Introduction:
Since the 1992 moratorium by the Food and Drug Administration (FDA), the debate on the association of breast implants with systemic illnesses has been ongoing. Breast implant-associated anaplastic large cell lymphoma has also raised significant safety concerns in recent years.
Methods:
A systematic search of the Manufacturer and User Facility Device Experience (MAUDE) database was performed to identify all cases of breast implant-associated deaths reported to the FDA.
Results:
The search identified 50 reported cases of apparent implant-related mortality; breast implant-associated anaplastic large cell lymphoma comprised the majority of fatal outcomes (n = 21, 42%), followed by lymphoma (n = 4, 8%), breast cancer (n = 3, 6%), pancreatic cancer (n = 2, 4%), implant rupture (n = 2, 4%), and postoperative infections (n = 2, 4%). Single cases (n = 1, 2% each) of leukemia, small bowel cancer, lung disease, pneumonia, autoimmune and joint disease, amyotrophic lateral sclerosis, liver failure, and sudden death, and 2 cases (4%) of newborn deaths, to mothers with breast implants, were also identified. A literature review demonstrated that 54% of alleged implant-related deaths were not truly associated with breast implant use: the majority of these reports (82%) originated from the public and third-party sources, rather than evidence-based reports by health-care professionals and journal articles.
Conclusions:
Although there exists a need for more comprehensive reporting in federal databases, the information available should be considered for a more complete understanding of implant-associated adverse outcomes. With only 46% of FDA-reported implant-related deaths demonstrated to be truly associated with breast implant use, there exists a need for public awareness and education on breast implant safety.
INTRODUCTION
Breast implants represent a multi-million dollar industry and a large proportion of aesthetic procedures presently performed by plastic surgeons worldwide.1 It is estimated that, each year, at least 1.5 million implants are used for aesthetic and reconstructive purposes.2 Modern-day implants are a product of constant evolution from the first generation developed by Dow Corning (Midland, Michigan, USA) in 1963, a process veiled with both controversy and safety concerns.1,3
In 1992, the Food and Drug Administration (FDA) issued a moratorium on silicone gel-based breast implants in response to culminating, well-publicized anecdotal reports linking implants to systemic diseases.4,5 Billions of dollars would be issued in lawsuit settlements in the coming years, fueled by public concerns that would continue to grow, and reinforced by the very issuing of the moratorium itself.5 This prompted scrutiny of the available evidence, refuting any association between breast implants and cancer, systemic diseases, immune dysregulation, connective tissue disorders, or other neurological dyscrasias.5–7 The moratorium lifting in 2006, conditional on large and longitudinal market studies by major producers, represented collaborative efforts between the FDA, industry, and medical professionals to respond to fathomable public concern in an evidence-based fashion.3,6,8
Several years later, however, in 2011, and in response to 34 reported cases of breast implant-associated anaplastic large cell lymphoma (BIA-ALCL), the FDA released a new safety communication.9,10 BIA-ALCL, a unique type of non-Hodgkin T-cell lymphoma, is associated with textured implants and commonly presents as a late peri-implant seroma, swelling, and tenderness of the affected breast.10–12 An update to the original safety communication regarding BIA-ALCL was issued by the FDA in 2016, reporting that a total of 258 cases had been submitted to their Manufacturer and User Facility Device Experience (MAUDE) database until that date.10 In 2019, a further update was issued reporting that, since 2010, the agency had received medical device reports on a total of 457 new cases, including 9 deaths.13 This reinstated concerns among the public and prompted further research to better understand the epidemiology and pathophysiology of BIA-ALCL, especially with regard to the safety of textured implants.2,10,14,15
The FDA requires all manufacturers to report on malfunctions or device-related adverse events to its MAUDE database; patients and health-care professionals may also author their own entries.2 This database thus represents a valuable resource for patients, consumers, and health-care professionals on medical device-related adverse outcomes.2,16 The primary aim of this study was to conduct a systematic search of the MAUDE database for all implant-related deaths reported to the FDA and assess the proportion attributable to adverse outcomes truly linked to breast implant use, as presently established in the literature.
METHODS
A systematic search of the MAUDE database for both silicone and saline breast implants (product class codes “Prosthesis, Breast, Inflatable, Internal, Saline-FWM AND Prosthesis, Breast, Non-Inflatable, Internal, Silicone Gel-Filled-FTR”) was performed to categorize the available entries pertaining to implant-related mortality. This was done using the search terms “dead” or “death” or “died” or “passed away.” In addition, all entries for which the “Event Type” was classified as either “death,” “NA” (not applicable), or “other” were included for review.
Two independent reviewers (JA and TS) evaluated the “Event Text” of retrieved entries for relevance and data usability. Duplicates were detected and excluded by cross-referencing of patient- and adverse outcome-related information, from both data categories and narrative texts, within the saline and silicone records independently. Duplicate records were largely a result of multiple submissions by different sources (health-care professionals, manufacturers, or patient relatives), or duplicate submissions pertaining to implant-related deaths that have been described previously, either in the literature or FDA reports. Data extracted comprised all aspects of patient, implant, and procedure information, and information pertaining to the initial presentation and latency of fatal outcomes. Treatment, treatment response, explantation, and implant testing information were also assessed, along with the official reported cause of death in each case. Entries were appraised for their comprehensiveness and sufficiency of information provided to the FDA.
