Supplemental Digital Content is available in the text.
Background:
Soft tissue free flap reconstruction of upper extremities has proven to be reliable and essential for limb salvage and function. Nevertheless, comparative data regarding flap outcome are still lacking. The present study aimed to compare procedural features and individual complication rates of different free flaps used for upper extremity reconstruction.
Methods:
The authors evaluated retrospectively the results of 164 free flaps in 149 patients with upper extremity defects. Chart reviews were performed from April 2000 to June 2014, analyzing flap choices, complication, and success rate assessment for patients >18 years old, with a soft tissue defect of the upper extremity. Chosen flap types were classified as fasciocutaneous (including adipocutaneous) and muscle-based, respectively. We comparatively analyzed total flap loss, flap survival after microsurgical revisions, and susceptibility rates for thromboses rates and partial flap necrosis.
Results:
Defect size was larger when muscle-based flaps were used (231 ± 38.6 versus 164 ± 13.7 cm2, P < 0.05). Outcome analysis revealed a tendency towards higher arterial thrombosis rates for muscle flaps (10.2% versus 4.3%) and venous thrombosis rates for fasciocutaneous flaps (2% versus 7%). Total flap loss (6.1% versus 7.8%) and flap survival after vascular revisions (75% versus 70.6%) showed comparable rates. Partial flap necrosis was generally higher in muscle-based flaps (22.4% versus 8.6%, P = 0.02) with impact on patients’ hospital stay (37.2 ± 4.69 and 27.11 ± 1.62 days, n = 115, P = 0.01), while no differences in partial necrosis rates were noted in flaps larger than 300 cm2 (25% versus 10%, P = 0.55). There was a trend over time towards using fasciocutaneous-based flaps more frequently with a final overall percentage of 83.7% between 2012 and 2014.
Conclusions:
Microsurgical tissue transfer to the upper extremity is safe and reliable, but flap-type specific procedural and measures should be taken into consideration. Total flap loss as well as flap survival after microsurgical revisions are not altered between these flaps. They differ, however, in their susceptibilities for thromboses rates, partial flap necrosis and thus require individual risk stratification and flap placement.
INTRODUCTION
Continuous optimizations in microsurgical techniques and equipment have facilitated the reconstruction of soft tissue defects of the upper extremity using microsurgical free flaps as a standard procedure.1,2 Due to the growing microsurgical expertise and safety as well as the versatility and variability of currently available free flap types and components eligible for upper extremity reconstruction, the amputation rate was largely reduced, while the overall functional outcome of the hand or arm was improved.3–6 Perioperative complications and functional recovery can differ widely in the clinical context and still pose a great challenge for the reconstructive microsurgeon.3,7–9 An individual risk stratification of the patient, a careful evaluation of free flaps types and knowledge of their specific characteristics, and individual complication rates are of paramount importance, when procedural features and the process of decision making are compared.1,10–12 Soft tissue free flap reconstruction of upper extremities has proven to be safe and reliable, while functional outcome in correlation to microsurgical outcome is currently underreported, as a recent meta-analysis of our group comparing 23 relevant studies with a mean of 20 patients (range: 10–79) has shown (Zhang et al., under review). Available studies suffer from limitations such as irregular inclusion criteria, small cohort sizes, and variable endpoints. The purpose of this article is to contribute to the preexisting literature with a large monocentric study and to provide principles for decision-making and risk stratification in microvascular upper extremity reconstruction, particularly in regard to the complications rates seen in various types of free flaps and their compounds being currently used for coverage.
