Abstract
Existing reports identify the involved roles of ZNF139 and its one circular RNA (circRNA), circZNF139, in the progression of various tumors. However, their relevance and function role in bladder cancer (BC) remain largely unexplored. Herein, we aimed to reconnoiter the role and potential mechanism of ZNF139 and circZNF139 in the progression of BC. Firstly, bioinformatics analyses indicated ZNF139 was upregulated in BC tissues and correlated with disease-free survival of BC patients. The subcellular localization and structural analyses of ZNF139 conveyed the possibility of ZNF139 functioning as a transcription factor. Secondly, circZNF139 was validated by bioinformatics analyses and RNase R tests. ZNF139 and circZNF139 were both significantly upregulated in BC cell lines. Functionally, ZNF139/circZNF139 had facilitated effects on the proliferative, clonal, migratory, and invasive potential of BC cells. Mechanistically, GO, KEGG pathway analyses and western blot assays altogether unveiled ZNF139/circZNF139 activated PI3K/AKT pathway in BC cells, supported by the alteration of AKT at phosphorylation level and PI3K at the protein level. Collectively, this work reveals ZNF139 and circZNF139 cooperate closely with each other to promote cell proliferation, migration and invasion via activation of PI3K/AKT pathway in BC.
Keywords: circular RNA, ZNF139, bladder cancer, PI3K/AKT signaling pathway, bioinformatics
INTRODUCTION
Ranked as the tenth most repeatedly diagnosed tumor in the global, bladder cancer (BC) is a heterogeneous group of tumors with various histological subgroups [1] and entails the highest cost for each patient among cancers of all stripes [2]. Most BCs are urothelial carcinomas, previously known as transitional cell carcinomas, which is a cancer sourced from the cells in the inner lining of the bladder. In term of the histopathological characteristics, urothelial carcinomas can be commonly subdivided into conventional urothelial carcinoma, urothelial carcinoma with diverging differentiation or variant urothelial carcinoma [3]. The gold standard for treatment is a radical cystectomy preceded, in combination with chemotherapy or bladder instillations of BCG or mitomycin when possible to prevent the distant spread [4]. Despite the advance in these invasive treatments, BCs still have recurrences with high rates and patients suffered have associated worse outcome. Currently, cystoscopy is the gold standard for BC diagnosis and surveillance. Unfortunately, its high frequency not only brings great pains to patients and lowers their life quality, but also causes high financial burden to individuals and nations [5]. Accordingly, it is still urgently needed to inspect the molecular pathogenesis of this disease and identify effective biomarkers.
Sourced from precursor mRNA, circular RNA (circRNA) was first identified in the early 1990s and represents a large number of non-coding RNAs [6]. After that, many circRNAs highly abundant and conserved among species have continuously been reported [7–10]. They are covalently closed with circular structure, have tissue-specific and cell-specific expression patterns, and are regulated by specific cis-acting elements and trans-acting factors [11]. Despite these valuable advances in understanding circRNAs, their functions remain largely unexplored. Numerous circRNAs are reported to exert vital biological effects by acting as microRNA or protein sponges, such as well-studied ciRS-7 and circHIPK3 [12, 13]. Additionally, other circRNAs can interact with RNA-binding proteins or be translated into proteins, as respectively exemplified by circMb1 [14] and circZNF609 [15]. Recent developments in the field of circRNAs have led to an increasing interest in characterizing their relevance in bountiful diseases. Specially, many studies focused on the role of circRNAs in tumor development and their biomarker potential reveal that circRNAs correlated with clinical pathological characteristics represent an attractive new class of biomarkers, such as for diagnosis, prognosis and prediction [16–19]. In spite of the emerging BC-associated circRNAs, such as circHiPK3 [20] and circBCRC-3 [21], other circRNAs’ relevance and function in BC have not been thoroughly addressed and remain largely unclear.
As one member of the zinc finger protein family, zinc finger with KRAB and SCAN domains 1 (ZNF139, also termed ZKSCAN1) plays regulatory roles in the transcriptional level of multifold genes and is related to the development and progression of tumors. Convincing results have appeared from studies of gastric cancer [22, 23] and hepatocellular carcinoma [24]. However, very little is currently known about the relevance and function of ZNF139 in BC. According to Jeck et al. [25] and Salzman et al. [26], a covalently linked 668-nt circRNA termed circZNF139 is generated by splicing exons 2 and 3 of ZNF139 together. This circRNA is particularly abundant in human brain and liver [27], and has been reported to be implicated in the development of hepatocellular carcinoma [28]. However, its function role in BC remains largely unknown.
In this work, we aimed to investigate the role and preliminary potential mechanism of ZNF139 and its circRNA (circZNF139) in regulating the progression of BC. First, the expression of ZNF139 in BC tissues, its correlation with the BC prognosis, as well as its localization and structure were assessed according to bioinformatics analyses. Second, co-expression genes related with ZNF139 in BC were evaluated by Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses. The circZNF139 was validated, and the effect of ZNF139/circZNF139 on the proliferative, clonal, migratory, and invasive potential of BC cells was investigated, respectively. Finally, the association of ZNF139/circZNF139 with the PI3K/AKT signaling pathway in BC was primarily explored.
RESULTS
Bioinformatics analyses of ZNF139
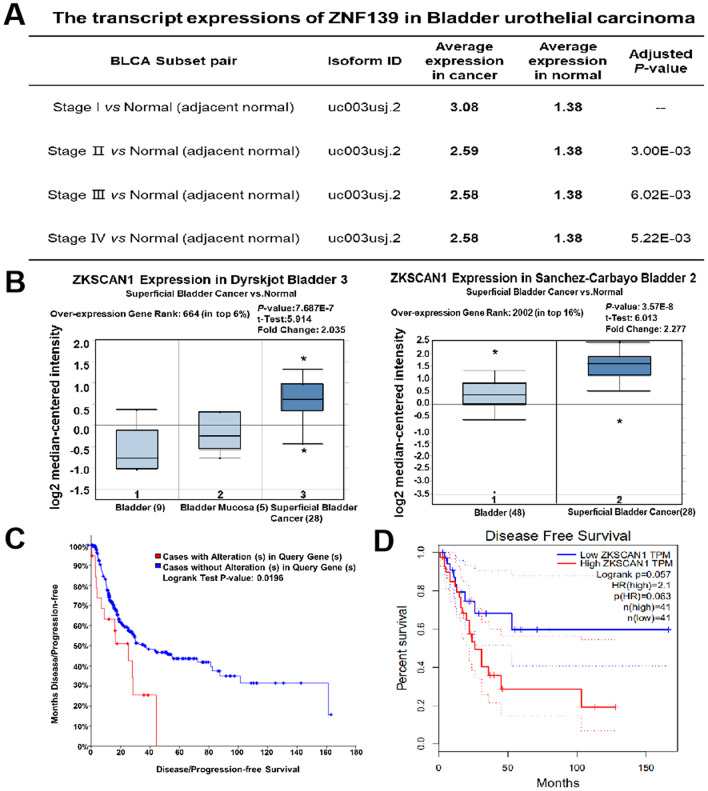
Initially, the transcription levels of ZNF139 in multifarious studies were evaluated from Cancer RNA-Seq Nexus (CRN, http://syslab4.nchu.edu.tw) and Oncomine (http://www.oncomine.org) databases. According to Data in CRN database, the transcript expressions of ZNF139 were significantly higher in bladder urothelial carcinoma at Stage I/II/III/IV than in adjacent normal (Figure 1A). As presented in Figure 1B, further analysis of multiple BC samples in Oncomine consistently showed higher expression of ZNF139 in superficial BC than normal. It thus followed that ZNF139 is upregulated in BC tissues. Next, the association of ZNF139 expression with the prognosis of BC patients was analyzed by cBioPortal database (http://cbioportal.org) and GEPIA database (http://gepia.cancer-pku.cn/). The results demonstrated that cases with alteration in query gene (namely ZNF139) had evidently worse disease-free survival than cases without alteration in ZNF139 (Figure 1C) and high expression of ZNF139 was associated with worse disease free survival (Figure 1D). Taken together, all results presented above indicated that ZNF139 is upregulated in BC tissues and correlated with the disease-free survival of BC patients according to bioinformatics analyses.