To verify the alleged association of reported adverse outcomes with breast implant use, a literature search was subsequently conducted using the PubMed database by 2 independent reviewers (JA-R and TS). Fatal outcomes were classified into 2 categories: those demonstrated to be associated with breast implant use and those for which an evidence-based association with breast implants has not been established in the literature. Any and all discrepancies between reviewers were resolved through consensus or discussion with the senior author (TD).
RESULTS
A flowchart of the database search is presented in Figure 1. A total of 20,539 records were retrieved from the MAUDE database inclusive through November 2018, representing 4,448 entries relating to silicone implants (21.7%) and 16,091 records relating to saline implants (78.3%). The search strategy identified a total of 294 entries with potential relevance to breast implant-related mortality (1.4%). Following de-deduplication and full-text screening, 43 unique reports were included in the final synthesis, representing a total 50 implant-related deaths reported to the FDA. These included 33 cases associated with silicone implants, and 17 associated with saline (Fig. 1).
Fig. 1.
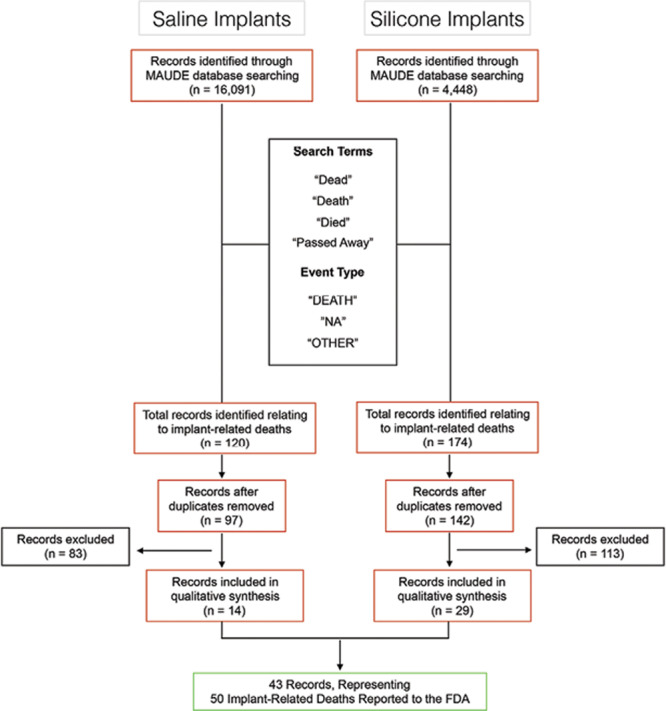
Search strategy of the FDA MAUDE database for entries pertaining to breast implant-associated mortality.
Implant Characteristics
Implant manufacturer was specified in 34 cases of death (68%); for all of which Allergan (Allergan Inc., Dublin, Ireland) was listed as the manufacturer (n = 34, 100%). Implant manufacturer was not specified for the remaining 16 cases (32%). Implant surface was specified for 6 silicone and 5 saline implants (n = 11, 22%); all cases were associated with BIA-ALCL, and a textured surface was listed for all (n = 11, 100%). Implant size was specified in only 1 case of a saline implant-related death (n = 1, 2%) and unspecified for the remaining 49 cases (Table 1).
Table 1.
Summary of Implant Characteristics, Identified from Entries Reporting on Breast Implant-associated Morality in the US MAUDE Database
| Silicone | Saline | Total | |
|---|---|---|---|
| Manufacturer | n | N | |
| Allergan | 22 | 12 | 34 (68%) |
| Style 410 | 6 | 0 | |
| Style 15 | 6 | 0 | |
| Style 20 | 5 | 0 | |
| Other | 4 | 4 | |
| Style not specified | 1 | 8 | |
| Manufacturer not specified | 11 | 5 | 16 (32%) |
| Implant surface | |||
| Textured | 6 | 5 | 11 (22%) |
| Smooth | 0 | 0 | 0 |
| Unknown | 27 | 12 | 39 (78%) |
| Total | 33(66%) | 17 (34%) | 50 |
Patient Information
Information pertaining to patient ages was provided for 7 implant-related deaths (14%). This comprised a mean of 49.7 years for 4 patients reported on by 1 entry, “early 60s” as the reported patient age in another entry, whereas 2 additional reports described the deaths of neonates to mothers with breast implants. A medical history before implantation was described in 6 cases (12%); this included breast cancer (n = 3, 6%), breast reconstruction following breast cancer (n = 2, 4%), and breast and endometrial cancer (n = 1, 2%). The indication for implantation was specified in 7 cases (14%); these included breast reconstruction (n = 4, 8%), breast augmentation (n = 2, 4%), or revision reconstruction (n = 1, 2%) (Table 2).
Table 2.
