Supplemental Digital Content is available in the text.
Background:
Ischemic complications after immediate breast reconstruction have devastating consequences; however, individual risk assessment remains challenging. We seek to develop an intraoperative assessment tool to assist in estimating risk of ischemic complications in immediate breast reconstruction.
Methods:
Patients undergoing immediate breast reconstruction were prospectively identified and evaluated with an intraoperative mastectomy flap ischemia risk assessment tool consisting of 8 binary questions. Breast measurements and patient demographics were recorded. Reconstructions were then prospectively evaluated postoperatively for ischemic complications. Outcomes were analyzed with significance set at P values <0.05.
Results:
Thirty-one patients underwent 45 immediate breast reconstruction. The majority of reconstructions were tissue expander based (64.4%) following therapeutic (62.2%) skin-sparing (93.3%) mastectomies. Average follow-up was 11.16 months. Sixteen reconstructions (35.6%) experienced an ischemic complication. The average total mastectomy flap ischemic risk score was 4.29. The correlation value of higher scores with increasing ischemic complications was 0.65. Reconstructions with scores greater than 5 had significantly higher rates of ischemic complications (P = 0.0025). Reconstructions with a score of >6 and >7 also had significantly higher rates of ischemic complications (P < 0.0001, each). The sensitivity and specificity of intraoperative mastectomy flap compromise were 81.25% and 62.07%.
Conclusions:
Ischemic complications after immediate breast reconstruction were positively correlated with higher scores using a clinical intraoperative mastectomy flap ischemia risk assessment tool. Scores greater than 5 seem to be a threshold value at which ischemic complications are significantly greater. This simple, easy-to-implement intraoperative tool may assist plastic surgeons in assessing risk and optimizing outcomes in immediate breast reconstruction.
INTRODUCTION
Ischemic complications after immediate breast reconstruction, whether implant- or autologous-based, carry significant clinical consequences including reconstructive failure.1–4 Rates of mastectomy flap necrosis after immediate reconstruction continue to be established but range from 3% to 40% depending on the severity.1 A myriad of patient-, operative-, and surgeon-specific factors influences the risk of ischemic complications.4,5 However, predicting individual patient risk remains challenging.
Various technological devices have been employed to assist in prediction of postoperative ischemic complications.6–9 Such modalities largely include tissue angiography and spectroscopy.6–9 Although these technologies have demonstrated variable effectiveness in reducing the incidence of postoperative ischemic complications in immediate breast reconstruction, they introduce a large cost burden that limits their implementation.6,7 Therefore, we sought to develop an intraoperative mastectomy flap risk assessment tool to assist in both estimating risk of ischemic complications and aid in clinical decision-making to optimize outcomes in immediate breast reconstruction.
METHODS
Patients undergoing immediate implant- or autologous-based breast reconstruction at a major metropolitan public medical center without availability of fluorescent angiography were prospectively identified. Patient demographics, comorbidities, and oncologic characteristics were recorded. Three breast surgeons and 5 plastic surgeons were involved in the care of these patients.
Intraoperatively, each reconstruction was evaluated with an intraoperative mastectomy flap ischemia risk assessment tool by an independent surgeon. The risk assessment tool consisted of 8 binary questions, as determined by the authors, to be answered by the independent surgeon that covered clinical assessments of flap perfusion and flap thickness, and use of methylene blue, electrocautery and infiltration of a 0.5% lidocaine, 1:200,000 epinephrine solution (Fig. 1). Higher scores were hypothesized to correlate with greater risk of ischemic complications. Location of any intraoperative mastectomy flap compromise and general breast measurements were also recorded. Mastectomy flap tissue was not routinely trimmed during the operation. Patients were then prospectively evaluated postoperatively for the occurrence of any ischemic complications at each postoperative follow-up office visit. Ischemic complications were defined as any impending or actual tissue/skin loss of the mastectomy flaps. Partial-thickness necrosis was defined as that managed with local wound care. Full-thickness necrosis was defined as that managed with debridement in either the office or the operating room. Locations of postoperative ischemic complications were also noted (see document, Supplemental Digital Content 1, which displays the worksheets for intra- and postoperative mastectomy flap evaluation, http://links.lww.com/PRSGO/B273).
Fig. 1.

Clinical intraoperative mastectomy flap risk assessment tool.
Descriptive statistics and measure of central tendency were used to describe absolute and mean results, respectively. Student’s t tests were used to analyze continuous data sets, whereas chi-square tests were used to compare proportional responses. Univariate analysis was performed to evaluate for independent risk factors for the occurrence of ischemic complications. The risk for occurrence of an ischemic complication for each total score of the risk assessment tool was calculated. A correlation value was also determined for the correlation between risk assessment score and rate of ischemic complications. Sensitivity, specificity, and positive and negative predictive values were calculated for correlation between the presence of intraoperative mastectomy flap compromise and postoperative mastectomy flap ischemic complications. A subgroup analysis was also performed comparing risk assessment scores and rate of ischemic complications between implant-based and autologous-based reconstructions.