Figure 1.
Upregulation of ZNF139 in BC tissues and its correlation with the prognosis of BC patients. (A) The transcript expressions of ZNF139 were significantly higher in bladder urothelial carcinoma at varied stages (Stage I, II, III, IV) than in adjacent normal as analyzed in CRN database (http://syslab4.nchu.edu.tw). (B) Box plots showed expression of ZKSCAN1 (namely ZNF139) at mRNA level in Dyrskjot Bladder 3 (left panel) and Sanchez-Carbayo Bladder 2 (right panel), respectively. The shown indicators include over-expression gene rank, associated P values, statistical analysis and fold change according to Oncomine (http://www.oncomine.org). The association of ZNF139 expression with the disease-free survival was analyzed by (C) cBioPortal database (http://cbioportal.org) and (D) GEPIA database (http://gepia.cancer-pku.cn/). ZNF139, zinc finger with KRAB and SCAN domains 1; BC, bladder cancer; CRN, CancerRNA-Seq Nexus.
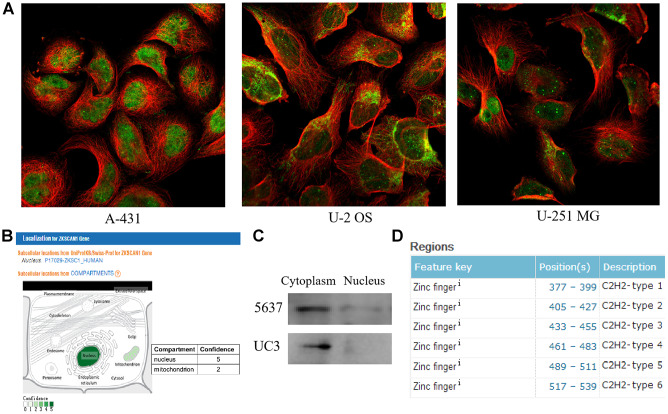
The Human Protein Atlas database (https://www.proteinatlas.org/) and GeneCards database (https://www.genecards.org/) were then employed to analyze the subcellular localization of ZNF139. As clearly shown in Figure 2A, ZNF139 was mainly localized to the nuclear bodies and its additional location was nucleoplasm and mitochondria in human epidermal cancer A-431 cells, human osteosarcoma U-2 OS cells, and human glioma U-251 MG cells. The result from GeneCards demonstrated two compartments, nucleus with confidence of 5 and mitochondrion with confidence of 2 (Figure 2B), indicating that ZNF139 was principally located in the nucleus. We then isolated nuclear and cytoplasmic proteins in BC cells, and detected the expression of ZNF139 in them by western blot assays. The results showed that in BC cells, ZNF139 was expressed in both the cytoplasm and the nucleus, and the expression in the cytoplasm was significantly higher than that in the nucleus (Figure 2C). As a member of the zinc finger protein family, ZNF139 protein contained C2H2- structure specific to transcription factors according to UniProt (https://www.uniprot.org/) as displayed in Figure 2D. Combined with the above-mentioned results, they pointed to the conjecture that ZNF139 may function as a transcription factor.
Figure 2.
The subcellular localization and structural analyses of ZNF139. (A) The subcellular localization for ZNF139 obtained from Human Protein Atlas database (https://www.proteinatlas.org/), green represents antibody and red represents microtubules. (B) The subcellular localization for ZNF139 obtained from GeneCards database (https://www.genecards.org/). (C) The subcellular localization for ZNF139 in BC cells as analyzed by western blot assays. (D) The structural analysis of ZNF139 based on UniProt database (https://www.uniprot.org/). BC, bladder cancer.
GO and KEGG pathway analyses of co-expression genes related with ZNF139
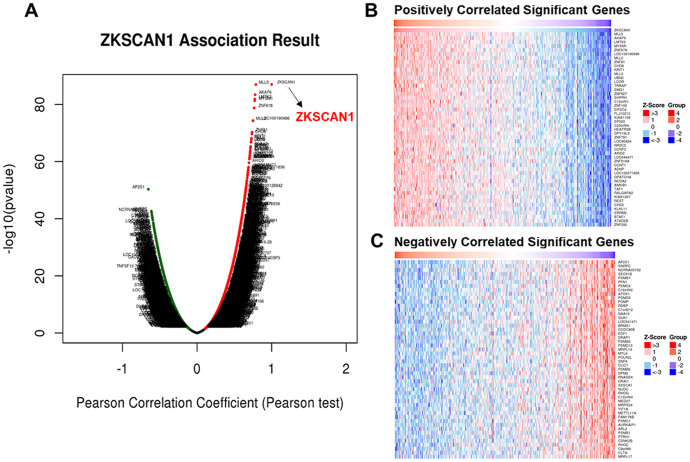
The mRNA sequencing data from 408 BC patients in the TCGA were analyzed by LinkFinder module of LinkedOmics (http://www.linkedomics.org/login.php) database. The volcano plot displayed 7,077 genes (dark red plots) were significantly positively correlated with ZNF139, while 5,942 genes (dark green plots) were significantly negatively correlated with ZNF139 (P-value<0.05), as clearly shown in Figure 3A. In addition, the top-50 significant genes positively and negatively correlated with ZNF139 were separately shown in the heat maps (Figure 3B, 3C), and also listed in Supplementary Tables 1 and 2.
Figure 3.
