Abstract
Bombyx mori-derived silk fibroin (SF) has recently gained interest for its intrinsic or engineered adhesive properties. In a previous study by our group, the mechanism of the protein’s intrinsic adhesiveness to biological substrates such as leather has been hypothesized to rely on hydrogen bond formation between amino acid side chains of SF and the substrate. In this study, the serine side chains of SF were chemically functionalized with substituents with different hydrogen bonding abilities. The effect of these changes on adhesion to leather was investigated along with protein structural assessments. The results confirm our hypothesis that adhesive interactions are mediated by hydrogen bonds and indicate that the length and nature of the side chains are important for both adhesion and secondary structure formation.
Keywords: silk fibroin, adhesive strength, hydrogen bonding
Graphical Abstract
1. Introduction
Surgical adhesives or glues have gained popularity in recent years in ophthalmic, neurosurgical, oral, plastic and reconstructive, and trauma and emergency surgery applications.1–9 Such adhesives are typically based on natural or synthetic biopolymers which impart important clinical properties such as biocompatibility and bioresorption/biodegradation. Recent advances in the field of adhesives include materials capable of wet adhesion.10–12 However, several significant unmet needs still exist. Specifically, mismatches between the target tissue and adhesives’ elastic properties cause serious adverse effects.2 In parallel, the lack of adhesion reversibility constitutes a major issue for the users; although reversible adhesives have been previously reported, currently there are no surgical adhesives with such property. Having a biocompatible, biomimetic adhesive that could be safely repositioned if or when needed would drastically ease the task of a surgeon or medic.
Silk fibroin (SF), a natural polymeric protein, lends itself well to the development of the next generation of adhesive. Performant, biocompatible silk-based irreversible adhesives have previously been reported.10,13–16 This polymer can be naturally sourced from silkworm cocoons (i.e. Bombyx mori) which would translate to a cost-effective raw material and manufacturing.17 It is easily processable and can be versatile in its physical presentation (i.e., as solution, film, gel or powder) which would mean product and application versatility.18 It has tunable mechanical properties and could be easily tailored to match tissue characteristics which would make a silk-based adhesive superior to existing adhesives.18–20 More importantly, it has intrinsic adhesive properties and would require minimal engineering/chemical tailoring which could mean faster regulatory approval and time to market.13,21
Fundamentally, SF has a molecular weight of ~416 kDa protein and consists of a light chain (~26 kDa) and a heavy chain (~390 kDa) connected via a single disulfide bond.22,23 The heavy chain is capable of forming beta-sheets and undergoing conformational changes under various external stimuli (i.e., temperature, pH, ionic strength, etc.) which translates to SF’s ability to be processed as solutions, gels, films, sponges, fibers and have versatile physical and mechanical properties.18,24
We have previously shown that adhesive interactions between SF and biological substrates are enabled by physical interactions that involve amino acid side chains capable of hydrogen bonding.25 Herein, we sought to confirm our previous hypothesis that SF amino acid side chains that drive beta-sheet formation via hydrogen bonding are also involved in adhesive binding to biological substrates. For this, serine side chains were chemically functionalized with substituents with different abilities to hydrogen bond (−COOH > −OH > −CH3). The serine side chains were selected for engineering based on the amino acid composition of SF. Specifically, serine is the most abundant amino acid in the protein’s primary sequence capable of hydrogen bond formation.26 The hydrophilic amino acids of SF consists of serine (~12%), tyrosine (~5.5%) , and aspartate and glutamate (~1.5%); all other constituent amino acids (glycine, alanine, valine, etc.) are hydrophobic and/or chemically inert. Modified silks were then assessed for their adhesive capabilities to sheep skin/leather via single lap sheer tests, in parallel with structural assessments. Our results support the aforementioned adhesion mechanism hypothesis, highlight the role of hydrogen bonding in adhesion and inform on subsequent design approaches necessary to impart reversibility into silk adhesives via temporary and controlled hydrogen bond disruption.
2. Materials and Methods
2.1. Materials
Sodium carbonate (Na2CO3) was purchased from EMD Chemicals Inc. (Gibbstown, NJ). Methanol (MeOH, HPLC grade), lithium bromide (LiBr), hydrochloric acid (HCl, 6N), phosphate buffered saline (PBS) and dialysis cassettes (Slide-A-Lyzer, MWCO 3500) were purchased from ThermoFisher Scientific (Waltham, MA). Sodium hydroxide (NaOH, pellets) was purchased from VWR International, LLC. (Radnor, PA). Tris-Acetate SDS Running Buffer and NuPAGE™ LDS Sample Buffer was purchased from Novex (Carlsbad, CA). NuPAGE™ 7% Tris-Acetate Gel, Sample Reducing Agent, and HiMark™ Pre-stained HMW Protein Standard were purchased from Invitrogen (Carlsbad, CA). Iodoacetic acid (97%) was purchased from ACROS Organics/Fisher Scientific (Hampton, NH), while 3-chloro-1-propanol (98%) and 1-chlorobutane (99%) were purchased from Sigma Aldrich (St. Louis, MO).
2.2. Silk Fibroin Extraction
Silk fibroin (SF) was extracted from commercial, medical device grade Bombyx mori silk yarn (Bratac, Brazil)27 according to a modified protocol.18 Specifically, 7.5 g of yarn was cut with scissors into 1–2-inch pieces and added to 3 L of boiling aqueous solution of 0.02 M Na2CO3 to remove sericin. The silk fibers were removed after 30 minutes, rinsed three times with deionized water and dried overnight under environmental conditions. The dried fibroin was then dissolved in 9.3 M LiBr solution at 20% w/v and placed in a 60°C oven for 4 h. The resulting solution was then transferred to dialysis cassettes and dialyzed against deionized water for 48 h.
2.3. Silk Solution Concentration Determination
The concentration of the extracted SF in aqueous solution was determined with an MJ33 moisture analyzer from Mettler Toledo (Columbus, OH). Briefly, the instrument was tared with an aluminum sample pan (Mettler Toledo, Columbus, OH). Subsequently, a 1 g (typically corresponding to 1 ml) aliquot of SF solutions was added into the pan and the weight of the sample was recorded. The sample drying process (temperature of 150 °C) was then started and when the instrument indicated cycle termination (detected as constant weight reached), the final weight of the sample was recorded. The concentration of the sample (8.3 w/w or w/v, considering the density of water as 1 g/mL) was determined by subtracting the final weight from the initial weight.