Statistical analysis was performed using GraphPad Software, Inc. (La Jolla, CA). A P value of less than 0.05 was considered significant.
RESULTS
Thirty-one patients underwent 45 immediate breast reconstructions after mastectomy. Average age and body mass index were 47.71 years and 27.77 kg/m2. Two (4.4%) and 3 (6.7%) patients, respectively, were active and former tobacco smokers. The rates of prior radiation and chemotherapy were 13.3% and 40.0%, respectively. Rates of adjuvant radiation and chemotherapy were 15.6% and 13.3%. The majority of mastectomies were skin sparing (93.3%) and for therapeutic indications (62.2%). Of all cases, 57.8% underwent concurrent sentinel lymph node biopsy or axillary lymph node dissection. The most common clinical breast cancer stages were stage 0 (46.7%), IA (24.4%), and IIA (11.1%). The majority of reconstructions were tissue expander based (64.4%) followed by autologous based (35.6%). The majority (69.0%) of tissue expander-based reconstructions utilized acellular dermal matrices. Average breast measurements were 22.93 cm for sternal notch to nipple, 9.36 cm for nipple to inframammary fold, 14.09 cm for breast width, 15.04 cm for incision to clavicle, and 6.39 cm for incision to inframammary fold. Average follow-up was 11.16 months (Table 1).
Table 1.
Overall Demographics and Surgical Characteristics of Patients Undergoing Immediate Breast Reconstruction after Mastectomy Assessed with the Mastectomy Flap Ischemia Risk Score
| No. (Patients) | 31 |
|---|---|
| No. (breasts) | 45 |
| Age, y | 47.71 |
| Body mass index, kg/m2 | 27.77 |
| Mastectomy indication | Therapeutic: 28 (62.2%); prophylactic: 17 (37.8%) |
| Smoking history | Active: 2 (4.4%); former: 3 (6.7%) |
| Prior radiation | 6 (13.3%) |
| Prior chemotherapy | 18 (40.0%) |
| Lymph node biopsy or dissection performed | 26 (57.8%) |
| Adjuvant radiation | 7 (15.6%) |
| Adjuvant chemotherapy | 6 (13.3%) |
| Pathologic breast cancer stage | 0: 21 (46.7%) |
| IA: 11 (24.4%) | |
| IB: 2 (4.4%) | |
| IIA: 5 (11.1%) | |
| IIB: 3 (6.7%) | |
| IIIA: 2 (4.4%) | |
| IIIC: 1 (2.2%) | |
| Mastectomy type | Skin-sparing mastectomy: 42 (93.3%) Nipple-sparing mastectomy: 3 (6.7%) |
| Mastectomy incision | Ellipse: 42 (93.3%) Inframammary fold: 3 (6.7%) |
| Sternal notch–nipple distance, cm | 22.93 (36) |
| Nipple–inframammary fold distance, cm | 9.36 (36) |
| Breast width, cm | 14.09 (41) |
| Incision–clavicle distance, cm | 15.04 (43) |
| Incision–inframammary fold distance, cm | 6.39 (43) |
| Reconstructive modality | Tissue expander: 29 (64.4%) Autologous: 16 (35.6%) |
| Acellular dermal matrix | 20 (69.0%) |
| Follow-up, mo | 11.16 |
Overall, 16 reconstructions (35.6%) experienced an ischemic complication with 7 (15.6%) and 9 (20.0%) incidences of full- and partial-thickness mastectomy flap necrosis, respectively. There were 2 incidences (4.4%) of reconstructive failure related to an ischemic complication. Nonischemic complications included minor cellulitis (2.2%) and hematoma (6.7%) (Table 2).
Table 2.
Overall Reconstructive Complications in Patients Undergoing Immediate Breast Reconstructions after Mastectomy Assessed with the Mastectomy Flap Ischemia Risk Score
| Ischemic Complications | |
|---|---|
| Full-thickness mastectomy flap necrosis | 7 (15.6%) |
| Partial-thickness mastectomy flap necrosis | 9 (20.0%) |
| Explantation (related to mastectomy flap necrosis) | 2 (4.4%) |
| Nonischemic complications | |
| Cellulitis (oral antibiotics) | 1 (2.2%) |
| Explantation (not related to mastectomy flap necrosis) | 3 (6.7%) |
| Breast hematoma | 3 (6.7%) |
| Abdominal wound breakdown | 2 (4.4%) |
| Pulmonary embolus | 2 (4.4%) |
In univariate analysis, the only factor found to be independently predictive of ischemic complications was having a sentinel lymph node biopsy performed (P = 0.0453). No other factors, including elevated body mass index (P = 0.1377), prior radiation (P = 0.4332) or chemotherapy (P = 0.7994), sternal notch–nipple distance ≥25 cm (P = 0.2689), or nipple–inframammary fold distance ≥10 cm (P = 0.8639), among others, were significantly predictive for the occurrence of an ischemic complication (Table 3).