METHODS
The present, retrospective study was designed in accordance to the Declaration of Helsinki and approved by the local ethics committee (Mainz, Germany). All medical records of patients who received a reconstruction of the upper extremities by means of free tissue transfer from April 2000 to June 2014 in our center were identified and recorded into a database. Approval by the local ethics committee was limited to data till 2014. Further, patient selection was then performed according to the following inclusion criteria: (a) soft tissue defect of the upper extremity, (b) age of patient ≥18 years, (c) microvascular transfer of a free autologous soft tissue flap as surgical technique, and (d) complete medical records. Conjoined flaps and composite osteocutaneous flaps, were excluded to increase the homogeneity of this study. Between April 2000 and June 2014, 164 free flaps were performed in 149 patients >18 years of age and eligible for the present study. The defect localization spanned from shoulder, upper arm, elbow, forearm to the hand. Each patient chart was reviewed for age, gender, type of injury, indication for free tissue transfer, flap choices, perioperative complications, flap outcome, and overall hospital stay. The article was not intended as a clinical follow-up study and focused solely on perioperative complications. A total of 9 different flap types were used and subdivided respectively as fasciocutaneous or muscle-based. We primarily opted for fasciocutaneous flaps for defect reconstruction, although sometimes forced to favor muscle-based flaps due to defect size.
Depending on comorbidities of the individual patient, perioperative anticoagulation was accomplished by semitherapeutic administration of low molecular weight heparin or by constant heparin perfusion. The remaining parameters of the perioperative protocol including the surgical techniques were the same. Patients with increased likelihoods of procedural-related comorbidities and patients with advanced age were postoperatively admitted to the intensive care unit for close follow-up and flap monitoring.
Data are presented with N (number), mean, standard error of the mean, or percentages and analyzed by Fisher’s exact test. All statistical analyses were 2-sided and P < 0.05 was considered significant. Statistical analyses were performed using GraphPad Prism (GraphPad Software Inc, San Diego, CA).
RESULTS
Patient Profiles and Hospital Stay
One hundred sixty-four flaps free were performed in 149 patients, which included 42 females and 107 males. The average age of the patients was 46.2 years (mean 46.2 years, range 7–88 years). The cause of the defect and injury was classified as trauma-, infection-, burn-, scar-, or tumor-related. Injuries and defects classified as others resulted from meningococcal septicemia or extravasation of cytostatic agents. From all the 164 free flaps included in our study, the majority was applied in a traumatized upper extremity followed by infection, tumor, and burn-related injuries (Table 1). The overall hospital stay in patients who received muscle-based free flap defect coverage was significantly longer when compared with fasciocutaneous flaps (37.2 ± 4.69 days, n = 49, range 8–191 days and 27.11 ± 1.62 days, n = 115, range 6–81 days, respectively, P = 0.01).
Table 1.
Patient Characteristics, Cause of the Injury and Defect, and Number of Free Flaps Applied
| Study Population | n (%) |
|---|---|
| Total no. patients | 149 |
| No. female patients | 42 (28.2) |
| No. male patients | 107 (71.8) |
| Mean age, years (range) | 46.2 (7–88) |
| No. of free flaps | 164 |
| Cause of defect | |
| Trauma | 57 (34.8) |
| Infection | 36 (22) |
| Acute burn | 15 (9.1) |
| Secondary scars | 11 (6.7) |
| Tumor | 16 (9.8) |
| Others | 14 (8.6) |
Types of Free Flaps and Trend Over Time
Nine different types of flaps were used (Table 2). The most frequently applied type of free flap was the Anterolateral Thigh (ALT) Flap (ALT, n = 66, corresponding to 40.2%) followed by the Latissimus Dorsi (LD, n = 33, corresponding to 20.1%) and Parascapular flap (PF, n = 28, corresponding to 17.1%). Each free flap was classified as fasciocutaneous or muscle-based, in regard to its anatomical characteristics (Table 2). Generally, for defect reconstruction fasciocutaneous flaps were used predominantly in 115 cases, corresponding to 70.1% of the flaps being used. Muscle-based flaps were used in 49 patients, corresponding to 29.9%. There was a complete ratio reversal of flaps being used when analyzing flap choices over 2-year periods as shown in Figure 1. As the overall number of flaps per time interval from 2000 to 2014 increased (ranging from 11 to 49 for a 2-year period), there was a drastic increase of fasciocutaneous flaps in relationship to muscle-based flaps that were performed (up to 83.7% from 2012 till 2014) [See figure, Supplemental Digital Content 1, which displays a 80-year-old male patient who suffered from wrist empyema (A) with a consecutive dorsal soft tissue defect of the hand with exposed extensor tendons (B). Defect coverage of the exposed tendons and hand was achieved by using an ALT flap, ALT (C and D), http://links.lww.com/PRSGO/B253] [See figure, Supplemental Digital Content 2, which displays a 61-year-old male patient who suffered from a machine crush injury of his forearm, resulting in a subtotal amputation with a comprehensive soft tissue defect as well as ulna bone and radius fractures and segmental injuries of the vessels and tendons. After primary stabilizing operations with osteosyntheses of the fractures and reconstruction of the vessels and tendons (A–C), defect coverage was performed by using an LD flap in combination with split-thickness skin grafts (D). Appearance of the reconstructed forearm at 3 months’ follow-up (E and F), http://links.lww.com/PRSGO/B254].