2.4. Chemical Modification of SF
SF chemical modifications were targeted to the serine side chains of the protein and were based on a previously published protocol.26 Halogenated substrates (iodoacetic acid, 3-chloro-1-propanol and 1-chlorobutane, respectively) were used for functionalization. The materials produced were a reaction control (RXN CTRL, in which SF was processed under the same experimental conditions as used for functionalization but without any halogenated substrates), carboxy-silk fibroin (carboxy-SF or SF-COOH), hydroxy-silk fibroin (hydroxy-SF or SF-OH) and methyl silk fibroin (methyl-SF or SF-CH3). Briefly, 2.6 mL of 10 M NaOH was added to 1.2 g of the desired halogenated substrate for modification and allowed to mix for 30 minutes. SF (6 ml, 8.3 % w/v) was then added to the solution and stirred for 1 h. Subsequently, the reaction was neutralized with 60 mg of NaH2PO4 and mixed for 15 minutes.28 The pH of the solution was adjusted to 7 using a 6 N HCl solution and a 1 M NaOH solution. The resulting modified silk solutions were placed in 3,500 MWCO dialysis cassettes and dialyzed against deionized water for 48 hours. Samples were then frozen at −80 °C overnight, then lyophilized.
2.5. Preparation of Films
Silk films were prepared by mixing SF (8 % w/v) with an equal volume (1:1 v/v ratio) of modified silk solution (0.5 % w/v). Aliquots of 0.5 ml were than cast into wells of a 24-well plate. Samples were left to dry on the bench overnight at environmental temperature and humidity. Thickness measurements were conducted with a 0–1” outside micrometer (Chicago Brand, Medford, OR).
2.6. Nuclear Magnetic Resonance Analysis of Modified Silks
To confirm the modifications of SF, proton nuclear magnetic resonance (1H NMR) spectral data were obtained using a Varian VNMRS 500 MHz (Agilent Technologies, Palo Alto, CA, USA) at 20°C. Samples were dissolved in D2O and all spectra were referenced to the residual solvent peak (H2O) at δ = 4.65 ppm. Peak assignments corresponding to the silk backbone: δ 0.72 (br, Val γ), 1.21 (br, Ala β), 1.90 (br, Val β), 2.74–2.85 (br, Asp/Tyr β), 3.77 (m, Ser β/Gly α), 4.14–4.29 (m, Ala α/Ser α), 6.61 (m, Tyr φ), 6.90 (m, Tyr φ), 7.03–7.12 (br, Phe φ). Peaks corresponding to the substituent protons in the carboxy-SF: 3.7 ppm (−CH2−COOH). Peaks corresponding to the substituent protons in the hydroxy-SF: 1.8 ppm (−CH2−CH2−CH2−OH), 3.55 ppm (−CH2−CH2−CH2−OH) and 3.6 ppm (−CH2−CH2−CH2−OH). Peaks corresponding to the substituent protons in the methyl-SF: 0.75 ppm (−CH2−CH2−CH2−CH3), 1.4 ppm (−CH2−CH2−CH2−CH3) and 3.4 ppm (−CH2−CH2−CH2−CH3), (the fourth peak expected at 1.67 ppm (−CH2−CH2−CH2−CH3) is not clearly detectable because of overlap with the SF backbone signals).
2.7. Gel Electrophoresis
The molecular weight distribution of modified silks was determined using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). For each condition, 5 μg/ml of sample was reduced with NuPAGE Sample reducing agent and loaded onto a 7% Tris-Acetate gel (NuPAGE, Life Technologies, Grand Island, NY). The gel was run under reducing conditions for 75 min at 150 V, with a high molecular weight ladder as reference (HiMark Prestained, Life Technologies) and stained with a SimplyBlue SafeStain staining solution (Thermo Fisher Scientific, Waltham, MA).
2.8. Fourier-Transform Infrared Spectroscopy
The structural conformations of modified silks (either as lyophilized powders or films) were analyzed with a Nicolet iS5 FT-IR equipped with an iD7 diamond attenuated total reflectance (ATR) accessory (Thermo Scientific, Waltham, MA). The absorbance of samples was measured between 4000 – 400 cm−1, with 64 scans, and a resolution of 4 cm−1. Background spectra were collected under the same conditions and subtracted from the sample. All samples were analyzed after 24 hours with or without methanol (100% v/v) treatment.
2.9. Amino Acid Analyses
Amino acid composition analyses were performed using the ninhydrin method.29 Acid hydrolyzed amino acids were characterized using High-Speed Amino Acid Analyzers, L-8900 and L8500A (Hitachi-HighTech, Tokyo, Japan).
2.10. Contact Angle Measurements
The wettability of the cast film surfaces was estimated by advancing contact angle measurements (θa) with distilled water using a FACE Contact Angle Meter CA-X (Kyowa Interface Science, Saitama, Japan). Ethanol (100% w/v, 24 h) treated cast films of the samples were prepared on glass substrates. The θ values were calculated as average of ten measurements obtained at different points on the surface (n = 5). Although ethanol was used as beta-sheet inducer for contact angle measurements, the mechanism of beta sheet induction by both methanol or ethanol is the same and has the same structural outcome, namely beta-sheet formation.
2.11. Cell Assays
Primary adult normal dermal fibroblasts (ATCC, Manassas, VA) were used for the assays. Modified silk solutions were sterile filtered through 0.45 μm syringe driven filter units (MIlliporeSigma, Burlington, MA) and 100 μl aliquots were used to cast films (n = 6) into wells of a 96-well plate. An n = 3 of each modified silk was treated with methanol (100% v/v, 2 h). The methanol was then removed and all films were allowed to air dry in the laminar flow hood. After 24 h, cells were plated on the films at a seeding density of 104 cells/well in 100 μl of serum-free fibroblast conditioned media (ATCC, Manassas, VA), then incubated for 24 h at 37°C/5% CO2. Subsequently, cell numbers were quantified with a methyltetrazolium salts colorimetric assay (Cell-Titer 96 Aqueous One Solution Cell Proliferation Assay, Promega, Madison, WI) and detected via absorbance at 450 nm with a microplate reader. A LIVE/DEAD viability/cytotoxicity kit for mammalian cells (ThermoFisher Scientific, Waltham, MA) was used to stain cells cultured on films.