Summary of Patient Information, Where Specified, Identified from Entries Reporting on Breast Implant-associated Mortality in the US MAUDE Database
| Patient, Adverse Event, and Report Information | |||
|---|---|---|---|
| Yes | No | Not Specified | |
| Medical history | |||
| History of cancer: n = 6 (12%) | |||
| Not applicable; neonate: n = 2 (4%) | |||
| Not specified: n = 42 (84%) | |||
| Indication for implant | |||
| Reconstruction: n = 5 (10%) | |||
| Augmentation: n = 2 (4%) | |||
| Not specified: n = 43 (86%) | |||
| Latency till adverse event | |||
| 2 wk; sepsis: n = 1 (2%) | |||
| 1 y; Burkitt’s lymphoma: n = 1 (2%) | |||
| 2 y; breast cancer: n = 1 (2%) | |||
| Mean 7.35 y; BIA-ALCL: n = 4 (8%) | |||
| 10 y; BIA-ALCL: n = 1 (2%) | |||
| 11 y; BIA-ALCL: n = 1 (2%) | |||
| 16 y; breast cancer: n = 1 (2%) | |||
| Not specified: n = 40 (80%) | |||
| Autopsy results reviewed | n = 1 (2%) | n = 11 (22%) | n = 38 (76%) |
| Manufacturer narrative | n = 36 (84%) | n = 7 (16%) | – |
| Adequate reporting | n = 6 (14%) | n = 37 (86%) | – |
Latency until the time of adverse outcome, from initial implantation, is also presented. Information pertaining to autopsy results, manufacturer narratives, and the comprehensiveness of the information provided to the FDA is presented as well.
Adverse Outcomes
Information pertaining to the date of adverse outcomes leading to death was available in 35 out of 43 entries reviewed (81.4%); event dates ranged from 1996 to 2018 (Fig. 2). Following implantation, the latency to symptom onset of fatal adverse outcomes ranged from 2 weeks, in a case of sepsis, to 16 years, in a case of breast cancer. The median latency was 7.35 years in 4 fatal cases of BIA-ALCL (Table 2).
Fig. 2.
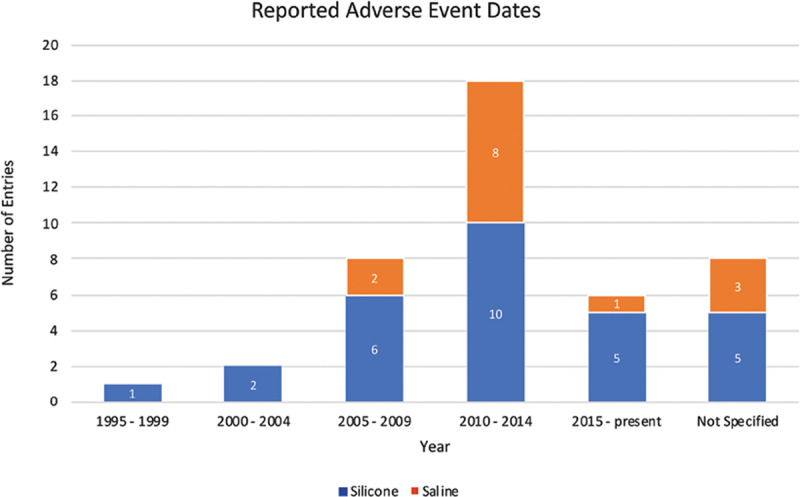
Adverse event dates. Timeline of fatal breast implant-associated adverse outcome dates, as identified from the FDA MAUDE database.
Mortality
A literature search was conducted to classify the reported fatal outcomes into 2 categories: those demonstrated in the literature to be associated with breast implant use and those for which an evidence-based association with breast implants has not been established (Fig. 3). Overall, of the 50 reported cases of implant-related deaths identified from the MAUDE database, only 23 deaths (46%) were shown to be truly associated with breast implant use, as currently established in the literature. The former group (n = 23, 46%) comprised 21 cases of BIA-ALCL (42%) and 2 cases of fatal postoperative infections (4%). Malignancies such as lymphoma (n = 4, 8%), breast cancer (n = 3, 6%), leukemia (n = 1, 2%), small bowel cancer (n = 1, 2%), and pancreatic cancer (n = 2, 4%) were classified into the latter group having no documented association. Additionally, single cases (n = 1 each, 2%) of sudden death, pneumonia, lung disease (in the setting of lupus and fibromyalgia), autoimmune and joint disease, amyotrophic lateral sclerosis, and liver failure were also identified. Finally, 2 cases of newborn deaths to mothers with breast implants (n = 2, 4%) and 2 cases of implant rupture (4%) represented the remaining cases of fatal outcomes for which an association with breast implants is not evidenced by the literature. The cause of death was unspecified in 5 cases (10%) (Table 3). Additional information pertaining to the initial presentation, treatment, and treatment response for the aforementioned adverse outcomes is presented in Table 4.
Fig. 3.
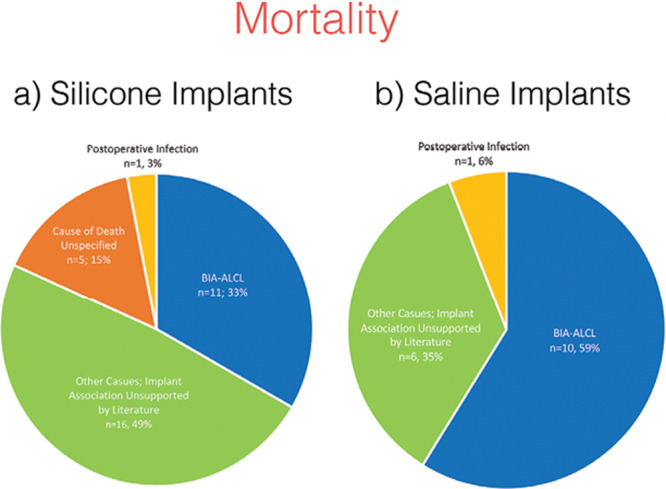
FDA-reported breast implant-associated mortality. Overview of the reported causes of mortality associated with (A) silicone and (B) saline breast implants; causes are classified according to whether an evidence-based association exists in the literature linking them to breast implant use.