Table 3.
Univariate Analysis of Risk Factors for Ischemic Complications after Mastectomy with Immediate Breast Reconstruction Including the Mastectomy Flap Ischemia Risk Score
| Variable | Total Mastectomies (N) | Mastectomies with Ischemic Complication (%) | Unadjusted OR (95% CI) | P |
|---|---|---|---|---|
| No. | 45 | – | – | |
| Age | ||||
| <50 y | 24 | 7 (29.2%) | 1.8214 (0.5305–6.2540) | 0.3407 |
| ≥50 y | 21 | 9 (42.9%) | ||
| BMI | ||||
| <30 kg/m2 | 29 | 8 (27.6%) | 2.6250 (0.7341–9.3864) | 0.1377 |
| ≥30 kg/m2 | 16 | 8 (50.0%) | ||
| Current smoking | ||||
| Yes | 2 | 1 (50.0%) | 1.8667 (0.1089–32.0108) | 0.6669 |
| No | 43 | 15 (34.9%) | ||
| Therapeutic indication | ||||
| Yes | 28 | 12 (42.9%) | 2.4375 (0.6334–9.3803) | 0.1950 |
| No | 17 | 4 (23.5%) | ||
| Sentinel lymph node biopsy performed | ||||
| Yes | 19 | 10 (52.6%) | 3.7037 (1.0278–13.3467) | 0.0453 |
| No | 26 | 6 (23.1%) | ||
| Axillary lymph node dissection performed | ||||
| Yes | 7 | 1 (14.3%) | 0.2556 (0.0279–2.3406) | 0.2273 |
| No | 38 | 15 (39.5%) | ||
| Prior radiation | ||||
| Yes | 6 | 3 (50.0%) | 2.0000 (0.3534–11.3186) | 0.4332 |
| No | 39 | 13 (33.3%) | ||
| Prior chemotherapy | ||||
| Yes | 18 | 6 (33.3%) | 0.8500 (0.2427–2.9763) | 0.7994 |
| No | 27 | 10 (37.0%) | ||
| Adjuvant radiation | ||||
| Yes | 7 | 2 (28.6%) | 0.6857 (0.1171–4.0151) | 0.6756 |
| No | 38 | 14 (36.8%) | ||
| Adjuvant chemotherapy | ||||
| Yes | 6 | 0 (0.0%) | 0.1096 (0.0058–2.0819) | 0.1410 |
| No | 39 | 16 (41.0%) | ||
| Nipple-sparing mastectomy | ||||
| Yes | 3 | 0 (0.0%) | 0.2294 (0.0111–4.7313) | 0.3404 |
| No | 42 | 16 (38.1%) | ||
| Implant-based reconstruction | ||||
| Yes | 29 | 11 (37.9%) | 1.3444 (0.3678–4.9146) | 0.6545 |
| No | 16 | 5 (31.3%) | ||
| Acellular dermal matrix | ||||
| Yes | 20 | 7 (35.0%) | 0.6731 (0.1353–3.3474) | 0.6286 |
| No | 9 | 4 (44.4%) | ||
| Sternal notch–nipple distance ≥25 cm | ||||
| Yes | 13 | 7 (53.8%) | 2.1875 (0.5462–8.7612) | 0.2689 |
| No | 23 | 8 (34.8%) | ||
| Nipple–inframammary fold distance ≥10 cm | ||||
| Yes | 15 | 6 (40.0%) | 0.8889 (0.2312–3.4181) | 0.8639 |
| No | 21 | 9 (42.9%) | ||
| Mastectomy flap ischemia risk score >5 | ||||
| Yes | 9 | 5 (55.6%) | 2.8409 (0.6378–12.6541) | 0.1707 |
| No | 36 | 11 (30.6%) | ||
| Mastectomy flap ischemia risk score >6 | ||||
| Yes | 6 | 4 (66.7%) | 4.5000 (0.7229–28.0120) | 0.1069 |
| No | 39 | 12 (30.8%) | ||
| Mastectomy flap ischemia risk score >7 | ||||
| Yes | 4 | 3 (75.0%) | 6.4615 (0.6120–68.2200) | 0.1207 |
| No | 41 | 13 (31.7%) |
Bold text indicates a significant P value.
BMI, body mass index.