Genes differentially expressed in association with ZNF139 in BC according to LinkedOmics. (A) The correlation between ZKSCAN1 (i.e.ZNF139) and genes differentially expressed in BC (413 samples) was evaluated by Pearson test. (B) Heat maps showed the top-50 significant genes positively and (C) negatively correlated with ZKSCAN1 (i.e.ZNF139) in BC. The red stands for positively correlated genes and the blue stands for negatively correlated genes. ZNF139/ZKSCAN1, zinc finger with KRAB and SCAN domains 1; BC, bladder cancer.
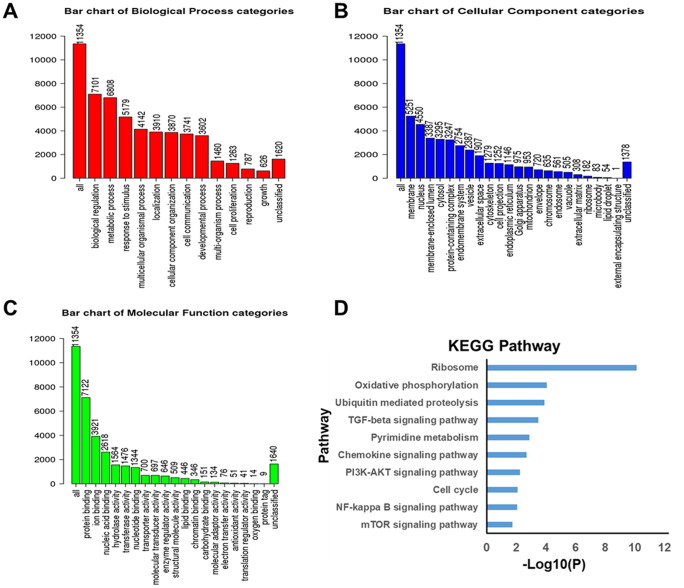
GO functional enrichment analysis is commonly involved in biological process, cellular component and molecular function. Here, GO functional enrichment of differentially expressed genes correlated with ZNF139 in BC was analyzed using Gene Set Enrichment Analysis (GSEA) with the help of the LinkFinder module of LinkedOmics. Results revealed that their biological processes were essentially enriched in biological regulation, metabolic process, response to stimulus and others (Figure 4A). With respect to cellular component, they were predominantly concentrated in membrane, nucleus, macromolecular complex and so forth (Figure 4B). As for molecular function, they were generally centralized on protein binding, ion binding, nucleic acid binding and the like (Figure 4C). Subsequently, KEGG analysis conducted as the same approach clearly shown in Figure 4D demonstrated that the significant pathways associated with these genes chiefly belonged to ribosome, oxidative phosphorylation and PI3K/AKT signaling pathway. Collectively, these findings suggested that ZNF139 may function as a transcription factor to affect related signaling pathways.
Figure 4.
Significantly enriched GO annotations and KEGG pathways of genes differentially expressed in correlation with ZNF139 in BC as analyzed using GSEA. (A) Biological processes. (B) Cellular component. (C) Molecular Function. (D) KEGG pathway analysis. GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes (KEGG); ZNF139, zinc finger with KRAB and SCAN domains 1; BC, bladder cancer; GSEA, Gene Set Enrichment Analysis.
The circZNF139, a circRNA form of ZNF139, was validated
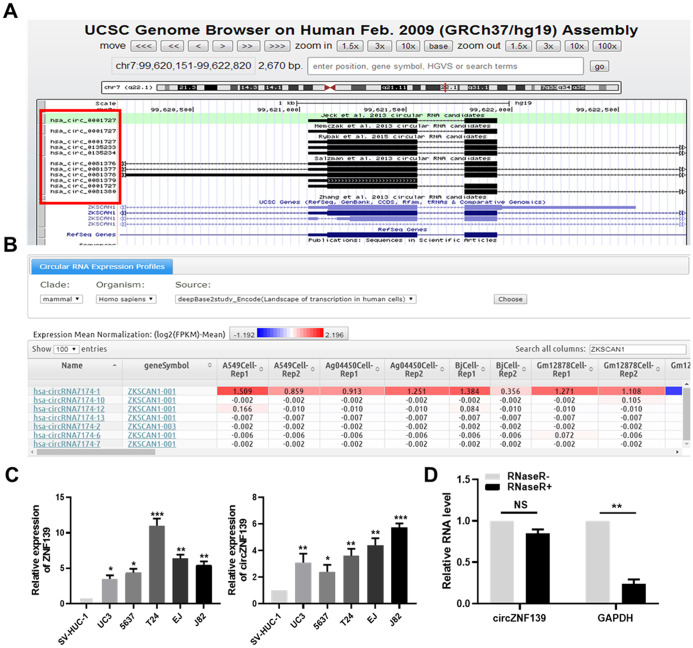
ZNF139 has protein-coding ability and may function as a transcription factor on the basis of aforementioned results. Next, we analyzed ZNF139 in the University of California, Santa Cruz (UCSC, http://genome.ucsc.edu/index.html) database and found some stable circRNAs formed from transcripts of ZNF139 gene (Figure 5A). Confirming to DeepBase (https://omictools.com/deepbase-tool) database, several circRNAs formed by ZNF139 were evidently differentially expressed in various tumor cells (Figure 5B). Additionally, the expression of circRNAs in various samples was summarized and presented in Supplementary Figure 1 in accordance with the search of ZNF139 in circBase (http://www.circbase.org/) database. Clearly, circZNF139 (circRNA ID: hsa_circ_0001727) with 668 nt spliced length had the widest expression spectrum, which was thus selected for the following work.
Figure 5.
The validation of circZNF139. (A) The circRNA-formed ability of ZNF139 evaluated by UCSC (http://genome.ucsc.edu/index.html) database. (B) Several circRNAs formed by ZNF139 were evidently differentially expressed in various tumor cells according to DeepBase (https://omictools.com/deepbase-tool) database. (C) ZNF139 (left panel) and circZNF139 (right panel) were both evidently upregulated in five BC cell lines (UC3, 5637, T24, EJ and J82) compared to one normal cell line SV-HUC-1 detected by qRT-PCR assay. (D) The RNA level of predicted circRNA and its control gene GAPDH were evaluated with or without RNase R treatment by qRT-PCR assay. The predicted circRNA, circZNF139, was resistant to RNase R treatment. *, P<0.05, **, P<0.01, ***, P<0.001.circ, circular; ZNF139, zinc finger with KRAB and SCAN domains 1; UCSC, University of California, Santa Cruz; BC, bladder cancer; qRT-PCR, quantitative real-time polymerase chain reaction; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
The expression of ZNF139 and its circRNA (circZNF139) was separately detected by qRT-PCR assay in five BC cell lines (UC3, 5637, T24, EJ and J82) and one normal cell line SV-HUC-1. What stands out in Figure 5C is that ZNF139 was significantly upregulated in five BC cell lines (UC3, 5637, T24, EJ and J82) in comparison with normal cell line SV-HUC-1 (P<0.05, P<0.01, P<0.001). So was the case of circZNF139 expressed in BC cells (Figure 5C, right panel, P<0.05, P<0.01, P<0.001). As a highly processive and hydrolytic 30 to 50 exoribonuclease that does not act on circRNA [29], the enzyme RNase R was used to further confirm the circular characteristics of circZNF139. As demonstrated in Figure 5D, level of the control gene GAPDH was obviously altered after RNase R+ treatment (P<0.01). As expected, level of circZNF139 showed no obvious change in RNase R+ group compared to RNase R- group, revealing circZNF139 was resistant to RNase R treatment. This also supported that apart from the ZNF139 mRNA, a natural circRNA form of the ZNF139 gene existed inside.