2.12. Wide Angle X-ray Scattering (WAXS)
Synchrotron WAXS measurements were conducted at the BL45XU beamline at SPring-8, Harima, Japan, according to a previous report.30 The X-ray energy was 12.4 keV at a wavelength of 0.1 nm, the sample-to-detector distance for the WAXS measurements was 258 mm, and the exposure time for each diffraction pattern was 10 s. The obtained diffraction data were converted into one-dimensional profiles using the software Fit2D.31
2.13. Adhesion Testing
A Discovery HR2 hybrid rheometer equipped with a DHR Film/Fiber Tension Accessory (TA Instruments, New Castle, DE) was used for single lap shear testing of all samples.32 Natural sheep skin chamois leather (Amazon, Seattle, WA) was washed with a mild detergent followed by 5 deionized water washes then allowed to dry for several days. The leather was then cut into individual 10 × 25 mm strips for testing. The silk film samples were cut into 10 × 10 mm squares and applied to a corresponding 10 × 10 mm terminal area of the leather that was pre-hydrated with 75 μL of deionized water or PBS, respectively. This controlled hydration procedure for the leather strips was found to provide sufficient moisture to mimic native tissues (i.e. intestines) while preventing the presence of excess solvent that could dissolve the adhesive, affect its local concentration and provide experimental data inconsistencies. To ensure complete contact between the surfaces, the two adhered strips were held together with a lead ring (~680 g) for either 30 min or 1.5 h prior to lap shear testing. For the tests, the grip to grip separation was set at 20 mm, and the crosshead speed was set to 50 mm/min. Results were quantified based on the maximum axial force (N) needed to break the adhesion between the strips.
2.14. Statistical Analyses
Values, represented as mean ± standard deviation (S.D.) were compared either with Student’s t test (2-tailed, type 3) (for data groups of two) or Single Factor ANOVA (for data groups of three) with p ≤ 0.05 considered statistically significant.
3. Results and Discussion
Chemical Functionalization of SF
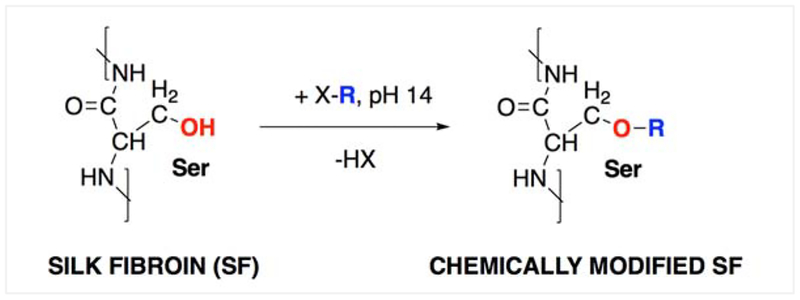
We have previously shown that adhesive interactions between SF and biological substrates seem to be enabled by physical interactions that involve amino acid side chains capable of hydrogen bonding.25 To further investigate this observation chemically modified silks were prepared by the covalent functionalization of serine side chains with halogenated reactants (Figure 1). The serine side chains were targeted for our approach given that they are the predominant amino acid moieties in the primary structure of SF capable of hydrogen bonding.
Figure 1.
Reaction scheme for the chemical modification of silk fibroin (SF), where X-R is a halogenated (X: chloro- or iodo-) reactant.
The reactants/serine side chain substituents were chosen to yield different hydrogen bonding abilities, but be similar in bulkiness/length and molecular weight to be able to account for potential steric effects (Table 1). Specifically, carboxymethyl has higher H-bonding ability than 3-hydroxypropyl while n-butyl cannot form hydrogen bonds. A reaction control (RXN CTRL) that had SF processed under the same experimental conditions that were used for functionalization but without any reactants was carried out in parallel, in order to account for any potential effects of the reaction conditions on the starting SF.
Table 1.
Substituents used for the chemical modification of SF.
| Substituent (SF-R) | Structural formula of R | Molecular weight of R (g/mol) | Side chain properties |
|---|---|---|---|
| R1 = carboxymethyl | −CH2−COOH | 59 | Polar, hydrophilic |
| R2 = 3-hydroxypropyl | −CH2−CH2−CH2−OH | 59 | Polar, hydrophilic |
| R3 = n-butyl | −CH2−CH2−CH2−CH3 | 57 | Non-polar, hydrophobic |
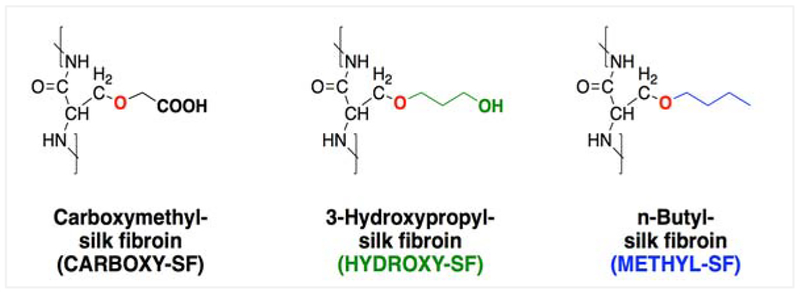
The structures of the chemically modified silks were analyzed by 1H-NMR and the peaks corresponding to the substituent atoms were identified (Figures S1–S4). The degree of substitution for each reaction was determined by amino acid analysis and was found to be comparable for all three reactions (Table S1). Overall, these data indicate that SF was successfully modified with iodoacetic acid, 3-chloro-1-propanol and 1-chlorobutane to yield carboxy-SF (SF-COOH), hydroxy-SF (SF-OH) and methyl-SF (SF-CH3), respectively (Figure 2).
Figure 2.
Structures of the three chemically modified SFs.
Characterization of Modified Silks
Molecular weight and charge evaluation.