Table 2.
Type of Free Flaps Used in All Patients and in Regard to the Underlying Cause of the Defect (Data in Total n and Percentage)
| Type of Free Flap | |||||||
|---|---|---|---|---|---|---|---|
| All (n = 164) | Trauma (n = 57) | Infection (n = 36) | Burn (n = 15) | Scar (n = 11) | Tumor (n = 16) | Others (n = 14) | |
| Muscle flaps (n = 49) | 49 (29.9) | ||||||
| Rectus abdominis | 1 (0.6) | 0 | 0 | 0 | 0 | 0 | 1 |
| LD | 33 (20.1) | 14 (8.5) | 4 (2.4) | 9 (5.4) | 2 (1.2) | 3 (1.8) | 1 (0.6) |
| Serratus | 13 (7.9) | 3 (1.8) | 2 (1.2) | 5 (3) | 1 (0.6) | 0 | 2 (1.2) |
| Gracilis | 2 (1.2) | 0 | 0 | 1 (0.6) | 0 | 0 | 1 (0.6) |
| Adipocutaneous flaps (n = 115) | 115 (70.1) | ||||||
| ALT | 66 (40.2) | 23 (14) | 18 (11) | 8 (4.9) | 5 (3) | 7 (4.3) | 5 (3) |
| Radial forearm | 2 (1.2) | 0 | 0 | 0 | 0 | 1 (0.6) | 1 (0.6) |
| Lateral arm | 17 (10.4) | 7 (4.3) | 5 (3) | 2 (1.2) | 1 (0.6) | 0 | 2 (1.2) |
| PF | 28 (17.1) | 9 (5.5) | 6 (3.7) | 5 (3) | 2 (1.2) | 5 (3) | 1 (0.6) |
| Medial sural artery perforator | 2 | 1 (0.6) | 1 (0.6) | 0 | 0 | 0 | 0 |
Fig. 1.
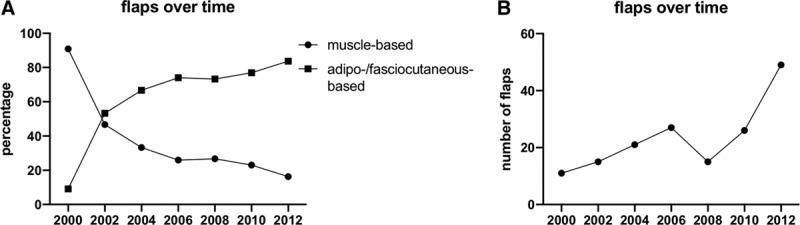
Trend over time. From 2000 to 2014, there was a drastic increase of fasciocutaneous-based flaps being performed, while muscle-based flaps correspondingly decreased (A). Simultaneously and in conjunction with this development, number of flaps increased (B). Number of flaps were calculated for 2 years, respectively.