The average total mastectomy flap ischemic risk score was 4.29 out of a maximum score of 8. The highest average item score was for utilizing cautery to perform the mastectomy (average score: 1.00), whereas the lowest average item score was for the presence of skin edge bleeding (average score: 0.27). The highest rates of ischemic complications were observed in reconstructions with total mastectomy flap ischemia risk scores of 5 (35.7%), 2 (42.9%), 7 (50.0%), and 8 (75.0%). The correlation value of higher mastectomy flap ischemia risk scores with increasing incidence of ischemic complications was 0.65 (Tables 4 and 5) (Fig. 2). The location of intraoperative mastectomy flap compromise correlated with the location of postoperative mastectomy flap ischemia in 75% of reconstructions with ischemic complications.
Table 4.
Average Overall Scoring Calculated with the Mastectomy Flap Ischemia Risk Score
| No. | Risk Factor | Average Score (Range 0.00–1.00) |
|---|---|---|
| 1 | Flap visibly mottled (0: no, 1: yes) | 0.29 |
| 2 | Dermis visible (0: no, 1: yes) | 0.62 |
| 3 | Capillary refill present (1: no, 0: yes) | 0.29 |
| 4 | Cautery utilized for mastectomy (0: no, 1: yes) | 1.00 |
| 5 | Methylene blue injected into dermis (0: no, 1: yes) | 0.42 |
| 6 | Skin edge bleeding present (1: no, 0: yes) | 0.27 |
| 7 | Pinch test <1 cm (0: no, 1: yes) | 0.71 |
| 8 | 0.5% lidocaine 1:200,000 epinephrine solution injected before mastectomy (0: no, 1: yes) | 0.69 |
| Sum 1–8 | Overall score | 4.29 |
Table 5.
Ischemic Complications Stratified by Overall Mastectomy Flap Ischemia Risk Score
| Overall Score | N | Ischemic Complications | Correlating Locations |
|---|---|---|---|
| 0 | 0 | N/A | N/A |
| 1 | 3 | Overall: 1 (33.3%) Full-thickness mastectomy flap necrosis: 0 (0.0%) Partial-thickness mastectomy flap necrosis: 1 (33.3%) |
0 (0.0%) |
| 2 | 7 | Overall: 3 (42.9%) Full-thickness mastectomy flap necrosis: 0 (0.0%) Partial-thickness mastectomy flap necrosis: 3 (42.9%) |
1 (33.3%) |
| 3 | 6 | Overall: 2 (33.3%) Full-thickness mastectomy flap necrosis: 1 (16.7%) Partial-thickness mastectomy flap necrosis: 1 (16.7%) |
1 (50.0%) |
| 4 | 6 | Overall: 0 (0.0%) Full-thickness mastectomy flap necrosis: 0 (0.0%) Partial-thickness mastectomy flap necrosis: 0 (0.0%) |
N/A |
| 5 | 14 | Overall: 5 (35.7%) Full-thickness mastectomy flap necrosis: 2 (14.3%) Partial-thickness mastectomy flap necrosis: 3 (21.4%) |
5 (100.0%) |
| 6 | 3 | Overall: 1 (33.3%) Full-thickness mastectomy flap necrosis: 1 (33.3%) Partial-thickness mastectomy flap necrosis: 0 (0.0%) |
1 (100.0%) |
| 7 | 2 | Overall: 1 (50.0%) Full-thickness mastectomy flap necrosis: 1 (50.0%) Partial-thickness mastectomy flap necrosis: 0 (0.0%) |
1 (100.0%) |
| 8 | 4 | Overall: 3 (75.0%) Full-thickness mastectomy flap necrosis: 2 (50.0%) Partial-thickness mastectomy flap necrosis: 1 (25.0%) |
3 (100.0%) |
N/A, not applicable.
Fig. 2.
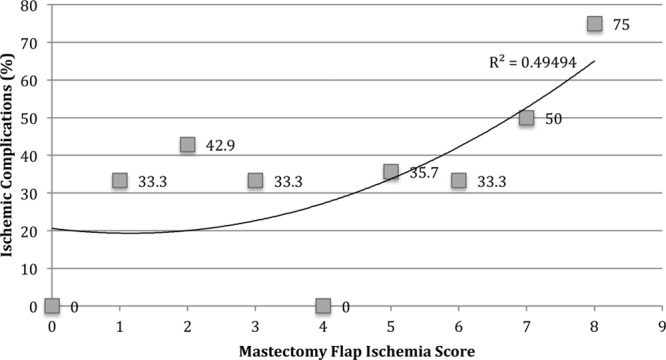
Overall ischemic complications stratified by mastectomy flap ischemia risk score.
Reconstructions with a total mastectomy flap ischemia risk score of greater than 5 had a significantly higher rate of ischemic complications compared to reconstructions with a score of 5 or less (P = 0.0025). Similarly, reconstructions with a score of >6 and >7 had significantly higher rates of ischemic complications (P < 0.0001, each) compared to reconstructions with scores of ≤6 and ≤7, respectively (Table 6). Total mastectomy flap ischemia risk scores of >5 [odds ratio (OR) = 2.8409; P = 0.1707], >6 (OR = 4. 500; P = 0.1069), and >7 (OR = 6.4615; P = 0.1207) all trended toward significance on univariate analysis.
