Background:
The changes in the mid face and lower face are among the most prominent features of the aging process. Intense focused ultrasound, known as the Ulthera System (Ulthera Inc., Mesa, AZ, USA), was designed to correct this process. It employs micro-focused ultrasound to cause discrete focal heating of the dermis and stimulates neocollagenesis and elastin remodeling.
Methods:
This study enrolled 50 adult patients of Indian ethnicity who opted for correction of mid face and lower face sagging by Ulthera. The subjects were treated using Ulthera 3.0 mm probes which targets deep dermis and 4.5 mm, which targets the superficial muscular aponeurotic system. All patients were evaluated for allergic reactions and side effects like scarring and nerve/muscle dysfunction. Investigators Global Aesthetic Improvement Scales Scores and Patients Global Aesthetic Improvement Scales Scores were used for analysis at 30 days, 60 days, 3 months, 6 months, and 1 year. Photographs were taken for detailed facial evaluation. Patients were asked to fill a self-assessment questionnaire.
Results:
At the end of 6 months, improvements in mid face and lower face were reported in 93% patients by blinded reviewers and 85% patients found the results to be satisfactory. The same results were maintained at the end of 1 year.
Conclusion:
Our study showed that substantial results can be achieved in overall aesthetic improvement of sagging of mid face and lower face with this modality using intense focused ultrasound which utilizes delivering of treatment at a single focal depth.
INTRODUCTION
The concept of replacing a surgeon’s scalpel with noninvasive procedures has attracted attention in medicine for more than half a century.1
In an effort to meet the patient’s demand of no downtime, many skin tightening procedures and nonablative skin resurfacing treatments have emerged (eg, monopolar, bipolar, tripolar radiofrequency) to induce collagen shrinkage and remodeling, while preserving the epidermis.2,3 Ultrasound has become a leading method, due to its ability to accurately focus energy into the body in the form of heat and selectively destroy small volumes of tissue.4
Intense focused ultrasound (IFUS) is an energy modality that propagates through the tissue, up to the depth of several millimeters. During the past decade, IFUS has been used as a clinical noninvasive surgical tool to treat tumors, including those of the liver, prostate, and uterus.5–8
Micro-focused ultrasound (MFU) can be focused on subcutaneous tissue where the temperature briefly reaches greater than 60°C, producing small (1 mm3) thermal coagulation points to a depth of up to 5 mm within the mid-to-deep reticular layer of the dermis and subdermis.9,10 The intervening papillary dermal and epidermal layers of skin remain unaffected. The application of heat at these discrete thermal coagulation points cause collagen fibers in the facial planes such as the superficial muscular aponeurotic system (SMAS) and platysma, as well as the deep reticular dermis, to become denatured, contracting and stimulating de novo collagen.11,12
A commercially available device combines MFU with high-resolution ultrasound imaging (MFU with visualization [MFU-V]), which enables visualization of tissue plane to a depth of 8 mm and allows the user to see where the MFU energy will be applied (Ultherapy; Ulthera Inc., Mesa, AZ, USA). Using different transducers, MFU-V treatment can be customized to meet the unique physical characteristics of each patient by adjusting energy and focal depth of the emitted ultrasound.
This study describes an investigation of Ultherapy for tightening facial skin. The purpose of this study was to assess both the safety and efficacy of this treatment.
Equipment Used
The MFU-V system (Ulthera; Ulthera Inc.) has the ability to deliver focused ultrasound energy at preselected depths of 4.5, 3, and 1.5 mm using different transducers, while providing real-time imaging to ensure accurate energy delivery to the intended tissue plane. In 2009, it received US Food and Drug Administration clearance for a noninvasive brow lift and subsequently to lift lax submental and neck skin.12
Facial and nonfacial areas have a wide range of thickness. Ulthera is an ideal treatment choice for all such anatomic areas. On the face, it targets the cutaneous layers on the skin such as the reticular dermis and fibromuscular layers such as the SMAS. Its range of action even includes tightening fibromuscular tissue encasing the muscles of the body.13
MATERIALS AND METHODS
Study Design
A prospective, double-blind study was carried out at The Esthetic Clinics from October 2017 to February 2019. Approval was obtained from the Institutional Review Board.
