of a collagen/fibroblast raft. After confluence is reached, epithelia are exposed to an air-liquid interface and high calcium concentration in order to induce terminal differentiation. The result is a stratified squamous epithelium with underlying stroma, which mimics in vivo tissue architecture.
More recently, direct methods of evaluating barrier integrity of the esophageal epithelium have been developed capitalizing on the ability of esophageal cells to differentiate when exposed to an air-liquid interface (ALI)(Sherrill et al., 2014; Ruffner et al., 2019; Nguyen et al., 2017). ALI culture involves growing epithelium on a porous membrane. After reaching confluence, the monolayers are exposed to high concentrations of calcium, and media is then removed from the upper chamber allowing exposure of the cells to air. These methods allow for histologic evaluation of stratified squamous epithelium as well as functional evaluation of mucosal integrity by measuring transepithelial resistance or Fluorescein Isothiocyanate (FITC) Dextran flux to assess permeability.
While ALI and OTC methods allow for evaluation of the esophageal epithelium in 3D, both require a large number of cells grown for multiple passages ex vivo prior to 3D culture development, and performing OTC and ALI cultures with primary patient-derived cells is challenging(Whelan et al., 2018). On the other hand, with the methods of organoid culture demonstrated here, a single biopsy, allows for immediate assessment and characterization of the epithelium in 3D within 10 days of procuring the biopsy.
DeWard et al first described esophageal epithelial organoid cultures from murine tissue(DeWard et al., 2014). Their methods involved utilizing Advance Dulbecco’s modified Eagle’s medium with multiple growth factors including: Glutamax, B27, p38 kinase inhibitor, epidermal growth factor, TGFβ inhibitor, R-spondin, Noggin, and Wnt3A. We found that human esophageal organoids did not grow or stratify in this enriched media(Kasagi et al., 2018a). Instead, we utilized a simplified media of KSFM with additional calcium that produced a stratified squamous epithelium from both murine and human tissue with almost 100% reliability.
Our recent publication demonstrates the use of this technique to recapitulate the unique epithelial changes associated with eosinophilic esophagitis (EoE) by stimulation with EoE-relevant cytokines (IL-13, IL-4) (Kasagi et al., 2018a). We evaluated the effect of the EoE milieu on Notch signaling and utilized genetic and pharmacologic inhibition of Notch signaling of organoids to simulate the reactive epithelial changes that occur in vivo. We expect that future work will utilize organoid technology to evaluate inherent differences in the epithelial tissue from disease state and healthy controls, as well as employ high-throughput drug screening protocols and genetic manipulation.
Critical Parameters/Troubleshooting
Biopsy Procurement
Biopsies procured from a normal esophagus tend to result in >85% cell viability. At times those from diseased tissue can contain exudate and dead cells, reducing the overall epithelial viability. In these cases, we suggest collecting 2 biopsies per patient to ensure an adequate number of organoids produced. Similarly, when patients are scoped with a pediatric endoscope due to critical stricture, small biopsy forceps are used, and 2 biopsies may be required.
Passage of primary specimens
We have found that the well described immortalized non-transformed EPC2-hTERT organoids passage indefinitely(Kasagi et al., 2018a). Organoids from biopsies do not grown beyond passage 4–5. Upon adding supplements Wnt 3A, Noggin/R-spondin, or A83–01 at the time of passage, there was improved organoid formation rate, however, it was not indefinite. We hypothesize that current culture conditions are permissive for keratinocyte progenitors with limited self-renewal capability.
Day of Stimulus or Harvest
It is critical to consider time points for stimulation and harvest for organoid culture. A critical question to consider is whether organoid formation rate (OFR), organoid size, differentiation, or proliferation will be evaluated. We strongly suggest using time course for stimulants, as early stimulation (Days 0–4) may affect OFR, whereas later stimulation (Days 5–7) may affect proliferation/differentiation.
Similarly, it is critical to harvest cells for evaluation at the same time points. Due to the presence of calcium in the media, terminal differentiation does occur. Harvesting one experiment on day 11 and another on day 12 may result in vastly different results that are not comparable.
Understanding Results
Organoid Formation Rate
The organoids are developed from a single cell suspension and each organoid arises from a keratinocyte progenitor with self-renewal capability. Thus, the organoid formation rate (OFR) is a quantifiable assessment of the self-renewing epithelial cells in a given population. For instance, we found that organoids from EoE patients and non-EoE controls had similar OFR despite the fact that EoE patients have marked expansion of the basal population. This signifies that despite expansion of the basal population, there is not an increase in the replicative cell population. OFR is calculated as the number of organoids formed, normalized to the number of primary keratinocytes seeded. For example, if 200 organoids were formed after seeding 2000 cells, OFR would be 0.1 (or 10%). In our hands, typical OFR from normal and EoE patient-derived biopsies is 2%.
Evaluating Epithelial Differentiation
Careful morphologic evaluation of the organoids provides information regarding epithelial differentiation. Organoids should be measured, and average organoid size per well should be assessed at each passage. Methodologies to evaluate differentiation may vary, and complementary methods may be utilized. In our recently published work(Kasagi et al., 2018a), we utilized an expert pathologist to evaluate the organoids for basaloid cell content and complimented this with immunohistochemistry and flow cytometry analysis for involucrin and CD29, respectively.