Table 3.
Overview of the Reported Causes of Mortality Associated with Silicone and Saline Breast Implants; Causes Are Classified According to Whether an Evidence-based Association Exists in the Literature Linking Them to Breast Implant Use
| Fatal Outcomes | n | Source of Reports | |||
|---|---|---|---|---|---|
| Silicone | Saline | Total | Health-care Professionals and Journal Publications | Other* | |
| Implant association supported by the literature | 23 | 20 (87%) | 3 (13%) | ||
| BIA-ALCL | 11 | 10 | 21 | 20 | 1 |
| Postoperative infection leading to sepsis | 1 | 1 | 2 | – | 2 |
| Implant association unsupported by the literature | 22 | 4 (18%) | 18 (82%) | ||
| Malignancies | |||||
| Lymphomas of unspecified origin | 1 | 2 | 3 | – | 3 |
| Breast cancer | 1 | 1 | 2 | 1 | 1 |
| Burkitt’s lymphoma | – | 1 | 1 | – | 1 |
| Leukemia | 1 | – | 1 | – | 1 |
| Small bowel cancer | 1 | – | 1 | – | 1 |
| Pancreatic cancer | 1 | – | 1 | – | 1 |
| De novo pancreatic cancer in BIA-ALCL patient | 1 | – | 1 | 1 | – |
| Other | |||||
| Newborn death | 2 | – | 2 | – | 2 |
| Sudden death | 1 | – | 1 | – | 1 |
| Pneumonia | – | 1 | 1 | – | 1 |
| Pulmonary fibrosis | 1 | – | 1 | – | 1 |
| Lupus, fibromyalgia, and lung disease leading to respiratory failure | – | 1 | 1 | – | 1 |
| Autoimmune and joint disease | 1 | – | 1 | – | 1 |
| Amyotrophic lateral sclerosis | 1 | – | 1 | – | 1 |
| Liver failure | 1 | – | 1 | – | 1 |
| Implant rupture and capsular contracture | 1 | – | 1 | 1 | – |
| Implant rupture, silicone leak, systemic migration, and breast cancer | 1 | – | 1 | 1 | – |
| Implant rupture, suspected silicone leak, and infection | 1 | – | 1 | – | 1 |
| Cause of death unspecified | 5 | – | 5 | 2 (40%) | 3 (60%) |
Reporting sources provided.
*Other sources include patients, patient families, and friends, third-party clinical research organizations, “company representatives,” and media reports.
Table 4.
Summary of the Initial Presentation, Treatment, and Treatment Response of Fatal Adverse Events, Where Specified
| Adverse Event Presentation, Treatment, and Treatment Response, Where Specified | ||
|---|---|---|
| Adverse Event | Initial Presentation | Treatment and Treatment Response |
| BIA-ALCL (n = 21) | Mass ± metastatic disease (n = 8, 38%) Seroma/effusion (n = 6, 29%) Swelling/breast enlargement (n = 3, 14%) Lymphadenopathy (supraclavicular, axillary) (n = 3, 14%) Capsular contracture (n = 1, 5%) Cutaneous changes (n = 2, 10%) Not specified (n = 8, 38%) |
Surgery (implant removal ± capsulectomy) + Chemotherapy (n = 7, 33%) Unspecified response (n = 7, 100%) Surgery (implant removal ± capsulectomy) (n = 4, 19%) Unspecified response (n = 4, 100%) Chemotherapy (n = 3, 14%) Poor treatment response (n = 1, 33%) Unspecified response (n = 2, 67%) Surgery (implant removal ± capsulectomy) + Chemotherapy + mastectomy (n = 1, 5%)Poor response (n = 1, 100%) Not specified (n = 6, 29%) |
| Unspecified lymphoma (n = 3) | Shortness of breath, capsular contracture, breast pain, and swelling, lymphadenopathy (n = 1, 33%) Not specified (n = 2, 67%) |
Surgery (implant removal + chemotherapy (n = 1, 33%)Moderate response (complications) (n = 1, 100%) Not specified (n = 2, 67%) |
| Breast cancer (n = 2) | Severe diarrhea and dehydration (n = 1, 50%) Not specified (n = 1, 50%) |
Steroids for suspected sarcoidosis (n = 1, 50%) Poor response (n = 1, 100%) Radiation therapy (n = 1, 50%) Unspecified response (n = 1, 100%) |
| Sepsis; toxic shock syndrome (n = 1) | Discomfort, pain, swelling, and inflammation (n = 1, 100%) | Not specified (n = 1, 100%) |
| Rupture, suspected silicone leak, and systemic migration; invasive breast ductal carcinoma (n = 1) | Capsular contracture, pain, lymphadenopathy, dysesthesia, gait, bowel and cognitive dysfunction (n = 1, 100%) | Surgery (implant removal) (n = 1, 100%) Poor response (n = 1, 100%) |
| Infection (n = 1) | Not specified (n = 1, 100%) | Antibiotic therapy (n = 1, 100%) Poor response (n = 1, 100%) |
| Other reported adverse events (n = 21) | Not specified (n = 21, 100%) | Not specified (n = 21, 100%) |
Overall implant explanation: yes (n = 19, 38%), no (n = 8, 16%), not specified (n = 23, 46%).