Inclusion and Exclusion Criteria
Fifty patients (women, 26; men, 24) in the age group of 25–55 years of age, with complaints of mild sagging of skin in the lower half of the face and neck region, were enrolled for the study. Exclusion criteria were active local infections or skin diseases that might alter wound healing, acne or keloidal scarring, significant ptotic skin or subcutaneous fat, recent ablative or nonablative skin procedures, and surgical procedures within a year to the proposed treatment sites.
Objective and Subjective Clinical Analysis
Good quality clinical photographs were clicked at baseline, 2 months, 3 months, 6 months, and 1 year and were compared for assessment. Efficacy from baseline to 6 months was rated quantitatively (objectively) by 2 independent investigators using the Investigator Assessment Scale on standardized photographs (0 = no change; 1 = mild improvement; 2 = moderate improvement; and 3 = significant improvement). The investigators were given pretreatment and posttreatment photographs of the patients in a paired manner to determine if discernable clinical improvement was noted. If a change was observed, the reviewer was asked to identify the posttreatment image. If the correct image was identified as posttreatment image, then only the assessment was considered as an improvement. Similarly, patients used the Subjective Assessment Scale to assess their results at the 6-month evaluation period. During their treatment procedure, patients assessed their levels of perception of pain and improvement on a 10-point scale (0 = no pain; 1–4 = mild pain; 5–8 = moderate pain; 9–10 = severe pain). The patients were weighed before starting the treatment and at every follow-up and were compared.
Treatment Protocol
Pretreatment
Topical anesthetic ointment (7%, lidocaine–prilocaine) was applied to the face and neck areas to be treated, for 45 minutes before the procedure. Patients washed their faces with a mild cleanser just before their procedure. All metallic jewelry was removed from the facial area.
Procedure
Currently available transducers emit frequencies of 10.0, 7.0, and 4.0 MHz with focal depths of 1.5, 3.0, and 4.5 mm, respectively. These transducers can be used in combination to target the dermis (1.5 mm), deep dermis (3.0 mm), or the subdermal tissues (4.5 mm) including the SMAS layer.
Selection of Ultrasound Hand Pieces
The forehead, temples, and the thin malar area were treated with the 7.5-MHz, 3.0-mm hand piece at the following energy settings: forehead, 0.3 to 0.35 J; malar, 0.35 J; and temple, 0.35 J. The cheeks and submental areas were treated with 4.4 MHz, 4.5 mm at the 1.2 J energy setting and at 7.5 MHz with the 3.0-mm hand piece at the highest energy setting, 0.45 J.
Treatment Settings
The spacing of pulses within each exposure line was set at 1.5 mm, allowing thermal coagulative zones to be created along each line. The operator moved the probe almost parallel to first exposure line, placing the second row of ultrasound exposures 3 to 5 mm away from the first line. This permitted a grid-like distribution of thermal coagulative zones, with closer spacing along each exposure line than between parallel exposure line.
Ultrasound Exposure Protocol
Markings were done on the mid face and neck regions as shown in Figure 1. These photographs are representative of the number of lines done in any particular patients. The numbers are variable depending on individual patient requirement. Ultrasound gel was applied to the skin. Then the probe was placed firmly on the targeted skin surface and pressed uniformly, so it was well coupled to the skin surface. The ultrasound imaging functionality was used to confirm that the probe was acoustically coupled to the skin tissue and that the geometric focal depth for therapy was in the mid-to-deep reticular dermis. If necessary, the probe was readjusted by further scanning of the region with imaging to satisfy these 2 conditions.
Fig. 1.
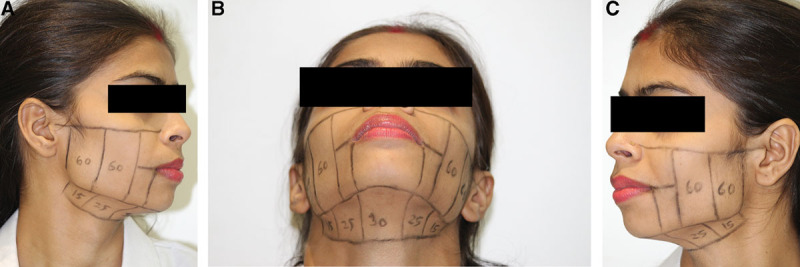
A–C, Markings for areas treated by Ulthera, in all patients. These photographs are representative of the number of lines done in any particular patient. The numbers are variable depending on individual patient requirements.