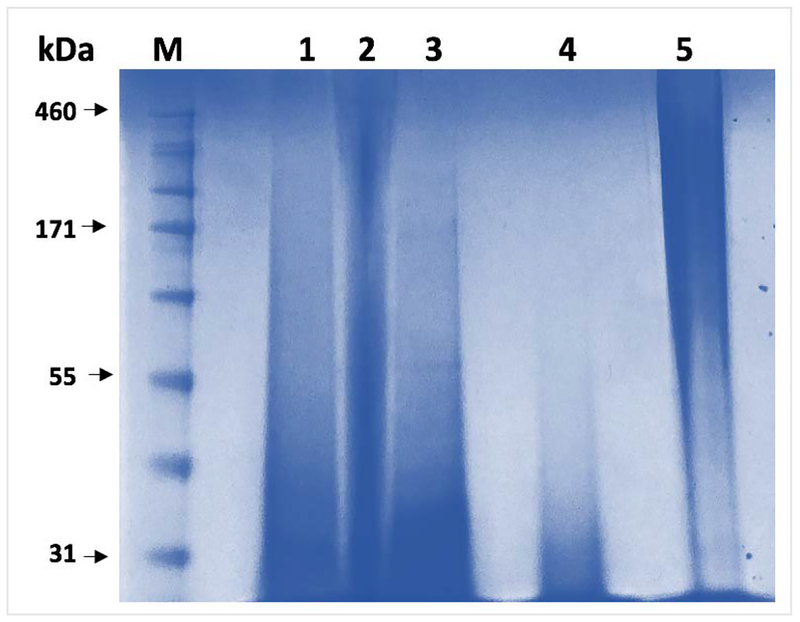
Albeit for a short duration the serine functionalization reaction was completed under highly alkaline pH. With the structures of the modified silks confirmed and degrees of substitution determined, we next employed a gel electrophoresis system to compare the behavior of the modified silks to the unmodified SF (Figure 3).
Figure 3.
Gel electrophoresis (SDS-PAGE) of silk samples. Lane 1 – carboxy-SF; lane 2 – hydroxy-SF; lane 3 – methyl-SF; lane 4 – reaction control (RXN CTRL) and lane 5 – SF. M – molecular weight marker.
Considering the polymeric nature of silk samples, their typical diffuse running pattern on electrophoresis gels due to the partial degradation of the fibroin during its extraction process14,33 and the fact that the electrophoretic separation process is a function of a sample’s molecular weight and charge, it is important to note that our analysis was primarily aimed at qualitatively comparing the samples. Compared to the unmodified SF (Figure 3, lane 5) the RXN CTRL sample (Figure 3, lane 4) seems to have reduced molecular weight, most likely due to the expected hydrolytic degradation of the protein backbone under the alkaline conditions of the reaction. All three functionalized silks (Figure 3, lanes 1–3) appear to have higher molecular weights than the RXN CTRL, which is expected given that the degree of substitution for the samples was in the 74–79% range (percent serine side chains modified per molecule of SF). Interestingly, the hydroxy-SF sample (Figure 3, lane 2) appears to be running at higher molecular weight than the carboxy- and methyl-SF, respectively, even though theoretically their molecular weights should be comparable. We attributed this electrophoretic pattern to the different interactions of the hydroxy- moieties of the sample with the sodium dodecyl sulfate of the electrophoresis gel, compared to the other modified counterparts. Chemically, the hydroxy-SF is expected to be similar to the RXN CTRL where the serine side chains display a similar hydroxy moiety to the hydroxy-SF; however, the 3-hydropxypropyl chain length probably enhances the exposure of the −OH groups to the electrophoretic environment. Cumulated with the aforementioned analytical data, the SDS-PAGE results additionally confirm that the chemical modification of SF with all three substituents was successful, and that based on the added substitutions the three novel compounds are electrophoretically different than the unmodified SF or the RXN CTRL. Additionally, this experiment confirms the expected hydrolytic degradation of SF under alkaline conditions.
Secondary structure analyses.
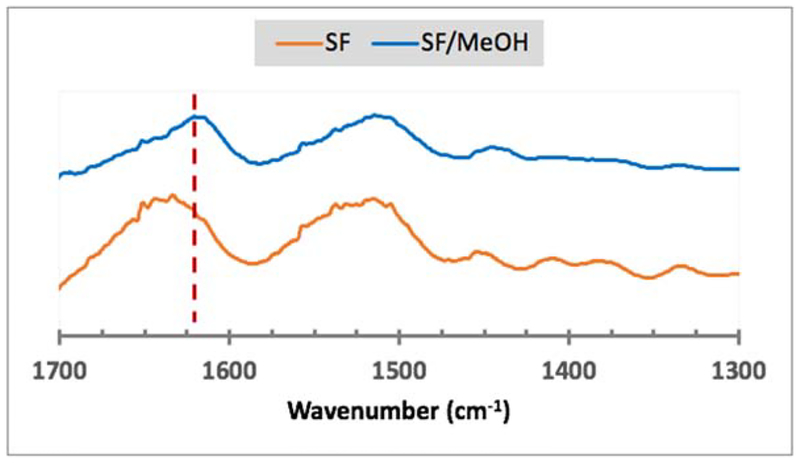
Formation of beta-sheets is a hallmark of silk fibroin; however, given that the functionalization of the serine side chains adds length and/or bulk to the primary sequence, the molecular alignment of the SF backbone that drives secondary structure formation could be affected. When samples were analyzed via wide angle X-ray scattering (WAXS), the carboxy-SF appeared to display low intensity peaks corresponding to beta-sheet crystallization; however, the intensities of the peaks were not obviously distinguishable above the background noise level (Figure S5). Subsequently, structural changes were exogenously induced in lyophilized samples via methanol treatment for 24 hours and monitored by infrared spectroscopy. In SF, as expected, the methanol treatment induced beta-sheet formation as evident by the appearance of the characteristic absorption peak at 1620 cm−1 (Figure 4).
Figure 4.
FTIR spectra of SF and SF treated with methanol (SF/MeOH). The red dotted line indicates the position of the 1620 cm−1 absorption peak corresponding to the C=O stretch of atoms involved in beta-sheet formation.
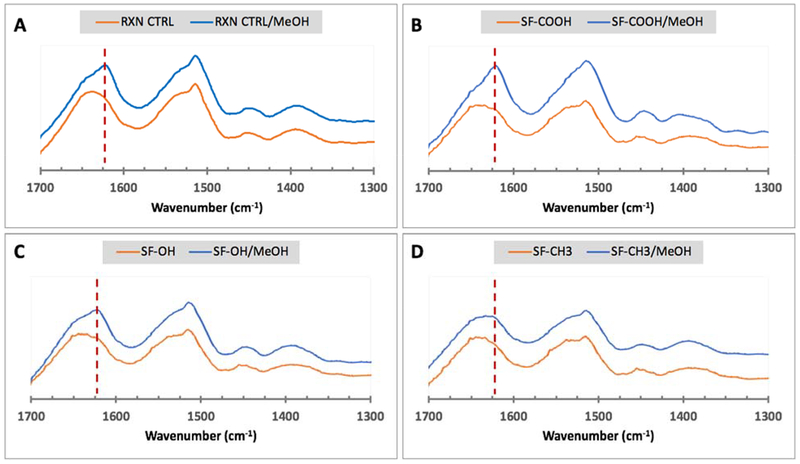
In response to methanol treatment the chemically modified silks formed beta-sheets only when the substituted molecules maintained their ability to form hydrogen bonds (Figure 5). Specifically, the RXN CTRL, SF-COOH and SF-OH formed beta-sheets as evident by the presence of 1620 cm−1 peaks in all inducer-treated samples (Figure 5A–C). In contrast, no obvious beta-sheet specific peak was detected in SF-CH3 after methanol treatment (Figure 5D).