Defect Localization and Defect Size
In our series of upper extremity reconstruction, free flaps were performed mainly to the hand (46.3%), forearm (23.2%), and elbow (17.7%) and only to a lesser degree to the upper arm and shoulder (1.8% and 2.4%, respectively). The number of fasciocutaneous and muscle-based flaps used for each defect localization is visualized in Figure 2. In general, mirroring the ratio between the 2 flap types used (115 fasciocutaneous and 49 muscle-based flaps, ratio of 2.35), fasciocutaneous flaps were more often used for defect coverage of the hand and forearm than muscle-based flaps (32.9% and 13.4%, ratio 2.46, as well as 18.9% and 4.3%, ratio 4.4 for hand and forearm defects, respectively). At the level of the elbow, 7.9% of the flaps being used were muscle-based, while 9.8% were fasciocutaneous flaps (ratio of 1.24). In 40 cases, the flaps being performed were combined with simultaneous osteosyntheses (24.4%). Nine of those were muscle-based flaps (18.4%), while 31 fasciocutaneous flaps were combined with a simultaneous osteosynthesis (27%).
Fig. 2.
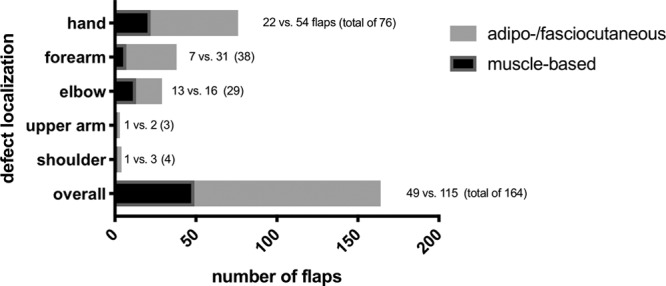
Defect localization of free flap reconstruction in the upper extremity. A subgroup analysis is made between reconstruction with muscle-based or fasciocutaneous flaps.
Defect sites were significantly larger in muscle-based flaps compared with defect sizes of fasciocutaneous flaps with a mean size of 231 ± 38.6 versus 164 ± 13.7 cm2 (P < 0.05).
Complications Analysis
A total number of 91 complications were observed, with an overall minor complication rate of 37% (n = 60) and overall major complication rate of 19% (n = 31). Major complications included total flap loss due to vascular complications and arterial and/or venous thromboses. A total number of 10 flaps (6.3%) were identified with an arterial thrombosis during postoperative monitoring, while 9 showed a venous thrombosis (5.6%). All patients with compromised flap perfusion were immediately taken to the operating room. Of all the patients included in this study, 12 (7.5%) showed complete flap loss due to arterial and or venous thrombosis. In 21 patients (13.1%), a partial flap necrosis occurred. Partial flap necrosis rate was significantly higher in muscle-based flaps with 22.4% (n = 11) when compared with fasciocutaneous-based flaps (8.6%, n = 10, P = 0.02). In a subsequent subgroup analysis with flaps >300 cm2, fasciocutaneous and muscle-based flaps showed no significant differences in partial flap necrosis rates. A total of 18 flaps were included in this subgroup analysis and 2 out of 8 (25%) muscle-based flaps showed partial flap necrosis, while 1 out of 10 fasciocutaneous flaps (10%) was affected (P = 0.55). Postoperative hematoma formation occurred in 14 patients corresponding to 8.8%. Hematoma formation did not affect the vascular supply or the perfusion of the flaps. Wound infection and wound dehiscence resulting in an additional revision were observed in 6 and 19 patients (3.8% and 11.9%), respectively. Hematoma formation, wound infection as well as dehiscence were classified as minor perioperative complications. A complete analysis of compromised flap perfusion due to thrombosis of the pedicle and a differentiation of fasciocutaneous-based versus muscle-based flaps is shown in Table 3 and Figure 3. In brief, outcome analysis revealed a tendency towards a higher rate of arterial thrombosis in muscle-based flaps (10.2% and 4.3%, respectively, P = 0.167) while fasciocutaneous flaps were more prone for venous thrombosis (2% and 7%, P = 0.28); however, these results were not statistically significant.