Treatment exposure was initiated, with a line of individual ultrasound pulses being delivered over approximately 2 seconds. The probe was then slid to the next location, and repositioned 3 to 5 mm laterally, such that it was adjacent and parallel to the previous treatment line. Each individual thermal coagulation zone had an inverted conical shape, pointed down.
Cone width at the point of maximal breadth (superior pole) was 0.5 to 0.75 mm for all probes and relatively wider for the 7-MHz probes than the 4-MHz probes. The energy delivery sequence was repeated. For patients who acknowledged their low pain threshold levels or experienced moderate discomfort during treatment, a pain management program was initiated in a graded fashion beginning with oral nonsteroidal anti-inflammatory drugs, pain and sedative medications, distractive hand/foot massages, and squeezy balls. Usage of topical analgesic gels for an hour before treatment lessened pain in a few patients. Finally, if required, infiltration of buffered lidocaine was offered for pain.
Posttreatment Care
Ultrasound gel was wiped off. Patients were instructed that mild redness and swelling might persist for several days, but that if they encountered any other effects, they should contact the investigator promptly. Icing and vigorous exercises were avoided for 3–5 days.
DATA COLLECTION AND STATISTICAL ANALYSIS
All the collected data were stored in the Microsoft excel version 2003, and GraphPad Prism v.6 statistical analysis Software (GraphPad Software, San Diego, CA, USA) was used to run the data analysis. Data were checked for the normality test before choosing the statistical tests and were found to be parametric. We used paired-t test, and covariance was measured.
RESULTS
Demographic variables including the mean age of participants, gender-wise distribution, and body mass index (BMI) are mentioned in Table 1. All the 50 patients completed treatment and received follow-up examinations (there were no drop outs from the study). Objectively, all patients demonstrated improvement, out of 50 patients, 12 (24%) of them showed improvement by 2 objective scores, 12 (24%) showed improvement by 3 objective score, and 26 (52%) showed improvement by 1 objective scores at the end of second month.
Table 1.
Demographic Distribution of Participants for Ulthera Treatment
| Participants Distribution (N = 50) | ||||
|---|---|---|---|---|
| Gender | Age Range (y) | N (%) | Age (Mean ± SD) | BMI (Mean ± SD) |
| Female | 25–55 | 26 (52%) | 38.4 ± 1.25 | 21.69 ± 2.19 |
| Male | 24 (48%) | 36.76 ± 2.36 | 23.7 ± 1.64 | |
P < 0.005 is considered as level of significance.
BMI, body mass index.
By the end of 6 months, 60% and 40% patients showed improvement by 3 and 2 scores, respectively. These changes were maintained at the end of 1-year follow-up (Table 2).
Table 2.
Objective Assessment by Investigators’ Assessment Scale
| Paired t test | ||||
|---|---|---|---|---|
| No. of Sessions | Mean ± SD | Covariance | t Value | Level of Significance |
| After 2 months | 1.6 ± 0.76 | 0.216 | 8.510 | P < 0.001 |
| After 6 months | 2.64 ± 0.48 | |||
| After 1 year | 2.60 ± 0.50 |
P < 0.005 is considered as level of significance.
Fourteen (25%), 8 (16%), and 28 (56%) patients showed improvement by 2, 3, and 1 grades, respectively, at the end of second month. By the end of 6 months, 64% and 36% patients showed improvement by 3 and 2 grades, respectively (Table 3).
Table 3.
Subjective Assessment by Patients’ Assessment Scale
| Paired t test | ||||
|---|---|---|---|---|
| No. of Sessions | Mean ± SD | Covariance | t Value | Level of Significance |
| After 2 months | 1.52 ± 0.65 | 0.148 | 5.73 | P < 0.001 |
| After 6 months | 2.56 ± 0.50 | |||
| After 1 year | 2.50 ± 0.48 |
Objectively, average improvement at 6 months was by 2.5 grades and subjectively by 2.8 grades. These results were maintained when evaluated at the end of 1 year. All patients experienced mild-to-moderate pain during the treatment session. Almost all the patients had swelling that persisted for 2 to 14 days. Thirty-two percent patients faced mild pain; 48% faced moderate pain, and 20% faced severe pain during the procedure.