Figure 5.
FTIR analyses of modified lyophilized silks either treated or untreated with methanol (MeOH). A – RXN CTRL; B – carboxy-SF; C – hydroxy-SF; and D – methyl-SF. The red dotted line corresponds to the expected position of the amide I peak at 1620 cm−1 generated via beta-sheet formation.
These results seem to be in agreement with previously published data that indicate that beta-sheet formation occurs via hydrogen bonding mediated by polar side chains.34,35 The methyl-SF lacks the ability to form hydrogen bonds and therefore is not expected to form beta-sheets. In contrast, the RXN CTRL has the same hydrogen bonding ability as SF as the serine side chains are unaltered. The carboxy-SF has increased ability to form hydrogen bonds with adjacent atoms, while the hydroxy-SF is similar to the unaltered serine but has a longer −OH bearing side chain capable of hydrogen bonding. These observations are important, especially in the context of our previous findings, where we showed that adhesive interactions with a substrate could be further enhanced via post-adhesion induction of beta-sheets.25 In the context of the FTIR data presented herein, the adhesive strength of all chemically modified silks with the exception of methyl-SF, could further be enhanced via secondary structure induction.
Hydrophilicity/hydrophobicity measurements.
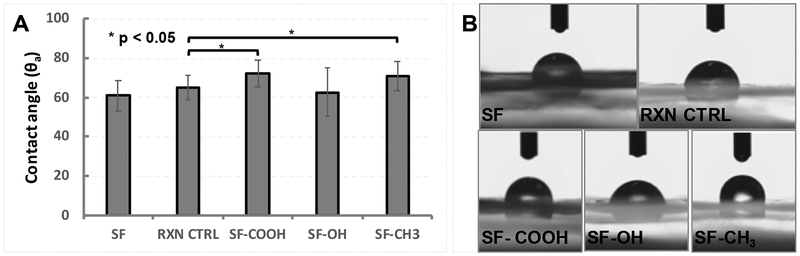
To further investigate the effect of the serine functionalization on material properties, ethanol treated modified silk films were tested for surface hydrophilicity/ hydrophobicity (Figure 6). Ethanol and methanol are comparable in their ability to induce secondary structural changes in silk by reorienting the molecule and enabling beta-sheet formation.
Figure 6.
Water contact angle measurements of silk films. A - Only the carboxy-SF and methyl-SF were statistically more hydrophobic than the RXN CTRL (TTEST p < 0.05). No statistically significant differences were observed between SF and RXN CTRL. ANOVA for the group: p = 0.003 for alpha = 0.05, F = 4.66 and F crit = 2.58. B – representative contact angle images for water droplets on ethanol treated modified silk films.
Compared to SF, the RXN CTRL seemed to be slightly more hydrophobic but the difference was not statistically significant. When compared to the RXN CTRL, the carboxy-SF and methyl-SF samples were significantly more hydrophobic while the hydroxy-SF yielded similar contact angle measurements. The increase in hydrophobicity for the carboxy-SF is most likely due to the formation of crystalline structures/beta-sheets, consistent with our WAXS and FTIR analyses.
Cytotoxicity evaluation.
As an initial assessment of the adhesives’ biocompatibility, we next sought to understand if the engineered silks induced any cytotoxicity. One standardized way to determine a material’s cytotoxicity is by assessing cell viability via metabolic activity.36 Per ISO 10993–5: 2009,36 cells exposed to or cultured on different materials can be incubated with a methyltetrazolium salt (MTS) that is reduced by metabolically active/live cells to formazan, a blue-violet colored compound. The intensity of the developed color, corresponding to the amount of formazan produced, is indicative of the number of viable cells. A material is deemed cytotoxic if the relative viability of cells exposed to or cultured on it, as determined by the MTS method, is lower than 70% of the relative viability of negative control.
In this context, to investigate the effect of the chemical modifications of SF on cells, we cultured primary human dermal fibroblasts both on methanol treated and untreated silk materials (Figure 7). We chose to assay our materials as films for ease of handling and to mimic the intended clinical silk adhesive presentation. Engineered silk solutions by themselves would not form films rather they would crystallize, most likely to the decreased length and molecular weight of the polymeric backbone. To this end, films were prepared as a defined mix of SF and modified silk as described in the Materials and Methods section. Determined film thickness values were 62 ± 15 μm. SF was used as negative control in all cell assays as its cyto-/biocompatibility is well documented.18,33,37
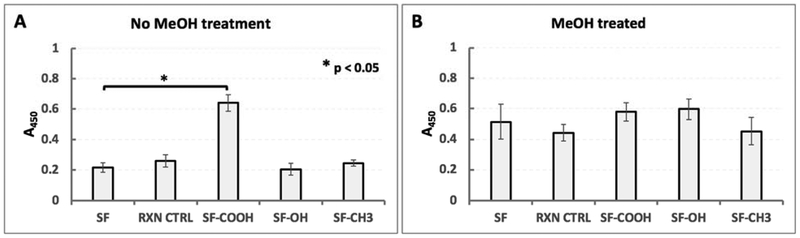
Figure 7.
Cytocompatibility evaluation of modified silks via MTS assay. A – primary human dermal fibroblasts were grown on films that were not treated with MeOH; only cells on carboxy-SF displayed a statistically significant difference in cell numbers compared to SF (TTEST p < 0.05, ANOVA for the group: p = 2.03E-13 for alpha = 0.05, F = 112.67 and F crit = 2.87); B - primary human dermal fibroblasts were grown on films that were treated with MeOH. No statistical significance was found between individual modified silks when compared to SF via TTEST (statistical significance set to p < 0.05); however, ANOVA analysis for the group indicated a slight variance between samples (p = 0.0006 for alpha = 0.05, F = 4.64 and F crit = 2.76).