Table 3.
Complications of Total and Muscle-Based or Adipo-/Fasciocutaneous Free Flap Reconstruction in the Upper Extremity (n and %)
| No. Complications and Complication Rate | |||
|---|---|---|---|
| Total (%) | Muscle-based (%) | Adipo-/Fasciocutaneous-based | |
| Perioperative major complication | |||
| Arterial thrombosis | 10 (6.3) | 5 (10.2) | 5 (4.3) |
| Venous thrombosis | 9 (5.6) | 1 (2) | 8 (7) |
| Total flap loss | 12 (7.5) | 3 (6.1) | 9 (7.8) |
| Partial flap necrosis | 20 (12.5) | 11 (22.4) | 9 (7.8) |
| Perioperative minor complication | |||
| Hematoma | 14 (8.8) | 1 (2) | 13 (11.3) |
| Wound infection | 6 (3.8) | 4 (8.2) | 2 (1.7) |
| Dehiscence | 19 (11.9) | 4 (8.2) | 15 (13) |
Fig. 3.
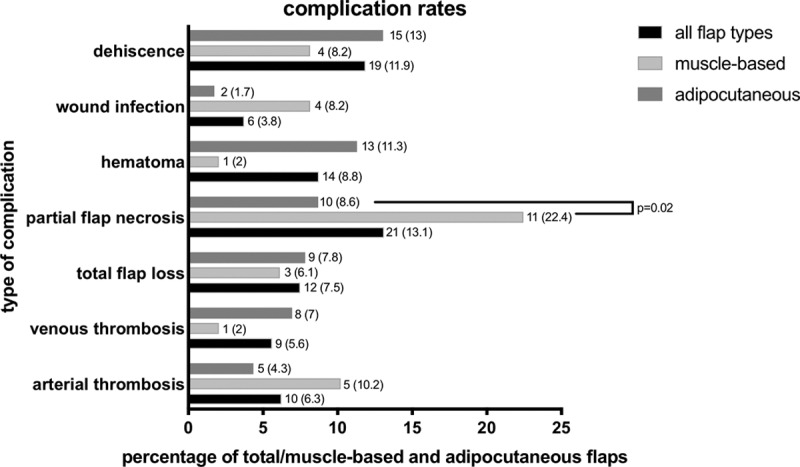
Visualization of complication rates of all flap types vs muscle-based vs fasciocutaneous free flap reconstruction in the upper extremity. The according data, total and flap-specific numbers as well as percentages are simultaneously presented in Table 3. Data are given as n (%).
Flap loss occurred with comparable rates in 6.1% of muscle-based flaps and 7.8% of fasciocutaneous-based flaps (P = 0.99). A total of 21 out of 164 flaps (12.8%) required secondary operations due to vascular complications (insufficiency of arterial and venous blood flow). In relationship to the type of free flap being applied, no significant differences in rates of vascular revisions were noticed when fasciocutaneous flaps were compared with muscle-based flaps (8.1 and 14.8%, respectively, P = 0.31). No significant differences in flap success rates after a successful microsurgical revision were noticed between these 2 groups (71.4% of the all revisions survived, 75%, n = 3 and 70.6%, n = 12 for muscle-based and fasciocutaneous-based flaps, respectively, P = 0.86).
DISCUSSION
Soft tissue reconstruction of the upper limb is challenging, and reconstructive surgeons aim to cover complex defects in conjunction with an optimal functional outcome.13–15 Hand and wrist defects are especially critical as these regions are seen as functional as well as aesthetical entities, and inconspicuous reconstruction may simultaneously impact the patients psychosocial and socioeconomical well-being. A recent meta-analysis based on 23 original studies published in the last 20 years included 283 flaps in 279 patients and increased the level of evidence on safety and reliability of free soft tissue transfer to the upper extremity by pooling cohorts (Zhang et al, unpublished data). However, original studies suffered from various limitations, including small sample cohorts (1079 patients), inconsistency of endpoints and variable inclusion criteria. In addition, discussion of reconstructive strategies, procedural features, decision-making, and assessment of individual complication rates were remarkably rare and underreported. We aimed to contribute to the preexisting literature by analyzing an upper extremity reconstruction cohort, solely from our center.16,17 To the best of our knowledge, the presented data constitutes the largest single-center series and outcome comparison of different types of free flaps used to reconstruct soft tissue defects of the upper extremity.