ZNF139/circZNF139 promotes the proliferative, clonal, migratory, and invasive potential of BC cells in vitro
With an aim to explore the effect of ZNF139 and circZNF139 on the biological behavior of BC cells, gain-of- and loss-of-function assays were performed in UC3 and 5637 cells. The 3.1-ZNF139 was constructed and transfected into cells for ZNF139 overexpression, while two si-ZNF139s (si1-ZNF139 and si2-ZNF139) were transfected into cells for ZNF139 knockdown. Similarly, 3.1-circZNF139 was for circZNF139 overexpression, whereas si1-circZNF139 and si2-circZNF139 were for its knockdown. After separate transfection with 3.1-ZNF139, si1-ZNF139, si2-ZNF139, 3.1-circZNF139, si1-circZNF139 or si2-circZNF139 into BC cells, their corresponding efficiencies were determined and confirmed by qRT-PCR assays (Supplementary Figure 2, P<0.01, P<0.001). The results of CCK-8 assays revealed the proliferative ability of cells in 3.1-ZNF139 or 3.1-circZNF139 groups was evidently enhanced, while that in si-ZNF139s or si-circZNF139s groups was notably attenuated (Figure 6A, 6B, P<0.05, P<0.01). Turning now to the experimental evidence on clonal assay, the colony formation of BC cells was promoted in UC3 and 5637 cells with ZNF139 or circZNF139 overexpression, while that was inhibited in those cells with ZNF139 or circZNF139 knockdown (Figure 6C, 6D). In addition, ZNF139 or circZNF139 overexpression obviously facilitated cell migration and invasion in UC3 and 5637 cells (Figure 6E–H). Conversely, ZNF139 or circZNF139 knockdown evidently suppressed cell migration and invasion in UC3 and 5637 cells (Figure 6E–H). These data collectively indicated that ZNF139/circZNF139 stimulated the proliferative, clonal, migratory, and invasive potential of BC cells.
Figure 6.
Cell proliferation, clone, migration, and invasion of UC3 and 5637 cells were evaluated after ZNF139/circZNF139 overexpression or knockdown. (A–B) CCK8 assay was employed to assess the proliferation of UC3 and 5637 cells with ZNF139/circZNF139 overexpression or knockdown. (C–D) Crystal violet staining was used to examine the colony formation of UC3 and 5637 cells with ZNF139/circZNF139 overexpression or knockdown. (E–F) Scratch wound healing assay was employed to evaluate the migration of UC3 and 5637 cells with ZNF139/circZNF139 overexpression or knockdown. Images of cell migration at 0 and 48 h transfection are shown at a magnification of 40×. (G–H) Transwell assay was used to analyze the invasion of UC3 and 5637 cells with ZNF139/circZNF139 overexpression or knockdown. Images are representative of the cells invading one field at a magnification of 100×. *, P<0.05; **, P<0.01. ZNF139, zinc finger with KRAB and SCAN domains 1; circ, circular; h, hour.
ZNF139/circZNF139 activates the PI3K/AKT signaling pathway in BC
Supportive by the aforementioned results, ZNF139 may function as a transcription factor to affect related signaling pathway, possibly such as PI3K/AKT signaling pathway. According to cBioPortal (http://cbioportal.org) database, it was apparent that the protein expression of AKT was higher in ZNF139-altered group than that in ZNF139-unaltered group, suggesting that the phosphorylation level of AKT was affected by ZNF139 expression (Figure 7A). We therefore hypothesized that ZNF139 and circZNF139 probably regulated PI3K/AKT signaling pathway in the progression of BC cells. Western blot assays were performed to determine the protein levels of AKT, PI3K and the phosphorylation level of AKT in UC3 and 5637 cells transfected with 3.1-ZNF139, si1-ZNF139, si2-ZNF139, 3.1-circZNF139, si1-circZNF139, si2-circZNF139 or matched controls (3.1/NC). Closer inspection of the results suggested that UC3 cells with 3.1-ZNF139 or 3.1-circZNF139 presented a promotion in the phosphorylation level of AKT and the protein level of PI3K, while cells with si-ZNF139s or si-circZNF139s exerted an opposite effect on their levels (Figure 7B). So was the case of 5637 cells (Figure 7C). By and large, these findings unearthed that ZNF139 and its circRNA (circZNF139) could activate PI3K/AKT signaling pathway in BC cells.
Figure 7.
ZNF139 and its circRNA (circZNF139) activates PI3K/AKT signaling pathway in BC cells. (A) The expression of AKT in ZNF139-altered and ZNF139-unaltered groups in three datasets as analyzed in cBioPortal (http://cbioportal.org) database. (B–C) The protein levels of AKT, p-AKT, and PI3K in UC3 and 5637 cells treated with 3.1-ZNF139, si1-ZNF139, si2-ZNF139, 3.1-circZNF139, si1-circZNF139, si2-circZNF139 or matched controls (3.1/NC) were determined by western blot assays, respectively. ZNF139/ZKSCAN1, zinc finger with KRAB and SCAN domains 1; circ, circular; si, small interfering; NC, negative control.
DISCUSSION
Reportedly, ZNF139 as a regulatory role in many genes at their transcriptional level is involved in the development and progression of various tumors. Notably, its roles are well studied in gastric cancer, including tumor metastasis, apoptosis, and multidrug resistance [22, 23, 30, 31]. However, no previous study has investigated the relevance and function of ZNF139 in BC. Through a series of bioinformatics analyses in the present study, we found that ZNF139 was upregulated in BC tissues, including bladder urothelial carcinoma and superficial BC. Meanwhile, its expression was obviously correlated with the disease-free survival of BC patients, but not with their overall survival. These findings possibly indicated that ZNF139 is involved in the occurrence and prognosis of BC.
As a member of the zinc finger protein family, ZNF139 is a transcription factor [32, 33]. Theoretically, it was located in the nucleus to a great extent. However, according to our bioinformatics analysis, ZNF139 was mainly located in the nucleus, and secondly common in mitochondrion. ZNF139 encodes a member of the Kruppel zinc-finger family of proteins with C2H2 type. According to UniProt, ZNF139 protein contained C2H2- structure specific to transcription factors. Later mRNA sequencing data sourced from TCGA outlined 7,077 positively genes and 5,942 negatively genes correlated with ZNF139. These supported or verified that ZNF139 could function as a transcription factor with a wide-ranging effect on the transcriptome.