On the non-treated films and compared to SF only cells on carboxy-SF displayed statistically higher metabolic activity when tested with a MTS colorimetric assay (Figure 7A). On the methanol treated films, cell metabolic activity appeared to be similar to those on SF films (Figure 7B).
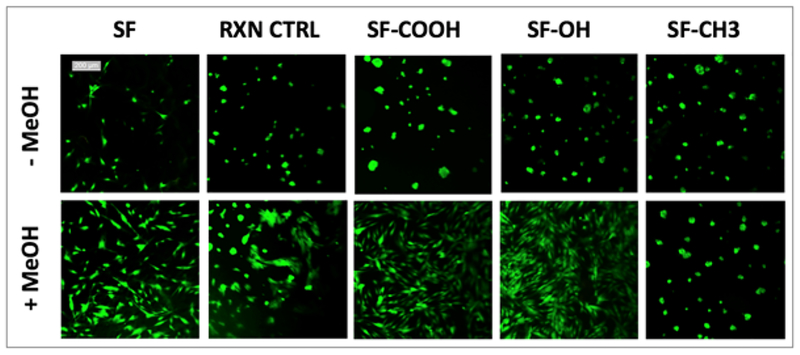
In addition to determining cellular metabolic activity, cells grown on methanol treated or untreated films were visually assessed via LIVE/DEAD assay (Figure 8).
Figure 8.
LIVE/DEAD viability/cytotoxicity assay of primary human dermal fibroblasts grown on MeOH treated or untreated films. Live cells are stained green, dead cells stained red – no significant number of dead cells was detected in any of the samples. Scale bar for all images is 200 μm.
In agreement with the MTS assay results, all films were well tolerated by cells and the viability of cells was high with no significant number of dead cells noted. Interestingly, cells on non-treated films appeared to mostly clustered in clumps while on methanol treated films, they displayed spindle-shaped, fibroblast-like phenotypes except for cells on SF-CH3 that maintained the same morphology as on untreated films. This observation agrees with the aforementioned FTIR data where we show that methyl-SF cannot undergo structural changes in response to methanol treatment. In summary, these cell assay results indicate that our chemically functionalized silks are not cytotoxic.
Adhesion testing.
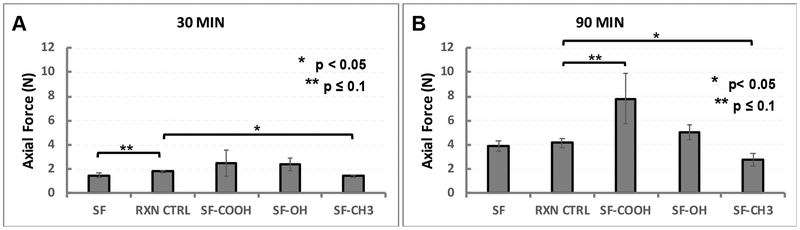
In order to assess the effects of the hydrogen bonding ability of silk amino acid side chains on adhesion to biological substrates, we performed a series of single lap shear tests with modified silk films (Figure 9). As substrate representative, we chose sheep skin chamois leather as our attempts to use intestinal tissues lead to data variations that correlated with tissue surface variations. When moist leather strips were adhered for 30 minutes, the RXN CTRL appeared to be more adhesive than SF (TTEST p = 0.05) while methyl-SF seemed less adhesive than the RXN CTRL (TTEST p = 0.01). However, ANOVA analysis of all the modified silks revealed no statistical differences most likely because of the sample variance in the carboxy-SF group (Figure 9A). At 90 minutes, there were no differences between SF and the RXN CTRL, but carboxy-SF was significantly more adhesive than SF or the RXN CTRL (TTEST p = 0.09) and methyl-SF was less adhesive than the RXN CTRL (TTEST p = 0.02) (Figure 9B).
Figure 9.
Lap shear testing of sheep skin with modified silk films. A – adhesion at 30 minutes. ANOVA for the group shows no statistically significant differences between groups (p = 0.09 for alpha = 0.05, F = 2.64 and F crit = 3.47); B – adhesion at 90 minutes shows statistical significance between groups (p = 0.09 for alpha = 0.00007, F = 11.11 and F crit = 3.35) with carboxy-SF being more adhesive than the RXN CTRL (TTEST p = 0.09) and the methyl-SF being less adhesive than the RXN CTRL (TTEST p = 0.02).
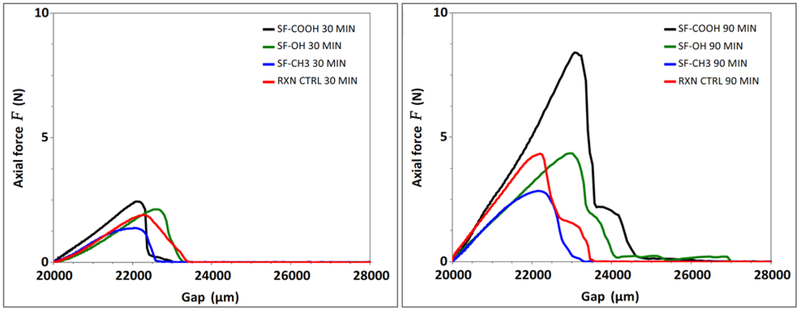
Representative shear strength profiles for the modified silks are shown in Figure 10. Overall, our cumulated data indicates that, in agreement with our hypothesis, hydrogen bonding is driving silk adhesion to our biological substrate.
Figure 10.
Representative shear strength graphs for modified silk films as determined via single lap shear tests. A – adhesion strength of modified silks at 30 min; B – adhesion strength of modified silks at 90 min.
The goal of this study was to investigate the mechanism of adhesion of silk fibroin to a biological substrate (moist sheep skin) in order to gain an understanding of subsequent silk-based adhesive design approaches. Based on our previous findings25 we postulated the adhesion mechanism is hydrogen-bonding mediated and that the serine side chains of the fibroin backbone are involved in these interactions. To test this hypothesis, we aimed to chemically modify serine side chains with functionalities with different hydrogen bonding abilities and compare the properties of these engineered silk with the unmodified silk fibroin control. We quantitatively showed via amino acid analysis that we were able to successfully modify ~ 75 of the serine side chains of the silk protein and we confirmed these findings by 1H-NMR. These changes to the serine side chains resulted in different abilities of the engineered silks to undergo structural changes as evident by FTIR, or adhere to sheep skin as determined via la-sheer testing. As highlighted by contact angle measurements, the chemical modifications translated to different surface properties of the engineered silks, also reflected by the different cell attachment profiles of primary cells cultured on these films. Taken together our data confirm our initial hypothesis and point to the conclusion that the serine side chains are critical for enabling adhesion to a biological substrate via hydrogen bonding. Further studies will focus on testing silk in application specific situations (i.e. for the development of skin adhesive, silks will be tested with commercial human skin grafts or organotypic skin models) and on developing reversible adhesives that could disrupt on-demand hydrogen bond-mediated adhesions.