For upper extremity reconstruction, we predominantly applied fasciocutaneous free flaps, which are more convenient and safer, considering a subsequent secondary flap elevation in the case of implant removal and/or combined secondary procedures like a teno-, arthro and neurolysis to improve range of motion and functional outcome. In addition, the fascial layer is believed to provide a better and more sufficient gliding plane for early exercises, although it has not yet been sufficiently proven. In contrast, muscle-based free flaps are often applied in supersized or multifocal defects.16–19 Muscle-based flaps are straight-forward to harvest and routinely used as residency training procedures.19–21 Muscle-based free flaps, especially the LD, are predominantly indicated when donor sites of fasciocutaneous free flaps are limited, too bulky, or would result in an imbalanced donor site morbidity. In our cohort, the LD was the most commonly used muscle-based free flap. Although it is not shown in literature, defect size may contribute to the specific complication rates of muscle-based flaps as shown in our data. Defect size and reconstruction by muscle-based free flaps increases the duration of hospital stay based on the presented data. Outcome analysis revealed a significantly higher rate of partial necrosis in muscle-based flaps when compared with fasciocutaneous flaps. These findings, however, seem to be unrelated to defect size as shown in our subgroup analysis. The rate of venous and arterial thromboses, as well as flap loss or success rate after vascular revision of the anastomoses was not significantly altered. As we could demonstrate, the rate of total flap loss after defect reconstruction of the upper extremity was 7.5% and comparable to existing data for reconstruction of the lower extremity.10 Microsurgical reconstruction of the upper limb may, however, be more challenging than lower extremity reconstruction, as partial flap necrosis is significantly higher compared with the available data for lower limb reconstruction suggesting a partial flap necrosis rate of 6%.10,13 Based on these findings, we advocate more and more and especially in large, articular regions and complex soft tissue defects to use composite reconstruction strategies and/or early evaluation and usage of AV loops, to reduce the risk of partial flap necrosis due to tension during flap fixation and in regions of the post-capillary “last meadow.”
Flow rates are generally considered higher in muscle-based flaps compared with fasciocutaneous flaps, and these physiological flap characteristics might ultimately impact thrombosis and complication rates, as shown in our analysis.22 Muscle-based flaps are more sensitive for temporary ischemia and tension at the post-capillary “last meadow,” which may result in a higher risk and higher rates for partial necrosis. An important issue for the management of muscle-based flaps and the prevention of partial necrosis, apart from pedicle-related malposition and thus potential malperfusion, is the sufficient trimming of the flap and its correlation to flap design, to overcome the temptation to involve zonal parts of the flap distant of the pedicle for defect coverage. A helpful strategy to overcome this temptation is to raise a small PF in conjunction with a LD flap as a composite flap and to use the PF part for coverage of the LD and main pedicle, which significantly reduces tension and facilitates flap placements and arc of rotation. The rise of microsurgical expertise led to the clinical establishment of modern perforator-based free flaps as standard procedures.21 Over the last 10 years, our department experienced a radical transition from using muscle-based flap choices initially to favoring fasciocutaneous flaps. Our data suggest that microsurgical reconstruction of defects in the upper limb with fasciocutaneous flaps may offer distinct advantages and may prove superior in regard to