Overall implant testing: yes (n = 2, 4%), no or not specified (n = 48, 96%).
Information on implant explantation before patient death and implant testing by manufacturers is presented as well.
The offending implants were explanted before death in 19 cases (38%) (Table 4). Autopsies were performed in 12 cases (24%), although information pertaining to the results was provided for only 1 patient. A narrative response by implant manufacturers to the identified reports was available for 36 out of 43 entries (84%), whereas only 6 entries were deemed to entail adequate reporting to the FDA (14%), as established through consensus among the authors following their review (Table 2).
Sources of Reports
Entries were authored by several sources; when classified according to total fatal outcomes (n = 50), the source of reports comprised journal articles (n = 15, 30%), patients, patient families and friends (n = 15, 30%), health-care professionals (n = 11, 22%), third-party clinical research organizations, and company representatives (n = 8, 16%), and media reports (n = 1, 2%) (Tables 3 and 5). When considering fatal outcomes which, through the authors’ literature search, were demonstrated to be truly associated with breast implant use (BIA-ALCL and postoperative infections, n = 23), 87% of cases were identified from reports of health-care professionals or journal articles, whereas only 13% were based on the reports of remaining sources; this difference was statistically significant (chi-square test, P < 0.001). In contrast, 82% of reports pertaining to fatal outcomes attributed to breast implants, but for which an evidence-based association has not been established in the literature, were provided by other sources discounting journal articles and health-care professionals; this association was also statistically significant (chi-square test, P < 0.001) (Fig. 4). A detailed presentation of reporting sources for each identified fatal outcome and a stratification based on outcomes with or without an evidence-based association to breast implants is presented in Tables 3 and 5, respectively.
Table 5.
Source of Reports for Entries Relating to Breast Implant-associated Mortality in the US MAUDE Database
| Source of Reports | Fatal Outcomes | ||
|---|---|---|---|
| Implant Association Established in the Literature | No Implant Association Established in the Literature | Not Specified | |
| Health-care professionals, n = 11 | 7 (64%) | 2 (18%) | 2 (18%) |
| Journal articles, n = 15 | 13 (87%) | 2 (13%) | – |
| Patients, n = 4 | – | 4 (100%) | – |
| Patient family and friends, n = 11 | 2 (18%) | 9 (82%) | – |
| Media, n = 1 | – | 1 (100%) | – |
| “Company representatives,” n = 1 | 1 (100%) | – | – |
| “Clinical research organization,” n = 7 | – | 4 (57%) | 3 (43%) |
| Total | |||
| Health-care professionals and journal articles, n = 26 | 20 (77%) | 4 (15%) | 2 (8%) |
| Other, n = 24 | 3 (13%) | 18 (75%) | 3 (12%) |
The proportion of reports, for which an evidence-based association with breast implant use either has or has not been established in the literature, relative to the total reports per given source, is also presented.
Fig. 4.
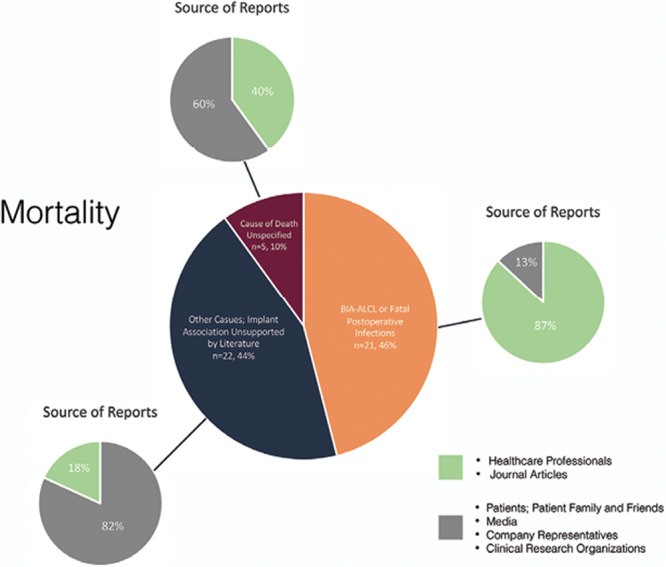
Mortality and reporting sources. Overall mortality associated with both silicone and saline breast implants; causes are classified according to whether an evidence-based association exists in the literature linking them to breast implant use. An overview of reporting sources for each category is presented independently. Entries reporting on outcomes unassociated with breast implant use originated predominantly from the public and third-party sources, whereas the majority of reporting on implant-associated adverse outcomes were based on reports from health-care professionals or journal articles.
DISCUSSION
The MAUDE database represents a useful resource for patients, consumers, and health-care professionals on both foreign and domestic adverse events related to medical devices sold in the United States.2,16 Data available in this database can give valuable device-related safety information and contribute to the risk–benefit considerations constantly deliberated by patients and health-care professionals, especially in the context of breast implants, their controversial history, and recent safety developments.2 In the present study, an analysis of the MAUDE database was performed to characterize entries pertaining to breast implant-related deaths. To the authors’ knowledge, this is the first study reporting on implant-associated mortality reported to the FDA. This study demonstrated that a total of 50 implant-related deaths have been submitted to the FDA through the MAUDE database, of which, only 23 (46%) were demonstrated to be truly associated with breast implant use based on available knowledge. The majority of these cases (87%) were based on the reports of health-care professionals and journal articles, whereas the majority of false reports (82%) originated from the public and third-party sources.