On an average, based on the skin laxity, 500 exposure lines (range: 480–700) were placed using the focused ultrasound system on the face and neck of each subject. The weight records of all the patients before and after the study were constant, demonstrating that the results noted in the area of Ulthera were due to the effect of the treatment on fibroblasts and collagen. Because facial size varied, the total numbers of lines were adjusted to ensure consistent density and spacing. No other adverse events, including but not limited to nerve and muscle dysfunction, facial fat deformity, scarring, or bleeding were observed. Pictures have been provided to demonstrate the entire spectrum of results seen (Figs. 2–4).
Fig. 2.
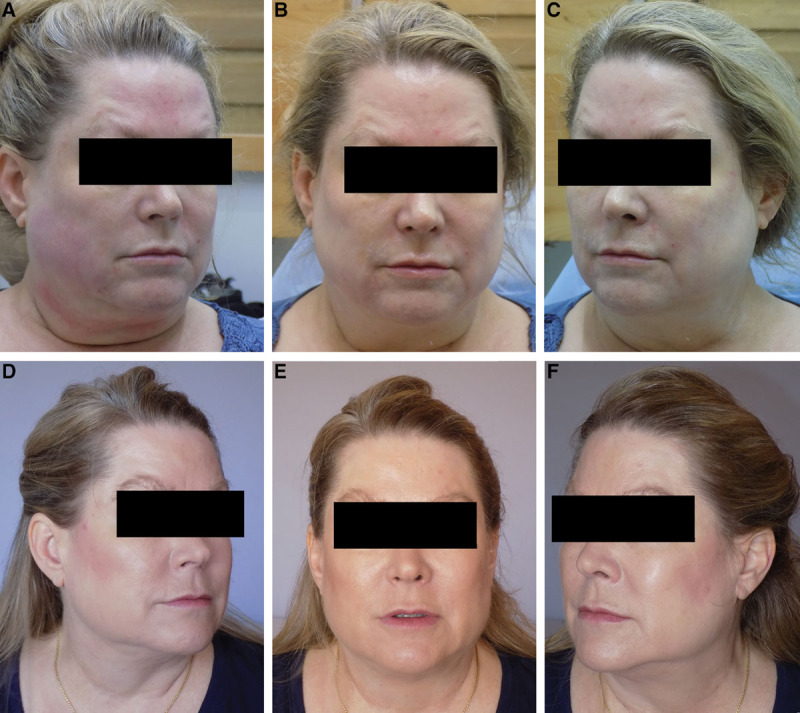
Patient 1: age of the patient: 50 years. A–C, Before Ultherapy. D–F, Results after 24 weeks of Ultherapy.
Fig. 4.
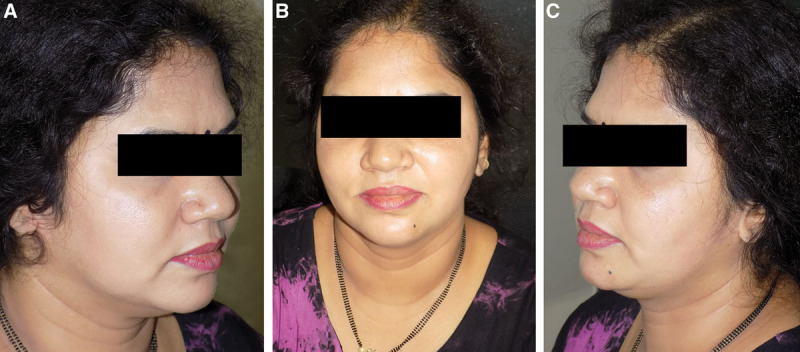
Results after 40 weeks of Ultherapy.
Fig. 3.