partial flap necrosis rates when compared with musculocutaneous flaps. With improved and simplified intraoperative tools to monitor fasciocutaneous flaps, as for example near-infrared indocyanine green video angiography (ICG-NIR-VA), these flaps and techniques offer distinct advantages over muscle-based free flaps in regards to surgical decisions, trimming, placement, and designing of the flap.22 This may prove essential when adequate and safe positioning of the flap is necessary, as in the case of a combined procedure with osteosynthesis and subsequent implant coverage or when functional structures are exposed. It was generally believed that muscle-based flaps provide better perfusion, which may reduce the risk of wound infection due to improved vascularity.23 Regarding minor complications, a higher rate of hematoma formation and wound dehiscence in total were seen, when compared with available data regarding the lower extremity.10 These results might partially be rooted in a more prone position and open exposure of the upper extremity, compared with the lower limb. However, considering the number of hematoma formation, wound dehiscence, and infection, these rates generally were relatively low. In regard to the complexity of microsurgical reconstruction of the upper extremity, the analyzed complication rates reflect the overall safety of the procedures and are in accordance to the complication rates of other recipient sites, like for example breast reconstruction, although there is still room for improvement.24,25
CONCLUSIONS
Microsurgical tissue transfer to the upper extremity has evolved into a safe and reliable procedure, and fasciocutaneous flaps are the most frequently applied flap type in upper extremity reconstruction in the last decade. Fasciocutaneous and muscle-based free flap differ in rates of partial flap necrosis with impact in hospital stay and thus, require individual risk stratification; however, flap loss as well as flap survival after microsurgical revisions are not altered between these groups statistically. Although not directly shown by our results, muscle-based free flaps may benefit from tension free fixation, additional composites for pedicle coverage as an adjunct during dissection and ICG-NIR-Angiography to define the post-capillary last meadow to reduce the rate of partial necrosis. These flaps are still indispensable workhorses for large defects of the upper extremity. Further studies should focus on functional outcome with comparable and matched cohorts, while assessment for functionality of upper extremity reconstruction should be internationally standardized to enable comparison.
Supplementary Material
Footnotes
Published online 31 December 2019.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Baccarani A, Follmar KE, De Santis G, et al. Free vascularized tissue transfer to preserve upper extremity amputation levels. Plast Reconstr Surg. 2007;120:971–981. [DOI] [PubMed] [Google Scholar]
- 2.Giessler GA, Erdmann D, Germann G. Soft tissue coverage in devastating hand injuries. Hand Clin. 2003;19:63–71, vi. [DOI] [PubMed] [Google Scholar]
- 3.Hamdi M, Van Landuyt K, Monstrey S, et al. A clinical experience with perforator flaps in the coverage of extensive defects of the upper extremity. Plast Reconstr Surg. 2004;113:1175–1183. [DOI] [PubMed] [Google Scholar]
- 4.Hing DN, Buncke HJ, Alpert BS. Use of the temporoparietal free fascial flap in the upper extremity. Plast Reconstr Surg. 1988;81:534–544. [DOI] [PubMed] [Google Scholar]
- 5.Erdmann D, Sundin BM, Yasui K, et al. Microsurgical free flap transfer to amputation sites: indications and results. Ann Plast Surg. 2002;48:167–172. [DOI] [PubMed] [Google Scholar]
- 6.McCabe SJ, Breidenbach WC. The role of emergency free flaps for hand trauma. Hand Clin. 1999;15:275–288, viii. [PubMed] [Google Scholar]
- 7.Chen HC, Tang YB, Mardini S, et al. Reconstruction of the hand and upper limb with free flaps based on musculocutaneous perforators. Microsurgery. 2004;24:270–280. [DOI] [PubMed] [Google Scholar]
