Abstract
Purpose: To evaluate the performance of peripheral intravascular lithotripsy (IVL) in a real-world setting during endovascular treatment of multilevel calcified peripheral artery disease (PAD). Materials and Methods: The Disrupt PAD III Observational Study (ClinicalTrials.gov identifier NCT02923193) is a prospective, nonrandomized, multicenter, single-arm observational study assessing the acute safety and effectiveness of the Shockwave Peripheral IVL System for the treatment of calcified, stenotic lower limb arteries. Patients were eligible if they had claudication or chronic limb-threatening ischemia and moderate or severe arterial calcification. Between November 2017 and August 2018, 200 patients (mean age 72.5±8.7 years; 148 men) were enrolled across 18 sites and followed through hospital discharge. Results: In the 220 target lesions, IVL was more commonly used in combination with other balloon-based technologies (53.8%) and less often with concomitant atherectomy or stenting (19.8% and 29.9%, respectively). There was a 3.4-mm average acute gain at the end of procedure; the final mean residual stenosis was 23.6%. Angiographic complications were rare, with only 2 type D dissections and a single perforation following drug-coated balloon inflation (unrelated to the IVL procedure). There was no abrupt closure, distal embolization, no reflow, or thrombotic event. Conclusion: Use of peripheral IVL to treat severely calcified, stenotic PAD in a real-world study demonstrated low residual stenosis, high acute gain, and a low rate of complications despite the complexity of disease.
Keywords: acute gain, calcification, chronic total occlusion, common femoral artery, complication, iliac artery, infrapopliteal arteries, lithoplasty, lithotripsy, occlusion, peripheral artery disease, popliteal artery, stenosis, superficial femoral artery
Introduction
In patients with peripheral artery disease (PAD), the presence of calcification indicates a worse prognosis and remains a challenge for even the most experienced endovascular interventionists.1 Intra-arterial calcium is associated with higher rates of acute angiographic complications such as dissections, perforations, slow or no reflow, distal embolization, and/or recoil.2–5 Heavily calcified arteries may be difficult to dilate with conventional balloon angioplasty (BA) and often require mechanical debulking and/or modification of the plaque with focal-force balloons or atherectomy. Despite advancements in calcium modification techniques, limitations remain in the ability to treat calcified lesions. High pressure balloon angioplasty may be of insufficient force to effectively modify the entire calcified lesion. Rotational and orbital atherectomy are able to achieve acute gain by affecting the superficial calcium but medial calcium is not affected.6
Intravascular lithotripsy (IVL) is a novel calcium modification tool that uses sonic pressure waves to modify both intimal and medial calcium. Similar to urologic lithotripsy, the sonic pressure waves pass harmlessly through the soft tissue and fracture the calcium, minimizing risk to the non-calcified portions of the vessel. While IVL has been studied as a standalone treatment, demonstrating safety and effectiveness,7–9 the use of IVL in the real-world setting has not been reported.
The aim of this first report from the Disrupt PAD III Observational Study was to evaluate the safety and efficacy of peripheral IVL in combination with adjunctive devices in an all-comers cohort of patients with calcified lower limb PAD.
Materials and Methods
Study Device
The Peripheral IVL System (Shockwave Medical, Fremont, CA, USA) is indicated for lithotripsy-enhanced, low-pressure balloon dilation of calcified, stenotic peripheral arteries. The system consists of a generator, a connector cable, and an IVL catheter that houses an array of lithotripsy emitters enclosed in an integrated balloon. Peripheral IVL catheters utilized in this initial analysis were 60 mm in length and ranged in diameter from 3.5 to 7.0 mm in 0.5-mm increments. IVL catheter selection is based on sizing, with the IVL catheter diameter being 1.1:1 to the reference vessel diameter (RVD). Once a calcified arterial lesion is crossed with a 0.014-inch guidewire, the IVL catheter is advanced across the lesion and positioned using radiopaque marker bands. The integrated balloon is expanded to 4 atm using a mixed saline and contrast solution to achieve balloon–vessel wall apposition (the low pressure decreases the risk of barotrauma). The generator is activated, producing 3 kV of energy that travels through the connector cable and catheter to the lithotripsy emitters at 1 pulse per second to vaporize the fluid within the balloon and create a rapidly expanding bubble. A series of sonic pressure waves travel through the fluid-filled balloon and pass through soft vascular tissue, selectively cracking the hardened intimal and/or medial calcified plaque. The emitters positioned along the length of the device create a localized field effect within the vessel. Lithotripsy is administered in 30-pulse increments. Following calcium disruption, the balloon is then inflated to nominal pressure (6 atm) to maximize lumen gain. This cycle is then repeated as often as needed until the desired diameter is obtained. The IVL catheter can then be moved to other lesion locations to deliver up to 180 total pulses per catheter. Additional catheters can be utilized in a target lesion. There is no set minimum or maximum number of pulses delivered to a given area.