Table 6.
Comparison of Ischemic Complications by Mastectomy Flap Ischemia Risk Score Thresholds
| Mastectomy Flap Ischemia Score (X) | Score ≤X | Score >X | P |
|---|---|---|---|
| 0 | 0/0 (N/A) | 16/45 (35.6%) | – |
| 1 | 1/3 (33.3%) | 15/42 (35.7%) | 0.9318 |
| 2 | 4/10 (40.0%) | 12/35 (34.3%) | 0.7042 |
| 3 | 6/16 (37.5%) | 10/29 (34.5%) | 0.8007 |
| 4 | 6/22 (27.3%) | 10/23 (43.5%) | 0.1247 |
| 5 | 11/36 (30.6%) | 5/9 (55.6%) | 0.0025 |
| 6 | 12/39 (30.8%) | 4/6 (66.7%) | <0.0001 |
| 7 | 13/41 (31.7%) | 3/4 (75.0%) | <0.0001 |
| 8 | 16/45 (35.6%) | 0/0 (N/A) | – |
Bold text indicates a significant P value.
N/A, not applicable.
The correlation between presence of intraoperative mastectomy flap compromise and postoperative mastectomy flap ischemic complications was then assessed. The sensitivity and specificity of intraoperative mastectomy flap compromise were 81.25% and 62.07%. The positive and negative predictive values were 54.17% and 85.71% (Table 7 and 8).
Table 7.
Correlation between Intraoperative Mastectomy Flap Compromise and Postoperative Mastectomy Flap Ischemic Complications
| Area of Postoperative Ischemic Complications (N = 16) | No Area of Postoperative Ischemic Complications (N = 29) | ||
|---|---|---|---|
| Area of intraoperative compromise (N = 24) | 13 | 11 | 24 |
| No Area of intraoperative compromise (N = 21) | 3 | 18 | 21 |
| 16 | 29 |
Sensitivity = 81.25% (95% CI: 54.35%–95.95%); specificity = 62.07% (95% CI: 42.26%–79.31%); positive predictive value = 54.17% (95% CI: 41.23%–66.57%); negative predictive value = 85.71% (95% CI: 67.54%–94.54%).
CI, confidence interval.
Table 8.
Subgroup Analysis Comparing Outcomes in Implant-based and Autologous Reconstructions
| Implant-based Reconstruction (N = 29) | Autologous Reconstruction (N = 16) | P | |
|---|---|---|---|
| Average score | 4.14 | 4.56 | 0.4881 |
| Ischemic complications | 11 (37.9%) | 5 (31.3%) | 0.5835 |
Lastly, a subgroup analysis was performed comparing risk assessment scores and rate of ischemic complications between implant-based and autologous-based reconstructions (Table 8). The average risk assessment scores for implant and autologous reconstructions were 4.14 and 4.56, respectively (P = 0.4881). Similarly, rates of ischemic complications between the groups were statistically comparable (37.9% versus 31.3%; P = 0.5835).
DISCUSSION
As extirpative breast techniques have evolved from the radical mastectomy of Halsted to nipple-sparing mastectomy, an emphasis has been placed on increasing preservation of the breast skin envelope with conserved oncologic safety.10–14 Such technical advances have enhanced aesthetic and reconstructive outcomes while also demonstrating improved patient-reported outcomes.15,16 However, increasing skin preservation places increased stress on the tissue itself given its relative hypovascular status after mastectomy.1 Post mastectomy, breast skin flap perfusion is largely based on the contribution of the superficial vasculature in the subdermal and subcutaneous tissues.17–21 When damage is incurred by this superficial vasculature, breast flap ischemia will ensue in the form of mastectomy flap necrosis, which may include the nipple-areola complex in nipple-sparing mastectomy.17
Ischemic complications after immediate breast reconstruction can have devastating consequences. Full-thickness mastectomy flap necrosis in implant-based reconstruction risks device exposure and reconstructive failure. In autologous breast reconstruction, full-thickness skin flap necrosis predisposes to poor wound healing, potential need for skin grafting, and ultimate compromise of final aesthetic result. In both reconstructive modalities, partial-thickness mastectomy flap necrosis will likewise impede wound healing, result in increased scarring, and potentially progress to full-thickness necrosis and reconstructive failure.17,22
A myriad of patient-, operative-, and surgeon-specific factors has been identified as risk factors for ischemic complications.4,5 These include prior surgery, mastectomy type, indication and incision, dissection technique, body mass index, breast size, and diabetes, among others.4,5,23,24 Perhaps the most important factor in minimizing risk of ischemic complications in immediate breast reconstruction is the quality of the postmastectomy breast skin envelope.17,25,26 Mastectomy flap quality can be optimized by performing dissection at the level of the breast capsule, preserving subcutaneous tissue to maximize oncologic resection and minimize damage to the superficial vascular plexus supplying the skin flaps.25,26 Despite these considerations, estimating individual patient risk for ischemic complications after immediate breast reconstruction remains challenging.