Commonly, circRNAs consist of a large class of non-coding RNAs, generated by a non-canonical back splicing event [11]. As newly discovered molecules among the non-coding RNA network, circRNAs have been identified to be implicated in various cancers [11, 34, 35]. As previously reported, ZNF139 gene could generate two types of RNA, including ZNF139 mRNA and circZNF139, which were both involved in the progression of hepatocellular carcinoma [24]. In our work, the circZNF139, a circRNA form of ZNF139, was validated by UCSC, DeepBase and circBase databases. This suggested that circZNF139 (circRNA ID: hsa_circ_0001727) with 668 nt spliced length had the widest expression spectrum, also supportive by the reported uncovers [25, 26]. On the other, qRT-PCR analysis revealed that ZNF139 and its circRNA (circZNF139) were significantly upregulated in BC cell lines. The circular characteristics of circZNF139 were confirmed by tests of the enzyme RNase R., equal to the results presented in previous study [24].
Numerous studies have concentrated on the role of circRNAs in tumors, in which individual circRNAs exert either suppressive or promoted effects on the progression of tumors [36, 37]. In this study, gain-of and loss-of-function assays collectively unearthed that ZNF139/circZNF139 had facilitated effects on the proliferative, clonal, migratory, and invasive potential of BC cells. As for its underlying regulatory mechanism, we could obtain some clues from GO and KEGG pathway analyses of co-expressed genes correlated with ZNF139 in BC. They clearly revealed that one of the significant pathways associated with these genes was PI3K/AKT signaling pathway. In addition, the apparent result of ZNF139 affecting the phosphorylation level of AKT, obtained from cBioPortal database, further supported the hypothesis that ZNF139 and its circZNF139 probably regulated PI3K/AKT signaling pathway in the progression of BC cells. Later western blot assays with overexpression or knockdown of ZNF139/circZNF139 unveiled that they activated PI3K/AKT signaling pathway in BC cells.
Ultimately, this research contributed to the knowledge regarding the relevance and function of ZNF139/circZNF139 in BC. The main finding emerging from this study is the possible regulatory mechanism in which ZNF139/circZNF139 promotes cell proliferation, migration and invasion via the activation of PI3K/AKT signaling pathway in BC. These findings, despite preliminary, first disclosed the synergistic effect of ZNF139/circZNF139 on BC. Undoubtedly, the few limitations characterizing this study cannot be ignored. First, related verification was only conducted in cell lines, not including animal models or patients’ samples. Second, the underlying mechanism about how circZNF139 regulates PI3K/AKT signaling pathway in BC is unexplored. Third, the relevance and function of circZNF139 in other tumors is absent. Further studies, which take these limitations into account, will need to be undertaken.
CONCLUSIONS
To sum up, this work reveals one possible regulatory mechanism in which ZNF139 and circZNF139 cooperate closely with each other to promote cell proliferation, migration and invasion via the activation of PI3K/AKT signaling pathway in BC. This provides a theoretical basis for BC pathogenesis, and potential therapeutic markers for BC treatment.
MATERIALS AND METHODS
CRN and Oncomine analyses
CRN, freely available at http://syslab4.nchu.edu.tw/CRN, is known as the first public database which provides phenotype-specific coding-transcript/long non-coding RNA expression profiles and mRNA–long non-coding RNA co-expressed networks in cancer cells [38]. It consists of cancer RNA-Seq data sets in the TCGA, SRA and GEO databases. In this work, this open source was employed to evaluate the transcript expressions of ZNF139 in bladder urothelial carcinoma at varied stages (Stage I, II, III, IV) relative to adjacent normal. As the world’s largest oncogene chip database and integrated data mining platform, Oncomine (http://www.oncomine.org) contains 715 datasets and 86,733 pairs of cancer and normal tissues [39]. The Oncomine Platform—from web applications to translational bioinformatics services—has laid a foundation for ground-breaking discoveries with scalability, high quality, consistency and standardized analysis. ZNF139 expression was assessed in Dyrskjot Bladder 3 and Sanchez-Carbayo Bladder 2 tissues, and associated P-value < 0.01 was considered significant.
GeneCards and UniProt analyses
Featured in a searchable, integrative database, GeneCards provides comprehensive, user-friendly information concerning all annotated and predicted human genes, which is accessed via the website https://www.genecards.org/. It automatically integrates gene-centric data from more than 80 digital sources, encompassing the following information—genome, transcriptome, proteome, genome, clinic and so forth [40]. Here, GeneCards was used to explore the subcellular localization for ZNF139. Characterized by entirety, high quality and free access, UniProt provides the scientific community with resource of protein sequence and functional information, which can be accessed at http://www.uniprot.org/ [41]. This database was therefore employed for structural analysis of ZNF139.
LinkedOmics analysis
Available at http://www.linkedomics.org/login.php, the LinkedOmics is well acknowledged as a publicly available portal for analyzing multi-omics data from all 32 TCGA Cancer types [42]. This web platform contains three analytical modules- LinkFinder, LinkInterpreter and LinkCompare. Among them, on the one hand, the LinkFinder module of LinkedOmics was employed for analyzing genes differentially expressed in correlation with ZNF139 in the TCGA BLCA cohort (n=408). Pearson’s correlation coefficient was used for statistical analysis. All results were visualized as volcano plots and heat maps. On the other hand, the LinkInterpreter module of LinkedOmics performs pathway and network analyses of differentially expressed genes to derive biological insights from the association results. Data obtained from the LinkFinder results were signed and ranked according to the following criterion: Rank Criteria: p-value; Minimum Number of Genes:3; Simulations:1000. GSEA was applied for analyses of GO and KEGG pathways about the genes co-expressed with ZNF139.
cBioPortal analysis
The cBio Cancer Genomics Portal (http://cbioportal.org) offers an open-access resource with more than 5,000 tumor samples for interactively exploring genomics data sets of multidimensional cancer [43]. c-BioPortal was employed to analyze the association of ZNF139 expression with the overall survival and disease-free survival. Moreover, the expressions of AKT1, AKT2, AKT3 in ZNF139-altered and ZNF139-unaltered groups were analyzed in this database.
UCSC analysis
UCSC Genome Browser (http://genome.ucsc.edu/), a web-based and open source platform, efficiently provides the speedy, scalable display of sequence alignments and annotations landscaped against a good deal of genome assemblies under quality reference [44]. This database was utilized to evaluate the circRNA-formed ability of ZNF139.