Study Design and Patient Enrollment
The Disrupt PAD III Observational Study is a prospective, nonrandomized, multicenter, single-arm study designed to assess the acute safety and effectiveness of the Shockwave Peripheral IVL System in combination with adjunctive devices in patients being treated for calcified lower limb lesions. Patients were eligible for enrollment if they had claudication or chronic limb-threatening ischemia (CLTI; defined as Rutherford category 4–6) and at least moderate calcification associated with de novo or restenotic stenoses in the iliofemoral, femoropopliteal, or infrapopliteal arteries. The threshold for moderate calcification was defined as fluoroscopic evidence of calcification on parallel sides of the vessel extending ≥50% of the lesion length in lesions ≥50 mm long or extending a minimum of 20 mm if the lesion length was <50 mm. A patient was considered enrolled once the IVL catheter insertion was attempted.
The study was conducted in accordance with the Declaration of Helsinki, ISO 14155:2011 guidelines, and Good Clinical Practices. An independent angiographic core laboratory (Yale Cardiovascular Research Group, New Haven, CT, USA) analyzed all angiograms; calcifications were graded by the laboratory using the Peripheral Academic Research Consortium (PARC) criteria (Figure 1).7 The ethics committee or institutional review board of each site approved the study protocol and informed consent form, which was signed by all patients prior to study enrollment. The study was registered on the National Institutes of Health website (ClinicalTrials.gov; identifier NCT02923193).
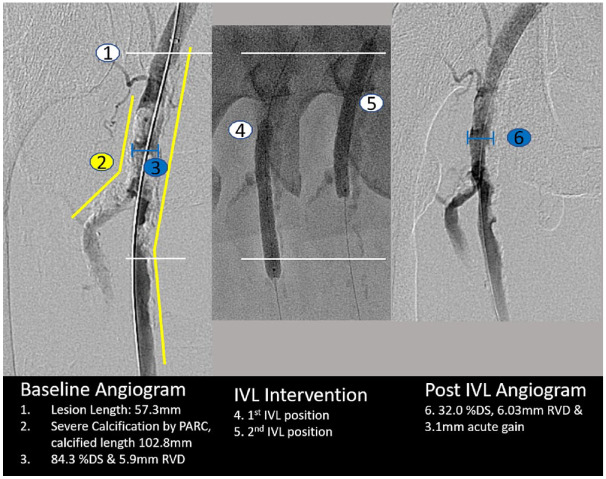
Figure 1.
Example of core laboratory angiographic assessment of a treated lesion. DS, diameter stenosis; IVL, intravascular lithotripsy; PARC, Peripheral Academic Research Consortium; RVD, reference vessel diameter.
Study Procedures
All investigators were trained in the use of the Peripheral IVL System. Adjunctive technologies, including drug-elution therapy, atherectomy, and stenting, were allowed per physician discretion to optimize treatment and outcomes. Vascular access, anticoagulation, introduction of guidewires, and catheter use were conducted according to each institution’s standard of care for endovascular procedures. Final angiography (including runoff views) was performed to assess the final procedure result.
Patient Population
Between November 2017 and August 2018, the first 200 patients were enrolled across 18 sites in the United States (n=16) and Europe and are the subject of this initial analysis. Baseline characteristics represent a complex patient population with risk factors for calcification, including increased age, diabetes, and renal insufficiency. Patients were being treated for CLTI in 30.1% of the cases. A total of 220 calcified lesions were treated with IVL in the iliac artery (14.8%), common femoral artery (CFA; 12.5%), superficial femoral artery (SFA; 56.0%), popliteal artery (14.4%), and infrapopliteal vessels (2.3%); nearly one-third (32.9%) were chronic total occlusions. Infrapopliteal lesions were not included in this analysis due to the small number. Baseline patient and lesion characteristics are summarized in Table 1.