Clinical examination of skin flap variability remains the gold standard of evaluation.1 However, clinical evaluation is limited by subtle changes in examination belying subclinical ischemia and limited ability for assessment of capillary refill in darker skin tones.1 Given the significant repercussions of poor skin flap perfusion, multiple adjunctive technological modalities to assess perfusion have been developed.6–9,27–30 These largely include various forms of tissue angiography and spectroscopy to provide additional information regarding impaired tissue perfusion to guide the surgeon in minimizing risk for postoperative mastectomy flap ischemia.6–9,27–30 Any decreased flow may lead the surgeon to remove devascularized tissue, decrease intraoperative tissue expander fill, change the operative plan from immediate permanent implant to tissue expander placement, or bank flap skin in autologous reconstruction, among other maneuvers to mitigate tissue loss.25–31 These technologies, however, add significant cost to the operation, which limits their implementation in all clinical scenarios and hospital systems. We therefore sought to develop a simple, easy-to-implement risk assessment tool to assist surgeons in predicting risk for mastectomy flap ischemic in immediate breast reconstruction.
The risk assessment tool consisted of 8 binary questions that the operating surgeon answered intraoperatively. Higher scores indicated a greater perceived risk of mastectomy flap compromise. Any areas of compromise were also localized on the mastectomy skin flaps. To assess this risk assessment tool, 45 immediate breast reconstructions were prospectively identified and evaluated at a large, public, and metropolitan medical center without access to adjunctive skin perfusion assessment technologies. The majority of reconstructions were tissue expander based after therapeutic skin-sparing mastectomy with an average follow-up of nearly 12 months. The overall rate of ischemic complications, being partial- or full-thickness mastectomy flap or nipple-areola complex necrosis, was 35.6%. Importantly, the rate of reconstructive failure secondary to ischemic complications was 4.4%.
On univariate analysis of individual risk factors analyzed in this cohort, the only variable significantly associated with ischemic complications was the performance of a sentinel lymph node biopsy (P = 0.0453). This may be explained as a sentinel lymph node biopsy will inherently involve increased dissection during mastectomy, especially if performed through the mastectomy incision rather than a separate axillary incision. This increased dissection may result in increased retraction on the skin flaps or inadvertent iatrogenic injury to the skin flaps during biopsy, leading to mastectomy flap compromise. Notably, in both univariate analysis and subgroup analysis, outcomes and risk scores in implant- or autologous-based reconstruction were equivalent.
The average risk assessment score for all reconstructions was 4.29/8. A positive correlation between higher scores and greater incidence of ischemic complications was established, confirming the designed objective of the tool. Importantly, risk assessment scores greater than 5, greater than 6, and greater than 7 all were significantly associated with higher rates of ischemic complications. Likewise, scores greater than these values all trended toward significance on univariate analysis. These findings indicate that a score of 6 or higher represents a threshold value above which a reconstruction may be expected to have a significantly higher risk of ischemic complications. Scores of 6 or higher therefore should encourage the surgeon to mitigate risk of ischemic complications by excising compromised tissue or decreasing stress on the mastectomy flaps.
Upon further evaluation of this risk assessment tool, the sensitivity and specificity of intraoperative mastectomy flap compromise based on the tool and postoperative ischemia were 81.25% and 62.07%. Positive and negative predictive values were 54.17% and 85.71%. These findings indicate that this risk assessment tool is effective at ruling out mastectomy flap ischemia and less effective in ruling in ischemic events. Thus, a low score and lack of clinical mastectomy flap compromise should reassure the surgeon that there is low risk for postoperative ischemia. These characteristics are advantageous in such a tool as the risk of a false-negative result, being unexpected postoperative ischemia, is much greater than a false-positive result, being lack of postoperative ischemia in the face of predicted intraoperative compromise.
Overall, we have developed a simple tool to assess risk for mastectomy flap ischemia after immediate breast reconstruction while demonstrating its effectiveness in accomplishing its aim. A threshold value of 6 was also established, above which the surgeon is recommended to take action in mitigating risk of postoperative ischemia and potential reconstructive compromise. While exhibiting worthy specificity, this tool displays particular sensitivity and efficacy in ruling out postoperative ischemia in the absence of intraoperative mastectomy flap compromise. This tool supplements basic clinical judgment by providing the surgeon with objective and binary points to measure in determining a final score. Further, our tool is not reliant on any adjunctive perfusion assessment technologies, which may be cost-prohibitive in certain circumstances such as the public medical center in which this study was performed. Although individual factors in the tool were not examined but rather assessed as a whole to increase reliability, each individual factor can be addressed in a manner to increase chances of a successful reconstruction. For example, the need for methylene blue dye or its judicious application should be discussed with the ablative surgeon. Similarly, use of electrocautery should be minimized. This tool may encourage and facilitate these collaborative approaches between the extirpative and reconstructive breast teams to optimize outcomes. Prior studies have retrospectively developed a scoring system to assess mastectomy flap necrosis.32 However, ours is the first to prospectively evaluate a clinical, intraoperative tool to assess mastectomy flap ischemia.