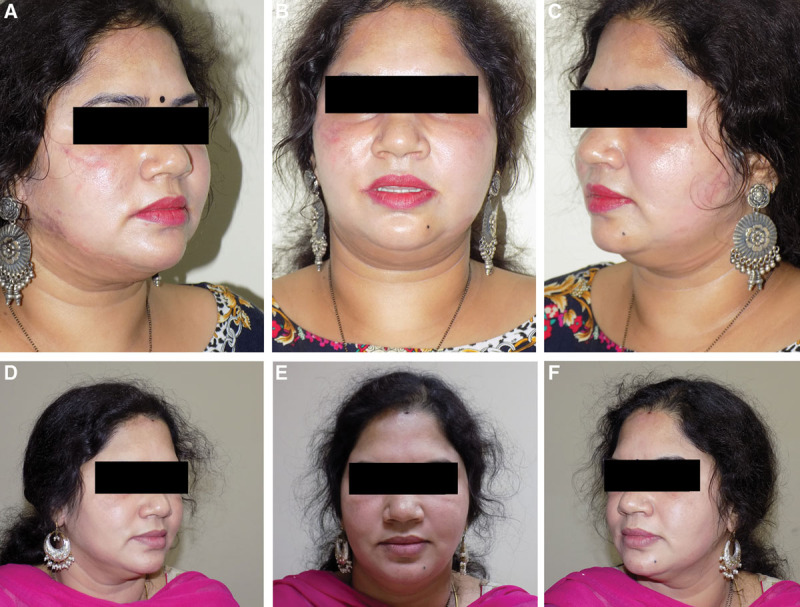
Patient 2: age of the patient: 45 years. A–C, Before Ultherapy. D–F, Results after 24 weeks of Ultherapy.
DISCUSSION
Various noninvasive procedures and devices have been developed in an effort to treat aging skin like laser (ablative and nonablative) carbon dioxide or erbium lasers, which induce sublethal thermal injury to the skin tissue, causing removal of the epidermis, contraction, and remodeling of the dermis.14 However, for patients requiring rejuvenation of the neck and lower two thirds of the face and for particularly lifting skin or tightening tissue, these treatments do not work.15
Although surgical rejuvenation remains the gold standard for many patients and physicians, Ulthera has clear advantages. This device provides dermal heating to induce collagen denaturation and subsequent synthesis. The epidermis is spared, and the patient’s downtime is minimized. This is unlike the other modalities which primarily focus on treating the superficial layers of the skin because of limitations in penetration depth.11
The epidermis-sparing properties of fractionated MFU devices such as the Ulthera System were demonstrated in a clinical study by Gliklich et al16 also showed that there was no effect on structures such as the facial nerve or its branches and there were discrete areas of coagulative damage. Furthermore, no thermal injury was apparent from histologic examination conducted 4 to 12 weeks posttreatment. In a cadaveric study, White et al10 found that ultrasonic energy deposited deep within the SMAS induces the most effective skin tightening.
Suh et al13 obtained biopsy specimens from 11 patients who had undergone treatment with the Ulthera device 2 months earlier and reported a statistically significant increase in dermal thickness secondary to increased dermal collagen fibers. Moreover, they found no evidence of epidermal changes or inflammatory reactions. These results support previous findings of White et al10 who reported sparing of the epidermis and focused thermal microablative damage, characterized histologically in human cadaveric skin.
The action of devices such as HIFU involves thermal as well as cavitation effects, to cause cell disruption and cell death. The injury that occurs when HIFU is applied to living tissue is the result of a thermomechanical process. As the name implies, this involves 2 distinct but inseparable mechanisms. The ultrasound energy which is absorbed by tissues causes molecular vibrations resulting in heat generation and a rapid rise in temperature at the focal zone. In addition, the repeated compressions and rarefactions that occur as waves of ultrasound propagation through living tissue result in powerful shear forces. On a cellular level, this microscopic shearing motion results in frictional heating.16
Lee et al17 examined the efficacy of Ulthera treatment and found that at 90 days, 80% of blinded assessors saw some clinical improvement in the 10 patients who completed the study, and 90% of patients noted improvement in skin laxity.
Study by Fabi3 (2014) for eyebrow lift showed that 35 out of 36 patients enrolled in the study had a mean improvement of 1.7 mm, in a time period of 90 days. Suh et al13 conducted a study on 22 Korean patients and noticed improvement in nasolabial fold in 77% patients. Seventy-three percent of his patients showed improvement in jaw lines.
Most recently, Kenkel18 focused specifically on treatment of the neck, which ultimately led to Food and Drug Administration approval for this indication. In our study also, we specifically performed the procedure in patient who had sagging of skin in lower half of the face and neck region.