Table 1.
Baseline Characteristics of the 200 Patients in the Study.a
| Age, y | 72.5±8.7 |
| Men | 148/200 (74.0) |
| Diabetes | 100/198 (50.5) |
| Hypertension | 189/198 (95.5) |
| Hyperlipidemia | 171/198 (86.4) |
| Current smoker | 42/197 (21.3) |
| Coronary artery disease | 133/197 (67.5) |
| Renal insufficiency | 35/197 (17.8) |
| Dialysis | 9/198 (4.5) |
| ABI | 0.7±0.3 |
| Rutherford category | |
| 2 | 19/193 (9.8) |
| 3 | 116/193 (60.1) |
| 4 | 21/193 (10.9) |
| 5 | 32/193 (16.6) |
| 6 | 5/193 (2.6) |
| Lesions/patientb | 1.1±0.3 |
| Lesion locationb | |
| Iliac | 32/216 (14.8) |
| CFA | 27/216 (12.5) |
| SFA | 121/216 (56.0) |
| Popliteal | 31/216 (14.4) |
| Infrapopliteal | 5/216 (2.3) |
| Lesion length,b mm | 103.4±71.9 |
| Calcified length,b mm | 140.9±89.6 |
| CTOb | 71/216 (32.9) |
| RVD,b mm | 5.7±1.6 |
| Diameter stenosisb | 80.8±17.9 |
| Severe calcificationb,c | 169/217 (77.9) |
| Eccentric calcificationb | 31/217 (14.3) |
Abbreviations: ABI, ankle-brachial index; CFA, common femoral artery; CTO, chronic total occlusion; RVD, reference vessel diameter; SFA, superficial femoral artery.
Continuous data are presented as the mean ± standard deviation; categorical data are given as the number/sample (percentage).
Adjudicated by the core laboratory.
Severe calcification defined by the Peripheral Academic Research Consortium criteria as ≥180° calcification or both sides of the vessel and greater than one-half of the total lesion length.
Statistical Analysis
No formal hypothesis testing was performed. Continuous variables are expressed as mean ± standard deviation and range. Categorical variables are described as the percentage.
Results
Procedural Details and Acute Outcomes
All 200 patients had successful IVL catheter delivery and received lithotripsy treatment with a mean 205.3±122.4 pulses. As shown in Table 2, the use of IVL with other balloon-based technologies (ie, conventional, drug-coated, and specialty balloons) was more common (53.8%) than IVL being utilized with atherectomy and/or stents (19.8% and 29.9%, respectively). The use of drug-coated balloons (DCBs) accounted for the majority of adjunctive therapy (77.7%), with BA being utilized 48.7% of the time, likely for pre- or postdilation; specialty balloon use was in a minority of procedures (6.1%). The use of adjunctive therapy varied across vessel beds (Figure 2). For calcified iliac artery lesions, IVL was primarily used as a preparation tool prior to deployment of various types of stents (Table 2). Embolic protection filters were used in 16.2% of total cases, more frequently with concomitant atherectomy (71.8%) than without atherectomy (2.5%).
Table 2.
Characteristics of the 200 Procedures.a
| Predilation | 61/197 (31.0) |
| Postdilation | 100/197 (50.8) |
| Successful IVL delivery, % | 100 |
| IVL pulses | 205.3±122.4 |
| Adjunctive technology | |
| Balloon angioplasty | 96/197 (48.7) |
| Drug-coated balloon | 153/197 (77.7) |
| Stent | 59/197 (29.9) |
| Atherectomy | 39/197 (19.8) |
| Specialty balloon | 12/197 (6.1) |
| Balloon onlyb | 106/197 (53.8) |
| Embolic protection | 32/198 (16.2) |
| With atherectomy | 28/39 (71.8) |
| Without atherectomy | 4/159 (2.5) |
| Iliac stent use | |
| None | 7/32 (21.9) |
| Stent placed per lesionc | 25/32 (78.1) |
| Covered | 10/32 (31.3) |
| Bare metal | 14/32 (43.8) |
| Drug-eluting | 2/32 (6.3) |
Abbreviation: IVL: intravascular lithotripsy.