Limitations of this study include its reliance on surgeon reporting of postoperative complications including the degree of postoperative mastectomy ischemia, if present. However, the prospective nature of this study ensures that patient identification and data collection were sufficiently accurate compared with retrospective study designs. Further, average follow-up was less than 1 year, at over 11 months. However, the primary outcomes evaluated are early complications that would be expected to occur well before the average follow-up period. Future directions will include refinement of the risk assessment tool and focus on comparison of outcomes in reconstructions assessed with and without this risk assessment tool to determine its broader efficacy in reducing ischemic risk via improved detection.
CONCLUSIONS
In conclusion, ischemic complications after immediate breast reconstruction were positively correlated with higher scores using the sensitive and effective clinical intraoperative mastectomy flap ischemia risk assessment tool established in this study. A threshold value of 6 or greater from a total possible score of 8 indicates significantly greater risk of postoperative mastectomy flap ischemia. This simple, easy-to-implement tool may assist plastic surgeons in assessing risk and optimizing outcomes in immediate postmastectomy breast reconstruction.
Supplementary Material
Footnotes
Published online 31 December 2019.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Khavanin N, Qiu C, Darrach H, et al. Intraoperative perfusion assessment in mastectomy skin flaps: how close are we to preventing complications? J Reconstr Microsurg. 2019;35:471–478. [DOI] [PubMed] [Google Scholar]
- 2.Andrade WN, Baxter N, Semple JL. Clinical determinants of patient satisfaction with breast reconstruction. Plast Reconstr Surg. 2001;107:46–54. [DOI] [PubMed] [Google Scholar]
- 3.Guyomard V, Leinster S, Wilkinson M. Systematic review of studies of patients’ satisfaction with breast reconstruction after mastectomy. Breast. 2007;16:547–567. [DOI] [PubMed] [Google Scholar]
- 4.Matsen CB, Mehrara B, Eaton A, et al. Skin flap necrosis after mastectomy with reconstruction: a prospective study. Ann Surg Oncol. 2016;23:257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salibian AA, Frey JD, Bekisz JM, et al. Ischemic complications after nipple-sparing mastectomy: predictors of reconstructive failure in implant-based reconstruction and implications for decision-making. Plast Reconstr Surg Glob Open. 2019;7:e2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mirhaidari S, Azouz V, Wagner DS. Routine laser-assisted indocyanine green angiography in immediate breast reconstruction: is it worth the cost? Plast Reconstr Surg Glob Open. 2019;7:e2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu EH, Zhu SL, Hu J, et al. Intraoperative SPY reduces post-mastectomy skin flap complications: A systematic review and meta-analysis. Plast Reconstr Surg Glob Open. 2019;7:e2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeon FHK, Varghese J, Griffin M, et al. Systematic review of methodologies used to assess mastectomy flap viability. BJS Open. 2018;2:175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munabi NC, Olorunnipa OB, Goltsman D, et al. The ability of intra-operative perfusion mapping with laser-assisted indocyanine green angiography to predict mastectomy flap necrosis in breast reconstruction: a prospective trial. J Plast Reconstr Aesthet Surg. 2014;67:449–455. [DOI] [PubMed] [Google Scholar]
- 10.Adam H, Bygdeson M, de Boniface J. The oncological safety of nipple-sparing mastectomy - a Swedish matched cohort study. Eur J Surg Oncol. 2014;40:1209–1215. [DOI] [PubMed] [Google Scholar]
- 11.Benediktsson KP, Perbeck L. Survival in breast cancer after nipple-sparing subcutaneous mastectomy and immediate reconstruction with implants: a prospective trial with 13 years median follow-up in 216 patients. Eur J Surg Oncol. 2008;34:143–148. [DOI] [PubMed] [Google Scholar]
- 12.Burdge EC, Yuen J, Hardee M, et al. Nipple skin-sparing mastectomy is feasible for advanced disease. Ann Surg Oncol. 2013;20:3294–3302. [DOI] [PubMed] [Google Scholar]
- 13.Coopey SB, Tang R, Lei L, et al. Increasing eligibility for nipple-sparing mastectomy. Ann Surg Oncol. 2013;20:3218–3222. [DOI] [PubMed] [Google Scholar]