The association of breast implants with systemic diseases has been a subject of deliberation since the 1992 FDA moratorium on silicone breast implants.4,5 Despite a comprehensive review by the Institute of Medicine, which demonstrated that there existed no association between breast implants and systemic diseases, the notion of “breast-implant-illness,” nonetheless, persists today.4,6,7,17 Additional studies have further negated any association between breast implants and connective tissue disorders, immune dysregulation, cancer (including breast cancer), and neurological diseases.18–21 Other concerns pertaining to breast implant interference with the detection of breast cancer and its impact on survival exist today.22 There is no evidence however of any association between breast implants and adverse pregnancy or postpartum outcomes in newborns.23 The present study identified 22 such cases of alleged breast implant-related deaths, including the deaths of 2 newborns, and 5 additional cases for which the cause of death was not specified, but was attributed to breast implants and submitted to the MAUDE database, nonetheless. The majority of these reports (82%) originated from the public and third-party sources, whereas 87% of entries, for which an association with breast implant use is established, were based on the reports of health-care professionals or journal articles. These findings attest to the present state of concern and prevailing misconceptions among the public pertaining to the safety of breast implants and accentuates the need for further evidence-based endeavors for public education.
Two out of 23 cases of death identified in this study relating directly to breast implant use arose following postoperative sepsis. As with any other surgical procedure, postoperative infections are a well-established risk.24 Several risk factors are reported to be associated with surgical site infections in breast surgery.25–27 In contrast to breast augmentation procedures, implant-based reconstructive surgeries following mastectomy are associated with significantly higher rates of infection (1.1%–2.5% versus 1%–35%, respectively).25,27–29 This is largely due to an increased risk of implant and tissue exposure to endogenous flora during reconstructive procedures, and the greater association of risk factors with breast cancer patients, relative to healthy patients seeking breast augmentation.27,29 Although the overall risk of infections among breast augmentation and breast reconstructions remains lower than that of other surgical procedures,24,29 adherence to strict prophylactic and intraoperative aseptic techniques, and postoperative monitoring, diagnostic, and treatment protocols, is essential to ensure patient safety and avoid the progression to sepsis and death.30
BIA-ALCL has drawn significant attention within the medical literature in recent years due to its strict iatrogenic nature and categorical association with textured breast implants.11,31 Recent reports have also emerged describing a case of implant-associated anaplastic large cell lymphoma arising in a woman with textured gluteal implants.32 In 2006, the FDA released a safety communication reporting on an increased risk of BIA-ALCL in women with textured breast implants; insight into disease pathophysiology and precise relative risk estimations however have since remained elusive.9,10 In a recent report, Collett et al conducted the most comprehensive, global review of the literature yet to provide an evidence-based assessment of the incidence, risk, and prevalence of BIA-ALCL.31 The authors encountered several barriers in their appraisal of the available data, pertaining generally to inadequate insight on both the total number of BIA-ALCL cases worldwide (numerator), and the total number of textured breast implants in use (denominator).31 The factors responsible comprised poor and underreporting of adverse outcomes, inadequate data registries, lack of awareness, cosmetic tourism, and the fear of litigation.31 The authors report on a BIA-ALCL incidence of 1 out of 2,832 among patients with high-textured, high-surfaced implants, representing an exponential rise from postulated incidence rates over the past decade.12,31,33
Medical device malfunction databases may serve as an additional useful source of information pertaining to BIA-ALCL and the overall safety of breast implants. A review of 40 international government authority database entries pertaining to BIA-ALCL was performed by Srinivasa et al in 2017; the MAUDE database was among those reviewed.2 The purpose of the study was to assess the adequacy and utility of federal databases with the goal of gaining further insight into BIA-ALCL epidemiology, treatment, and associated outcomes.2 Srinivasa et al identified 363 unique cases of BIA-ALCL, 258 of which were identified from the MAUDE database (as of September 2015). The authors reported on a statistically significant association with textured implants (P < 0.0001), the efficacy of various treatment modalities with respect to their associated outcomes, and the documentation of only 5 BIA-ALCL-related deaths.2 However, following their analysis, the authors concluded that federal medical device registries presented with significant shortcomings pertaining to their ability to accurately capture and analyze implant-associated adverse outcomes. They discussed the need for creating a global medical device registry with tissue bank centralization (such as the presently adopted Patient Registry and Outcomes for Breast Implants and ALCL Etiology and Epidemiology14) and more detailed insight into country-specific total and textured implant sales information, for a more accurate assessment of BIA-ALCL incidence and prevalence.2
In the present study, the authors report on 21 BIA-ALCL-related deaths identified from the MAUDE database, inclusive through November 2018. The reported cases comprised 11 instances associated with silicone implants and 10 cases associated with saline. In a 2019 update on BIA-ALCL released by the FDA, 9 new patient deaths were identified since 2010.13 The data from the present study corroborate these findings, with 6 silicone BIA-ALCL-related deaths and 3 saline BIA-ALCL cases identified since 2010. In the most recent report detailing risk estimates of BIA-ALCL, a total of 17 BIA-ALCL-related deaths were reported following a comprehensive review of the literature by Collett et al.31 The present study however identified a total of 21 BIA-ALCL-related deaths: 13 cases from entries for which journal articles were listed as sources, and 8 additional cases submitted directly to the FDA, for which health-care professionals (n = 7) and “company representative” (n = 1) were listed as entry sources. This demonstrates the disparity that exists between information available on implant-associated adverse outcomes in literature and federal databases and accentuates the need to consider, document, and report on the available data within federal databases for a more comprehensive assessment of breast implant-associated adverse outcomes.