Time Considerations
Procuring the biopsies and making the organoids on Day 0 in the simplified media takes 2 hours. Having the reagents thawed and ready makes it a more efficient process. Replenishing spent media and stimulating the organoids with cytokines takes about 15 minutes, and harvesting also takes approximately 2 hours. The organoids need to be fixed overnight after harvesting for paraffin embedding.
Significance Statement.
The esophagus connects the oral cavity and the stomach. The surface is lined by layers of stratified squamous epithelium, and renews through continuous cell division (proliferation) and differentiation followed by loss (desquamation) from the outmost layer into the lumen. This provides barrier against the luminal contents including acid, microorganisms, and food allergens. Under disease conditions, a variety of leukocytes are recruited to the esophagus to cause inflammation and produce cytokines. In response to cytokines, epithelial cells show reactive changes, resulting in impaired barrier function and aggravated inflammation. We describe protocols to reconstitute human and mouse esophageal epithelial structures in a highly efficient novel 3D cell culture to model and analyze epithelial homeostasis and perturbation under disease conditions such as esophagitis.
ACKNOWLEDGEMENT
This study was supported by the following NIH Grants: K01DK103953 (KAW), K08DK106444 and R03DK118310 01A1 (ABM), P01CA098101 (HN, TK, KAW, VG), U54CA163004 (TK, HN), R01DK114436 and R01AA026297 (HN), P30DK050306, P30CA013696, P30ES013508 University of Pennsylvania Center of Excellence in Environmental Toxicology (HN), R01AI092135 and K24AI135034 University of California San Diego (SA), Children’s Hospital of Philadelphia Epithelial Biology Center of Excellence (ABM, TK, KH), HL132996-02S1 (DB), NASPGHAN Young Investigator Award/George Ferry Award, T32 DK083256 (DB, Timothy Wang PI, and gifts from the Phyllis and Ivan Seidenberg Family Fund for Children’s Digestive Health). CEGIR (U54 AI117804) (ABM, JMS, GWF, DB) is part of the Rare Disease Clinical Research Network (RDCRN), an initiative of the Office of Rare Diseases Research (ORDR), NCATS, and is funded through collaboration between NIAID, NIDDK, and NCATS. CEGIR is also supported by patient advocacy groups including American Partnership for Eosinophilic Disorders (APFED), Campaign Urging Research for Eosinophilic Diseases (CURED), and Eosinophilic Family Coalition (EFC). VG holds the tier 2 Canada Research Chair in Gastrointestinal Stem Cell Biology. We thank the University of Pennsylvania NIH/NIDDK Center for Molecular Studies in Digestive and Liver Diseases.
Contributor Information
Hiroshi Nakagawa, Division of Digestive and Liver Diseases, Department of Medicine and Irving Cancer Research Center, Columbia University Medical Center, 1130 St. Nicholas Avenue, Room 201, New York, NY 10032-3802.
Yuta Kasagi, Department of Surgery, Fukuoka Higashi Medical Center, Adress: 1-1-1 Chidori Koga Fukuoka Fukuoka 811-3113 Japan.
Tatiana A. Karakasheva, Epithelial Biology Center, Division of Pediatric Gastroenterology, Hepatology, and Nutrition, The Children’s Hospital of Philadelphia, Abramson Research Center, 3615 Civic Center Blvd, Philadelphia, PA 19104-6160
Takeo Hara, Division of Pediatric Gastroenterology, Hepatology, and Nutrition, The Children’s Hospital of Philadelphia, Abramson Research Center, 3615 Civic Center Blvd 902E, Philadelphia, PA 19104-6160.
Bailey Aaron, Division of Pediatric Gastroenterology, Hepatology, and Nutrition, The Children’s Hospital of Philadelphia, Perlman School of Medicine, University of Pennsylvania, Abramson Research Center, 3615 Civic Center Blvd 902E, Philadelphia, PA 19104-6160.
Takashi Kijima, Division of Digestive and Liver Disease, Department of Medicine, Herbert Irving Comprehensive Cancer Center, Columbia University, 1130 St. Nicholas Avenue, Room 202B, New York, NY 10032-3802.
Veronique Giroux, Département d’anatomie et biologie cellulaire, Faculté de Médecine et des Sciences de la Santé, Université de Sherbrooke, (819) 821-8000 #72456.
Dominique Bailey, Division of Digestive and Liver Diseases, Columbia University Medical Center.
Benjamin Wilkins, Department of Pathology, The Children’s Hospital of Philadelphia, Perlman School of Medicine, University of Pennsylvania, 34th and Civic Center Blvd., Philadelphia, PA 19104.
Julian A. Abrams, Division of Digestive and Liver Diseases, Columbia University Medical Center.
Gary W. Falk, Division of Gastroenterology, University of Pennsylvania Perelman School of Medicine, PCAM South Pavilion, 7th Floor, 3400 Civic Center Boulevard, Philadelphia, Pennsylvania 19104-4311.