Unlike many laser devices, ultrasound therapy does not target melanin and therefore is safe for all skin types. A significant advantage of the dermatologic use of IFUS in Asian patients is that the absorption of ultrasound energy is independent of the melanin of skin. Instead the microscopic and bulk mechanical properties of the tissue determine the absorption in the skin. Therefore, in contrast to light-based devices, the action of IFUS is independent of skin color and chromophores.19 In the study done by Laubach et al20 in 2008, post mortem, human skin samples with Fitzpatrick skin type II–V were treated with HIFU, and well-defined skin lesions were created in the deeper dermis and subcutaneous tissue, without any damage to epidermis and dermis. It is thus helpful in overcoming the difficulties encountered by light-based treatment of darker individuals.20
The number of lines per treatment area depends on the baseline skin laxity of the patient. Fabi,4 in his study for complete face/neck lift, advocated giving a total of 500–600 lines for mild skin laxity, 600–700 lines for moderate skin laxity, and as high as 800 lines for severe skin laxity.4 In our study, all the patients had mild skin laxity, and satisfactory results were achieved by giving a total of 500 lines per patient.
The beneficial effects of Ulthera even include the longevity of its action. In the study by Fabi4 2015, as per Physician Global Aesthetic improvement scale, 67% of the patient showed improvement in appearance at 180 days. In our study, objective improvement by 2.5 grade and subjective improvement by 2.8 grade were seen at the end of 6 months. The same results were maintained by the end of 1 year.
Ulthera is a relatively safe procedure. The most common adverse effect reported by the patients is slight amount of discomfort during the procedure. Topical local anesthetic application before the procedure significantly reduces the discomfort. Adverse effects like transient erythema, edema, and occasional bruising have been reported in the past.9,11,13,21 Uncommon complications include postoperative hyperpigmentation, striated linear skin patterns or wheels. These wheels can be attributed to the improper treatment technique and are mostly associated with 1.5 and 3 mm transducer.4,11,13
A rare complication is transient numbness around the perioral area. Suh et al13 in his study mentioned that 4 out of 22 patients developed numbness around the mandible which was resolved within 2–3 weeks. Similar case has been reported by Jeong et al,22 and he mentioned that thermal injury to the branches of trigeminal nerve can lead to transient numbness along it distribution. However, no such adverse effects were seen in our study. Our results are especially gratifying, as in the Asian Indian population, lower face and neck aging is the earliest and worst determinant of aging23 and impacting this positively has a gratifying impact on the aging of the entire face.
CONCLUSIONS
In today’s scenario where more and more patients are opting for nonsurgical procedures for facial rejuvenation, Ulthera proves to be the most significant, noninvasive procedure. The 1-year follow-up done in our study reflects the longevity of its action. It also highlights the safety profile, because no major adverse effects were noted. In addition, it can be used in all the skin types (Fitzpatrick I–V), which in turn increases its versatility. To the best of our knowledge, this is the longest reported case series follow-up post Ulthera. For the patient with mild-to-moderate skin and soft tissue laxity, Ulthera undoubtedly provides adequately good results. Longer-term follow-up studies, with larger sets of population and more severe skin laxity, will be the paths for future research.