Continuous data are presented as the mean ± standard deviation; categorical data are given as the number/sample (percentage).
Balloon-based technology refers to conventional, drug-coated, or specialty balloons.
Two stents were placed in a single lesion.
Figure 2.
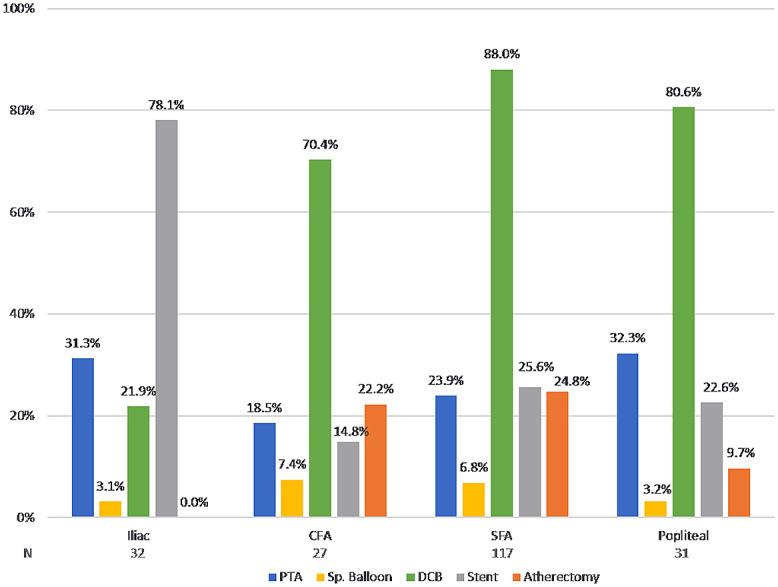
Adjunctive therapy utilization across multiple vessels.
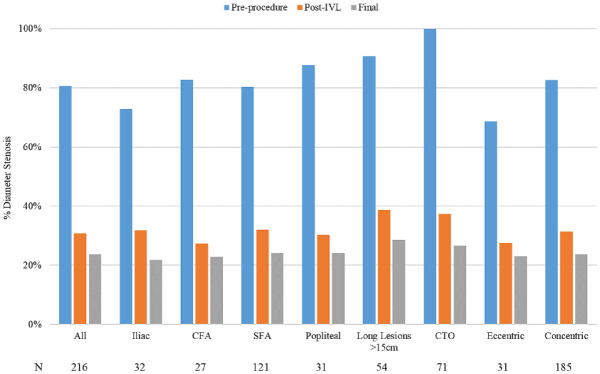
Final results and angiographic complications are summarized in Table 3. The average acute gain at the end of the procedure was 3.4±1.2 mm. The final mean residual stenosis was 23.6%±9.7% and was consistently <30% across all lesion types, with the majority of reduction in stenosis occurring following IVL treatment as demonstrated in Figure 3. A representative case is shown in Figure 4.
Table 3.
| Diameter stenosis | 23.6±9.7 |
| Concentric lesions | 23.7±10.1 |
| Eccentric lesions | 23.0±6.8 |
| Acute gain, mm | 3.4±1.2 |
| Concentric lesions | 3.5±1.2 |
| Eccentric lesions | 3.2±1.2 |
| Dissection, type D/E/F | 2/187c (1.1) |
| Concentric lesions | 2/162 (1.4) |
| Eccentric lesions | 0 |
| Perforation | 1/187d (0.5) |
| Distal embolization | 0 |
| Thrombus | 0 |
| No reflow | 0 |
| Abrupt closure | 0 |
Continuous data are presented as the mean ± standard deviation; categorical data are given as the number/sample (percentage).
Adjudicated by the core laboratory.
Type D concentric superficial femoral artery lesions.
Following drug-coated balloon inflation, unrelated to intravascular lithotripsy.
Figure 3.
Baseline and residual stenosis across subgroups.