DeepBase and circBase analyses
Freely accessible at https://omictools.com/deepbase-tool, deepBase is a platform designed for annotating and discovering small RNAs, long non-coding RNAs as well as circRNAs according to sequencing data of next generation [45]. Here, the expression level of several circRNAs formed by ZNF139 in various tumor cells was predicted in this database. Moreover, the expression level of several circRNAs formed by ZNF139 in various tumor samples was further evaluated in circBase database, which provides scripts to identify known and novel circRNAs in sequencing data with free access at http://www.circbase.org/ [46].
Cell culture and transfection
The human normal SV-HUC-1 cell line and five BC cell lines (UC3, 5637, T24, EJ and J82) were purchased from Cell Bank of Type Culture Collection of Chinese Academy of Sciences (Shanghai, China). They were maintained in Dulbecco’s modified Eagle’s medium (Thermo Fisher Scientific, Waltham, MA, USA) with 10% (v/v) fetal bovine serum (Invitrogen, Carlsbad, CA, USA) in a humidified atmosphere of 5% (v/v) CO2 at 37°C.
The pcDNA 3.1, 3.1-ZNF139 and 3.1-circZNF139 were separately constructed by Sangon Biotech (Shanghai, China). Small interfering (si) RNAs against ZNF139 (si1-ZNF139 and si2-ZNF139), circZNF139 (si1-circZNF139 and si2-circZNF139) and their corresponding NCs were also synthesized by SangonBiotech (Shanghai, China). The sequences used in this work were listed as follows: si1-ZNF139: 5’-GCACUAUUCACAGCGGAUU-3’ (sense); si2-ZNF139: 5’-GCCACAGUUCCAAUCUCAU-3’ (sense); si1-circZNF139: 5’-AGUGCUCCUUAUCAUUUCU-3’ (sense); si2-circZNF139: 5’-GUCAGUGCUCCUUAUCAUU-3’ (sense). The UC3 and 5637 cell lines were plated in 6-well platesfor18 h, followed by being transfected with indicated siRNAs or plasmids using Lipofectamine 2000 (Invitrogen) in line with the manufacturer’s instructions. Approximately 48 h post transfection, cells were collected for the following assays.
RNA isolation and qRT-PCR assay
In accordance with the manufacturer’s instructions, total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA) and then reversely transcribed to a single-stranded cDNA using Reverse Transcription SystemKit (Takara, Dalian, China). qRT-PCR assays were performed by using SYBR Premix Ex Taq (Takara Biotech, Japan) on an ABI 7900 system (Applied Biosystems, Foster City, CA, USA). Relative gene expression was calculated using the 2-ΔΔCt method. The housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the internal control. The PCR primers were listed as follows: ZNF139, forward 5’-TGTAATGAGTGCGGGAAGG-3’ and reverse 5’-AATCAGGTATGAGTTTCGGTTG-3’; circZNF139, forward 5’-AGTCCCACTTCAAACATTCGTCT-3’ and reverse 5’-CACCTTCACTATTACGATACCATCC-3’; GAPDH, forward 5’-ACGGATTTGGTCGTATTGGGCG-3’ and reverse 5’-GCTCCTGGAAGATGGTGATGGG-3’.
RNase R treatment
The RNase R treatment was performed in reference to the previous report [24]. In brief, 5 μg of total RNA was incubated for 15 min at 37°C in the presence or absence of RNase R (Epicentre Biotechnologies, Madison, WI, USA) at a concentration of 3 U·μg-1. They were separately labeled as RNase R+ group and RNase R- group. After purified by phenol–chloroform extraction, the RNA was treated for qRT-PCR assay.
Cell proliferation, colony formation, migration, and invasion assays
Cell Counting Kit-8 (CCK-8) assays were performed to evaluate the proliferative ability of UC3 and 5637 cells. Cells were cultured in 96-well plates and incubated for 24, 48 and 72 hours (h). The CCK-8 solution (Beyotime Institute of Biotechnology, Shanghai, China) was added to measure the optical density. The absorbance values at each point were measured at 450 nm. At least triplicate samples were evaluated for each treatment.
For colony formation assays, UC3 and 5637 cells (5 × 102 cells per well) were seeded in a 6-well plate and cultured for 14 days after treatment. Colonies were fixed with 10% formaldehyde for 5 min, and stained with 1% crystal violet for 20 min and counted.
The scratch wound-healing assays were conducted to evaluate the migratory ability of UC3 and 5637 cells. The cells were plated on 6-well plates and scraped by a pipette tip to produce uniform wounds prior to transfection. The exfoliated cells were removed by washing each well thrice with PBS. The initial distance (0 h) and the distances after 48 h of scratching were microscopically detected. The images were presented at a magnification of 40× for each group. At least three biological replicates were conducted.
For the invasion assay, the transwell system and Matrigel BD Biosciences (New York, NY, USA) were employed in reference to the manufacturers’ protocols. The upper chamber was treated by Matrigel at 37°C for 2 h and subsequently, transfected cells suspended in serum-free medium were added in. After incubation for 48 h, the cells remaining in the upper chamber were removed by cotton tip carefully, and the cells at the bottom of the insert were fixed, stained with 1% crystal violet, and counted under a microscope. The images of stained cells (invaded cells) in random fields were shown at a magnification of 100× for each group. At least three biological replicates were conducted.
Western blot assay
Western blot assay was performed using the standard procedure as reported [47]. Briefly, cells were lysed by cell lysis buffer (Beyotime Biotechnology Shanghai, China). Afterwards, the collected protein samples were separated by SDS-PAGE. After blocking, the membrane was incubated with primary antibodies—AKT (1:1000, Catalog: A10605, ABclonal Technology); p-AKT-s473 (1:1000, Catalog: AP0140, ABclonal Technology); PI3K (1:1000, Catalog: A11177, ABclonal Technology); GAPDH (1:1000, Catalog: AC002, ABclonal Technology). The incubation was next followed with the horseradish peroxidase-conjugated goat anti-mouse (1:10000, Catalog: AS003, ABclonal Technology) or anti-rabbit (1:10000, Catalog: AS014, ABclonal Technology). GAPDH was used as internal control.
Statistics
Data in this study are presented as the mean ± SD. A Student’s t-test was used to analyze the statistical significance among groups using GraphPad Prism 8.0 (La Jolla, CA, USA). Value of P< 0.05 was considered to be statistically significant. All experiments were independently conducted at least three times.
Supplementary Material
Footnotes
AUTHOR CONTRIBUTIONS: S. L. conceived and designed the study, J. Y., D. Y., K. Q., C. C., and X. L. performed the analysis procedures, X. Y., K. Q., C. C., and T. L. analyzed the results, X. L., S. L., and T. L. contributed analysis tools, J. Y., and S. L. contributed to the writing of the manuscript. All authors reviewed the manuscript.
CONFLICTS OF INTEREST: The authors declare that there are no conflicts of interest.
FUNDING: This work was supported by the National Natural Science Foundation of China (81802541).