Footnotes
Published online 31 December 2019.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.Clement GT. Perspectives in clinical uses of high-intensity focused ultrasound. Ultrasonics. 2004;42:1087–1093. [DOI] [PubMed] [Google Scholar]
- 2.Kapoor R, Shome D, Ranjan A. Use of a novel combined radiofrequency and ultrasound device for lipolysis, skin tightening and cellulite treatment. J Cosmet Laser Ther. 2017;19:266–274. [DOI] [PubMed] [Google Scholar]
- 3.Fabi SG. Microfocused ultrasound with visualization for skin tightening and lifting: my experience and a review of the literature. Dermatol Surg. 2014;40suppl 12):S164–S167. [DOI] [PubMed] [Google Scholar]
- 4.Fabi SG. Noninvasive skin tightening: focus on new ultrasound techniques. Clin Cosmet Investig Dermatol. 2015;8:47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim KH, Geronemus RG. Nonablative laser and light therapies for skin rejuvenation. Arch Facial Plast Surg. 2004;6:398–409. [DOI] [PubMed] [Google Scholar]
- 6.Foster RS, Bihrle R, Sanghvi NT, et al. High-intensity focused ultrasound in the treatment of prostatic disease. Eur Urol. 1993;23 (suppl 1):29–33. [DOI] [PubMed] [Google Scholar]
- 7.Gianfelice D, Khiat A, Amara M, et al. MR imaging-guided focused US ablation of breast cancer: histopathologic assessment of effectiveness– initial experience. Radiology. 2003;227:849–855. [DOI] [PubMed] [Google Scholar]
- 8.Kennedy JE, Wu F, ter Haar GR, et al. High-intensity focused ultrasound for the treatment of liver tumours. Ultrasonics. 2004;42:931–935. [DOI] [PubMed] [Google Scholar]
- 9.Bozec L, Odlyha M. Thermal denaturation studies of collagen by microthermal analysis and atomic force microscopy. Biophys J. 2011;101:228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White WM, Makin IR, Barthe PG, et al. Selective creation of thermal injury zones in the superficial musculoaponeurotic system using intense ultrasound therapy: a new target for noninvasive facial rejuvenation. Arch Facial Plast Surg. 2007;9:22–29. [DOI] [PubMed] [Google Scholar]
- 11.Alam M, White LE, Martin N, et al. Ultrasound tightening of facial and neck skin: a rater-blinded prospective cohort study. J Am Acad Dermatol. 2010;62:262–269. [DOI] [PubMed] [Google Scholar]
- 12.Minkis K, Alam M. Ultrasound skin tightening. Dermatol Clin. 2014;32:71–77. [DOI] [PubMed] [Google Scholar]
- 13.Suh DH, Shin MK, Lee SJ, et al. Intense focused ultrasound tightening in Aasian skin: clinical and pathologic results. Dermatol Surg. 2011;37:1595–1602. [DOI] [PubMed] [Google Scholar]
- 14.Ortiz AE, Tremaine AM, Zachary CB. Long-term efficacy of a fractional resurfacing device. Lasers Surg Med. 2010;42:168–170. [DOI] [PubMed] [Google Scholar]
- 15.Ogden S, Griffiths TW. A review of minimally invasive cosmetic procedures. Br J Dermatol. 2008;159:1036–1050. [DOI] [PubMed] [Google Scholar]
- 16.Gliklich RE, White WM, Slayton MH, et al. Clinical pilot study of intense ultrasound therapy to deep dermal facial skin and subcutaneous tissues. Arch Facial Plast Surg. 2007;9:88–95. [DOI] [PubMed] [Google Scholar]
- 17.Lee HS, Jang WS, Cha YJ, et al. Multiple pass ultrasound tightening of skin laxity of the lower face and neck. Dermatol Surg. 2012;38:20–27. [DOI] [PubMed] [Google Scholar]
- 18.Kenkel J. Evaluation of the Ulthera system for improving skin laxity and tightening. Paper presented at: ASAPS Annual Meeting; May 7, 2012; Vancouver, Canada: 3–8. [Google Scholar]
- 19.Goss SA, Johnston RL, Dunn F. Comprehensive compilation of empirical ultrasonic properties of mammalian tissues. J Acoust Soc Am. 1978;64:423–457. [DOI] [PubMed] [Google Scholar]
- 20.Laubach HJ, Makin IR, Barthe PG, et al. Intense focused ultrasound: evaluation of a new treatment modality for precise microcoagulation within the skin. Dermatol Surg. 2008;34:727–734. [DOI] [PubMed] [Google Scholar]
- 21.Chan NP, Shek SY, Yu CS, et al. Safety study of transcutaneous focused ultrasound for non-invasive skin tightening in asians. Lasers Surg Med. 2011;43:366–375. [DOI] [PubMed] [Google Scholar]
- 22.Jeong KH, Suh DH, Shin MK, et al. Neurologic complication associated with intense focused ultrasound. J Cosmet Laser Ther. 2014;16:43–44. [DOI] [PubMed] [Google Scholar]
- 23.Shome D, Khare S, Ayyar A, et al. Aging and the Indian face: an analytical study of aging in the Asian Indian face. J Plast Reconstruct Surg Glob Open. 2019. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]