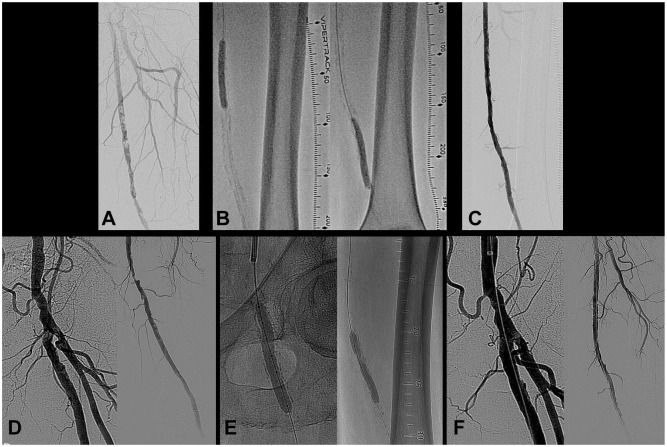
Figure 4.
(A) Severe concentric calcification of a left superficial femoral artery (SFA) with 94.3% stenosis [reference vessel diameter (RVD) 4.95 mm]. (B) The lesion was treated with 180 pulses from a 6.5-mm intravascular lithotripsy (IVL) catheter, followed by drug-coated balloon (DCB) angioplasty. (C) The treatment achieved a 24.9% residual stenosis and 3.83-mm acute gain. (D) Severe eccentric calcification of a left SFA with an 88.7% ostial stenosis (RVD 6.83 mm) a second 80.4% mid SFA lesion (RVD 5.88 mm). (E) The lesions were treated with 210 pulses from a 7.0-mm IVL catheter, followed by DCB angioplasty. (F) Treatment achieved a 17.3% residual stenosis in the ostial SFA (4.91-mm acute gain) and a 19.3% residual stenosis (3.49-mm acute gain) in the mid-vessel lesion.
Angiographic complications included 2 type D dissections and a single perforation that occurred after DCB use unrelated to the IVL procedure. There were no instances of abrupt closure, no reflow, distal embolization, or thrombotic events. There was no clinically meaningful difference in outcomes between concentric and eccentric calcified lesions, including residual stenosis (23.7%±10.1% vs 23.0%±6.8%), acute gain (3.5±1.2 vs 3.2±1.2 mm), or flow-limiting dissections (1.4% vs 0%).
Discussion
To our knowledge, this initial analysis from the Disrupt PAD III Observational Study represents the largest real-world clinical experience with IVL used for the treatment of severely calcified lesions in lower limb PAD. Since the Disrupt PAD I and II trials reported the initial IVL experience in a controlled trial setting, the aim of this first report was to understand the acute safety and effectiveness of IVL therapy in the everyday practice setting with no restriction on adjunctive therapy use. Based on this initial assessment, IVL safety and effectiveness in the real-world setting was consistent with outcomes reported in the smaller controlled Disrupt PAD I, PAD II, and below-the-knee clinical studies.8–10 IVL treatment resulted in good clinical outcomes despite the inclusion of additional vessel beds, complex lesions, and adjunctive technologies.
The all-comer design of the Disrupt PAD III Observational Study included iliac, CFA, and infrapopliteal lesions, all of which were excluded from the Disrupt PAD I and II studies (Figure 5A). The current study also included patients with a greater proportion of long lesions, CTOs, and CLTI (Figure 5B). Notably, this analysis represents the initial experience of nearly all of the investigators participating in the study. Since access to IVL was limited to Europe and New Zealand in the Disrupt PAD I and II trials, the Disrupt PAD III study represents the initial experience with IVL in the United States.
Figure 5.
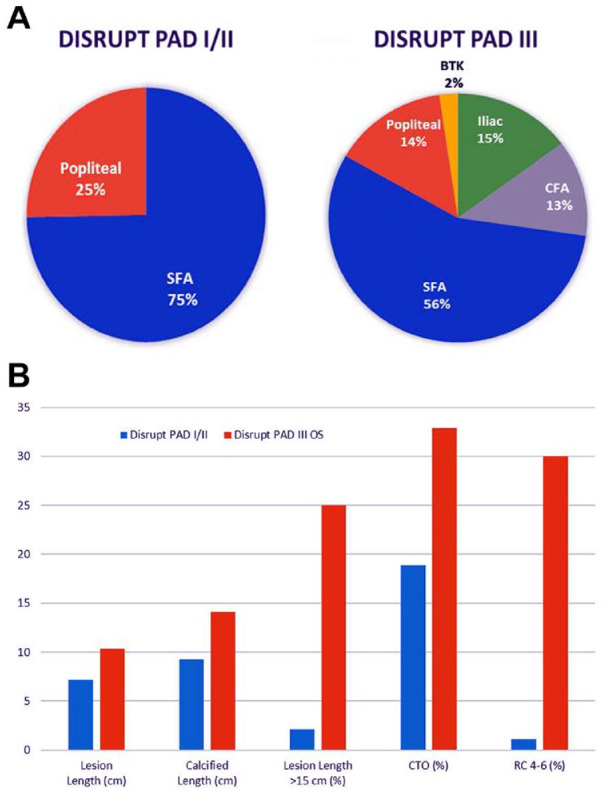
Lesion complexity comparison between Disrupt PAD I/II and Disrupt PAD III Observational Study. (A) Peripheral arteries included and (B) complexity of lesions treated.