- 8.Hsu CC, Lin YT, Lin CH, et al. Immediate emergency free anterolateral thigh flap transfer for the mutilated upper extremity. Plast Reconstr Surg. 2009;123:1739–1747. [DOI] [PubMed] [Google Scholar]
- 9.Gupta A, Lakhiani C, Lim BH, et al. Free tissue transfer to the traumatized upper extremity: risk factors for postoperative complications in 282 cases. J Plast Reconstr Aesthet Surg. 2015;68:1184–1190. [DOI] [PubMed] [Google Scholar]
- 10.Xiong L, Gazyakan E, Kremer T, et al. Free flaps for reconstruction of soft tissue defects in lower extremity: a meta-analysis on microsurgical outcome and safety. Microsurgery. 2016;36:511–524. [DOI] [PubMed] [Google Scholar]
- 11.Bumbasirevic M, Stevanovic M, Lesic A, et al. Current management of the mangled upper extremity. Int Orthop. 2012;36:2189–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herter F, Ninkovic M, Ninkovic M. Rational flap selection and timing for coverage of complex upper extremity trauma. J Plast Reconstr Aesthet Surg. 2007;60:760–768. [DOI] [PubMed] [Google Scholar]
- 13.Yildirim S, Taylan G, Eker G, et al. Free flap choice for soft tissue reconstruction of the severely damaged upper extremity. J Reconstr Microsurg. 2006;22:599–609. [DOI] [PubMed] [Google Scholar]
- 14.Cerkes N, Erer M, Sirin F. The combined scapular/parascapular flap for the treatment of extensive electrical burns of the upper extremity. Br J Plast Surg. 1997;50:501–506. [DOI] [PubMed] [Google Scholar]
- 15.Chen HC, Buchman MT, Wei FC. Free flaps for soft tissue coverage in the hand and fingers. Hand Clin. 1999;15:541–554. [PubMed] [Google Scholar]
- 16.Cho EH, Shammas RL, Carney MJ, et al. Muscle versus fasciocutaneous free flaps in lower extremity traumatic reconstruction: a multicenter outcomes analysis. Plast Reconstr Surg. 2018;141:191–199. [DOI] [PubMed] [Google Scholar]
- 17.Salgado CJ, Mardini S, Jamali AA, et al. Muscle versus nonmuscle flaps in the reconstruction of chronic osteomyelitis defects. Plast Reconstr Surg. 2006;118:1401–1411. [DOI] [PubMed] [Google Scholar]
- 18.Guzman-Stein G, Fix RJ, Vasconez LO. Muscle flap coverage for the lower extremity. Clin Plast Surg. 1991;18:545–552. [PubMed] [Google Scholar]
- 19.Demirtas Y, Kelahmetoglu O, Cifci M, et al. Comparison of free anterolateral thigh flaps and free muscle-musculocutaneous flaps in soft tissue reconstruction of lower extremity. Microsurgery. 2010;30:24–31. [DOI] [PubMed] [Google Scholar]
- 20.Yazar S, Lin CH, Lin YT, et al. Outcome comparison between free muscle and free fasciocutaneous flaps for reconstruction of distal third and ankle traumatic open tibial fractures. Plast Reconstr Surg. 2006;117:2468–2475; discussion 2476. [DOI] [PubMed] [Google Scholar]
- 21.Hirche C, Kneser U, Xiong L, et al. Microvascular free flaps are a safe and suitable training procedure during structured plastic surgery residency: a comparative cohort study with 391 patients. J Plast Reconstr Aesthet Surg. 2016;69:715–721. [DOI] [PubMed] [Google Scholar]
- 22.Selber JC, Garvey PB, Clemens MW, et al. A prospective study of transit-time flow volume measurement for intraoperative evaluation and optimization of free flaps. Plast Reconstr Surg. 2013;131:270–281. [DOI] [PubMed] [Google Scholar]
- 23.Mathes SJ, Alpert BS, Chang N. Use of the muscle flap in chronic osteomyelitis: experimental and clinical correlation. Plast Reconstr Surg. 1982;69:815–829. [DOI] [PubMed] [Google Scholar]
- 24.Serletti JM, Higgins JP, Moran S, et al. Factors affecting outcome in free-tissue transfer in the elderly. Plast Reconstr Surg. 2000;106:66–70. [DOI] [PubMed] [Google Scholar]
- 25.Momoh AO, Ahmed R, Kelley BP, et al. A systematic review of complications of implant-based breast reconstruction with prereconstruction and postreconstruction radiotherapy. Ann Surg Oncol. 2014;21:118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]