- 14.De La Cruz L, Moody AM, Tappy EE, et al. Overall survival, disease-free survival, local recurrence, and nipple-areolar recurrence in the setting of nipple-sparing mastectomy: a meta-analysis and systematic review. Ann Surg Oncol. 2015;22:3241–3249. [DOI] [PubMed] [Google Scholar]
- 15.Howard MA, Sisco M, Yao K, et al. Patient satisfaction with nipple-sparing mastectomy: a prospective study of patient reported outcomes using the BREAST-Q. J Surg Oncol. 2016;114:416–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoon-Flannery K, DeStefano LM, De La Cruz LM, et al. Quality of life and sexual well-being after nipple sparing mastectomy: a matched comparison of patients using the breast Q. J Surg Oncol. 2018;118:238–242. [DOI] [PubMed] [Google Scholar]
- 17.Frey JD, Salibian AA, Choi M, et al. Mastectomy flap thickness and complications in nipple-sparing mastectomy: objective evaluation using magnetic resonance imaging. Plast Reconstr Surg Glob Open. 2017;5:e1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seitz IA, Nixon AT, Friedewald SM, et al. “Nacsomes”: a new classification system of the blood supply to the nipple areola complex (NAC) based on diagnostic breast MRI exams. J Plast Reconstr Aesthet Surg. 2015;68:792–799. [DOI] [PubMed] [Google Scholar]
- 19.Cunningham L. The anatomy of the arteries and veins of the breast. J Surg Oncol. 1977;9:71–85. [DOI] [PubMed] [Google Scholar]
- 20.van Deventer PV. The blood supply to the nipple-areola complex of the human mammary gland. Aesthetic Plast Surg. 2004;28:393–398. [DOI] [PubMed] [Google Scholar]
- 21.O’Dey Dm, Prescher A, Pallua N. Vascular reliability of nipple-areola complex-bearing pedicles: an anatomical microdissection study. Plast Reconstr Surg. 2007;119:1167–1177. [DOI] [PubMed] [Google Scholar]
- 22.Wagner RD, Braun TL, Zhu H, et al. A systematic review of complications in prepectoral breast reconstruction. J Plast Reconstr Aesthet Surg. 2019;72:1051–1059. [DOI] [PubMed] [Google Scholar]
- 23.Frey JD, Salibian AA, Karp NS, et al. The impact of mastectomy weight on reconstructive trends and outcomes in nipple-sparing mastectomy: progressively greater complications with larger breast size. Plast Reconstr Surg. 2018;141:795e–804e. [DOI] [PubMed] [Google Scholar]
- 24.Frey JD, Salibian AA, Levine JP, et al. Incision choices in nipple-sparing mastectomy: a comparative analysis of outcomes and evolution of a clinical algorithm. Plast Reconstr Surg. 2018;142:826e–835e. [DOI] [PubMed] [Google Scholar]
- 25.Frey JD, Salibian AA, Choi M, et al. The importance of tissue perfusion in reconstructive breast surgery. Plast Reconstr Surg. 2019;1441S Utilizing a Spectrum of Cohesive Implants in Aesthetic and Reconstructive Breast Surgery):21S–29S. [DOI] [PubMed] [Google Scholar]
- 26.Frey JD, Salibian AA, Choi M, et al. Optimizing outcomes in nipple-sparing mastectomy: mastectomy flap thickness is not one size fits all. Plast Reconstr Surg Glob Open. 2019;7:e2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reintgen C, Leavitt A, Pace E, et al. Risk factor analysis for mastectomy skin flap necrosis: implications for intraoperative vascular analysis. Ann Plast Surg. 2016;76suppl 4):S336–S339. [DOI] [PubMed] [Google Scholar]
- 28.Duggal CS, Madni T, Losken A. An outcome analysis of intraoperative angiography for postmastectomy breast reconstruction. Aesthet Surg J. 2014;34:61–65. [DOI] [PubMed] [Google Scholar]
- 29.Diep GK, Hui JY, Marmor S, et al. Postmastectomy reconstruction outcomes after intraoperative evaluation with indocyanine green angiography versus clinical assessment. Ann Surg Oncol. 2016;23:4080–4085. [DOI] [PubMed] [Google Scholar]
- 30.Agochukwu NB, Huang C, Zhao M, et al. A novel noncontact diffuse correlation spectroscopy device for assessing blood flow in mastectomy skin flaps: a prospective study in patients undergoing prosthesis-based reconstruction. Plast Reconstr Surg. 2017;140:26–31. [DOI] [PubMed] [Google Scholar]
- 31.Kovach SJ, Georgiade GS. The “banked” TRAM: a method to insure mastectomy skin-flap survival. Ann Plast Surg. 2006;57:366–369. [DOI] [PubMed] [Google Scholar]
- 32.Lemaine V, Hoskin TL, Farley DR, et al. Introducing the SKIN score: a validated scoring system to assess severity of mastectomy skin flap necrosis. Ann Surg Oncol. 2015;22:2925–2932. [DOI] [PubMed] [Google Scholar]