Transition from the controlled clinical study environment to expanded use of IVL in everyday practice with highly complex lesions and operators new to IVL therapy resulted in acute effectiveness and safety outcomes that were consistent with previous studies. In these subjects typically excluded from contemporary peripheral studies due to the large calcium burden, IVL treatment resulted in a large acute gain and low residual stenosis across all lesions. Vascular complication rates were rare in this all-comers cohort, consisting of only 2 flow-limiting dissections and a perforation that was attributed to an adjunctive therapy.
In contrast to Disrupt PAD I and II, the unrestricted use of adjunctive therapies the Disrupt PAD III cohort provided new insight into the incorporation of IVL into broader clinical practice. Over three-quarters of patients were treated with DCBs following IVL treatment. This initial cohort was enrolled prior to the release of the meta-analysis that signaled an increase in mortality following paclitaxel DCB usage in the femoropopliteal arteries.11 It will be interesting to see if the rate of DCB utilization remains high in the full cohort given this is a high-risk patient group where benefits may outweigh the long-term risks.
Atherectomy was safely used in combination with IVL in a fifth of the procedures in this study, with the highest utilization observed in the SFA, followed by the CFA and popliteal arteries. There was no atherectomy performed in the iliac arteries. While IVL effectively modifies arterial calcium through sonic pressure waves delivered spherically outward from the center of the balloon, atherectomy devices are designed to mechanically debulk and are limited to superficial calcium and susceptible to wire bias. A potential synergy exists when utilizing IVL in conjunction with atherectomy in that atherectomy may be able to facilitate IVL catheter target lesion crossing, if necessary, allowing IVL to treat areas of medial calcification or areas of angulation or bifurcation in which atherectomy may not be effective or have a greater risk of complications. This approach has previously been reported in the coronary IVL literature with rotational atherectomy.12–14 It is also possible that for experienced operators, atherectomy was used to debulk superficial calcium while IVL was utilized for medial calcification modification. Only 4 sites accounted for 82% of the atherectomy cases, suggesting that operators at these institutions may currently use atherectomy as a first-line therapy. Additionally, as hospital economics are of concern, atherectomy plus IVL may have been utilized to optimize outcomes and reimbursement.
Interestingly, embolic protection filter use in this study was higher than previously reported in peripheral IVL studies.8–10,15 However, filter deployment appeared to be used primarily in the setting of concomitant atherectomy use whereas embolic filters were only utilized in <3% of cases when IVL was not used with atherectomy. Since this cohort represents the initial investigator experience with IVL, embolic filters may have been utilized out of an abundance of caution. However, regardless of the presence or absence of an embolic filter, there were no reports of distal embolization, no reflow, or abrupt closure in this study.