The use of the MAUDE database is not without its limitations; as cautioned by the FDA, the interpretation of information provided must only be done in the context of additional and distinct information given its author-dependent nature.2,34,35 Furthermore, the protection of some of the information under the Freedom of Information Act may render the interpretation of some aspects of the narrative text difficult, whereas a direct cause–effect relationship between reported adverse events and the medical device in question may not necessarily be established despite the availability of entries on the topic.35 The latter points were of notable relevance in the present study, with only 23 out of 50 deaths reported to be implant-related were shown to be truly associated to breast implant use. Finally, only 6 out of 43 entries reviewed were deemed to be adequate with respect to the comprehensiveness of information provided; this, especially in the context of rare implant-related adverse events such as BIA-ALCL and death, accentuates the need for more complete reporting by manufacturers and health-care professionals, whereas entries provided by the public may require some degree of vetting to ensure accuracy and avoid duplicates.2
CONCLUSIONS
Information gathered from federal databases is critical and should be considered for a more complete understanding of breast implant-associated adverse outcomes. A systematic assessment of the MAUDE database identified 50 cases of implant-related deaths reported to the FDA, of which only 23 arose following adverse events truly associated with breast implants. Concerns pertaining to the association of breast implants with systemic diseases thus persist today, necessitating evidence-based public awareness and education on breast implant safety.
Footnotes
Published online 26 December 2019.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.Chang EI, Hammond DC. Clinical results on innovation in breast implant design. Plast Reconstr Surg. 2018;1424S The Science of Breast Implants):31S. [DOI] [PubMed] [Google Scholar]
- 2.Srinivasa DR, Miranda RN, Kaura A, et al. Global adverse event reports of breast implant-associated ALCL: an international review of 40 government authority databases. Plast Reconstr Surg. 2017;139:1029–1039. [DOI] [PubMed] [Google Scholar]
- 3.Spear SL, Parikh PM, Goldstein JA. History of breast implants and the food and drug administration. Clin Plast Surg. 2009;36:15–21, v. [DOI] [PubMed] [Google Scholar]
- 4.Coroneos CJ, Selber JC, Offodile AC, II, et al. US FDA breast implant postapproval studies: long-term outcomes in 99,993 patients. Ann Surg. 2019;269:30–36. [DOI] [PubMed] [Google Scholar]
- 5.Cole NM. Consequences of the U.S. Food and drug administration-directed moratorium on silicone gel breast implants: 1992 to 2006. Plast Reconstr Surg. 2018;141:1137–1141. [DOI] [PubMed] [Google Scholar]
- 6.Colwell AS, Mehrara B. Editorial: US FDA breast implant postapproval studies-long-term outcomes in 99,993 patients. Ann Surg. 2019;269:39–40. [DOI] [PubMed] [Google Scholar]
- 7.Bondurant S, Ernster V, Herdman R; Institute of Medicine Committee on the Safety of Silicone Breast Implants. The National Academies Collection: Reports funded by National Institutes of Health. In: Safety of Silicone Breast Implants. 1999Washington, DC: National Academies Press (US) National Academy of Sciences. [Google Scholar]
- 8.FDA Update on the Safety of Silicone Gel-Filled Breast Implants. Center for Devices and Radiological Health U.S. Food and Drug Administration; 2011. https://www.fda.gov/media/80685/download. Accessed March 1, 2019 [Google Scholar]
- 9.Anaplastic large cell lymphoma (ALCL) in women with breast implants: Preliminary FDA findings and analyses. 2011. U.S. Food and Drug Administration; https://www.nvpc.nl/uploads/stand/NVPC110126DOC-FN-ASPS_Final_ALCL_White_Paper_Clean_Version_1-18-1177.pdf. Accessed March 1, 2019. [Google Scholar]
- 10.Doren EL, Miranda RN, Selber JC, et al. U.S. Epidemiology of breast implant-associated anaplastic large cell lymphoma. Plast Reconstr Surg. 2017;139:1042–1050. [DOI] [PubMed] [Google Scholar]
- 11.Brody GS, Deapen D, Taylor CR, et al. Anaplastic large cell lymphoma occurring in women with breast implants: analysis of 173 cases. Plast Reconstr Surg. 2015;135:695–705. [DOI] [PubMed] [Google Scholar]
- 12.Clemens MW, Miranda RN. Coming of age: breast implant-associated anaplastic large cell lymphoma after 18 years of investigation. Clin Plast Surg. 2015;42:605–613. [DOI] [PubMed] [Google Scholar]
- 13.Statement from Binita Ashar, M.D., of the FDA’s Center for Devices and Radiological Health on agency’s continuing efforts to educate patients on known risk of lymphoma from breast implants [press release]. 2016. Food and Drug Administration; https://www.fda.gov/news-events/press-announcements/statement-binita-ashar-md-fdas-center-devices-and-radiological-health-agencys-continuing-efforts. Accessed March 1, 2019. [Google Scholar]