REFERENCES
- 1.Alifrangis C, McGovern U, Freeman A, Powles T, Linch M. Molecular and histopathology directed therapy for advanced bladder cancer. Nat Rev Urol. 2019; 16:465–83. 10.1038/s41585-019-0208-0 [DOI] [PubMed] [Google Scholar]
- 2.Leow JJ, Cole AP, Seisen T, Bellmunt J, Mossanen M, Menon M, Preston MA, Choueiri TK, Kibel AS, Chung BI, Sun M, Chang SL, Trinh QD. Variations in the costs of radical cystectomy for bladder cancer in the USA. Eur Urol. 2018; 73:374–82. 10.1016/j.eururo.2017.07.016 [DOI] [PubMed] [Google Scholar]
- 3.Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE. The 2016 WHO classification of tumours of the urinary system and male genital organs-part B: prostate and bladder tumours. Eur Urol. 2016; 70:106–19. 10.1016/j.eururo.2016.02.028 [DOI] [PubMed] [Google Scholar]
- 4.Dueñas M, Pérez-Figueroa A, Oliveira C, Suárez-Cabrera C, Sousa A, Oliveira P, Villacampa F, Paramio JM, Martínez-Fernández M. Gene expression analyses in non muscle invasive bladder cancer reveals a role for alternative splicing and Tp53 status. Sci Rep. 2019; 9:10362. 10.1038/s41598-019-46652-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turo R, Cross W, Whelan P. Bladder cancer. Medicine. 2012; 40:14–19. 10.1016/j.mpmed.2011.09.008 [DOI] [Google Scholar]
- 6.Cocquerelle C, Mascrez B, Hétuin D, Bailleul B. Mis-splicing yields circular RNA molecules. FASEB J. 1993; 7:155–60. 10.1096/fasebj.7.1.7678559 [DOI] [PubMed] [Google Scholar]
- 7.Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer M, Loewer A, Ziebold U, Landthaler M, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013; 495:333–38. 10.1038/nature11928 [DOI] [PubMed] [Google Scholar]
- 8.Wang PL, Bao Y, Yee MC, Barrett SP, Hogan GJ, Olsen MN, Dinneny JR, Brown PO, Salzman J. Circular RNA is expressed across the eukaryotic tree of life. PLoS One. 2014; 9:e90859. 10.1371/journal.pone.0090859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ivanov A, Memczak S, Wyler E, Torti F, Porath HT, Orejuela MR, Piechotta M, Levanon EY, Landthaler M, Dieterich C, Rajewsky N. Analysis of intron sequences reveals hallmarks of circular RNA biogenesis in animals. Cell Rep. 2015; 10:170–77. 10.1016/j.celrep.2014.12.019 [DOI] [PubMed] [Google Scholar]
- 10.Xia S, Feng J, Lei L, Hu J, Xia L, Wang J, Xiang Y, Liu L, Zhong S, Han L, He C. Comprehensive characterization of tissue-specific circular RNAs in the human and mouse genomes. Brief Bioinform. 2017; 18:984–92. 10.1093/bib/bbw081 [DOI] [PubMed] [Google Scholar]
- 11.Kristensen LS, Andersen MS, Stagsted LV, Ebbesen KK, Hansen TB, Kjems J. The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet. 2019; 20:675–91. 10.1038/s41576-019-0158-7 [DOI] [PubMed] [Google Scholar]
- 12.Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature. 2013; 495:384–88. 10.1038/nature11993 [DOI] [PubMed] [Google Scholar]
- 13.Zheng Q, Bao C, Guo W, Li S, Chen J, Chen B, Luo Y, Lyu D, Li Y, Shi G, Liang L, Gu J, He X, Huang S. Circular RNA profiling reveals an abundant circHIPK3 that regulates cell growth by sponging multiple miRNAs. Nat Commun. 2016; 7:11215. 10.1038/ncomms11215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ashwal-Fluss R, Meyer M, Pamudurti NR, Ivanov A, Bartok O, Hanan M, Evantal N, Memczak S, Rajewsky N, Kadener S. circRNA biogenesis competes with pre-mRNA splicing. Mol Cell. 2014; 56:55–66. 10.1016/j.molcel.2014.08.019 [DOI] [PubMed] [Google Scholar]
- 15.Pamudurti NR, Bartok O, Jens M, Ashwal-Fluss R, Stottmeister C, Ruhe L, Hanan M, Wyler E, Perez-Hernandez D, Ramberger E, Shenzis S, Samson M, Dittmar G, et al. Translation of CircRNAs. Mol Cell. 2017; 66:9–21.e7. 10.1016/j.molcel.2017.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kristensen LS, Hansen TB, Venø MT, Kjems J. Circular RNAs in cancer: opportunities and challenges in the field. Oncogene. 2018; 37:555–65. 10.1038/onc.2017.361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang F, Nazarali AJ, Ji S. Circular RNAs as potential biomarkers for cancer diagnosis and therapy. Am J Cancer Res. 2016; 6:1167–76. [PMC free article] [PubMed] [Google Scholar]
- 18.Smid M, Wilting SM, Uhr K, Rodríguez-González FG, de Weerd V, Prager-Van der Smissen WJ, van der Vlugt-Daane M, van Galen A, Nik-Zainal S, Butler A, Martin S, Davies HR, Staaf J, et al. The circular RNome of primary breast cancer. Genome Res. 2019; 29:356–66. 10.1101/gr.238121.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang P, Qiu Z, Jiang Y, Dong L, Yang W, Gu C, Li G, Zhu Y. Silencing of cZNF292 circular RNA suppresses human glioma tube formation via the Wnt/β-catenin signaling pathway. Oncotarget. 2016; 7:63449–55. 10.18632/oncotarget.11523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Zheng F, Xiao X, Xie F, Tao D, Huang C, Liu D, Wang M, Wang L, Zeng F, Jiang G. CircHIPK3 sponges miR-558 to suppress heparanase expression in bladder cancer cells. EMBO Rep. 2017; 18:1646–59. 10.15252/embr.201643581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie F, Li Y, Wang M, Huang C, Tao D, Zheng F, Zhang H, Zeng F, Xiao X, Jiang G. Circular RNA BCRC-3 suppresses bladder cancer proliferation through miR-182-5p/p27 axis. Mol Cancer. 2018; 17:144. 10.1186/s12943-018-0892-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Dekken H, Tilanus HW, Hop WC, Dinjens WN, Wink JC, Vissers KJ, van Marion R. Array comparative genomic hybridization, expression array, and protein analysis of critical regions on chromosome arms 1q, 7q, and 8p in adenocarcinomas of the gastroesophageal junction. Cancer Genet Cytogenet. 2009; 189:37–42. 10.1016/j.cancergencyto.2008.08.018 [DOI] [PubMed] [Google Scholar]