IVL utilizes a unique mechanism of action that does not rely on high-pressure balloon dilation to crack calcium but instead utilizes sonic pressure waves. These sonic pressure waves effectively fracture both intimal and medial calcification, improving vessel compliance and allowing safer and more effective modification of vascular calcium. The acoustic waves pass through soft tissue, leaving the inner elastic lamina intact, and attenuate toward the hard calcium. The fractured or compressed calcium remains confined within the vessel lining. Given the mechanism of action and the reported safety and effectiveness of IVL, successful use of IVL for calcium modification has facilitated other procedures, including transfemoral passage of large-bore aortic valve delivery systems16,17 and stent placement in calcified carotid arteries,18 as well as treatment of stent underexpansion due to severe coronary calcification.19–21
Limitations
First, the Disrupt PAD III Observational Study is a single-arm study without a control group and thus no definitive comparisons can be made to other interventions regarding safety and effectiveness. While the purpose of the observational study was to assess acute outcomes following IVL treatment in the real-world setting, the Disrupt PAD III randomized controlled trial will directly assess safety and effectiveness outcomes in IVL plus DCB compared with BA plus DCB. Second, by design, only acute procedure results are reported in this study. The PAD III randomized controlled trial will follow subjects through 2 years, which will provide data on the longer-term safety and effectiveness of IVL. Third, the infrapopliteal lesions treated in this cohort were too few to analyze. During early enrollment in the observational study, there was limited commercial availability of the S4 IVL catheter sized for use in infrapopliteal lesions. Thus, data availability was limited for this lesion subset. However, the PAD III Observational Study has recently been expanded to 1500 subjects with a minimum of 200 patients treated with the S4 IVL catheter. This allowance should ensure a robust infrapopliteal lesion cohort for evaluation.
Conclusion
This analysis of the PAD III Observational Study represents the largest report of IVL utilization in daily clinical practice. The use of peripheral IVL to treat severely calcified stenotic lower limb lesions continued to demonstrate consistent acute safety and effectiveness outcomes comparable to prior IVL controlled trials. These consistent results were achieved even though this cohort included the initial IVL experience in the United States, additional vessel beds not studied in prior IVL trials, and highly complex lesions and adjunctive therapies.
Acknowledgments
The authors thank Jason Hokama, PhD, and Suzanne Wallace, MSN, CRNP, of Shockwave Medical for support in manuscript preparation.
Footnotes
Authors’ Note: A portion of this dataset was presented at the Charing Cross Symposium (April 15–18, 2019; London, UK).
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: George Adams reports receiving research support from Boston Scientific, CSI, Medtronic, and Volcano and is a consultant to Abbott, Bard, Cook, CSI, Shockwave Medical, and Volcano. Nicolas Shammas reports receiving research support from Boston Scientific, Bard, Phillips, Intact Vascular, and VentureMed Group and is an Advisory Board member or consultant to Intact Vascular, VentureMed Group, Boston Scientific, and Bard. Sahil Parikh is a consultant to Terumo, Meril Life Sciences, and eFemoral and an Advisory Board member for Abbott, Boston Scientific, Medtronic, Philips, and CSI. Ehrin Armstrong is a consultant to Abbott Vascular, Boston Scientific, Cardiovascular Systems, Gore Medical, Intact Vascular, Medtronic, Philips, PQ Bypass, and Shockwave Medical. Peter Soukas receives research support from C.R. Bard, Intact Vascular, PQ Bypass, Philips, Shockwave Medical, and Gore Medical. Gunnar Tepe receives research support from Braun, Biotronic, Bard, Gore Medical, Boston Scientific, Medtronic, Verian, Shockwave Medical, and Phillips and is on the Advisory Board for Medtronic and Phillips. William Gray is a consultant to Shockwave Medical.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: All authors’ institutions received funding for the Disrupt PAD III Study from Shockwave Medical.