Conclusion
The research described herein aimed to test our previously formulated hypothesis that silk’s adhesion to biological substrates, specifically sheep skin, is mediated by hydrogen bonding. The results presented support our hypothesis and set the foundation for the subsequent development of reversible silk-based adhesives. The ability to attach/detach surgical adhesives on demand is still a challenge in the medical field and we believe that with a fundamental understanding of silk’s innate adhesion mechanism to biological tissues, the development of reversible silk-based adhesives can be achieved in the near future.
Supplementary Material
Acknowledgements
Dr. Serban’s funding for this project was provided by a pilot project grant from the Center for Biomolecular Structure and Dynamics COBRE, NIH Grant P20GM103546. Dr. Numata’s funding was provided by JST ERATO Grant JPMJER1602. Dr. Hiroyasu Masunaga and Dr. Takaaki Hikima are acknowledged for their technical support at BL45XU SPring-8 synchrotron, Japan.
Footnotes
Supporting information
Proton NMR (1H-NMR) analyses of modified silks. Amino acid analyses of modified silks. Cell viability/number assessment on control materials. Structural analyses of modified silks via wide angle X-ray scattering.
References
- (1).Sanders L; Nagatomi J Clinical Applications of Surgical Adhesives and Sealants. Crit Rev Biomed Eng 2014, 42 (3–4), 271–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Jain R; Wairkar S Recent Developments and Clinical Applications of Surgical Glues: An Overview. International Journal of Biological Macromolecules 2019. 10.1016/j.ijbiomac.2019.06.208. [DOI] [PubMed] [Google Scholar]
- (3).Jarrett P; Coury A Tissue Adhesives and Sealants for Surgical Applications In Joining and Assembly of Medical Materials and Devices; Elsevier, 2013; pp 449–490. 10.1533/9780857096425.4.449. [DOI] [Google Scholar]
- (4).Borie E; Rosas E; Kuramochi G; Etcheberry S; Olate S; Weber B Oral Applications of Cyanoacrylate Adhesives: A Literature Review. BioMed Research International 2019, 2019, 1–6. 10.1155/2019/8217602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Chiara O; Cimbanassi S; Bellanova G; Chiarugi M; Mingoli A; Olivero G; Ribaldi S; Tugnoli G; Basilicò S; Bindi F; Briani L; Renzi F; Chirletti P; Di Grezia G; Martino A; Marzaioli R; Noschese G; Portolani N; Ruscelli P; Zago M, Sgardello S; Stagnitti F; Miniello S A Systematic Review on the Use of Topical Hemostats in Trauma and Emergency Surgery. BMC Surgery 2018, 18 (1). 10.1186/s12893-018-0398-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Chibbaro S; Tacconi L Use of Skin Glue versus Traditional Wound Closure Methods in Brain Surgery: A Prospective, Randomized, Controlled Study. Journal of Clinical Neuroscience 2009, 16 (4), 535–539. 10.1016/j.jocn.2008.08.004. [DOI] [PubMed] [Google Scholar]
- (7).Coulthard P; Worthington H; Esposito M; van der Elst M; van Waes OF Tissue Adhesives for Closure of Surgical Incisions In Cochrane Database of Systematic Reviews; The Cochrane Collaboration, Ed.; John Wiley & Sons, Ltd: Chichester, UK, 2002. 10.1002/14651858.CD004287. [DOI] [Google Scholar]
- (8).Giordano S; Koskivuo I; Suominen E; Veräjänkorva E Tissue Sealants May Reduce Haematoma and Complications in Face-Lifts: A Meta-Analysis of Comparative Studies. Journal of Plastic, Reconstructive & Aesthetic Surgery 2017, 70 (3), 297–306. 10.1016/j.bjps.2016.11.028. [DOI] [PubMed] [Google Scholar]
- (9).Guhan S; Peng S-L; Janbatian H; Saadeh S; Greenstein S; Al Bahrani F; Fadlallah A; Yeh T-C; Melki SA Surgical Adhesives in Ophthalmology: History and Current Trends. British Journal of Ophthalmology 2018, 102 (10), 1328–1335. 10.1136/bjophthalmol-2017-311643. [DOI] [PubMed] [Google Scholar]
- (10).Burke KA; Roberts DC; Kaplan DL Silk Fibroin Aqueous-Based Adhesives Inspired by Mussel Adhesive Proteins. Biomacromolecules 2016, 17 (1), 237–245. 10.1021/acs.biomac.5b01330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Li J; Celiz AD; Yang J; Yang Q; Wamala I; Whyte W; Seo BR; Vasilyev NV; Vlassak JJ; Suo Z; Mooney DJ Tough Adhesives for Diverse Wet Surfaces. Science 2017, 357 (6349), 378–381. 10.1126/science.aah6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Li L; Zeng H Marine Mussel Adhesion and Bio-Inspired Wet Adhesives. Biotribology 2016, 5, 44–51. 10.1016/j.biotri.2015.09.004. [DOI] [Google Scholar]
- (13).Serban MA Silk Medical Devices. US20150056261A1, 2015. [Google Scholar]
- (14).Heichel DL; Burke KA Dual-Mode Cross-Linking Enhances Adhesion of Silk Fibroin Hydrogels to Intestinal Tissue. ACS Biomaterials Science & Engineering 2019. 10.1021/acsbiomaterials.9b00786. [DOI] [PubMed] [Google Scholar]
- (15).Serban MA; Panilaitis B; Kaplan DL Silk Fibroin and Polyethylene Glycol-Based Biocompatible Tissue Adhesives. J Biomed Mater Res A 2011, 98 (4), 567–575. 10.1002/jbm.a.33149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Sogawa H; Ifuku N; Numata K 3,4-Dihydroxyphenylalanine (DOPA)-Containing Silk Fibroin: Its Enzymatic Synthesis and Adhesion Properties. ACS Biomaterials Science & Engineering 2019. 10.1021/acsbiomaterials.8b01309. [DOI] [PubMed] [Google Scholar]