- 14.McCarthy CM, Loyo-Berrios N, Qureshi AA, et al. Patient Registry and Outcomes for Breast Implants and Anaplastic Large Cell Lymphoma Etiology and Epidemiology (PROFILE): Initial Report of Findings, 2012-2018. Plast Reconstr Surg. 2019;1433S A Review of Breast Implant-Associated Anaplastic Large Cell Lymphoma65s–73s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gidengil CA, Predmore Z, Mattke S, et al. Breast implant-associated anaplastic large cell lymphoma: a systematic review. Plast Reconstr Surg. 2015;135:713–720. [DOI] [PubMed] [Google Scholar]
- 16.Brown SL, Todd JF, Luu HM. Breast implant adverse events during mammography: reports to the food and drug administration. J Womens Health (Larchmt). 2004;13:371–378; discussion 379. [DOI] [PubMed] [Google Scholar]
- 17.Magnusson MR, Cooter RD, Rakhorst H, McGuire PA, Adams WP, Jr, Deva AK. Breast implant illness: a way forward. Plast Reconstr Surg. 2019;1433S A Review of Breast Implant-Associated Anaplastic Large Cell Lymphoma):74s–81s. [DOI] [PubMed] [Google Scholar]
- 18.Janowsky EC, Kupper LL, Hulka BS. Meta-analyses of the relation between silicone breast implants and the risk of connective-tissue diseases. N Engl J Med. 2000;342:781–790. [DOI] [PubMed] [Google Scholar]
- 19.Brinton LA, Lubin JH, Burich MC, et al. Breast cancer following augmentation mammoplasty (United States). Cancer Causes Control. 2000;11:819–827. [DOI] [PubMed] [Google Scholar]
- 20.Singh N, Picha GJ, Hardas B, et al. Five-year safety data for more than 55,000 subjects following breast implantation: comparison of rare adverse event rates with silicone implants versus national norms and saline implants. Plast Reconstr Surg. 2017;140:666–679. [DOI] [PubMed] [Google Scholar]
- 21.Balk EM, Earley A, Avendano EA, et al. Long-term health outcomes in women with silicone gel breast implants: a systematic review. Ann Intern Med. 2016;164:164–175. [DOI] [PubMed] [Google Scholar]
- 22.Lavigne E, Holowaty EJ, Pan SY, et al. Breast cancer detection and survival among women with cosmetic breast implants: systematic review and meta-analysis of observational studies. BMJ. 2013;346:f2399. [DOI] [PubMed] [Google Scholar]
- 23.Kjøller K, Friis S, Lipworth L, et al. Adverse health outcomes in offspring of mothers with cosmetic breast implants: a review. Plast Reconstr Surg. 2007;1207 suppl 1):129S–134S. [DOI] [PubMed] [Google Scholar]
- 24.Korol E, Johnston K, Waser N, et al. A systematic review of risk factors associated with surgical site infections among surgical patients. PLoS One. 2013;8:e83743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Washer LL, Gutowski K. Breast implant infections. Infect Dis Clin North Am. 2012;26:111–125. [DOI] [PubMed] [Google Scholar]
- 26.Olsen MA, Lefta M, Dietz JR, et al. Risk factors for surgical site infection after major breast operation. J Am Coll Surg. 2008;207:326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sinha I, Pusic AL, Wilkins EG, et al. Late surgical-site infection in immediate implant-based breast reconstruction. Plast Reconstr Surg. 2017;139:20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saha D, Davila AA, Ver Halen JP, et al. Post-mastectomy reconstruction: a risk-stratified comparative analysis of outcomes. Breast. 2013;22:1072–1080. [DOI] [PubMed] [Google Scholar]
- 29.Pittet B, Montandon D, Pittet D. Infection in breast implants. Lancet Infect Dis. 2005;5:94–106. [DOI] [PubMed] [Google Scholar]
- 30.Phillips BT, Bishawi M, Dagum AB, et al. A systematic review of antibiotic use and infection in breast reconstruction: what is the evidence? Plast Reconstr Surg. 2013;131:1–13. [DOI] [PubMed] [Google Scholar]
- 31.Collett DJ, Rakhorst H, Lennox P, et al. Current risk estimate of breast implant-associated anaplastic large cell lymphoma in textured breast implants. Plast Reconstr Surg. 2019;1433S A Review of Breast Implant-Associated Anaplastic Large Cell Lymphoma):30S–40S. [DOI] [PubMed] [Google Scholar]
- 32.Shauly O, Gould DJ, Siddiqi I, Patel KM, Carey J. The first reported case of gluteal implant-associated Anaplastic Large Cell Lymphoma (ALCL). Aesthet Surg J. 2019;39:NP253–NP258. [DOI] [PubMed] [Google Scholar]
- 33.Magnusson M, Beath K, Cooter R, et al. The epidemiology of breast implant-associated anaplastic large cell lymphoma in Australia and New Zealand confirms the highest risk for grade 4 surface breast implants. Plast Reconstr Surg. 2019;143:1285–1292. [DOI] [PubMed] [Google Scholar]
- 34.Administration USFD. MAUDE - Manufacturer and User Facility Device Experience. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/search.cfm. Accessed March 20, 2019.
- 35.Edwards MC. Comments on “long-term safety of textured and smooth breast implants” and a plea to abandon the use of the MAUDE database. Aesthet Surg J. 2018;38:NP64–NP65. [DOI] [PubMed] [Google Scholar]