Seema Aceves, Division of Allergy Immunology, Rady Children’s Hospital San Diego, University of California, San Diego, 3020 Children’s Way, San Diego, CA.
Jonathan M. Spergel, Division of Allergy and Immunology, Children’s Hospital of Philadelphia, 34th and Civic Center Blvd, Philadelphia, PA.
Kathryn E. Hamilton, Division of Pediatric Gastroenterology, Hepatology, and Nutrition, The Children’s Hospital of Philadelphia, Perlman School of Medicine, University of Pennsylvania, Abramson Research Center, 3615 Civic Center Blvd 902D, Philadelphia, PA 19104-6160.
Kelly A. Whelan, Fels Institute for Cancer Research & Molecular Biology, Lewis Katz School of Medicine at Temple University, 3307 N. Broad Street, Room 203, Philadelphia, PA 19140.
Amanda Muir, Division of Pediatric Gastroenterology, Hepatology, and Nutrition, The Children’s Hospital of Philadelphia, Perlman School of Medicine, University of Pennsylvania, Abramson Research Center, 3615 Civic Center Blvd 902E, Philadelphia, PA 19104-6160.
LITERATURE CITED
- Blanchard C, Mingler MK, Vicario M, Abonia JP, Wu YY, Lu TX, Collins MH, Putnam PE, Wells SI, and Rothenberg ME 2007. IL-13 involvement in eosinophilic esophagitis: transcriptome analysis and reversibility with glucocorticoids. The Journal of allergy and clinical immunology 120:1292–1300. [DOI] [PubMed] [Google Scholar]
- DeWard AD, Cramer J, and Lagasse E 2014. Cellular heterogeneity in the mouse esophagus implicates the presence of a nonquiescent epithelial stem cell population. Cell reports 9:701–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada H, Nakagawa H, Oyama K, Takaoka M, Andl CD, Jacobmeier B, von Werder, A., Enders GH, Opitz OG, and Rustgi AK 2003. Telomerase induces immortalization of human esophageal keratinocytes without p16INK4a inactivation. Molecular cancer research : MCR 1:729–738. [PubMed] [Google Scholar]
- Kalabis J, Wong GS, Vega ME, Natsuizaka M, Robertson ES, Herlyn M, Nakagawa H, and Rustgi AK 2012. Isolation and characterization of mouse and human esophageal epithelial cells in 3D organotypic culture. Nature Protocols 7:235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasagi Y, Chandramouleeswaran PM, Whelan KA, Tanaka K, Giroux V, Sharma M, Wang J, Benitez AJ, DeMarshall M, Tobias JW, et al. 2018a. The Esophageal Organoid System Reveals Functional Interplay Between Notch and Cytokines in Reactive Epithelial Changes. Cellular and molecular gastroenterology and hepatology 5:333–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasagi Y, Dods K, Wang JX, Chandramouleeswaran PM, Benitez AJ, Gambanga F, Kluger J, Ashorobi T, Gross J, Tobias JW, et al. 2018sb. Fibrostenotic eosinophilic esophagitis may reflect epithelial lysyl oxidase induction by fibroblasts-derived tumor necrosis factor-α. The Journal of allergy and clinical immunology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim DM, Narasimhan S, Michaylira CZ, and Wang M-L 2009. TLR3-mediated NF-{kappa}B signaling in human esophageal epithelial cells. American journal of physiology. Gastrointestinal and liver physiology 297:G1172–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir AB, Lim DM, Benitez AJ, Modayur Chandramouleeswaran P, Lee AJ, Ruchelli ED, Spergel JM, and Wang M-L 2013. Esophageal epithelial and mesenchymal cross-talk leads to features of epithelial to mesenchymal transition in vitro. Experimental cell research 319:850–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen N, Fernando SD, Biette KA, Hammer JA, Capocelli KE, Kitzenberg DA, Glover LE, Colgan SP, Furuta GT, and Masterson JC 2017. TGF-β1 alters esophageal epithelial barrier function by attenuation of claudin-7 in eosinophilic esophagitis. Mucosal immunology 1:729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosekrans SL, Baan B, Muncan V, and van den Brink GR 2015. Esophageal development and epithelial homeostasis. American journal of physiology. Gastrointestinal and liver physiology 309:G216–28. [DOI] [PubMed] [Google Scholar]
- Ruffner MA, Song L, Maurer K, Shi L, Carroll MC, Wang JX, Muir AB, Spergel JM, and Sullivan KE 2019. Toll-like receptor 2 stimulation augments esophageal barrier integrity. Allergy 1:729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrill JD, Kiran KC, Blanchard C, Stucke EM, Kemme KA, Collins MH, Abonia JP, Putnam PE, Mukkada VA, Kaul A, et al. 2014. Analysis and expansion of the eosinophilic esophagitis transcriptome by RNA sequencing. Genes and immunity 15:361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan KA, Muir AB, and Nakagawa H 2018. Esophageal 3D Culture Systems as Modeling Tools in Esophageal Epithelial Pathobiology and Personalized Medicine. Cellular and molecular gastroenterology and hepatology 5:461–478. [DOI] [PMC free article] [PubMed] [Google Scholar]