- (17).Holland C; Numata K; Rnjak-Kovacina J; Seib FP The Biomedical Use of Silk: Past, Present, Future. Advanced Healthcare Materials 2019, 8 (1), 1800465 10.1002/adhm.201800465. [DOI] [PubMed] [Google Scholar]
- (18).Rockwood DN; Preda RC; Yücel T; Wang X; Lovett ML; Kaplan DL Materials Fabrication from Bombyx Mori Silk Fibroin. Nat Protoc 2011, 6 (10), 1612–1631. 10.1038/nprot.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Bai S; Han H; Huang X; Xu W; Kaplan DL; Zhu H; Lu Q Silk Scaffolds with Tunable Mechanical Capability for Cell Differentiation. Acta Biomaterialia 2015, 20, 22–31. 10.1016/j.actbio.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Wang X; Ding Z; Wang C; Chen X; Xu H; Lu Q; Kaplan DL Bioactive Silk Hydrogels with Tunable Mechanical Properties. Journal of Materials Chemistry B 2018, 6 (18), 2739–2746. 10.1039/C8TB00607E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Serban MA Translational Biomaterials-the Journey from the Bench to the Market-Think “Product.” Curr. Opin. Biotechnol 2016, 40, 31–34. 10.1016/j.copbio.2016.02.009. [DOI] [PubMed] [Google Scholar]
- (22).Yamaguchi K; Kikuchi Y; Takagi T; Kikuchi A; Oyama F; Shimura K; Mizuno S Primary Structure of the Silk Fibroin Light Chain Determined by CDNA Sequencing and Peptide Analysis. J. Mol. Biol 1989, 210 (1), 127–139. [DOI] [PubMed] [Google Scholar]
- (23).Zhou C-Z; Confalonieri F; Jacquet M; Perasso R; Li Z-G; Janin J Silk Fibroin: Structural Implications of a Remarkable Amino Acid Sequence. Proteins: Structure, Function, and Genetics 2001, 44 (2), 119–122. 10.1002/prot.1078. [DOI] [PubMed] [Google Scholar]
- (24).Serban MA; Kaplan DL Silks In Tissue engineering: Principles and practices; CRC Press, 2012; pp 1–16. [Google Scholar]
- (25).Johnston ER; Miyagi Y; Chuah J-A; Numata K; Serban MA Interplay between Silk Fibroin’s Structure and Its Adhesive Properties. ACS Biomaterials Science & Engineering 2018, 4 (8), 2815–2824. 10.1021/acsbiomaterials.8b00544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Serban MA; Kaplan DL PH-Sensitive Ionomeric Particles Obtained via Chemical Conjugation of Silk with Poly(Amino Acid)s. Biomacromolecules 2010, 11 (12), 3406–3412. 10.1021/bm100925s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Jewell M; Daunch W; Bengtson B; Mortarino E The Development of SERI ® Surgical Scaffold, an Engineered Biological Scaffold: The Development of SERI ® Surgical Scaffold. Annals of the New York Academy of Sciences 2015, 1358 (1), 44–55. 10.1111/nyas.12886. [DOI] [PubMed] [Google Scholar]
- (28).Hermanson GT Bioconjugate Techniques, 2nd edition; Academic Press: San Diego, 2008. [Google Scholar]
- (29).Moore S; Stein WH Photometric Ninhydrin Method for Use in the Chromatography of Amino Acids. J. Biol. Chem 1948, 176 (1), 367–388. [PubMed] [Google Scholar]
- (30).Yazawa K; Ishida K; Masunaga H; Hikima T; Numata K Influence of Water Content on the β-Sheet Formation, Thermal Stability, Water Removal, and Mechanical Properties of Silk Materials. Biomacromolecules 2016, 17 (3), 1057–1066. 10.1021/acs.biomac.5b01685. [DOI] [PubMed] [Google Scholar]
- (31).Hammersley AP; Svensson SO; Hanfland M; Fitch AN; Hausermann D Two-Dimensional Detector Software: From Real Detector to Idealised Image or Two-Theta Scan. High Pressure Research 1996, 14 (4–6), 235–248. 10.1080/08957959608201408. [DOI] [Google Scholar]
- (32).Bochyńska AI; Hannink G; Buma P; Grijpma DW Adhesion of Tissue Glues to Different Biological Substrates: Adhesion of Tissue Glues to Different Biological Substrates. Polymers for Advanced Technologies 2017, 28 (10), 1294–1298. 10.1002/pat.3909. [DOI] [Google Scholar]
- (33).Wray LS; Hu X; Gallego J; Georgakoudi I; Omenetto FG; Schmidt D; Kaplan DL Effect of Processing on Silk-Based Biomaterials: Reproducibility and Biocompatibility. Journal of Biomedical Materials Research Part B: Applied Biomaterials 2011, 99B (1), 89–101. 10.1002/jbm.b.31875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Asakura T; Ohgo K; Ishida T; Taddei P; Monti P; Kishore R Possible Implications of Serine and Tyrosine Residues and Intermolecular Interactions on the Appearance of Silk I Structure of Bombyx Mori Silk Fibroin-Derived Synthetic Peptides: High-Resolution 13C Cross-Polarization/Magic-Angle Spinning NMR Study. Biomacromolecules 2005, 6 (1), 468–474. 10.1021/bm049487k. [DOI] [PubMed] [Google Scholar]
- (35).Asakura T; Suita K; Kameda T; Afonin S; Ulrich AS Structural Role of Tyrosine in Bombyx Mori Silk Fibroin, Studied by Solid-State NMR and Molecular Mechanics on a Model Peptide Prepared as Silk I and II. Magn Reson Chem 2004, 42 (2), 258–266. 10.1002/mrc.1337. [DOI] [PubMed] [Google Scholar]
- (36).International Organization for Standardization. ISO 10993–5:2009 BIOLOGICAL EVALUATION OF MEDICAL DEVICES -- PART 5: TESTS FOR IN VITRO CYTOTOXICITY. 2009.
- (37).Altman GH; Diaz F; Jakuba C; Calabro T; Horan RL; Chen J; Lu H; Richmond J; Kaplan DL Silk-Based Biomaterials. Biomaterials 2003, 24 (3), 401–416. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.