ORCID iDs: Nicolas Shammas  https://orcid.org/0000-0001-8279-0111
https://orcid.org/0000-0001-8279-0111
Ehrin J. Armstrong  https://orcid.org/0000-0002-1381-4754
https://orcid.org/0000-0002-1381-4754
Gunnar Tepe  https://orcid.org/0000-0002-1239-994X
https://orcid.org/0000-0002-1239-994X
References
- 1. Walker KL, Nolan BW, Columbo JA, et al. Lesion complexity drives the cost of superficial femoral artery endovascular interventions. J Vasc Surg. 2015;62:998–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Babaev A, Zavlunova S, Attubato MJ, et al. Orbital atherectomy plaque modification assessment of the femoropopliteal artery via intravascular ultrasound (TRUTH Study). Vasc Endovascular Surg. 2015;49:188–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Freed M, Grines C, Safian RD. The New Manual of Interventional Cardiology. Physicians’ Press; 1997. [Google Scholar]
- 4. Lee MS, Canan T, Rha SW, et al. Pooled analysis of the CONFIRM registries: impact of gender on procedure and angiographic outcomes in patients undergoing orbital atherectomy for peripheral artery disease. J Endovasc Ther. 2015;22:57–62. [DOI] [PubMed] [Google Scholar]
- 5. Mintz GS, Popma JJ, Pichard AD, et al. Patterns of calcification in coronary artery disease. A statistical analysis of intravascular ultrasound and coronary angiography in 1155 lesions. Circulation. 1995;91:1959–1965. [DOI] [PubMed] [Google Scholar]
- 6. Dini CS, Tomberli B, Mattesini A, et al. Intravascular lithotripsy for calcific coronary and peripheral artery stenoses. EuroIntervention. 2019;15:714–721. [DOI] [PubMed] [Google Scholar]
- 7. Patel MR, Conte MS, Cutlip DE, et al. Evaluation and treatment of patients with lower extremity peripheral artery disease: consensus definitions from Peripheral Academic Research Consortium (PARC). J Am Coll Cardiol. 2015;65:931–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brodmann M, Holden A, Zeller T. Safety and feasibility of intravascular lithotripsy for treatment of below-the-knee arterial stenoses. J Endovasc Ther. 2018;25:499–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brodmann M, Werner M, Brinton TJ, et al. Safety and performance of lithoplasty for treatment of calcified peripheral artery lesions. J Am Coll Cardiol. 2017;70:908–910. [DOI] [PubMed] [Google Scholar]
- 10. Brodmann M, Werner M, Holden A, et al. Primary outcomes and mechanism of action of intravascular lithotripsy in calcified, femoropopliteal lesions: Results of Disrupt PAD II. Catheter Cardiovasc Interv. 2019;93:335–342. [DOI] [PubMed] [Google Scholar]
- 11. Katsanos K, Spiliopoulos S, Kitrou P, et al. Risk of death following application of paclitaxel-coated balloons and stents in the femoropopliteal artery of the leg: a systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc. 2018;7:e011245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. De Maria GL, Scarsini R, Banning AP. Management of calcific coronary artery lesions: is it time to change our interventional therapeutic approach? JACC Cardiovasc Interv. 2019;12:1465–1478. [DOI] [PubMed] [Google Scholar]
- 13. Jurado-Román A, Gonzálvez A, Galeote G, et al. RotaTripsy: Combination of rotational atherectomy and intravascular lithotripsy for the treatment of severely calcified lesions. JACC Cardiovasc Interv. 2019;12:e127–e129. [DOI] [PubMed] [Google Scholar]
- 14. Vainer J, Lux A, Ilhan M, et al. Smart solution for hard times: successful lithoplasty of an undilatable lesion. Neth Heart J. 2019;27:216–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brodmann M, Schwindt A, Argyriou A, et al. Safety and feasibility of intravascular lithotripsy for treatment of common femoral artery stenoses. J Endovasc Ther. 2019;26:283–287. [DOI] [PubMed] [Google Scholar]
- 16. Di Mario C, Goodwin M, Ristalli F, et al. A prospective registry of intravascular lithotripsy-enabled vascular access for transfemoral transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2019;12:502–504. [DOI] [PubMed] [Google Scholar]
- 17. Di Mario C, Chiriatti N, Stolcova M, et al. Lithotripsy-assisted transfemoral aortic valve implantation. Eur Heart J. 2018;39:2655. [DOI] [PubMed] [Google Scholar]
- 18. Grillo P, Tripolino C, Tassone EJ, et al. Critical calcified carotid stenosis treated with shockwave lithoplasty. Arch Med Sci Atheroscler Dis. 2018;3:e164–e165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Watkins S, Good R, Hill J, et al. Intravascular lithotripsy to treat a severely underexpanded coronary stent. EuroIntervention. 2019;15:124–125. [DOI] [PubMed] [Google Scholar]
- 20. Ali ZA, McEntegart M, Hill JM, et al. Intravascular lithotripsy for treatment of stent underexpansion secondary to severe coronary calcification. Eur Heart J. 2020;41:485–486. [DOI] [PubMed] [Google Scholar]
- 21. Gorla R, Casenghi M, Bedogni F. Commentary: Intravascular lithoplasty for the treatment of calcified plaques: a new tool for the interventionist. J Endovasc Ther. 2019;26:288–290. [DOI] [PubMed] [Google Scholar]