- 23.Tan B, Li Y, Zhao Q, Fan L, Wang D. ZNF139 increases multidrug resistance in gastric cancer cells by inhibiting miR-185. Biosci Rep. 2018; 38:BSR20181023. 10.1042/BSR20181023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yao Z, Luo J, Hu K, Lin J, Huang H, Wang Q, Zhang P, Xiong Z, He C, Huang Z, Liu B, Yang Y. ZKSCAN1 gene and its related circular RNA (circZKSCAN1) both inhibit hepatocellular carcinoma cell growth, migration, and invasion but through different signaling pathways. Mol Oncol. 2017; 11:422–437. 10.1002/1878-0261.12045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeck WR, Sorrentino JA, Wang K, Slevin MK, Burd CE, Liu J, Marzluff WF, Sharpless NE. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA. 2013; 19:141–57. 10.1261/rna.035667.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salzman J, Chen RE, Olsen MN, Wang PL, Brown PO. Cell-type specific features of circular RNA expression. PLoS Genet. 2013; 9:e1003777. 10.1371/journal.pgen.1003777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang D, Wilusz JE. Short intronic repeat sequences facilitate circular RNA production. Genes Dev. 2014; 28:2233–47. 10.1101/gad.251926.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Q, Cai Y, Xiong H, Deng Y, Dai X. CCRDB: a cancer circRNAs-related database and its application in hepatocellular carcinoma-related circRNAs. Database (Oxford). 2019; 2019:baz063. 10.1093/database/baz063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vincent HA, Deutscher MP. Insights into how RNase R degrades structured RNA: analysis of the nuclease domain. J Mol Biol. 2009; 387:570–83. 10.1016/j.jmb.2009.01.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fan L, Tan B, Li Y, Zhao Q, Liu Y, Wang D, Zhang Z. Silencing of ZNF139-siRNA induces apoptosis in human gastric cancer cell line BGC823. Int J Clin Exp Pathol. 2015; 8:12428–36. [PMC free article] [PubMed] [Google Scholar]
- 31.Nie H, Mu J, Wang J, Li Y. miR-195-5p regulates multi-drug resistance of gastric cancer cells via targeting ZNF139. Oncol Rep. 2018; 40:1370–78. 10.3892/or.2018.6524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y, Tan BB, Zhao Q, Fan LQ, Wang D, Liu Y. ZNF139 promotes tumor metastasis by increasing migration and invasion in human gastric cancer cells. Neoplasma. 2014; 61:291–98. 10.4149/neo_2014_037 [DOI] [PubMed] [Google Scholar]
- 33.Li Y, Zhao Q, Fan LQ, Wang LL, Tan BB, Leng YL, Liu Y, Wang D. Zinc finger protein 139 expression in gastric cancer and its clinical significance. World J Gastroenterol. 2014; 20:18346–53. 10.3748/wjg.v20.i48.18346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nie JH, Li TX, Zhang XQ, Liu J. Roles of non-coding RNAs in normal human brain development, brain tumor, and neuropsychiatric disorders. Noncoding RNA. 2019; 5:36. 10.3390/ncrna5020036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okholm TL, Nielsen MM, Hamilton MP, Christensen LL, Vang S, Hedegaard J, Hansen TB, Kjems J, Dyrskjøt L, Pedersen JS. Circular RNA expression is abundant and correlated to aggressiveness in early-stage bladder cancer. NPJ Genom Med. 2017; 2:36. 10.1038/s41525-017-0038-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeng K, Chen X, Xu M, Liu X, Hu X, Xu T, Sun H, Pan Y, He B, Wang S. CircHIPK3 promotes colorectal cancer growth and metastasis by sponging miR-7. Cell Death Dis. 2018; 9:417. 10.1038/s41419-018-0454-8 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Barbagallo D, Caponnetto A, Cirnigliaro M, Brex D, Barbagallo C, D'Angeli F, Morrone A, Caltabiano R, Barbagallo GM, Ragusa M, Di Pietro C, Hansen TB, Purrello M. CircSMARCA5 inhibits migration of glioblastoma multiforme cells by regulating a molecular axis involving splicing factors SRSF1/SRSF3/PTB. Int J Mol Sci. 2018; 19:480. 10.3390/ijms19020480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li JR, Sun CH, Li W, Chao RF, Huang CC, Zhou XJ, Liu CC. Cancer RNA-seq nexus: A database of phenotype-specific transcriptome profiling in cancer cells. Nucleic Acids Res. 2016; 44:D944–51. 10.1093/nar/gkv1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rhodes DR, Kalyana-Sundaram S, Mahavisno V, Varambally R, Yu J, Briggs BB, Barrette TR, Anstet MJ, Kincead-Beal C, Kulkarni P, Varambally S, Ghosh D, Chinnaiyan AM. Oncomine 3.0: genes, pathways, and networks in a collection of 18,000 cancer gene expression profiles. Neoplasia. 2007; 9:166–80. 10.1593/neo.07112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Safran M, Dalah I, Alexander J, Rosen N, Iny Stein T, Shmoish M, Nativ N, Bahir I, Doniger T, Krug H, Sirota-Madi A, Olender T, Golan Y, et al. GeneCards version 3: the human gene integrator. Database (Oxford). 2010; 2010:baq020. 10.1093/database/baq020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.The UniProt Consortium. UniProt: the universal protein knowledgebase. Nucleic Acids Res. 2017; 45:D158–69. 10.1093/nar/gkw1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vasaikar SV, Straub P, Wang J, Zhang B. LinkedOmics: analyzing multi-omics data within and across 32 cancer types. Nucleic Acids Res. 2018; 46:D956–63. 10.1093/nar/gkx1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, Antipin Y, Reva B, Goldberg AP, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012; 2:401–04. 10.1158/2159-8290.CD-12-0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tyner C, Barber GP, Casper J, Clawson H, Diekhans M, Eisenhart C, Fischer CM, Gibson D, Gonzalez JN, Guruvadoo L, Haeussler M, Heitner S, Hinrichs AS, et al. The UCSC genome browser database: 2017 update. Nucleic Acids Res. 2017; 45:D626–34. 10.1093/nar/gkw1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang JH, Shao P, Zhou H, Chen YQ, Qu LH. deepBase: A database for deeply annotating and mining deep sequencing data. Nucleic Acids Res. 2010; 38:D123–30. 10.1093/nar/gkp943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Glažar P, Papavasileiou P, Rajewsky N. circBase: A database for circular RNAs. RNA. 2014; 20:1666–70. 10.1261/rna.043687.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang P, Ma X, Song E, Chen W, Pang H, Ni D, Gao Y, Fan Y, Ding Q, Zhang Y, Zhang X. Tubulin cofactor A functions as a novel positive regulator of ccRCC progression, invasion and metastasis. Int J Cancer. 2013; 133:2801–11. 10.1002/ijc.28306 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.